Abstract
Chronic use of heparin as an anti-coagulant for the treatment of thrombosis or embolism invokes many adverse systemic events including thrombocytopenia, vascular reactions and osteoporosis. Here, we addressed whether adverse effects might also be directed to mesenchymal stem cells that reside in the bone marrow compartment. Harvested human bone marrow-derived mesenchymal stem cells (hMSCs) were exposed to varying doses of heparin and their responses profiled. At low doses (<200 ng/ml), serial passaging with heparin exerted a variable effect on hMSC proliferation and multipotentiality across multiple donors, while at higher doses (≥100 µg/ml), heparin supplementation inhibited cell growth and increased both senescence and cell size. Gene expression profiling using cDNA arrays and RNA-seq analysis revealed pleiotropic effects of low-dose heparin on signaling pathways essential to hMSC growth and differentiation (including the TGFβ/BMP superfamily, FGFs, and Wnts). Cells serially passaged in low-dose heparin possess a donor-dependent gene signature that reflects their altered phenotype. Our data indicate that heparin supplementation during the culturing of hMSCs can alter their biological properties, even at low doses. This warrants caution in the application of heparin as a culture supplement for the ex vivo expansion of hMSCs. It also highlights the need for careful evaluation of the bone marrow compartment in patients receiving chronic heparin treatment.
Keywords: mesenchymal stem cells, glycosaminoglycans, multipotency, cell proliferation, microarray
1. Introduction
Heparin, a highly sulfated heparan glycosaminoglycan variant produced and stored primarily by mast cells [1], possesses the highest net negative charge density of all known biological molecules [2]. Its negative charge binds to positively charged, heparin-binding domains (HBDs) present in a large number of extracellular proteins. This group of proteins includes fibroblast growth factors (FGFs), vascular endothelial growth factors (VEGFs), bone morphogenetic proteins (BMPs) and large extracellular structural molecules such as fibronectin and laminin, as well as its main clinical target antithrombin III [3]. Both unfractionated (UFH) and low molecular weight (LMWH) heparin have been widely used as anticoagulants to enable surgery and dialysis, as well as to treat pathological conditions such as thrombosis and embolism.
The ability of heparin to interact with many proteins renders it a potential therapeutic agent beyond its use as an anti-thrombotic [4]. Heparin’s high affinity for protein has resulted in its application in cell culture to enhance the desirable activity of critical extracellular biomolecules used as supplements for the expansion of human stem cells. For example, heparin has been reported to promote both Wnt and FGF signaling in human embryonic stem cells (hESCs), thereby increasing their proliferation [5,6]. Similarly, heparin has been shown to enhance Wnt-induced differentiation signals in osteogenic cells [7], further highlighting its diverse effects. Tissue culture surfaces coated with glycosaminoglycans such as heparin support greater proliferation of MSCs [8,9]. Heparin-functionalized hydrogels and heparinized nanoparticles have also been developed to support the viability and differentiation of hMSCs [10,11].
The widespread use of heparin in both clinical and research practice makes a detailed study of both its mechanism of action and long-term effects in cell culture highly advisable. The abiding usefulness of heparin as a general preserver of growth factor/cytokine protein conformation and bioactivity derives from the comprehensiveness of its binding profile, its easy availability and its low cost. Heparins facilitate the binding of many proteins to high-affinity receptors on cells, particularly within the endothelium [12–14]. Factors such as VEGF165 and FGF-2 normally associate with heparan sugars on cell surfaces to form ligand:sugar:receptor complexes that induce proliferative signals [15,16]. Furthermore, VEGF165 affinity-selected sugar has been shown to exert proangiogenic effects on endothelial cells [17]. In contrast, short heparin fragments (~5.0-kDa) purified from porcine intestinal mucosa can suppress VEGF165-mediated angiogenesis when delivered subcutaneously [18]. Also, heparin derivatives as well as heparan sulfate (HS) isolated from bone marrow stromal cells and BMP-2 affinity-selected HS have been shown to increase the osteogenic potential of BMP-2 [9,19,20]. Genes encoding the synthesis of ECM proteins can also be regulated by heparin [21]. Heparin has been shown to activate metalloproteinase-2, leading to remodeling of the ECM [22] and can act as a heparanase inhibitor and is known to affect the in vitro tubular morphogenesis of microvessels [23]. In certain fibroblasts, inflammatory cells, and tumor cells (most prominently), heparanase activity is enhanced, where the expression of heparanase mRNA is know to correlate with increased metastatic potential [24]. Furthermore, type 1 diabetes has been shown to be a heparanase-dependent disease [25]. These broad biological effects of heparin and heparin-degrading enzymes are consistent with the multiplicity of proteins that interact with its hyper-sulfated sugar chains and maintain tissue homeostasis.
In most tissues, heparin-binding proteins are usually controlled by physiologically relevant and tissue-specific HS on the cell surface. There are notable differences in the structure between heparin and HS; most importantly heparin contains 3-O-sulfation and lacks discrete protein-binding domains [26]. Excess heparin with its greater negative-charge density can out-compete physiologically relevant HS-protein interactions and thus disrupt a number of biological processes associated with tissue development and repair that require proper maintenance of stem cell pools. Also, safety concerns ascribed to heparin’s binding promiscuity are evident from patients presenting with heparin-induced thrombocytopenia [27], osteoporosis [28,29] and vascular reactions [30,31]. Indeed, heparin has been shown to enhance osteoclastic bone resorption through an interaction with osteoprotegerin (OPG) [32], whilst other HS variants have been shown to exert anti-osteoclastic effects [33]. Mastocytosis, a disorder characterized by increased numbers of mast cells that produce excessive heparin, is associated with osteoporosis, which again indicates the generally adverse effect of heparin on skeletal tissue [34]. Even though chronic heparin use is associated with unwanted clinical events, it is widely used as a stem cell culture supplement without a clear understanding of its effects on stem cell phenotypes.
Adult stem cells are a key driver of natural tissue replenishment, and are amongst the small number of cells that can both undergo proliferation and differentiate into the various lineages needed to repair or regenerate damaged tissue [35,36]. Heparin supplementation in medium has been reported to promote hMSC proliferation [37]. Heparin-functionalized hydrogels have been formulated in such a way that they are able to retain combinations of FGFs and ECM proteins and so support the growth, adhesion or differentiation of hMSCs [10,11,38,39]. However, we lack precise knowledge of the biological effects of heparin on hMSCs.
This study set out to determine whether heparin, over a range of doses, could change the intrinsic properties of hMSCs in vitro. It proved capable of altering the molecular profiles of hMSCs, even at low doses, and affected their potential for growth and differentiation in a donor-dependent manner. Our findings suggest that caution should be exercised whenever stem cells are serially passaged in heparin-supplemented media.
2. Materials and Methods
Throughout the study, control data generated in hMSCs grown in maintenance conditions [40] was compared to heparin-treated hMSCs in Figures 1A, 1B, 2 and 3. Experimentation with heparin was performed in parallel with studies using HS [40]. Data obtained with heparin and HS were analyzed separately to address distinct findings on different biological properties of hMSCs that emerged from the two datasets.
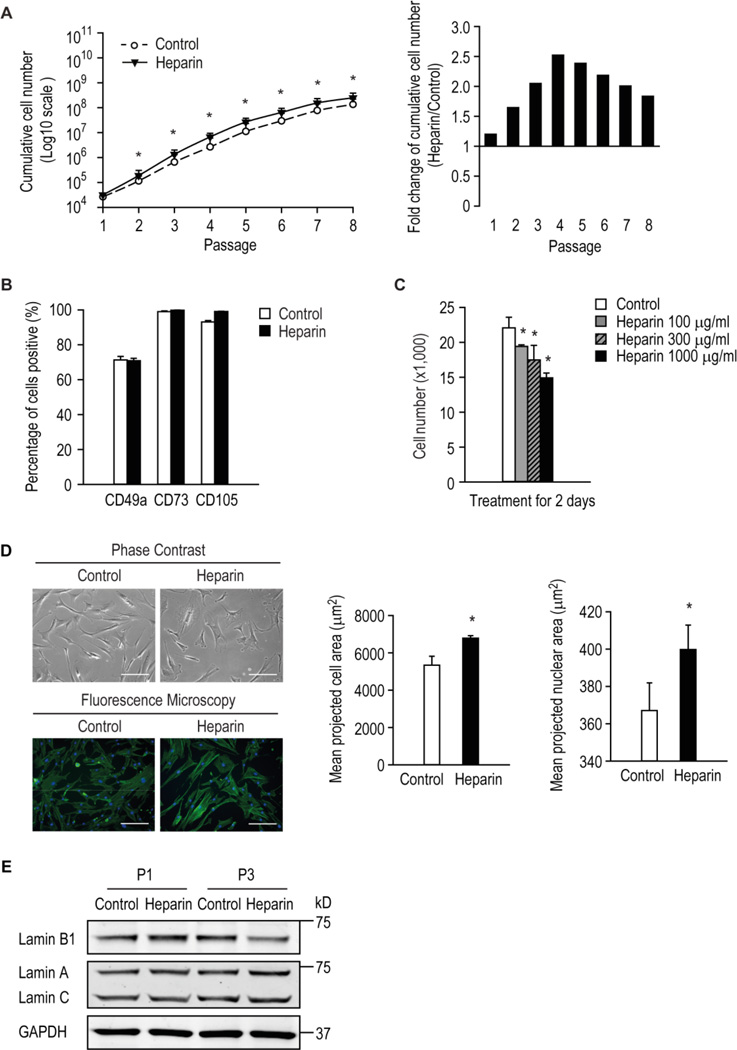
Figure 1. The response of hMSCs to various doses of heparin.
