Abstract
Purpose
We aimed to evaluate the adoption of hypofractionated whole breast irradiation (HF-WBI) over time and factors related to its adoption for patients undergoing lumpectomy. We also examined whether HF-WBI can increase the overall use of radiotherapy.
Methods
Using data from the National Cancer Database between 2004 and 2013, we identified 528,051 invasive and 190,431 ductal carcinoma in situ (DCIS) patients who underwent lumpectomy. HF-WBI was defined as 2.5-3.33 Gy/fraction to the breast, whereas conventional therapy (CF-WBI) was defined as 1.8-2.0 Gy/fraction.
Results
The usage of HF-WBI among invasive cancer patients increased from 0.7% in 2004 to 15.6% in 2013 and among DCIS patients, HF-WBI increased from 0.4% in 2004 to 13.4% in 2013. However, these changes only leaded to a slight increase in the overall use of radiotherapy. Interestingly, for DCIS patients who lived ≥50 miles from hospitals, the uptake of HF-WBI translated to a moderate increase in the overall use of radiotherapy (58% in 2004 to 63% in 2013). Multivariable logistic regression showed that older age, node negative or smaller tumor, living in mountain states, rural area or ≥50 miles from hospitals, and treated in large or academic cancer centers were associated with elevated HF-WBI use. The median duration of finishing radiotherapy for HF-WBI was 26 days, compared to 47 days for CF-WBI.
Conclusions
Although HF-WBI can save 3 weeks of patient time, its adoption remained low in the US. There was only a slight increase in the overall use of radiotherapy among patients undergoing lumpectomy.
Keywords: hypofractionated radiation therapy, breast cancer, regimens, trend
INTRODUCTION
Conventional fractionated whole-breast irradiation (CF-WBI) therapy, delivered over 5–7 weeks, has been the standard early stage breast cancer treatment after breast-conserving surgery (BCS), as established by multiple randomized trials comparing adjuvant WBI to non-adjuvant treatment [1]. A modified version of the treatment, hypofractionated whole breast irradiation (HF-WBI), is usually delivered in 16 fractions over about three weeks, and has been shown to be as effective as the conventional treatment. Four randomized trials have concluded that HF-WBI has local recurrence rates and disease-free survival rates that are comparable to CF-WBI [2–6]. Additionally, similar rates of toxicity have been reported between the two fractionation schemes [2, 5, 6]. These trials evaluated several variation of HF-WBI dose schedule, including 42.5 Gy in 16 fractions over 3 weeks [5], 39 Gy, 42.9 Gy or 41.6 Gy in 13 fractions over 5 weeks [2, 4], and 40 Gy in 15 fractions over 3 weeks [3]. The initial results of the four randomized trials were published between 2002 and 2008. ASTRO published guidelines in 2011, supporting the use of HF-WBI for patients who are older than 50 years, have pT1-2N0 tumor, and are not treated with chemotherapy [7].
Several studies have showed that the use of HF-WBI therapy has increased in the United States [8–10], but detailed information are lacking as to which HF-WBI doses were adopted in practice, how long HF-WBI can be finished in practice, and whether boost radiation was taken after HF-WBI. In addition, previous studies have examined trend of HF-WBI uptake 2011 or earlier in general population or Medicare population [8, 10], but it is unclear if the uptake of HF-WBI has changed after the publication of the ASTRO guidelines. NCCN guidelines support post-BCS irradiation except for women ≥70 years of age with lymph node-negative, estrogen receptor-positive, and T1 breast cancer (https://www.nccn.org), and the percentage of post-BCS irradiation is one of quality care measures endorsed by National Quality Forum (http://www.qualityforum.org). Therefore, it is important to know if the update of HF-WBI can lead to higher overall use of radiotherapy among patients undergoing BCS or just a redistribution of CF-WBI.
We analyzed trends in HF-WBI use and implementation in the US using the National Cancer Database (NCDB), a joint project between the American College of Surgeons and the American Cancer Society that collects hospital registry data covering approximately 70% of new breast cancer cases in the US. The NCDB has extensive information on radiation dosages, treatment schedules, fractions, radiation fields, boost fields, and therefore is an ideal dataset to examine HF-WBI radiation. With this database we are able to evaluate trends in HF-WBI use and factors related to its use compared to CF-WBI. We are also able to document the variation in the dose, fraction, boost regimen, and duration of HF-WBI among different facility types and locations across the country. The findings from this study will help clinicians understand which patients are more likely to receive HF-WBI, the fractionation schemes most commonly used (with and without boost), and the factors related to lower frequencies of HF-WBI implementation.
The aims of this study were three-fold. First, we analyzed the trend in HF-WBI implementation in the US between 2004 and 2013, and examined whether an increase in usage of the HF-WBI modality resulted in an increase in overall radiotherapy usage among breast cancer patients that had received breast-conserving surgery. Second, we investigated factors related to the adoption of HF-WBI by comparing patients receiving HF-WBI and those receiving CF-WBI. Finally, we documented the variation in the dose, fraction, boost regimen, and duration of HF-WBI in the practices in the US.
MATERIALS/METHODS
Study Samples
For this study, data from the NCDB from 2004 to 2013 were used. The study included women who had first cancer diagnosis of breast cancer, had all or part of their first course treatment performed at the reporting facility, and received breast conserving surgery. Patients with ductal carcinoma in situ (DCIS) and patients with invasive breast cancer who had AJCC pT1-2N0-1 disease were included. Patients who underwent neoadjuvant therapy, had pT3-4N2-3 disease and underwent mastectomy were excluded.
Outcome Measures
In the NCDB, total fractions of radiation, dose to the breast, boost dose, and total days undergoing radiotherapy were recorded. For each patient, we separated the total fractions of radiation therapy into fractions to the breast and fractions in boost radiotherapy, by evaluating the doses to breast and boost, and total days of radiotherapy. Then, we calculated dose per fraction to the breast and dose per fraction in the boost radiotherapy. HF-WBI was defined as 2.5-3.33 Gy per fraction to the breast, whereas CF-WBI was defined as 1.8-2.0 Gy per fraction.
Statistical Analysis
We conducted the analysis in three steps. First, we examined the trend of all radiation modalities (including HF-WBI) use from 2004 to 2013 among all patients receiving lumpectomy. In this analysis, women ≥70 years of age with clinically lymph node-negative, estrogen receptor-positive, and T1 breast cancer were excluded because studies have showed that this group of patients is not always necessary to receive whole breast irradiation [11, 12] (https://www.nccn.org). In order to examine whether an increase in implementation of the HF-WBI modality resulted in an increase in overall radiotherapy usage, we analyzed the overall trend of all radiotherapy implementation. The analysis was separated for patients with invasive cancer, DCIS, and patients who were age ≥50 years, pT1-2N0 disease, and did not receive chemotherapy. The last subgroup represents patients who had been recommended for HF-WBI by ASTRO in 2011 [7]. We also conducted a trend analysis for patients who lived 50 miles or more away from reporting facilities to gauge if adoption of HF-WBI resulted in an increase in overall radiotherapy usage because inconvenience may be an important reason for low radiotherapy usage in these patients.
Next, we compared patients undergoing HF-WBI with patients undergoing CF-WBI using logistic regression, in order to identify factors related to HF-WBI use. Patient demographic, clinical, and pathologic characteristics were examined, as well as facility-related factors. The analysis for invasive cancer and DCIS was stratified separately. From these multivariable logistic regressions, adjusted odds ratios (OR) and 95% confidence intervals (CI) were calculated. Finally, to find the most common HF-WBI radiotherapy regimens in practice, we tabulated all the combination of radiation dose/fraction to the breast, and dose/fraction during boost. All statistical analyses were conducted using Stata 14 (Stata Corporation, College Station, TX).
RESULTS
Trend of HF-WBI and Other Radiotherapy Modalities
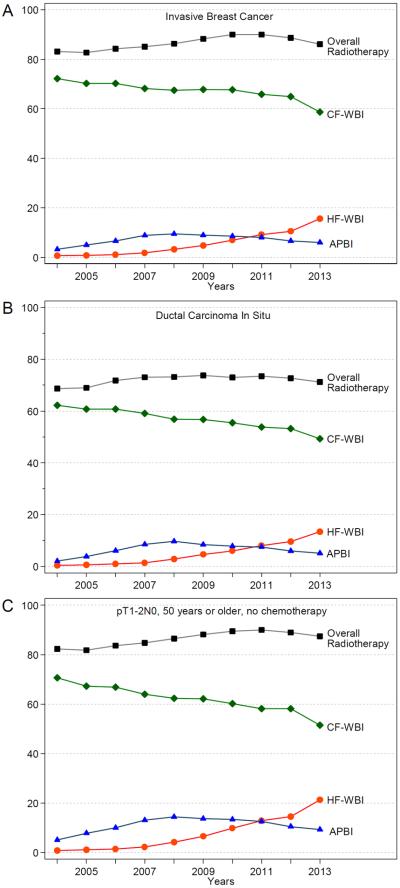
A total of 718,482 post-lumpectomy breast cancer patients (528,051 invasive breast cancer and 190,431 DCIS patients) from 2004 to 2013 were included in this analysis. Figure 1 shows the trend of different types of radiotherapy over time among breast cancer patients who received lumpectomy. The usage of HF-WBI post-BCS among invasive cancer patients increased from 0.7% in 2004 to 15.6% in 2013 (p<0.001, Figure 1A). Because of a corresponding decrease in CF-WBI, the increased usage of HF-WBI only slightly increased the overall use of radiotherapy (83.1% in 2004 to 90.0% in 2011 and 86.1% in 2013, p<0.001). Among DCIS patients (Figure 1B), HF-WBI therapy increased from 0.4% in 2004 to 13.4% in 2013 (p<0.001). The increase in usage of HF-WBI was largely offset by a corresponding reduction in CF-WBI, so that the increase in overall RT usage in DCIS patients was modest (68.7% in 2004 to 73.5% in 2011 and 71.2% in 2013, p<0.001). Figure 1C presents the trend in radiotherapy among invasive breast cancer patients who are 50 or older with a pathologic T1-2N0 disease without chemotherapy. In these patients, 0.9% received HF-WBI in 2004, 12.9% in 2011, and 21.4% in 2013. In addition, the percentage of accelerated partial breast irradiation (mainly brachytherapy) increased before 2008 but decreased thereafter.
Figure 1.
Trend of post-lumpectomy radiation therapy over time in patients with invasive breast cancer who received lumpectomy (A), patients with ductal carcinoma in situ (B), and patients aged 50 years or older with pT1-2N0 breast cancer, without prior chemotherapy (C). APBI, accelerated partial breast irradiation; CF-WBI, conventional fractionated whole breast irradiation; HF-WBI, hypofractionated whole breast irradiation.
For patients who lived 50 miles or more away from the hospitals (a patient population with low radiotherapy use), the percentage of HF-WBI usage in DCIS patients increased from 0.5% in 2004 to 14.8% in 2013, which corresponded to a moderate increase of the overall RT usage in DCIS patients (from 58.0% in 2003 to 67.7% in 2011 and 63.1% in 2013; Supplementary Figure 1).
Characteristics Related to the Use of HF-WBI
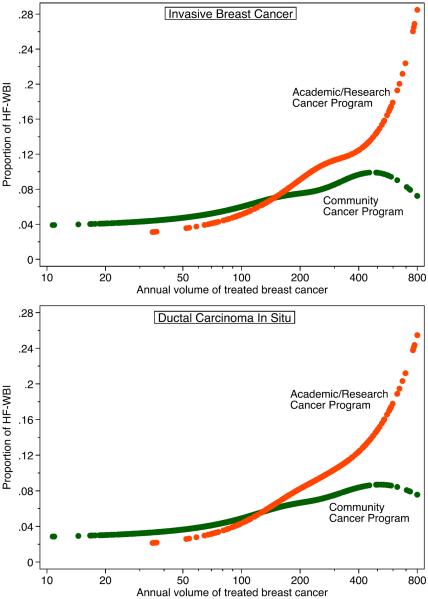
Patient demographic, tumor, and facility factors were compared between 37,619 invasive breast cancer patients who underwent HF-WBI and 430,379 patients who underwent CF-WBI (Table 1). Age at diagnosis was positively and strongly associated with the HF-WBI use, with 19.5% in women 80 years or older, yielding an adjusted odds ratio (aOR) of 8.40 compared to women under 40 years old. Compared with White Americans, African Americans were less likely to receive HF-WBI and Asian Americans were more likely to receive HF-WBI. There was no significant difference in HF-WBI use across insurance statuses in multivariable analysis adjusting for age and other factors. Patients with node negative, small, or low grade cancers, were more likely to receive HF-WBI. There were weak associations between laterality, histology and HF-WBI use, with lobular histology or left-side cancers being less likely to be treated with HF-WBI. Patients who had estrogen receptor positive cancers but did not receive hormone therapy were more likely to undergo HF-WBI than patients who had estrogen receptor negative cancers. HF-WBI was less likely to be used after chemotherapy. There was a big variation in HF-WBI use across treating facilities as reflected in the region, type, and volume of facilities. Facilities in mountain census region were the more likely to give patients HF-WBI, followed by facilities in pacific region. As illustrated in Figure 2, the use of HF-WBI depends on both facility type and volume: large academic/research cancer programs gave more HF-WBI than large community cancer programs. Among large (≥200 patients per year) academic/research cancer programs, the use of HF-WBI increased from 1.6% in 2004 to 21.5% in 2013. Both urban/rural continuum and distance from a patient's home to a cancer facility were associated with HF-WBI use in multivariable analysis and, interestingly, the association was bimodal. Patients living in large metropolitan areas or rural areas were more likely to receive HF-WBI. Patients who lived <5 miles or ≥50 miles away were more likely to use HF-WBI.
Table 1.