Human MSCs were serially passaged in the presence or absence of 160 ng/ml heparin and cumulative growth assessed (A) and the expression of surface antigens common to hMSCs by flow cytometry at late passage (B). * p < 0.05 versus control. (C) Number of hMSCs cultured with or without heparin at the indicated doses for 2 days. (D) left panel, hMSCs were treated for 3 days with or without 500 µg/ml heparin and imaged using phase contrast or fluorescence microscopy after cells were stained with DAPI and phalloidin to visualize nuclei in blue and actin cytoskeleton in green, respectively; right panel, image cytometric quantification of cell and nuclear projected area. * p < 0.05 versus control. (E) Human MSCs were grown in the presence or absence of 500 µg/ml heparin for one (P1) or three (P3) passages. The levels of target proteins were detected by Western blot analysis.
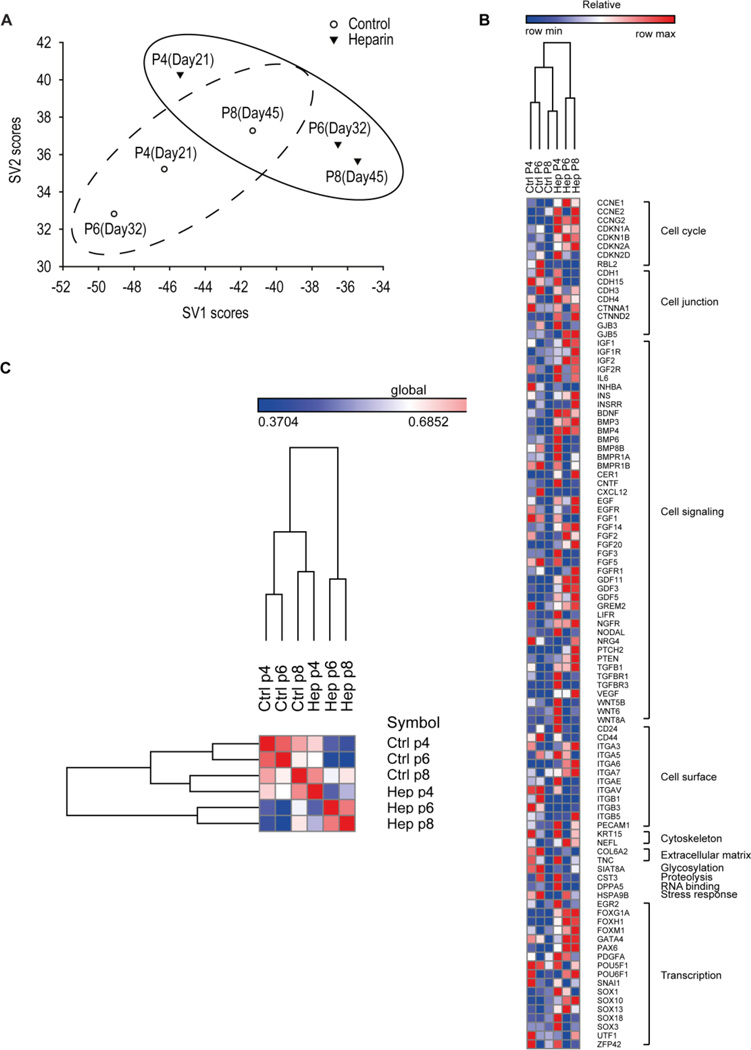
Figure 2. Human MSCs grown in the presence of heparin exhibited an altered gene expression signature.
Human MSCs were serially passaged with or without 160 ng/ml heparin. Gene expression signatures of hMSCs at P4, P6 and P8 were obtained using a stem cell-related chemiluminescent cDNA array. Expression profiles were analyzed by (A) singular value decomposition, or (B and C) hierarchical clustering and Pearson correlation distance/similarity approaches.
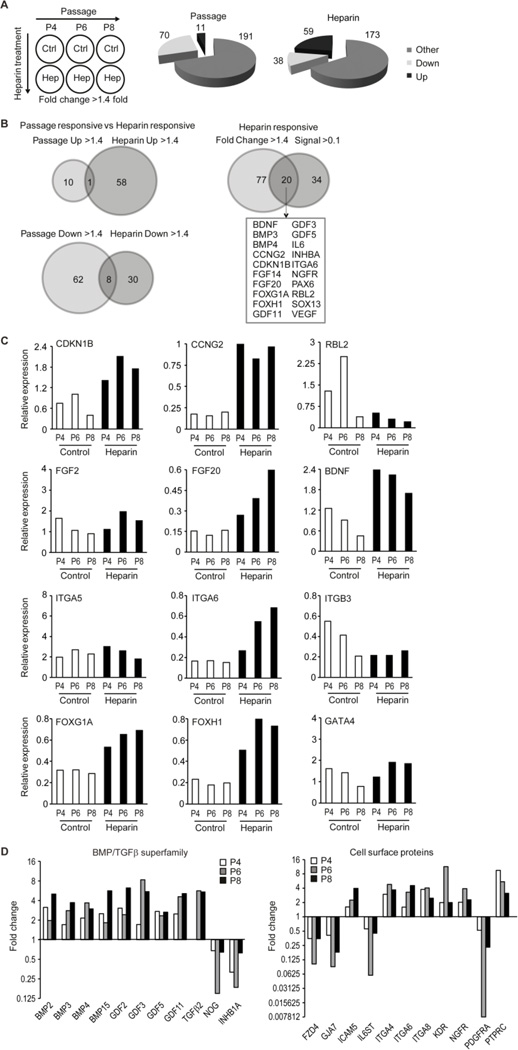
Figure 3. Heparin controls a broad set of genes involved in signal transduction in hMSCs.
Human MSCs were serially passaged with or without 160 ng/ml heparin. (A) Stem cell related gene expression data were analyzed for changes during passaging or in response to heparin at each passage as a six component matrix. The pie chart depicts the number of genes showing relevant changes during passage (left) or heparin treatment (right) for genes that are up- or down-regulated (>1.4 fold change; “Up” or “Down”) or shows no appreciable change (“Other”). (B) Venn diagrams illustrate the number of genes that are consistently up- or down-regulated at the three passages (P4, P6 or P8) and/or in response to heparin. The Venny diagram to the right compares all genes that are up (59) or down (38) regulated with genes that are robustly expressed (Signal >0.1). This analysis yields 20 genes that meet both criteria. (C) Expression analysis of selected genes to illustrate changes in expression during passage or heparin-treatment. (D) Bar graphs depict the fold change in expression in response to heparin for selected members of the BMP2/TGFβ family (left) or cell surface proteins (right). Gene expression was analyzed at three different passages (P4, P6 or P8) as indicated.
2.1. Cell culture
Human MSCs were either directly purchased from Lonza or isolated in our laboratory from human bone marrow mononuclear cells from young healthy male donors aged 20–30 years provided by Lonza (Donors 1 to 3) as previously described [36,41]. Cells were expanded and maintained in complete Dulbecco’s Modified Eagle’s Medium using our standard protocols [40] and we reported the number of passages under various treatment conditions (experimental passage number). Unless otherwise indicated, hMSCs were treated with 160 ng/ml of heparin (Sigma-Aldrich), a glycosaminoglycan dosage that is within the range used in other related stem cell studies [37,40].