Characteristics in invasive breast cancer patients treated with HF-WBI vs. CF-WBI
| CF-WBI | HF-WBI | % HF-WBI | AOR (95%CI)* | Chi-square† | |
|---|---|---|---|---|---|
| Age at diagnosis | |||||
| <40 | 13,351 | 361 | 2.6 | 1.0 (ref.) | 4536.03 |
| 40–44 | 25,951 | 925 | 3.4 | 1.19 (0.71–2.00) | |
| 45–49 | 44,993 | 2,155 | 4.6 | 1.48 (0.88–2.48) | |
| 50–54 | 53,388 | 3,423 | 6.0 | 1.87 (1.12–3.14) | |
| 55–59 | 58,453 | 4,453 | 7.1 | 2.16 (1.29–3.62) | |
| 60–64 | 60,338 | 5,834 | 8.8 | 2.54 (1.52–4.26) | |
| 65–69 | 53,630 | 6,214 | 10.4 | 2.95 (1.76–4.94) | |
| 70–74 | 37,364 | 5,304 | 12.4 | 3.94 (2.35–6.60) | |
| 75–79 | 26,585 | 4,425 | 14.3 | 5.17 (3.09–8.67) | |
| 80+ | 18,707 | 4,525 | 19.5 | 8.40 (5.01–14.09) | |
|
| |||||
| Race/ethnicity | |||||
| White | 319,902 | 30,865 | 8.8 | 1.0 (ref.) | 160.86 |
| Black | 39,426 | 2,738 | 6.5 | 0.91 (0.86–0.96) | |
| Hispanic | 16,337 | 1,640 | 9.1 | 1.07 (1.00–1.15) | |
| Asian | 11,106 | 1,718 | 13.4 | 1.49 (1.40–1.60) | |
| Other/unknown | 5,989 | 658 | 9.9 | 1.07 (0.96–1.20) | |
|
| |||||
| Insurance Status | |||||
| Not Insured | 6,495 | 461 | 6.6 | ||
| Private Insurance | 234,479 | 17,577 | 7.0 | ||
| Medicaid | 19,493 | 1,395 | 6.7 | ||
| Medicare | 123,735 | 17,513 | 12.4 | ||
| Other Government | 3,436 | 284 | 7.6 | ||
| Insurance Status Unknown | 5,122 | 389 | 7.1 | ||
|
| |||||
| Charlson/Deyo comorbidity index | |||||
| 0 | 344,043 | 32,351 | 8.6 | ||
| 1 | 41,581 | 4,401 | 9.6 | ||
| 2 | 7,136 | 867 | 10.8 | ||
|
| |||||
| N stage in invasive cancer | |||||
| pN0 | 310,959 | 33,846 | 9.8 | 1.0 (ref.) | 1485.91 |
| pN1 | 75,522 | 2,602 | 3.3 | 0.38 (0.36–0.40) | |
|
| |||||
| T stage in invasive cancer | |||||
| <=2.0 cm | 307,427 | 32,227 | 9.5 | 1.0 (ref.) | 163.30 |
| 2.1–5.0 cm | 83,424 | 5,271 | 5.9 | 0.78 (0.75–0.81) | |
|
| |||||
| Laterality | |||||
| Right | 193,956 | 19,285 | 9.0 | 1.0 (ref.) | 23.15 |
| Left | 198,465 | 18,325 | 8.5 | 0.94 (0.91–0.96) | |
|
| |||||
| Histology in invasive cancer | |||||
| Ductal | 317,730 | 29,665 | 8.5 | 1.0 (ref.) | 26.45 |
| Lobular | 29,400 | 3,396 | 10.4 | 0.92 (0.87–0.97) | |
| Ductal & lobular | 20,102 | 2,080 | 9.4 | 1.10 (1.04–1.17) | |
| Others | 25,528 | 2,478 | 8.8 | 0.95 (0.90–1.01) | |
|
| |||||
| Grade | |||||
| 1 | 101,086 | 12,381 | 10.9 | 1.0 (ref.) | 127.63 |
| 2 | 161,934 | 16,123 | 9.1 | 0.87 (0.85–0.90) | |
| 3 | 107,080 | 6,632 | 5.8 | 0.79 (0.76–0.83) | |
|
| |||||
| Surgical margin | |||||
| Negative | 374,676 | 36,335 | 8.8 | 1.0 (ref.) | 18.52 |
| Positive | 15,237 | 1,109 | 6.8 | 0.84 (0.78–0.91) | |
|
| |||||
| Estrogen receptor & hormone therapy | |||||
| Negative | 65,749 | 3,792 | 5.5 | 1.0 (ref.) | 65.22 |
| Positive, no hormone therapy | 40,986 | 4,809 | 10.5 | 1.24 (1.16–1.32) | |
| Positive, with hormone therapy | 270,010 | 27,934 | 9.4 | 1.05 (1.00–1.11) | |
|
| |||||
| Chemotherapy | |||||
| No | 228,768 | 30,339 | 11.7 | 1.0 (ref.) | 456.96 |
| Yes | 154,322 | 6,405 | 4.0 | 0.65 (0.62–0.68) | |
|
| |||||
| Facility location | |||||
| New England | 30,403 | 2,105 | 6.5 | 1.0 (ref.) | 2266.99 |
| Middle Atlantic | 59,297 | 6,956 | 10.5 | 1.56 (1.47–1.66) | |
| South Atlantic | 78,068 | 6,946 | 8.2 | 1.51 (1.41–1.61) | |
| East North Central | 75,877 | 7,377 | 8.9 | 1.77 (1.66–1.88) | |
| East South Central | 19,700 | 1,207 | 5.8 | 1.22 (1.12–1.34) | |
| West North Central | 29,442 | 2,648 | 8.3 | 1.45 (1.35–1.56) | |
| West South Central | 20,585 | 931 | 4.3 | 0.83 (0.75–0.92) | |
| Mountain | 15,703 | 3,152 | 16.7 | 4.18 (3.88–4.51) | |
| Pacific | 50,334 | 5,936 | 10.5 | 2.09 (1.96–2.24) | |
|
| |||||
| Facility Type & Volume | 4051.35 | ||||
| In community cancer program | |||||
| <120 | 82,172 | 4,623 | 5.3 | 1.0 (ref.) | |
| 120–207 | 70,939 | 5,825 | 7.6 | 1.53 (1.46–1.60) | |
| 208–342 | 60,871 | 4,932 | 7.5 | 1.49 (1.41–1.56) | |
| 343+ | 35,525 | 3,699 | 9.4 | 1.64 (1.55–1.73) | |
| In academic/research program | |||||
| <120 | 5,808 | 324 | 5.3 | 1.0 (ref.) | |
| 120–207 | 15,381 | 1,361 | 8.1 | 1.73 (1.49–2.02) | |
| 208–342 | 34,656 | 4,007 | 10.4 | 2.11 (1.83–2.43) | |
| 343+ | 45,586 | 9,287 | 16.9 | 3.71 (3.23–4.27) | |
|
| |||||
| Median income | |||||
| <$30000 | 53,606 | 3,934 | 6.8 | 1.0 (ref.) | 224.52 |
| $30000–34999 | 83,090 | 6,584 | 7.3 | 1.07 (1.02–1.13) | |
| $35,000–45999 | 105,882 | 9,537 | 8.3 | 1.17 (1.11–1.23) | |
| $46,000+ | 146,574 | 17,390 | 10.6 | 1.37 (1.31–1.45) | |
|
| |||||
| Urban/Rural | |||||
| Large metropolis (pop. ≥1 million) | 201,912 | 22,897 | 10.2 | 1.0 (ref.) | 511.32 |
| Metropolis (pop. <1 million) | 126,909 | 9,411 | 6.9 | 0.75 (0.73–0.78) | |
| Urban (pop. ≥20000) | 22,577 | 1,399 | 5.8 | 0.67 (0.63–0.72) | |
| Urban (pop. 2500–19999) | 24,853 | 2,408 | 8.8 | 1.14 (1.06–1.21) | |
| Rural | 5,313 | 572 | 9.7 | 1.34 (1.20–1.50) | |
|
| |||||
| Distance to facility | |||||
| < 5.0 miles | 130,101 | 12,396 | 8.7 | 1.0 (ref.) | 155.68 |
| 5.0–9.9 miles | 101,001 | 9,262 | 8.4 | 0.89 (0.86–0.92) | |
| 10.0–19.9 miles | 86,287 | 8,096 | 8.6 | 0.92 (0.89–0.96) | |
| 20.0–49.9 miles | 55,160 | 5,350 | 8.8 | 1.02 (0.97–1.06) | |
| >=50.0 miles | 16,938 | 2,366 | 12.3 | 1.32 (1.24–1.41) | |
Calculated from multivariable logistic regression, adjusting for year of diagnosis and all other variables with adjusted odds ratios in the table. Variables without adjusted odds ratios were not included in the final logistic regression model.
All p-values <0.001
Abbreviations: HF-WBI, hypofractionated whole breast irradiation; CF-WBI, conventional fractionated whole breast irradiation; pop., population; AOR, adjusted odds ratio; CI, confidence interval
Figure 2.
Estimated probability of receiving hypofractionated whole breast irradiation (HF-WBI) in patients with invasive breast cancer or ductal carcinoma in situ, by type and volume of cancer programs.
In DCIS patients, 9,415 received HF-WBI and 107,866 received CF-WBI. Table 2 displays characteristics for DCIS patients receiving HF-WBI as compared to CF-WBI. Once again, the category with the largest percent of patients receiving the hypofractionated treatment was the 80+ age category (18.5%). The characteristics related to HF-WBI use in DCIS patients were similar to those in invasive breast cancer patients, except for laterality, histology, and grade, which appeared to have no effect.
Table 2.
Characteristics in DCIS patients treated with HF-WBI vs. CF-WBI
| CF-WBI | HF-WBI | % HF-WBI | AOR (95% CI)* | Chi-square† | |
|---|---|---|---|---|---|
| Age at diagnosis | |||||
| <40 | 2,275 | 91 | 3.8 | 1.0 (ref.) | 1140.16 |
| 40–44 | 9,117 | 388 | 4.1 | 0.45 (0.16–1.25) | |
| 45–49 | 14,819 | 754 | 4.8 | 0.55 (0.20–1.53) | |
| 50–54 | 16,566 | 1,091 | 6.2 | 0.68 (0.25–1.88) | |
| 55–59 | 17,435 | 1,352 | 7.2 | 0.90 (0.33–2.48) | |
| 60–64 | 15,733 | 1,495 | 8.7 | 1.00 (0.36–2.75) | |
| 65–69 | 13,774 | 1,516 | 9.9 | 1.25 (0.45–3.44) | |
| 70–74 | 9,351 | 1,183 | 11.2 | 1.67 (0.61–4.61) | |
| 75–79 | 5,870 | 879 | 13.0 | 2.34 (0.85–6.46) | |
| 80+ | 2,926 | 666 | 18.5 | 3.80 (1.37–10.52) | |
|
| |||||
| Race/ethnicity | |||||
| White | 84,040 | 7,177 | 7.9 | 1.0 (ref.) | 37.75 |
| Black | 12,961 | 999 | 7.2 | 0.85 (0.76–0.94) | |
| Hispanic | 5,066 | 502 | 9.0 | 1.02 (0.89–1.17) | |
| Asian | 4,084 | 567 | 12.2 | 1.39 (1.22–1.58) | |
| Other/unknown | 1,715 | 170 | 9.0 | 1.06 (0.84–1.35) | |
|
| |||||
| Insurance status | |||||
| Not Insured | 1,689 | 166 | 8.9 | ||
| Private Insurance | 69,658 | 4,965 | 6.7 | ||
| Medicaid | 4,717 | 396 | 7.7 | ||
| Medicare | 29,333 | 3,704 | 11.2 | ||
| Other Government | 1,094 | 79 | 6.7 | ||
| Insurance Status Unknown | 1,375 | 105 | 7.1 | ||
|
| |||||
| Charlson/Deyo comorbidity index | |||||
| 0 | 95,873 | 8,191 | 7.9 | ||
| 1 | 10,371 | 1,058 | 9.3 | ||
| 2 | 1,622 | 166 | 9.3 | ||
|
| |||||
| Tumor size in DCIS | |||||
| <= 1.0 cm | 42,610 | 4,185 | 8.9 | 1.0 (ref.) | 73.77 |
| 1.1–2.0 cm | 19,994 | 1,825 | 8.4 | 0.80 (0.74–0.85) | |
| 2.1–3.0 cm | 6,159 | 606 | 9.0 | 0.76 (0.68–0.85) | |
| >3.0 cm | 5,464 | 506 | 8.5 | 0.71 (0.63–0.80) | |
|
| |||||
| Laterality | |||||
| Right | 52,530 | 4,699 | 8.2 | ||
| Left | 55,276 | 4,713 | 7.9 | ||
|
| |||||
| Histology in DCIS | |||||
| Ductal | 81,169 | 7,183 | 8.1 | ||
| Ductal & lobular | 3,202 | 277 | 8.0 | ||
| Comedocarcinoma | 10,393 | 670 | 6.1 | ||
| Cribriform | 9,511 | 989 | 9.4 | ||
| Papillary | 2,610 | 228 | 8.0 | ||
| Paget's disease | 273 | 21 | 7.1 | ||
| Others | 708 | 47 | 6.2 | ||
|
| |||||
| Grade | |||||
| 1 | 13,453 | 1,098 | 7.5 | ||
| 2 | 34,804 | 3,375 | 8.8 | ||
| 3 | 37,059 | 3,261 | 8.1 | ||
|
| |||||
| Estrogen receptor & hormone therapy | |||||
| Negative | 13,956 | 1,206 | 8.0 | 1.0 (ref.) | 27.01 |
| Positive, no hormone therapy | 27,264 | 3,176 | 10.4 | 1.19 (1.09–1.31) | |
| Positive, with hormone therapy | 47,521 | 4,087 | 7.9 | 1.02 (0.93–1.11) | |
|
| |||||
| Surgical margin | |||||
| Negative | 103,126 | 9,108 | 8.1 | ||
| Positive | 3,840 | 250 | 6.1 | ||
|
| |||||
| Facility location | |||||
| New England | 9,282 | 400 | 4.1 | 1.0 (ref.) | 807.42 |
| Middle Atlantic | 17,025 | 1,732 | 9.2 | 1.61 (1.38–1.88) | |
| South Atlantic | 21,889 | 1,910 | 8.0 | 1.97 (1.69–2.29) | |
| East North Central | 21,932 | 1,996 | 8.3 | 2.09 (1.80–2.43) | |
| East South Central | 5,181 | 300 | 5.5 | 1.22 (0.98–1.51) | |
| West North Central | 8,232 | 633 | 7.1 | 1.72 (1.45–2.04) | |
| West South Central | 5,530 | 285 | 4.9 | 1.45 (1.18–1.77) | |
| Mountain | 3,803 | 798 | 17.3 | 5.72 (4.82–6.80) | |
| Pacific | 12,717 | 1,270 | 9.1 | 2.39 (2.04–2.79) | |
|
| |||||
| Facility type & volume | 578.50 | ||||
| In community cancer program | |||||
| <120 | 22,125 | 985 | 4.3 | 1.0 (ref.) | |
| 120–207 | 19,954 | 1,425 | 6.7 | 1.74 (1.56–1.93) | |
| 208–342 | 17,222 | 1,198 | 6.5 | 1.55 (1.38–1.73) | |
| 343+ | 9,387 | 877 | 8.5 | 1.85 (1.63–2.09) | |
| In academic/research program | |||||
| <120 | 1,637 | 72 | 4.2 | 1.0 (ref.) | |
| 120–207 | 4,650 | 395 | 7.8 | 1.55 (1.11–2.17) | |
| 208–342 | 10,400 | 1,020 | 8.9 | 1.87 (1.36–2.58) | |
| 343+ | 12,300 | 2,448 | 16.6 | 3.02 (2.21–4.14) | |
|
| |||||
| Median income | |||||
| <$30000 | 14,509 | 1,048 | 6.7 | 1.0 (ref.) | 58.9 |
| $30000–34999 | 21,970 | 1,657 | 7.0 | 1.16 (1.04–1.30) | |
| $35,000–45999 | 28,691 | 2,347 | 7.6 | 1.28 (1.15–1.43) | |
| $46,000+ | 41,768 | 4,319 | 9.4 | 1.49 (1.34–1.67) | |
|
| |||||
| Urban/Rural | |||||
| Large metropolis (pop. ≥1 million) | 57,221 | 5,952 | 9.4 | 1.0 (ref.) | 180.40 |
| Metropolis (pop. <1 million) | 34,551 | 2,167 | 5.9 | 0.72 (0.67–0.77) | |