2.2. Cell proliferation and cumulative growth analysis
Human MSCs were plated in triplicate at 5,000 cells/cm2 in the presence or absence of heparin as indicated and proliferation determined by monitoring viable cell number using the GUAVA PCA-96 benchtop flow cytometer as per manufacturer’s instructions (Millipore). Briefly, cells were dislodged by trypsinization and stained with GUAVA Flex dye. Cell suspensions were counted using the GUAVA Viacount program.
To monitor the cumulative growth, cells were plated at the same density (5,000 cells/cm2) with or without heparin as indicated and sub-cultured upon reaching 70–80 % confluency. The cells were re-plated under the same condition at each passage and the viable cells were counted using GUAVA system as decribed above.
2.3. Image cytometry
Human MSCs were seeded in triplicate into chamber slides at 3,000 cells/cm2 and allowed to attach overnight. Cells were then treated with or without 500 µg/ml heparin for 3 days. Cells were fixed with 4 % paraformaldehyde and stained with Rhodamine-conjugated phalloidin and DAPI (Life Technologies). Samples were imaged with an Olympus IX83 inverted microscope with a Cool Pix HQ2 camera and MetaMorph software, using slide scanning mode. Image sets were then processed using CellProfiler 2.0 software (http://cellprofiler.org) [42,43]. Briefly, the analysis pipeline corrected illumination uniformity, segmented nuclei and cell areas using DAPI and actin staining, respectively, and measured the area and shape parameters of the identified objects. 500–2000 cells were analysed per sample.
2.4. Immunoblotting
Cells were treated as indicated and the levels of lamin A, B1 and C protein detected as described previously [44]. Antibodies against lamin A/C and lamin B1 were purchased from Millipore and YenZym, respectively, and the antibody against house-keeping protein GAPDH was from Sigma.
2.5. Flow cytometry
Cells were dislodged with TrypLE™ (Life Technologies) and washed with PBS. The expression of stem cell surface antigens of interest was assayed as described previously [36,40,41]. Stained cells were analyzed with the BD FACSArray™ Bioanalyzer and FlowJo software (Tree Star Inc). All antibodies were purchased from BD Biosciences. For Donor 1–3 samples, STRO-1 was kindly supplied by Prof Stan Gronthos, School of Medical Sciences, Faculty of Health Sciences, University of Adelaide, Australia, and isotype control IgM (µ) (Caltag Laboratories).
2.6. Stem cell microarray
RNA was extracted from serially-passaged hMSCs (+/− heparin supplementation), converted to cDNA and probed using the GEArray S Series Human Stem Cell Gene Array (SABiosciences) as per manufacturer’s instructions. The array was scanned using a Chemi-Smart 3000 image acquisition system (Vilber Lourmat). The stem cell signature was created by using Singular Value Decomposition (SVD) [45,46], which reveals the relatedness of different biological samples. Graphical clustering of expression signatures indicates biological similarities.
The gene expression data were further analysed using GeneSpring GC 11.0 software and DAVID Bioinformatics Resources 6.7 (http://david.abcc.ncifcrf.gov) [47]. The overlapping of gene targets in different cells was acquired using VENNY software (http://bioinfogp.cnb.csic.es/tools/venny/index.html). Hierarchical clustering was carried out using GENE-E (version 3.0.228; Broad Institute, Cambridge, MA, USA).
2.7. Multilineage differentiation
Following serial passaging in the presence or absence of heparin, hMSCs then induced for osteogenic, chondrogenic, or adipogenic differentiation and lineage commitment assessed by histochemical staining [36,40,41]. For clarity, heparin was not included in any of the induction media.
Osteogenic (Alizarin Red staining) adipogenic (Oil Red O staining) and chondrogenic (Alcian Blue) staining was performed as previously described [36,40]. Intensities of the colorimetric dyes were quantified from digitally scanned images using the “Unmix Colors” module in CellProfiler 2.1.1. Areas of interest were isolated by masking (for culture wells) or manual tracing (pellet sections). Total dye intensity was then integrated in the area of interest, and normalised to the area (for pellet sections of varying size).
2.8 Next generation RNA sequencing and bioinformatic analysis
Human MSCs were harvested with TRIzol® Reagent (Invitrogen) and RNA was isolated according the manufacturer’s protocol. RNA sequencing and bioinformatics was performed as detailed previously [47]. Briefly, oligo dT magnetic beads were used to select polyadenylated mRNAs for the TruSeq RNA method (Illumina). Samples were indexed using TruSeq Kits (12-Set A and 12-Set B) for multiplexing on the flow cells of an Illumina HiSeq 2000 sequencer. Library preparation and concentration was test for quality control using an Agilent Bioanalyzer DNA 1000 chip and Qubit fluorometer (Invitrogen) respectively. Sequencing data was processed using MAPRSeq (v.1.2.1), TopHat 2.0.6, HTSeq, and edgeR 2.6.2 work flows [47,48]. Gene expression values were normalized to 1 million reads and corrected for gene length (reads per kilobasepair per million mapped reads, RPKM). Functional gene annotation analysis and overlapping genes were performed as described above.
2.9. Statistical analysis
Experiments were performed with at least three biological replicates for every condition and the data expressed as mean ± S.D. unless stated otherwise. Differences among treatments were analyzed by a two-tailed unpaired t test. Significant differences were considered as those with a p value of < 0.05 (*).
3. Results
3.1. Heparin supplementation enhanced hMSC proliferation without affecting the expression of stem cell markers
Heparin binds and activates a large number of mitogenic factors and morphogens that mediate proliferation and lineage commitment of progenitor cells. We evaluated its mitogenic properties on hMSCs by monitoring cumulative cell growth. This was consistently enhanced when cells were serially passaged in the presence of 160 ng/ml heparin compared with the control (Fig. 1 A). Interestingly, at earlier passages (≤ passage 4) the proliferative effect of heparin was greatest after which a decline was observed (Fig. 1A, right panel). Heparin has been previously reported to accelerate proliferation of genetically modified hMSCs [37], consistent with our observations at early passage.
Having established the effect of heparin on the culture-expansion of hMSCs, we next examined the stem cell quality after serially-passaging by assessing the expression of particular stem cell surface antigens. The expression of surface antigens CD49a, CD73 and CD105, standard markers for the identification of MSCs, did not change appreciably on serially-passaged cells in the presence of heparin (Fig. 1B). These results suggest that routine expansion of hMSCs in the presence of heparin may not affect the selected cell surface antigens of a given donor.
3.2. High-dose heparin significantly inhibits hMSC growth
Having established that low-dose heparin (160 ng/ml) modestly increased hMSC proliferation, we next examined their response to a range of higher heparin concentrations. The results showed that growth of hMSCs were inhibited by heparin at 100–1000 µg/ml (Fig. 1C). Cells treated with 500 µg/ml heparin for 3 days also exhibited an altered morphology; these cells were flatter and larger than control cells, which preserved their fibroblast-like spindle shape (Fig. 1D, left panel). Quantification by image cytometry of the mean projected cell and nuclear areas confirmed that high-dose heparin treatment resulted in significantly larger cells and nuclei (Fig. 1D, right panel), morphological features that are consistent with onset of a dormant (quiescent or senescent) cellular state. Also, hMSCs cultured with 500 µg/ml heparin for three passages have reduced level of lamin B1 (Fig. 1E), a senescence marker reported previously [44], whilst the levels of lamin A or C were not affected. Thus, although heparin has positive effects on proliferation at low-doses, it is a potent growth inhibitor and might cause premature cellular senescence at higher doses.
3.3. Heparin supplementation in culture supports expansion of hMSCs but may compromise cell fate decisions
Because chronic heparin treatment appears to influence the naïve undifferentiated state of hMSCs, we next examined molecular pathways involved in this response using cDNA arrays. RNA samples were isolated from control and heparin-treated hMSCs at passages (P4, 6 or 8) and mRNA levels were determined using a customized cDNA array that monitors expression of a large set of extracellular ligands (e.g., growth factors, cytokines), cell surface proteins (e.g., growth factor receptors, integrins), cytoplasmic proteins and nuclear transcription factors (Figs. 2 and 3).