| Urban (pop. ≥20000) | 5,682 | 335 | 5.6 | 0.72 (0.61–0.85) | |
| Urban (pop. 2500–19999) | 6,052 | 575 | 8.7 | 1.39 (1.20–1.61) | |
| Rural | 1,317 | 161 | 10.9 | 1.91 (1.51–2.41) | |
|
| |||||
| Distance to facility | |||||
| < 5.0 miles | 37,038 | 3,073 | 7.7 | 1.0 (ref.) | 19.08 |
| 5.0–9.9 miles | 28,754 | 2,394 | 7.7 | 0.93 (0.86–1.00) | |
| 10.0–19.9 miles | 23,820 | 2,066 | 8.0 | 0.94 (0.86–1.02) | |
| 20.0–49.9 miles | 13,718 | 1,306 | 8.7 | 1.01 (0.91–1.12) | |
| ≥ 50.0 miles | 3,708 | 538 | 12.7 | 1.28 (1.09–1.49) | |
Calculated from multivariable logistic regression, adjusting for year of diagnosis and all other variables with adjusted odds ratios in the table. Variables without adjusted odds ratios were not included in the final logistic regression model.
All p-values <0.001
Abbreviations: HF-WBI, hypofractionated whole breast irradiation; CF-WBI, conventional fractionated whole breast irradiation; DCIS, ductal carcinoma in situ; pop., population; AOR, adjusted odds ratio; CI, confidence interval
Regimens and Delivery of HF-WBI
As radiotherapy regimens for invasive breast cancer and DCIS were similar, we combined them together for the analysis of radiation regimens. Among patients who received HF-WBI, 50.7% also received boost radiation to the tumor bed. By contrast, the majority of patients undergoing CF-WBI received boost radiation (90.5%).
Table 3 lists the common HF-WBI regimens. Although the top 10 most common regimens accounted for 78.2% of all HF-WBI, there were 39 distinct regimens each with at least 100 patients being treated. The most common HF-WBI regimen was 42.56 Gy in 16 fractions without a boost (41.4%), followed by 42.56 Gy in 16 fractions with a 10 Gy boost in 5 fractions (13.0%) and 42.56 Gy in 16 fractions with a 10 Gy boost in 4 fractions (12.7%).
Table 3.
Frequency of hypofracationated whole breast irradiation regimens*
| Dose and delivery | Boost | No. of patients | Percent |
|---|---|---|---|
| 42.56 Gy in 16 fractions | No | 19462 | 41.4 |
| 42.56 Gy in 16 fractions | 10 Gy in 5 fractions | 6106 | 13.0 |
| 42.56 Gy in 16 fractions | 10 Gy in 4 fractions | 5959 | 12.7 |
| 42.56 Gy in 16 fractions | 10.64 Gy in 4 fractions | 1358 | 2.9 |
| 40 Gy in 16 fractions | 10 Gy in 4 fractions | 769 | 1.6 |
| 40.05 Gy in 15 fractions | No | 714 | 1.5 |
| 43.2 Gy in 16 fractions | No | 682 | 1.5 |
| 40.05 Gy in 15 fractions | 10 Gy in 5 fractions | 632 | 1.3 |
| 42.56 Gy in 16 fractions | 8 Gy in 4 fractions | 564 | 1.2 |
| 42.56 Gy in 16 fractions | 7.98 Gy in 3 fractions | 544 | 1.2 |
|
| |||
| 42.56 Gy in 16 fractions | 12 Gy in 6 fractions | 475 | 1.0 |
| 42.56 Gy in 16 fractions | 14 Gy in 7 fractions | 420 | 0.9 |
| 42.5 Gy in 17 fractions | No | 418 | 0.9 |
| 42.56 Gy in 16 fractions | 9.6 Gy in 4 fractions | 309 | 0.7 |
| 40.05 Gy in 15 fractions | 7.95 Gy in 2 fractions | 281 | 0.6 |
| 42.56 Gy in 16 fractions | 7.5 Gy in 3 fractions | 276 | 0.6 |
| 40 Gy in 16 fractions | 12.5 Gy in 5 fractions | 274 | 0.6 |
| 42.56 Gy in 16 fractions | 5 Gy in 2 fractions | 253 | 0.5 |
| 42.56 Gy in 16 fractions | 16 Gy in 8 fractions | 227 | 0.5 |
| 42.56 Gy in 16 fractions | 9 Gy in 4 fractions | 219 | 0.5 |
| 40 Gy in 16 fractions | No | 194 | 0.4 |
| 42.5 Gy in 17 fractions | 4.5 Gy in 2 fractions | 192 | 0.4 |
| 42.56 Gy in 16 fractions | 10.6 Gy in 4 fractions | 190 | 0.4 |
| 40.5 Gy in 15 fractions | 10.8 Gy in 4 fractions | 177 | 0.4 |
| 42.56 Gy in 16 fractions | 5.32 Gy in 2 fractions | 165 | 0.4 |
| 42.56 Gy in 16 fractions | 12.5 Gy in 5 fractions | 164 | 0.3 |
| 40.5 Gy in 15 fractions | No | 161 | 0.3 |
| 40 Gy in 16 fractions | 13.75 Gy in 5 fractions | 155 | 0.3 |
| 40.5 Gy in 15 fractions | No | 152 | 0.3 |
| 45 Gy in 17 fractions | 11 Gy in 3 fractions | 145 | 0.3 |
| 40.05 Gy in 15 fractions | 8.01 Gy in 3 fractions | 143 | 0.3 |
| 35 Gy in 14 fractions | 15 Gy in 6 fractions | 141 | 0.3 |
| 42.56 Gy in 16 fractions | 5.3 Gy in 2 fractions | 124 | 0.3 |
| 42.56 Gy in 16 fractions | 7.95 Gy in 3 fractions | 117 | 0.2 |
| 42.56 Gy in 16 fractions | 9 Gy in 3 fractions | 117 | 0.2 |
| 40.5 Gy in 15 fractions | 7.5 Gy in 3 fractions | 116 | 0.2 |
| 40.05 Gy in 15 fractions | 10 Gy in 4 fractions | 112 | 0.2 |
| 40.5 Gy in 15 fractions | 6 Gy in 3 fractions | 107 | 0.2 |
| 39.9 Gy in 15 fractions | No | 100 | 0.2 |
|
| |||
| Other less common regimens | No | 4320 | 9.2 |
42.4 – 42.72 Gy in 16 fractions (2.65–2.67 Gy per fraction) were lumped into 42.56 Gy in 16 fractions as they are clinically the same
The most common delivery technique for HF-WBI was standard external beam irradiation (72.1%), but the proportions of 3D conformal (17.2%) and intensity-modulated radiation therapy (10.7%) for HF-WBI were higher than those for CF-WBI (3D conformal, 12.2%; intensity-modulated radiation therapy, 9.9%). There was an increasing trend that more 3D conformal radiation therapy was used to deliver HF-WBI in recent years: in 2004, 1.9% of HF-WBI was delivered using 3D conformal and 19.2% delivered using intensity-modulated radiation therapy, while the proportions of using 3D conformal and intensity-modulated radiation therapy were 21.0% and 9.0% in 2013, respectively.
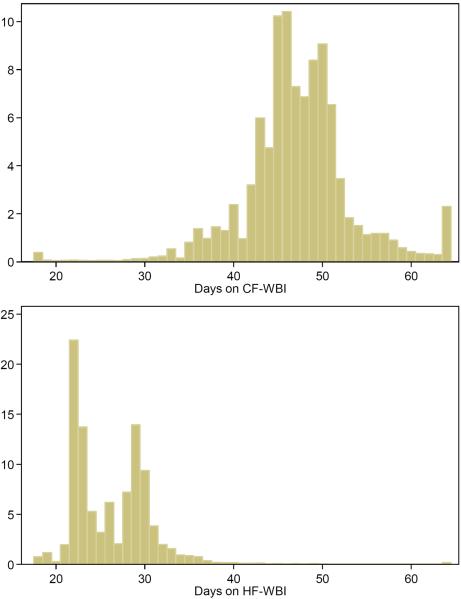
Figure 3 presents the distribution of days from the first to the last radiotherapy. The median days of finishing radiotherapy for patients receiving HF-WBI was 26 days (mean=26.4, 95% range: 21–36 days), as compared to 47 days (mean=47.6, 95% range: 35–63 days) for patients receiving CF-WBI. For HF-WBI patients with a boost, the median days on radiotherapy was 29 days and the median days was 23 days for HF-WBI patients without a boost. For CF-WBI patients with and without a boost, the median days on radiotherapy were 47 and 41 days, respectively.
Figure 3.
Histogram of days on radiotherapy for patients undergoing conventional fractionated whole breast irradiation (CF-WBI) and hypofractionated whole breast irradiation (HF-WBI).
DISCUSSION
The use of HF-WBI has been gradually increasing in the US in recent years in the centers contributing to the NCDB. The increases have occurred both in DCIS patients (from 0.4% in 2004 to 13.4% in 2013) and in invasive breast cancer patients (from 0.7% in 2003 to 15.6% in 2013). Various factors affect the likelihood of a patient receiving HF-WBI; perhaps the most notable factors are the patient's age and the type of treatment facility, i.e., academic/research cancer facilities with a large volume of patients tend to use HF-WBI more. Our study also showed that the adoption of HF-WBI did not increase the overall use of radiotherapy, although there was a moderate increase among patients who lived far away from hospitals. Apparently, the increase in HF-WBI has been offset by the reduction in CF-WBI and APBI use in recent years.
For invasive breast cancer patients, randomized clinical trials have demonstrated that HF-WBI is as effective as CF-WBI for treating breast cancer after lumpectomy [2–6]. For DCIS patients, several observational studies did not find differential risk of local recurrence between HF-WBI and conventional radiotherapy [13, 14]. Despite all the research on the effectiveness of HF-WBI, conventional radiotherapy remained the most common form of radiotherapy for post-lumpectomy breast cancer patients in the US. In this study, we found there was an increased uptake of HF-WBI from 2004 to 2011 (when ASTRO guideline was published) and a continued increase to 2013, but only 21.4% of patients who are recommended by the guideline received HF-WBI and only 13.4% of DCIS patients received HF-WBI in 2013. A study conducted in the Medicare population found 14.2% of invasive and 11.6% of DCIS patients received HF-WBI in 2011 [10], while a study conducted in commercially insured population found 34.5% of hypofractionation-endorsed patients received HF-WBI in 2013 [9]. Another study analyzed NCDB dataset and documented the rate of invasive patients undergoing WF-WBI was 13.05% in 2011 [8]. By contrast, HF-WBI has already become the standard form of treatment in the United Kingdom and over 70% of eligible women in Ontario, Canada received HF-WBI in 2008 [15, 16].
American physicians have been reluctant to adopt HF-WBI for a variety of reasons. One reason is a fear of increased toxicity, especially late effects of radiotherapy. It has been common belief in oncology that small radiation doses allow normal tissues to repair between fractions, and therefore physicians may be hesitant to go against their traditional beliefs and practices [17]. Many oncologists were waiting for a significant long-term follow-up study, which was not published until 2010 [5]. The two UK trials also showed that HF-WBI has similar or less late toxicities to normal breast tissues, compared to conventional radiotherapy [6]. Recently, two studies conducted in the US demonstrated that HF-WBI had significantly lower rates of early toxicities than conventional radiotherapy [18, 19], and a 2-year follow-up of the clinical trial in the US showed that patient-reported quality of life and physician-assessed cosmetic outcomes were similar between HF-WBI and CF-WBI arms [20]. Therefore, there should be no fear for toxicities to adopt HF-WBI.