The expression profiles of hMSCs were analyzed and the resulting hMSC signatures were evaluated by Singular Value Decomposition (SVD) [45,46] (Fig. 2A). These studies were modeled on similar approaches previously used to distinguish tumor subtypes [49,50]. The microarray data compared the expression of 262 genes across 6 samples. Each expression value was log-transformed and centered across samples before application of Singular Value Decomposition analysis. Projection was onto the first 2 right singular vectors, which together capture about 70% of the variation in the original data. Thereafter, each sample was defined by its score - a linear combination of the gene expression values and the coefficients of the 2 singular vectors. Samples with similar phenotypes tend to cluster on a score plot. The analysis revealed that the temporal expression of genes in cells cultured under control conditions are different in short-term versus long-term culture (Fig. 2A). These differences indicate that heparin alters the molecular phenotype of hMSCs as a function of time in culture. Human MSCs at passage 4 (the lowest passage analyzed), appear to have a signature resembling that of naïve hMSCs. Parallel cultures of hMSCs exposed to heparin have their own unique signature when compared to control cells, which reflect stem cell related alterations in their molecular characteristics phenotype. These observations were corroborated by hierearchical clustering (Fig. 2B) and Pearson correlation distance/similarity analysis (Fig. 2C) of representative genes that exhibit changes in gene expression (Fig. 3). Taken together, culture expansion in the presence of heparin modifies the passage-related gene expression phenotype of hMSCs.
3.4. Heparin controls a broad set of genes involved in signal transduction in hMSCs
We analyzed changes in gene expression over passage (independent of the effect of heparin) or upon heparin treatment (independent of the effect of passaging) in hMSCs using arbitrary cut-offs (>1.4 fold or <0.7 fold) to filter for biologically relevant changes (Fig. 3A). In addition, we applied optional filters related to relative expression levels (signal >1 or >0.1 arbitrary units) as indicated to focus on genes with prominent levels of expression. The arbitrary cut-offs for signal strength >1 or 0.1 were selected to obtain a manageable number of genes that illustrate what genes are robustly expressed in the basal state in the absence of heparin (signal >1) and/or which set of genes shows biologically interesting changes in gene expression in response to heparin (signal > 0.1 and fold change >1.4 or <0.7). Of the 262 genes analyzed, there are only 8 genes that are consistently expressed at high levels (signal > 1) in hMSCs regardless of passage or heparin treatment in all six conditions. These genes are the glycosylated cell surface protein CD24, the intracellular proteinase inhibitor Cystatin-3 (CST3), the extracellular ligand Wnt8A, integrin α5 (ITGA5), three different transcription factors (SOX1, EGR2/Krox20 and POU5F1/Oct4) and the cell cycle inhibitor CDKN2D. The abundant expression of these genes is consistent with the multi-lineage potential of hMSCs. CD24, SOX1 and POU5F1/OCT4 are markers of progenitor cells [51–53], while EGR2, Wnt8A and ITGA5 are linked to differentiation in distinct cell lineages [54–56].
During successive passaging from P4 to P8, 70 genes are down-regulated and 11 are up-regulated, reflecting modulations in proliferative potential and lineage phenotype (Fig. 3A, left pie chart). Heparin treatment consistently up-regulates 59 genes and down-regulates 38 genes by at least 1.4 fold, regardless of the passage number (Fig. 3A, right pie chart). The vast majority of genes regulated by heparin (~90 %) are different from those regulated as a consequence of passage. Only 1 of the 11 genes that were up-regulated and only 8 of the 70 genes that were down-regulated (~10 %) during passage were also modulated by heparin (Fig. 3B, left panel). Taken together, these findings indicate that heparin selectively alters expression of genes that are distinct from those related to continuous culture of hMSCs.
Of the 97 (i.e., 38 plus 59) genes that are up- or down-regulated by heparin, at least 20 genes had robust basal expression (signal >0.1) (Fig. 3B, right panel). Three of these have known roles in cell cycle control. The cell cycle regulators CDKN1B (p27) and CCNG2 (cyclin G2), both associated with cell growth inhibition, are each up-regulated, while the tumor suppressor protein RBL2 (p130) was down-regulated (Fig. 3C). In addition, heparin significantly increases expression of a series of growth factors, including FGF14, FGF20, BDNF, IL6 and VEGF, but not FGF2 (Fig. 3C and data not shown) as well as the expression of a number of cell surface proteins, including ITGA6 (integrin α6) and NGFR, but not ITGA5 or ITGB3 (Fig. 3C and data not shown). In addition, heparin increases the mRNA levels of a series of transcription factors, including FOXG1A, FOXH1, PAX6 and SOX13, but not GATA4 (Fig. 3C and data not shown). Thus, heparin selectively stimulates expression of a number of different cell surface proteins and nuclear effectors; such pleiotropic effects are expected based on its biochemical activity as a multivalent co-ligand.
Heparin’s most striking gene expression effect is the major induction of multiple members of the TGFβ/BMP/GDF superfamily including BMP3, BMP4, GDF3, GDF5 and GDF11 (Fig. 3D). In addition, heparin suppresses the corresponding inhibitors NOG (noggin) and INHB1A (inhibin 1A) (Fig. 3D). These changes are consistent with auto- or paracrine enhancement of TGFβ/BMP/GDF signaling. Heparin also increases the expression of selected Wnt and FGF members (Fig. 3C and data not shown), as well as modulates (up or down) cell surface markers including FZD4, NGFR, PDGFRA and PTPRC (Fig. 3D). We conclude that heparin compromises expression of mRNA transcripts encoding proteins known to play a role in cell fate decisions.
3.5. Heparin exhibits variable effects on the proliferation of hMSCs from different donors
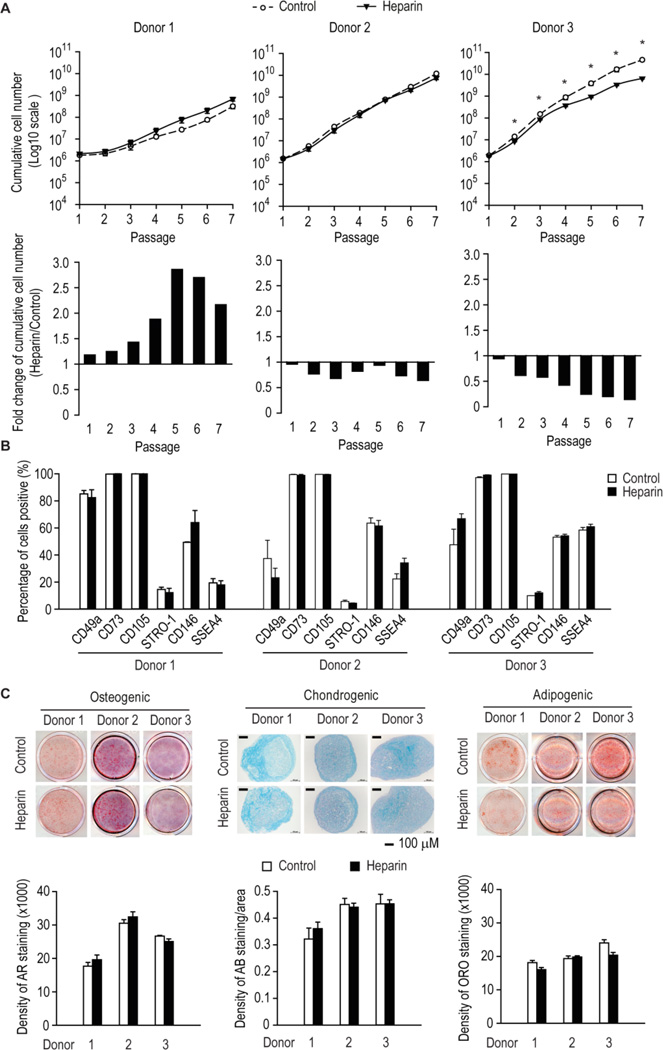
Having established that heparin at 160 ng/ml increased the number of hMSCs in culture and negatively affects their molecular signature, we next sought to determine whether this finding was similar for hMSCs freshly isolated from human bone marrow mononuclear cells. Human MSCs from different donors responded variably to long-term heparin treatment, as their growth was either slightly increased at late passages (≥ P5), not affected, or significantly inhibited (Fig. 4A).
Figure 4. The long-term effect of heparin on hMSCs from multiple donors.
(A) Human MSCs serially passaged in the presence or absence of 160 ng/ml heparin. Cumulative cell numbers were calculated. Significance is reported (* p < 0.05) when all 6 passages showed a difference. (B and C) Human MSCs were serially passaged in the presence or absence of 160 ng/ml heparin and assessed at late passage for the expression of cell surface antigens by flow cytometry, or their ability to differentiate into the osteogenic, adipogenic and chondrogenic lineages as assessed by histochemical staining for Alizarin Red, Oil-Red-O or Alcian Blue and the intensity of stain quantified by densitometry.