There may be a financial incentive to continue CF-WBI if reimbursement to physicians are based on number of treatment. In the UK and Canada, where the reimbursement system does not provide incentives, the adoption of HF-WBI was high [15, 16, 21], though this could be in part due to the fact that the major trials of HF-WBI were performed in these countries. However, an early survey found that in European countries other than the UK, the adoption of HF-WBI has been slow [21]. More contemporary studies of HF-WBI use in European countries are needed. Two interesting findings of our study are the regional variation of HF-WBI adoption and a higher update of HF-WBI in academic or large hospitals. It is possible that doctors in academic centers are more open to the research findings and less prone to financial incentives. This regional variation in HF-WBI and variation in insurance policies in the US could facilitate investigations on the impact of financial incentives on HF-WBI adoption. To achieve standardized HF-WBI in the US, patients should be thoroughly informed of HF-WBI as an encouraged option, and strategies to limit the financial incentives of delivering high fractions could be implemented, such as a method of reimbursing based on a factor other than the number of fractions. In 2013, as part of national Choosing Wisely campaign [22], ASTRO stated, “Don't initiate whole breast radiotherapy as a part of breast conservation therapy in women age ≥50 with early stage invasive breast cancer without considering shorter treatment schedules.” It is important to continue monitoring whether HF-WBI will be widespread adopted in the US in future.
There was a wide variation in terms of regimens of HF-WBI in the US. The most prevalent regimens were 42.4–42.72 Gy in 16 fractions without or with a boost, which model the dose schedule from the Canadian clinical trial [5]. Few patients received 40.05 Gy or 40.5 Gy in 15 fractions with or without a boost, which are similar to the regimen in the UK START B trial [3]. HF-WBI schedules given in 13 fractions over 5 weeks were seldom used in the US practices, possibly because of their long duration of treatment [2, 4]. There was no consensus on whether to give a boost to treat the tumor bed after HF-WBI, and about half of the patients received a boost radiation in practice. On average, hypofractionation schedules significantly shortened the duration of radiotherapy by 3 weeks.
This study has several strengths, including a large national sample representing both old and young patients, both invasive and DCIS patients. Another strength of this study is its precise definition of hypofractionated radiotherapy. Previous studies defined HF-WBI either according to total number of radiation fractions only (≤ 24 fractions) [9, 10], or based on the ratio of total dose to total number of fractions (2.2–4.0 Gy per fraction) [8]. Whether or not having a boost radiation and early stop of a conventional fractionated RT could misclassify WF-WBI as HF-WBI or vice versa. We took a tedious approach of listing all the combination of dose to the breast, boost dose, number of fraction, and total days on radiation therapy in order to avoid misclassification in radiotherapy type. Using this precise definition, we are able to provide accurate estimate of HF-WBI use patterns in the US, and identify factors associated to HF-WBI use with confidence.
The study also has several limitations. First, there is potential underreporting of radiotherapy in cancer registries as previous studies compared registry with medical chart or insurance claim data and found that radiotherapy was underreported by 12–26% [23–26]. Although a recent study showed that underreporting was less serious for hospitals accredited by the American College of Surgeons, on which the NCDB is based [26], our estimates of HF-WBI and overall radiotherapy use could be lower than the actual use because of potential underreporting. The underreporting of radiotherapy was higher in rural counties [25], so the use of HF-WBI for patients living in rural countries might be higher than reported in the NCDB. Second, about 5% of patients were reported to receive radiotherapy but the type of radiotherapy cannot be determined as dose and fraction were missing. This could have a slightly downward bias on the estimate of HF-WBI use. Third, hospitals that contributed data to the NCDB are more likely to be large or academic centers and less likely to be located in rural area compared to hospitals that did not contribute data to NCDB [27]. We found patients in large/academic centers and in rural area were more likely receive HF-WBI, so our estimate of HF-WBI use might be an underestimate (due to less rural hospital in NCDB) or overestimate (due to more academic centers in NCDB) of the true HF-WBI use for the entire US. Since the NCDB captures approximately 70% of all newly diagnosed malignant cancers in the US, the bias will not affect our conclusions.
In summary, use of HF-WBI after lumpectomy was rising between 2004 and 2013 in the US, and magnitude of increase was largest in patients who are 50 or older with node negative invasive breast cancer, the subgroup recommended for HF-WBI by ASTRO in 2011 [7]. However, the adoption of HF-WBI was far from complete in the US. We observed that HF-WBI reduced the time for radiotherapy by 21 days. Further studies are needed to continue monitor the trend of HF-WBI and investigate the impact of financial incentives on HF-WBI adoption in the US. Promotion of HF-WBI at community cancer facilities and rural areas could increase the overall appropriate use of radiotherapy for breast cancer.
Supplementary Material
Acknowledgments
This study used de-identified data and was exempt from human protection oversight by the institutional review board. The project was partially supported by a University of Chicago Comprehensive Cancer Center pilot grant from the National Cancer Institute of the National Institutes of Health (CA014599). Dr. Huo is an American Cancer Society scholar (MRSG-13-063-01).
Footnotes
Compliance with Ethical Standards All authors declared that they have no conflicts of interest.
Bibliography
- 1.Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y, Peto R. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owen JR, Ashton A, Bliss JM, Homewood J, Harper C, Hanson J, Haviland J, Bentzen SM, Yarnold JR. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. The lancet oncology. 2006;7:467–471. doi: 10.1016/S1470-2045(06)70699-4. [DOI] [PubMed] [Google Scholar]