Similar to the data in Figure 1B, the effect of long-term passaging with heparin on the expression of CD49a, CD73 and CD105 in hMSCs from different donors was also minimal (Fig. 4B). Expression of three additional stem cell markers STRO-1, CD146 and SSEA4 [57–59] was similar, except that heparin slightly increased SSEA4 expression in hMSCs from donor 2 (Fig. 4B). Multilineage staining for hMSCs from these three donors showed that long-term culturing with heparin supplementation also had little effect on the multipotency of hMSCs (Fig. 4C).
3.6. RNA-seq analysis reveals genome-wide differences that modify cell growth characteristics of MSCs upon chronic heparin treatment
Because hMSCs from three different donors exhibit different cell growth responses to heparin treatment, we investigated how heparin affects the molecular properties of hMSCs using transcriptomic analysis. We focused our RNA-seq analysis on Donor 3 in which heparin exhibits a clear negative effect on cell proliferation, and compared our dataset with expression results obtained for Donor 2 which is refractory to the growth modulatory effects of heparin (see Fig. 4A). As such, Donor 2 represents a negative control that permits elimination of gene expression effects of heparin independent of biological effects on cell proliferation. We compared gene expresion patterns of late cultured cells in the presence or absence of heparin (Fig. 5). We filtered the datasest for genes with reads per kilobasepair per million mapped reads (RPKMs) greater than 0.3, which reduces false discovery of mRNA detection, as well as for fold-changes in expression values greater than 2-fold (up or down) upon heparin treatment for each donor. Strikingly, we observed very limited overlaps in the groups of genes that are heparin responsive between the two donors (Fig. 5A), consistent with the observation that heparin has donor-dependent pleiotropic effects on cell growth (see Fig.4A).
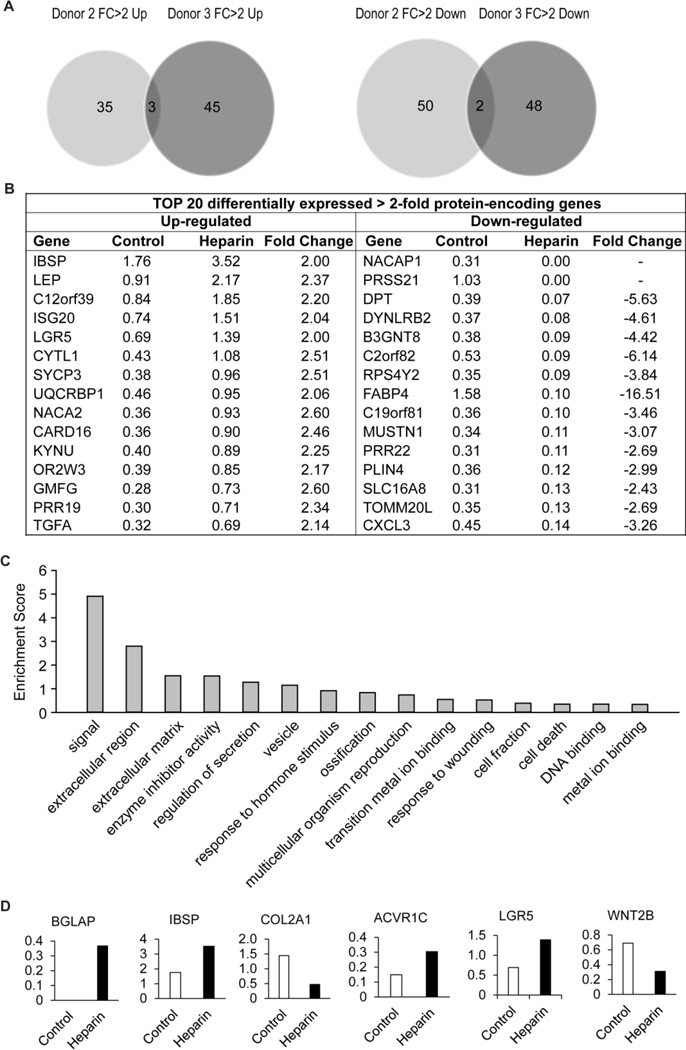
Figure 5. High-resolution gene expression analysis of the effects of heparin on long-term culture.
RNA-seq gene expression analysis was performed on hMSCs cultured with or without heparin (160ng/ml) for 7 passages. (A) Protein encoding genes with greater than 2-fold change in expression under heparin treatment were compared for both Donor 2 and 3. (B) Genes differentially expressed (>2-fold under heparin treatment) for Donor 3 were ranked from highest to lowest and the top 20 genes tabulated. (C) Functional annotation analysis of genes differentially expressed in Donor 3 grouped into the 15 most enriched categories. (D) Representative RNA-seq expression genes enriched in the functional category “Signal”.
We observed that there are 45 genes that are uniquely upregulated by heparin in Donor 3 only, while 48 genes are differentially down-regulated by heparin compared to Donor 2 (Fig. 5A). To identify the most prominently regulated genes within these groups, we sorted genes for highest expression values (in RPKM) yielding a set of 15 heparin-responsive up- or down-regulated genes with most robust expression (Fig. 5B). None of this diverse set of genes appear to have obvious functions that can directly account for the cell growth perturbations that are evident in biological assays (see Fig. 4). Hence, unique cell proliferative effects of heparin on donor 3 may affect primarily translational or post-translational mechanisms that do not perturb gene expression at the mRNA level (e.g., miRNAs, protein stability, protein phosphorylation).
To understand the biological activities of the entire set of genes both up- and down-regulated by heparin, we performed gene ontology analysis (Fig. 5C). The two most enriched categories are genes involved in signaling and extracellular region (enrichment scores of ~5 and ~3, respectively). Interestingly, these two categories are directly related to the main activities of heparin that control the interaction of signaling ligands with extracellular proteoglycans to modulate intracellular kinase pathways. Among the category signaling, heparin modulates the expression of at least two receptors (i.e., ACVR1C and LGR5), a ligand (Wnt2B) and three skeletal ECM proteins (i.e., BGLAP, ISBP and COL2A1). Compared to our earlier qPCR profiling studies with one original donor (Figs. 1 to 3) that prompted later studies with three additional donors (Figs. 4 and 5), we observed that different sets of genes are modulated. This result is expected from the observation that different donors respond both biologically (Figs. 1A and 4A) and molecularly (Figs. 2 and 5A) to heparin. Taken together, the RNA-seq results suggests that heparin may affect cell growth at least in part by altering mRNA levels of distinct sets of cell growth-related receptor signaling pathways in a donor dependent manner.
4. Discussion
Although widely used as an anti-clotting agent for patients at risk of thrombosis or embolism, heparin’s broad ability to bind and potentiate the activity of a plethora of factors unrelated to blood coagulation has rendered it a versatile agent that is widely used as a culture supplement for many mammalian cell types, including hMSCs and hESCs. For example, heparin has been used as an additive for stem cell expansion and hydrogel formulation to promote stem cell pluripotency [5].
Notwithstanding its widespread use as a stem cell culture additive, the results here show that heparin supplementation invites potential risk to ex vivo cultured stem cells from the bone marrow compartment. The adverse effect of long-term heparin therapy as an anti-coagulant on skeletal tissues is widely recognized, adding further caution to its use as a culture reagent. Heparin reduces bone density either through increasing bone resorption or decreasing bone formation [60]. The high affinity of heparin for BMPs can dysregulate the activity of those osteogenic growth factors, and thus osteoblast-induced bone formation. A number of studies have demonstrated that heparin restricts the signaling and inhibit the activity of BMPs 2, 4, 6 and 7 [61–64].
Our data suggests that heparin supplementation might modestly increase hMSC proliferation at low doses, but strongly suppresses growth at high doses. High dose heparin also significantly altered cell morphology, including cell and nuclear area and transformed the hMSCs to a more senescent phenotype. During osteogenic differentiation, MSCs gradually lose their original morphology, by flattening and spreading [65], which correlates with loss of proliferative ability [66], findings supported by our current study. Notably, heparin at high dose was reported to be deleterious to hESCs [5].
Continuous supplementation of hMSCs with a growth-permissive dose of heparin (as seen in Figure 1), resulted in changes in mRNA expression of multiple lineage-specific markers. These effects collectively represents a major drawback for the exploitation of hyper-sulfated heparin as a stem cell culture additive. Moreover, cultures supplemented with heparin show an altered molecular signature. Ultimately, hMSCs serially-passaged in heparin must be tested for their in vivo efficacy in well-accepted animal models. For example, rodent models of bone fracture repair have been used to assess hMSC quality [36,40,67] and provide a robust assessment of the effect of ex vivo expansion strategies on their therapeutic utility.
The analysis here demonstrates that heparin exerts long-term effects on cells that are reflected by changes in gene expression. Global mRNA expression profiles of control versus heparin-expanded cells as measured by cDNA arrays or RNA-seq are distinct in several donors at representative passages. Importantly, heparin alters the gene expression profiles of multiple ligands, receptors and downstream transcription factors in a donor-dependent manner. For example, heparin increases expression of several FGFs and lineage commitment-related factors such as BMP4, VEGF, GDF5 and Wnt2B, depending on the donor and/or method of analysis (i.e., cDNA array versus RNA-seq). Because FGF, BMP and Wnt signaling are central to the proliferation and differentiation capacities of hMSCs, the observed modulation of these transcripts by heparin during long-term culture may account for at least some of the changes in cell proliferation and ability to control cell fate decisions.
Another prominent finding of the gene expression profiling in response to heparin is the donor-dependent coordinate up-regulation of multiple members of the TGFβ/BMP/GDF superfamily and concomitant down-regulation of pathway inhibitors (i.e., noggin and inhibin 1A). TGFp signaling inhibits osteogenic differentiation and promotes senescence [5,68,69]. In contrast, BMPs stimulate stem cell differentiation [70]. Therefore, changes in the auto- or paracrine production of TGFβ/BMP family members may directly contribute to loss of multipotentiality and ‘stemness’.
We note that donor-to-donor variability contributes significantly towards the observed effects of heparin at both biological and molecular levels. Heparin treatment of hMSCs from four different donors revealed growth enhancement, inhibition or no difference. The pleiotropic effects of heparin may biologically amplify the inherent donor-to-donor variability of hMSCs. Indeed, analyses by cDNA arrays or high resolution RNA-seq revealed donor-dependent differences in gene expression in hMSCs from different donors following long term culture in heparin.
Although our study indicates that heparin may not be suitable as an additive for long-term proliferative expansion of naïve multipotent hMSCs, there are HS variants that may be more suitable for routine culturing of hMSCs. Both heparin and HS consist of a repeating disaccharide unit with highly variable sulfation patterns. Sulfation most often occurs on N-, 2-O-, and 6-O-groups and these sulfated groups are responsible for the interaction with growth factors or adhesion proteins. Though heparin is closely related to HS, it is primarily distinguished from HS by its higher sulfation, lack of domain structure, higher frequency of N-sulfation, presence of 3-O-sulfation, and more O-sulfation than N-sulfation [71]. Differences in the temporal expression of HS variants as tissues grow and mature may permit selective binding and co-activation of distinct growth factor-dependent signalling pathways that regulate cell growth and differentiation [72].
Indeed, leveraging this biochemical diversity, we have isolated HS variants that possess unique domains structures that target protein ligands controlling the phenotypic properties of hMSCs (e.g., FGF2, BMP2, VEGF165) [17,20,40,73–75]. We anticipate that better preparations of HS, rather than heparin, may be useful for routine culture of hMSCs without adversely affecting the biological properties of the cells.
5. Conclusions
This study assessed the effects of low-dose heparin on hMSCs in vitro, and highlights the potential risk to patients undergoing chronic heparin therapy – the possible impairment of naïve stem cell replenishment and subsequent maintenance of tissue homeostasis from the bone marrow. Our work demonstrates the hazard of using heparin and suggests caution in its use as an adjuvant during stem cell bioprocessing.
Highlights.
Heparin is a common cell culture adjuvant for the expansion of human stem cells.
Heparin affects multiple cell signaling components that can alter human mesenchymal stem cell responsiveness.
Heparin exerts donor-dependent effects on gene expression and proliferation of human mesenchymal stem cells.
Heparin is contra-indicated as a culture supplement for the expansion and maintenance of naïve human mesenchymal stem cells.
Acknowledgments
We thank all members of the Glycotherapeutics Group from the Institute of Medical Biology (IMB), Agency for Science, Technology and Research (A*STAR) and the Orthopedic Research Laboratories at Mayo Clinic for stimulating discussions. In particular, we thank Ms Phua Zer Cheng, and A*STAR’s Advanced Molecular Pathology Laboratory. We acknowledge that data was initially collected at the Institute of Molecular and Cell Biology, A*STAR and continued at IMB. This work was also supported by National Medical Research Council of Singapore (NMRC) and in part by National Institutes of Health Grants AR49069 (AvW).
Abbreviations
- hMSCs
human bone marrow-derived mesenchymal stem cells
- RNA-seq
high throughput next generation RNA sequencing
- FGF
fibroblast growth factor
- HBD
heparin-binding domain
- VEGFs
vascular endothelial factors
- UFH
unfractionated heparin
- LMWH
low-molecular-weight heparin
- hESC
human embryonic stem cell
- HS
heparan sulfate
- ECM
extracellular matrix
- FCS
fetal calf serum
- BMMNCs
human bone marrow mononuclear cells
- BrdU
5-bromo-2-deoxyuridine
- SVD
singular value decomposition
- ALP
alkaline phosphatase
- BSPII
bone sialoprotein II
- C/EBP
CCAAT/enhancer binding protein
- ALBP
adipocyte lipid binding protein
- CST3
Cystatin-3
- ITGA
integrin
- CCNG2
cyclin G2
- INHB
inhibin
- RPKM
reads per kilobasepair per million mapped reads
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ronnberg E, Pejler G. Serglycin: the master of the mast cell. Methods Mol Biol. 2012;836:201–217. doi: 10.1007/978-1-61779-498-8_14. [DOI] [PubMed] [Google Scholar]
- 2.Alter CS, Metcalfe DD, Bradford TR, Schwartz LB. Regulation of human mast cell tryptase. Effects of enzyme concentration, ionic strength and the structure and negative charge density of polysaccharides. Biochem J. 1987;248:821–827. doi: 10.1042/bj2480821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naimy H, Leymarie N, Bowman MJ, Zaia J. Characterization of heparin oligosaccharides binding specifically to antithrombin III using mass spectrometry. Biochemistry. 2008;47:3155–3161. doi: 10.1021/bi702043e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lane DA, Adams L. Non-anticoagulant uses of heparin. N Engl J Med. 1993;329:129–130. doi: 10.1056/NEJM199307083290212. [DOI] [PubMed] [Google Scholar]
- 5.Furue MK, Na J, Jackson JP, Okamoto T, Jones M, Baker D, Hata R, Moore HD, Sato JD, Andrews PW. Heparin promotes the growth of human embryonic stem cells in a defined serum-free medium. Proc Natl Acad Sci U S A. 2008;105:13409–13414. doi: 10.1073/pnas.0806136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasaki N, Okishio K, Ui-Tei K, Saigo K, Kinoshita-Toyoda A, Toyoda H, Nishimura T, Suda Y, Hayasaka M, Hanaoka K, Hitoshi S, Ikenaka K, Nishihara S. Heparan sulfate regulates self-renewal and pluripotency of embryonic stem cells. J Biol Chem. 2008;283:3594–3606. doi: 10.1074/jbc.M705621200. [DOI] [PubMed] [Google Scholar]
- 7.Ling L, Dombrowski C, Foong KM, Haupt LM, Stein GS, Nurcombe V, van Wijnen AJ, Cool SM. Synergism between Wnt3a and heparin enhances osteogenesis via a phosphoinositide 3-kinase/Akt/RUNX2 pathway. J Biol Chem. 2010;285:26233–26244. doi: 10.1074/jbc.M110.122069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uygun BE, Stojsih SE, Matthew HW. Effects of immobilized glycosaminoglycans on the proliferation and differentiation of mesenchymal stem cells. Tissue Eng Part A. 2009;15:3499–3512. doi: 10.1089/ten.tea.2008.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ratanavaraporn J, Tabata Y. Enhanced osteogenic activity of bone morphogenetic protein-2 by 2-O-desulfated heparin. Acta Biomater. 2012;8:173–1782. doi: 10.1016/j.actbio.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 10.K Na, Kim S, Park K, Kim K, Woo DG, Kwon IC, Chung HM, Park KH. Heparin/poly(l-lysine) nanoparticle-coated polymeric microspheres for stem-cell therapy. J Am Chem Soc. 2007;129:5788–5789. doi: 10.1021/ja067707r. [DOI] [PubMed] [Google Scholar]