- 3.Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bentzen SM, Bliss JM, Brown J, Dewar JA, Dobbs HJ, Haviland JS, Hoskin PJ, Hopwood P, Lawton PA, Magee BJ, Mills J, Morgan DA, Owen JR, Simmons S, Sumo G, Sydenham MA, Venables K, Yarnold JR. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371:1098–1107. doi: 10.1016/S0140-6736(08)60348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bliss JM, Brown J, Dewar JA, Dobbs HJ, Haviland JS, Hoskin PJ, Hopwood P, Lawton PA, Magee BJ, Mills J, Morgan DA, Owen JR, Simmons S, Sumo G, Sydenham MA, Venables K, Yarnold JR. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. The lancet oncology. 2008;9:331–341. doi: 10.1016/S1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie R, Parpia S, Shelley W, Grimard L, Bowen J, Lukka H, Perera F, Fyles A, Schneider K, Gulavita S, Freeman C. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 6.Haviland JS, Owen JR, Dewar JA, Agrawal RK, Barrett J, Barrett-Lee PJ, Dobbs HJ, Hopwood P, Lawton PA, Magee BJ, Mills J, Simmons S, Sydenham MA, Venables K, Bliss JM, Yarnold JR, Group ST. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. The lancet oncology. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 7.Smith BD, Bentzen SM, Correa CR, Hahn CA, Hardenbergh PH, Ibbott GS, McCormick B, McQueen JR, Pierce LJ, Powell SN, Recht A, Taghian AG, Vicini FA, White JR, Haffty BG. Fractionation for whole breast irradiation: an American Society for Radiation Oncology (ASTRO) evidence-based guideline. International journal of radiation oncology, biology, physics. 2011;81:59–68. doi: 10.1016/j.ijrobp.2010.04.042. doi: 10.1016/j.ijrobp.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 8.Wang EH, Mougalian SS, Soulos PR, Rutter CE, Evans SB, Haffty BG, Gross CP, Yu JB. Adoption of hypofractionated whole-breast irradiation for early-stage breast cancer: a National Cancer Data Base analysis. International journal of radiation oncology, biology, physics. 2014;90:993–1000. doi: 10.1016/j.ijrobp.2014.06.038. doi: 10.1016/j.ijrobp.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 9.Bekelman JE, Sylwestrzak G, Barron J, Liu J, Epstein AJ, Freedman G, Malin J, Emanuel EJ. Uptake and costs of hypofractionated vs conventional whole breast irradiation after breast conserving surgery in the United States, 2008–2013. Jama. 2014;312:2542–2550. doi: 10.1001/jama.2014.16616. doi: 10.1001/jama.2014.16616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jagsi R, Falchook AD, Hendrix LH, Curry H, Chen RC. Adoption of hypofractionated radiation therapy for breast cancer after publication of randomized trials. International journal of radiation oncology, biology, physics. 2014;90:1001–1009. doi: 10.1016/j.ijrobp.2014.09.032. doi: 10.1016/j.ijrobp.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 11.Hughes KS, Schnaper LA, Bellon JR, Cirrincione CT, Berry DA, McCormick B, Muss HB, Smith BL, Hudis CA, Winer EP, Wood WC. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31:2382–2387. doi: 10.1200/JCO.2012.45.2615. doi: 10.1200/JCO.2012.45.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes KS, Schnaper LA, Berry D, Cirrincione C, McCormick B, Shank B, Wheeler J, Champion LA, Smith TJ, Smith BL, Shapiro C, Muss HB, Winer E, Hudis C, Wood W, Sugarbaker D, Henderson IC, Norton L. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351:971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 13.Lalani N, Paszat L, Sutradhar R, Thiruchelvam D, Nofech-Mozes S, Hanna W, Slodkowska E, Done SJ, Miller N, Youngson B, Tuck A, Sengupta S, Elavathil L, Chang MC, Jani PA, Bonin M, Rakovitch E. Long-term outcomes of hypofractionation versus conventional radiation therapy after breast-conserving surgery for ductal carcinoma in situ of the breast. International journal of radiation oncology, biology, physics. 2014;90:1017–1024. doi: 10.1016/j.ijrobp.2014.07.026. doi: 10.1016/j.ijrobp.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson C, Valachis A. The role of boost and hypofractionation as adjuvant radiotherapy in patients with DCIS: a meta-analysis of observational studies. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2015;114:50–55. doi: 10.1016/j.radonc.2015.01.001. doi: 10.1016/j.radonc.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Mitin T, Kubicky CD. The Use of Hypofractionated Whole Breast Irradiation in Treatment of Patients With Early-Stage Breast Cancer in the United States. JAMA oncology. 2015;1:245–246. doi: 10.1001/jamaoncol.2014.321. doi: 10.1001/jamaoncol.2014.321. [DOI] [PubMed] [Google Scholar]
- 16.Ashworth A, Kong W, Whelan T, Mackillop WJ. A population-based study of the fractionation of postlumpectomy breast radiation therapy. International journal of radiation oncology, biology, physics. 2013;86:51–57. doi: 10.1016/j.ijrobp.2012.12.015. doi: 10.1016/j.ijrobp.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Coye MJ, Kell J. How hospitals confront new technology. Health affairs (Project Hope) 2006;25:163–173. doi: 10.1377/hlthaff.25.1.163. [DOI] [PubMed] [Google Scholar]
- 18.Shaitelman SF, Schlembach PJ, Arzu I, Ballo M, Bloom ES, Buchholz D, Chronowski GM, Dvorak T, Grade E, Hoffman KE, Kelly P, Ludwig M, Perkins GH, Reed V, Shah S, Stauder MC, Strom EA, Tereffe W, Woodward WA, Ensor J, Baumann D, Thompson AM, Amaya D, Davis T, Guerra W, Hamblin L, Hortobagyi G, Hunt KK, Buchholz TA, Smith BD. Acute and Short-term Toxic Effects of Conventionally Fractionated vs Hypofractionated Whole-Breast Irradiation: A Randomized Clinical Trial. JAMA oncology. 2015;1:931–941. doi: 10.1001/jamaoncol.2015.2666. doi: 10.1001/jamaoncol.2015.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jagsi R, Griffith KA, Boike TP, Walker E, Nurushev T, Grills IS, Moran JM, Feng M, Hayman J, Pierce LJ. Differences in the Acute Toxic Effects of Breast Radiotherapy by Fractionation Schedule: Comparative Analysis of Physician-Assessed and Patient-Reported Outcomes in a Large Multicenter Cohort. JAMA oncology. 2015;1:918–930. doi: 10.1001/jamaoncol.2015.2590. doi: 10.1001/jamaoncol.2015.2590. [DOI] [PubMed] [Google Scholar]
- 20.Swanick CW, Lei X, Shaitelman SF, Schlembach PJ, Bloom ES, Fingeret MC, Strom EA, Tereffe W, Woodward WA, Stauder MC, Dvorak T, Thompson AM, Buchholz TA, Smith BD. Longitudinal analysis of patient-reported outcomes and cosmesis in a randomized trial of conventionally fractionated versus hypofractionated whole-breast irradiation. Cancer. 2016 doi: 10.1002/cncr.30121. doi: 10.1002/cncr.30121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Laan HP, Hurkmans CW, Kuten A, Westenberg HA, Party E-RBW. Current technological clinical practice in breast radiotherapy; results of a survey in EORTC-Radiation Oncology Group affiliated institutions. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2010;94:280–285. doi: 10.1016/j.radonc.2009.12.032. doi: 10.1016/j.radonc.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 22.ASTRO Choosing Wisely List. 2013 https://www.astro.org/Clinical-Practice/Choosing-Wisely/2013-Choosing-Wisely-List.aspx. In.
- 23.Malin JL, Kahn KL, Adams J, Kwan L, Laouri M, Ganz PA. Validity of cancer registry data for measuring the quality of breast cancer care. J Natl Cancer Inst. 2002;94:835–844. doi: 10.1093/jnci/94.11.835. [DOI] [PubMed] [Google Scholar]
- 24.Du X, Freeman JL, Goodwin JS. Information on radiation treatment in patients with breast cancer: the advantages of the linked medicare and SEER data. Surveillance, Epidemiology and End Results. Journal of clinical epidemiology. 1999;52:463–470. doi: 10.1016/s0895-4356(99)00011-6. [DOI] [PubMed] [Google Scholar]
- 25.Walker GV, Giordano SH, Williams M, Jiang J, Niu J, MacKinnon J, Anderson P, Wohler B, Sinclair AH, Boscoe FP, Schymura MJ, Buchholz TA, Smith BD. Muddy water? Variation in reporting receipt of breast cancer radiation therapy by population-based tumor registries. International journal of radiation oncology, biology, physics. 2013;86:686–693. doi: 10.1016/j.ijrobp.2013.03.016. doi: 10.1016/j.ijrobp.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jagsi R, Abrahamse P, Hawley ST, Graff JJ, Hamilton AS, Katz SJ. Underascertainment of radiotherapy receipt in Surveillance, Epidemiology, and End Results registry data. Cancer. 2012;118:333–341. doi: 10.1002/cncr.26295. doi: 10.1002/cncr.26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bilimoria KY, Bentrem DJ, Stewart AK, Winchester DP, Ko CY. Comparison of commission on cancer-approved and -nonapproved hospitals in the United States: implications for studies that use the National Cancer Data Base. J Clin Oncol. 2009;27:4177–4181. doi: 10.1200/JCO.2008.21.7018. doi: 10.1200/JCO.2008.21.7018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.