- 11.Benoit DS, Durney AR, Anseth KS. The effect of heparin-functionalized PEG hydrogels on three-dimensional human mesenchymal stem cell osteogenic differentiation. Biomaterials. 2007;28:66–77. doi: 10.1016/j.biomaterials.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol. 1999;237:1–30. doi: 10.1007/978-3-642-59953-8_1. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J, Shing Y. Control of angiogenesis by heparin and other sulfated polysaccharides. Adv Exp Med Biol. 1992;313:355–364. doi: 10.1007/978-1-4899-2444-5_34. [DOI] [PubMed] [Google Scholar]
- 14.Gengrinovitch S, Greenberg SM, Cohen T, Gitay-Goren H, Rockwell P, Maione TE, Levi BZ, Neufeld G. Platelet factor-4 inhibits the mitogenic activity of VEGF121 and VEGF165 using several concurrent mechanisms. J Biol Chem. 1995;270:15059–15065. doi: 10.1074/jbc.270.25.15059. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209:139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- 16.Turnbull J, Powell A, Guimond S. Heparan sulfate: decoding a dynamic multifunctional cell regulator. Trends Cell Biol. 2001;11:75–82. doi: 10.1016/s0962-8924(00)01897-3. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Poon S, Murali S, Koo CY, Bell TJ, Hinkley SF, Yeong H, Bhakoo K, Nurcombe V, Cool SM. Engineering a vascular endothelial growth factor 165-binding heparan sulfate for vascular therapy. Biomaterials. 2014;35:6776–6786. doi: 10.1016/j.biomaterials.2014.04.084. [DOI] [PubMed] [Google Scholar]
- 18.Norrby K, Ostergaard P. A 5.0-kD heparin fraction systemically suppresses VEGF165-mediated angiogenesis. Int J Microcirc Clin Exp. 1997;17:314–321. doi: 10.1159/000179246. [DOI] [PubMed] [Google Scholar]
- 19.Bramono DS, Murali S, Rai B, Ling L, Poh WT, Lim ZX, Stein GS, Nurcombe V, van Wijnen AJ, Cool SM. Bone marrow-derived heparan sulfate potentiates the osteogenic activity of bone morphogenetic protein-2 (BMP-2) Bone. 2012;50:954–964. doi: 10.1016/j.bone.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murali S, Rai B, Dombrowski C, Lee JL, Lim ZX, Bramono DS, Ling L, Bell T, Hinkley S, Nathan SS, Hui JH, Wong HK, Nurcombe V, Cool SM. Affinity-selected heparan sulfate for bone repair. Biomaterials. 2013;34:5594–5605. doi: 10.1016/j.biomaterials.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Hitraya EG, Tan EM, Rudnicka L, Jimenez SA. Expression of extracellular matrix genes in adult human dermal microvascular endothelial cells and their regulation by heparin and endothelial cell mitogens. Lab Invest. 1995;73:393–402. [PubMed] [Google Scholar]
- 22.Tyagi SC, Kumar S, Katwa L. Differential regulation of extracellular matrix metalloproteinase and tissue inhibitor by heparin and cholesterol in fibroblast cells. J Mol Cell Cardiol. 1997;29:391–404. doi: 10.1006/jmcc.1996.0283. [DOI] [PubMed] [Google Scholar]
- 23.Sweeney SM, Guy CA, Fields GB, San Antonio JD. Defining the domains of type I collagen involved in heparin- binding and endothelial tube formation. Proc Natl Acad Sci U S A. 1998;95:7275–7280. doi: 10.1073/pnas.95.13.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hulett MD, Freeman C, Hamdorf BJ, Baker RT, Harris MJ, Parish CR. Cloning of mammalian heparanase, an important enzyme in tumor invasion and metastasis. Nat Med. 1999;5:803–809. doi: 10.1038/10525. [DOI] [PubMed] [Google Scholar]
- 25.Dudakovic A, Camilleri E, Riester SM, Lewallen EA, Kvasha S, Chen X, Radel DJ, Anderson JM, Nair AA, Evans JM, Krych AJ, Smith J, Deyle DR, Stein JL, Stein GS, Im HJ, Cool SM, Westendorf JJ, Kakar S, Dietz AB, van Wijnen AJ. High-resolution molecular validation of self-renewal and spontaneous differentiation in clinical-grade adipose-tissue derived human mesenchymal stem cells. J Cell Biochem. 2014;115:1816–1828. doi: 10.1002/jcb.24852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langeslay DJ, Beni S, Larive CK. Detection of the 1H and 15N NMR resonances of sulfamate groups in aqueous solution: a new tool for heparin and heparan sulfate characterization. Anal Chem. 2011;83:8006–8010. doi: 10.1021/ac202144m. [DOI] [PubMed] [Google Scholar]
- 27.Bambrah RK, Pham DC, Zaiden R, Vu H, Tai S. Heparin-induced thrombocytopenia. Clin Adv Hematol Oncol. 2012;9:594–599. [PubMed] [Google Scholar]
- 28.Mazziotti G, Canalis E, Giustina A. Drug-induced osteoporosis: mechanisms and clinical implications. Am J Med. 2010;123:877–884. doi: 10.1016/j.amjmed.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 29.Sackler JP, Liu L. Heparin-induced osteoporosis. Br J Radiol. 1973;46:548–550. doi: 10.1259/0007-1285-46-547-548. [DOI] [PubMed] [Google Scholar]
- 30.Bounameaux H, Schneider PA, Mossaz A, Suter P, Vasey H. Severe vasospastic reactions (ergotism) during prophylactic administration of heparin-dihydroergotamine. Vasa. 1987;16:370–372. [PubMed] [Google Scholar]
- 31.Gollub S, Ulin AW. Heparin-induced thrombocytopenia in man. J Lab Clin Med. 1962;59:430–435. [PubMed] [Google Scholar]
- 32.Irie A, Takami M, Kubo H, Sekino-Suzuki N, Kasahara K, Sanai Y. Heparin enhances osteoclastic bone resorption by inhibiting osteoprotegerin activity. Bone. 2007;41:165–174. doi: 10.1016/j.bone.2007.04.190. [DOI] [PubMed] [Google Scholar]
- 33.Ling L, Murali S, Stein GS, van Wijnen AJ, Cool SM. Glycosaminoglycans modulate RANKL-induced osteoclastogenesis. J Cell Biochem. 2010;109:1222–1231. doi: 10.1002/jcb.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benucci M, Bettazzi C, Bracci S, Fabiani P, Monsacchi L, Cappelletti C, Manfredi M, Ciolli S. Systemic mastocytosis with skeletal involvement: a case report and review of the literature. Clin Cases Miner Bone Metab. 2009;6:66–70. [PMC free article] [PubMed] [Google Scholar]
- 35.Prockop DJ, Oh JY. Medical therapies with adult stem/progenitor cells (MSCs): A backward journey from dramatic results in vivo to the cellular and molecular explanations. J Cell Biochem. 2012;113:1460–1469. doi: 10.1002/jcb.24046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samsonraj RM, Rai B, Sathiyanathan P, Puan KJ, Rotzschke O, Hui JH, Raghunath M, Stanton LW, Nurcombe V, Cool SM. Establishing criteria for human mesenchymal stem cell potency. Stem Cells. 2015 doi: 10.1002/stem.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mimura S, Kimura N, Hirata M, Tateyama D, Hayashida M, Umezawa A, Kohara A, Nikawa H, Okamoto T, Furue MK. Growth factor-defined culture medium for human mesenchymal stem cells. Int J Dev Biol. 2011;55:181–187. doi: 10.1387/ijdb.103232sm. [DOI] [PubMed] [Google Scholar]
- 38.Benoit DS, Anseth KS. Heparin functionalized PEG gels that modulate protein adsorption for hMSC adhesion and differentiation. Acta Biomater. 2005;1:461–470. doi: 10.1016/j.actbio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Bhakta G, Rai B, Lim ZX, Hui JH, Stein GS, van Wijnen AJ, Nurcombe V, Prestwich GD, Cool SM. Hyaluronic acid-based hydrogels functionalized with heparin that support controlled release of bioactive BMP-2. Biomaterials. 2012;33:6113–6122. doi: 10.1016/j.biomaterials.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helledie T, Dombrowski C, Rai B, Lim ZX, Lee I, Rider DA, Stein GS, Hong W, van Wijnen AJ, Hui JH, Nurcombe V, Cool SM. Heparan Sulfate Enhances the Self-Renewal and Therapeutic Potential of Mesenchymal Stem Cells from Human Adult Bone Marrow. Stem Cells Dev. 2012;21:1897–1910. doi: 10.1089/scd.2011.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rider DA, Dombrowski C, Sawyer AA, Ng GH, Leong D, Hutmacher DW, Nurcombe V, Cool SM. Autocrine fibroblast growth factor 2 increases the multipotentiality of human adipose-derived mesenchymal stem cells. Stem Cells. 2008;26:1598–1608. doi: 10.1634/stemcells.2007-0480. [DOI] [PubMed] [Google Scholar]
- 42.Kamentsky L, Jones TR, Fraser A, Bray MA, Logan DJ, Madden KL, Ljosa V, Rueden C, Eliceiri KW, Carpenter AE. Improved structure, function and compatibility for CellProfiler: modular high-throughput image analysis software. Bioinformatics. 2011;27:1179–1180. doi: 10.1093/bioinformatics/btr095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, Golland P, Sabatini DM. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dreesen O, Chojnowski A, Ong PF, Zhao TY, Common JE, Lunny D, Lane EB, Lee SJ, Vardy LA, Stewart CL, Colman A. Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J Cell Biol. 2013;200:605–617. doi: 10.1083/jcb.201206121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holter NS, Mitra M, Maritan A, Cieplak M, Banavar JR, Fedoroff NV. Fundamental patterns underlying gene expression profiles: simplicity from complexity. Proc Natl Acad Sci U S A. 2000;97:8409–8414. doi: 10.1073/pnas.150242097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alter O, Brown PO, Botstein D. Singular value decomposition for genome-wide expression data processing and modeling. Proc Natl Acad Sci U S A. 2000;97:10101–1016. doi: 10.1073/pnas.97.18.10101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 48.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bild AH, Yao G, Chang JT, Wang Q, Potti A, Chasse D, Joshi MB, Harpole D, Lancaster JM, Berchuck A, Olson JA, Jr, Marks JR, Dressman HK, West M, Nevins JR. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439:353–357. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- 50.Pomeroy SL, Tamayo P, Gaasenbeek M, Sturla LM, Angelo M, McLaughlin ME, Kim JY, Goumnerova LC, Black PM, Lau C, Allen JC, Zagzag D, Olson JM, Curran T, Wetmore C, Biegel JA, Poggio T, Mukherjee S, Rifkin R, Califano A, Stolovitzky G, Louis DN, Mesirov JP, Lander ES, Golub TR. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415:436–442. doi: 10.1038/415436a. [DOI] [PubMed] [Google Scholar]
- 51.Qiu Q, Hernandez JC, Dean AM, Rao PH, Darlington GJ. CD24-positive cells from normal adult mouse liver are hepatocyte progenitor cells. Stem Cells Dev. 2011;20:2177–2188. doi: 10.1089/scd.2010.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elkouris M, Balaskas N, Poulou M, Politis PK, Panayiotou E, Malas S, Thomaidou D, Remboutsika E. Sox1 maintains the undifferentiated state of cortical neural progenitor cells via the suppression of Prox1-mediated cell cycle exit and neurogenesis. Stem Cells. 2011;29:89–98. doi: 10.1002/stem.554. [DOI] [PubMed] [Google Scholar]
- 53.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lejard V, Blais F, Guerquin MJ, Bonnet A, Bonnin MA, Havis E, Malbouyres M, Bidaud CB, Maro G, Gilardi-Hebenstreit P, Rossert J, Ruggiero F, Duprez D. EGR1 and EGR2 involvement in vertebrate tendon differentiation. J Biol Chem. 2011;286:5855–5867. doi: 10.1074/jbc.M110.153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erter CE, Wilm TP, Basler N, Wright CV, Solnica-Krezel L. Wnt8 is required in lateral mesendodermal precursors for neural posteriorization in vivo. Development. 2001;128:3571–3583. doi: 10.1242/dev.128.18.3571. [DOI] [PubMed] [Google Scholar]
- 56.Hamidouche Z, Fromigue O, Ringe J, Haupl T, Marie PJ. Crosstalks between integrin alpha 5 and IGF2/IGFBP2 signalling trigger human bone marrow-derived mesenchymal stromal osteogenic differentiation. BMC Cell Biol. 2010;11:44. doi: 10.1186/1471-2121-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sacchetti B, Funari A, Michienzi S, Cesare SDi, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 58.Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW, Perlingeiro RC. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743–1751. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- 59.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 60.Rajgopal R, Bear M, Butcher MK, Shaughnessy SG. The effects of heparin and low molecular weight heparins on bone. Thromb Res. 2008;122:293–298. doi: 10.1016/j.thromres.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 61.Irie A, Habuchi H, Kimata K, Sanai Y. Heparan sulfate is required for bone morphogenetic protein-7 signaling. Biochem Biophys Res Commun. 2003;308:858–865. doi: 10.1016/s0006-291x(03)01500-6. [DOI] [PubMed] [Google Scholar]
- 62.Brkljacic J, Pauk M, Erjavec I, Cipcic A, Grgurevic L, Zadro R, Inman GJ, Vukicevic S. Exogenous heparin binds and inhibits bone morphogenetic protein 6 biological activity. Int Orthop. 2013;37:529–541. doi: 10.1007/s00264-012-1714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohkawara B, Iemura S, ten Dijke P, Ueno N. Action range of BMP is defined by its N-terminal basic amino acid core. Curr Biol. 2002;12:205–209. doi: 10.1016/s0960-9822(01)00684-4. [DOI] [PubMed] [Google Scholar]
- 64.Fisher MC, Li Y, Seghatoleslami MR, Dealy CN, Kosher RA. Heparan sulfate proteoglycans including syndecan-3 modulate BMP activity during limb cartilage differentiation. Matrix Biol. 2006;25:27–39. doi: 10.1016/j.matbio.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 65.Matsuoka F, Takeuchi I, Agata H, Kagami H, Shiono H, Kiyota Y, Honda H, Kato R. Morphology-based prediction of osteogenic differentiation potential of human mesenchymal stem cells. PLoS One. 2013;8:e55082. doi: 10.1371/journal.pone.0055082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol. 2000;28:707–715. doi: 10.1016/s0301-472x(00)00160-0. [DOI] [PubMed] [Google Scholar]
- 67.Rai B, Lin JL, Lim ZX, Guldberg RE, Hutmacher DW, Cool SM. Differences between in vitro viability and differentiation and in vivo bone-forming efficacy of human mesenchymal stem cells cultured on PCL-TCP scaffolds. Biomaterials. 2010;31:7960–7970. doi: 10.1016/j.biomaterials.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 68.Samsonraj RM, Raghunath M, Hui JH, Ling L, Nurcombe V, Cool SM. Telomere length analysis of human mesenchymal stem cells by quantitative PCR. Gene. 2013;519:348–355. doi: 10.1016/j.gene.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 69.Galindo M, Pratap J, Young DW, Hovhannisyan H, Im HJ, Choi JY, Lian JB, Stein JL, Stein GS, van Wijnen AJ. The bone-specific expression of Runx2 oscillates during the cell cycle to support a G1-related antiproliferative function in osteoblasts. J Biol Chem. 2005;280:20274–20285. doi: 10.1074/jbc.M413665200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.RH Xu, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 71.Gallagher JT, Walker A. Molecular distinctions between heparan sulphate and heparin. Analysis of sulphation patterns indicates that heparan sulphate and heparin are separate families of N-sulphated polysaccharides. Biochem J. 1985;230:665–674. doi: 10.1042/bj2300665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nurcombe V, Goh FJ, Haupt LM, Murali S, Cool SM. Temporal and functional changes in glycosaminoglycan expression during osteogenesis. J Mol Histol. 2007;38:469–481. doi: 10.1007/s10735-007-9123-4. [DOI] [PubMed] [Google Scholar]
- 73.Nurcombe V, Smart CE, Chipperfield H, Cool SM, Boilly B, Hondermarck H. The proliferative and migratory activities of breast cancer cells can be differentially regulated by heparan sulfates. J Biol Chem. 2000;275:30009–30018. doi: 10.1074/jbc.M003038200. [DOI] [PubMed] [Google Scholar]
- 74.Murali S, Leong DF, Lee JJ, Cool SM, Nurcombe V. Comparative assessment of the effects of gender-specific heparan sulfates on mesenchymal stem cells. J Biol Chem. 2011;286:17755–17765. doi: 10.1074/jbc.M110.148874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bramono DS, Rider DA, Murali S, Nurcombe V, Cool SM. The effect of human bone marrow stroma-derived heparan sulfate on the ex vivo expansion of human cord blood hematopoietic stem cells. Pharm Res. 2011;28:1385–1394. doi: 10.1007/s11095-010-0352-y. [DOI] [PubMed] [Google Scholar]