In patients with hematologic malignancies and acute myocardial infarction with severe thrombocytopenia (platelet count < 50,000 cells/mL), guideline‐recommended medical therapy is often withheld because of the fear of major bleeding. In this study, aspirin therapy was associated with improved survival without an increase in major bleeding in this high‐risk patient cohort.
Keywords: Thrombocytopenia, Cancer, Acute myocardial infarction, Aspirin
Abstract
Background.
Patients with hematologic malignancies are at risk for severe thrombocytopenia (sTP). The risk and benefit of aspirin are not known in thrombocytopenic cancer patients experiencing acute myocardial infarction (AMI).
Materials and Methods.
Medical records of patients with hematologic malignancies diagnosed with AMI at Memorial Sloan Kettering Cancer Center during 2005–2014 were reviewed. sTP was defined as a platelet count <50,000 cells per µL within 7 days of AMI.
Results.
Of 118 patients with hematologic malignancies who had AMI, 58 (49%) had sTP. Twenty‐five patients (43%) with sTP received aspirin as a treatment for AMI. Compared with patients without sTP with AMI, patients with sTP with AMI were less likely to receive aspirin (83% vs. 43%; p = .0001) and thienopyridine treatment (27% vs. 3%; p = .0005). During median follow‐up of 3.7 years after AMI, survival was lower in patients with sTP than in those with no sTP (23% vs. 50% at 1 year; log rank p = .003). Patients with sTP who received aspirin for AMI had improved survival compared with those who did not (92% vs. 70% at 7 days, 72% vs. 33% at 30 days, and 32% vs. 13% at 1 year; log rank p = .008). In multivariate regression models, aspirin use was associated with improved 30‐day survival both in the overall patient cohort and in sTP patients. No fatal bleeding events occurred. Major bleeding was not associated with sTP or aspirin use.
Conclusion.
Treatment of AMI with aspirin in patients with hematologic malignancies and sTP is associated with improved survival without increase in major bleeding.
Implications for Practice.
In patients with hematologic malignancies and acute myocardial infarction with severe thrombocytopenia (platelet count < 50,000 cells/µL), guideline‐recommended medical therapy is often withheld because of the fear of major bleeding. In this study, aspirin therapy was associated with improved survival without an increase in major bleeding in this high‐risk patient cohort.
Introduction
Bleeding due to thrombocytopenia is a feared complication in patients with hematologic malignancies. The incidence and severity of thrombocytopenia vary widely by cancer type, but it is more common with hematologic malignancies than with solid tumors, affecting 5%–33% of this patient population. [1]. However, cancer also creates a prothrombotic state, and the rate of thrombotic complications is as high if not higher in hematologic malignancies when compared with solid tumors [2]. Underlying mechanisms involve increased expression of procoagulant factors (e.g., tissue factor and cancer procoagulant), hyperleukocytosis leading to aberrant blood flow, side effects of certain chemotherapeutic agents and/or antiangiogenic drugs, and cytokine‐induced stimulation of cellular adhesion molecule expression by endothelial cells, resulting in increased platelet activation and aggregation [3]. Patients with hematologic malignancies are at risk for acute coronary syndrome (ACS) because of shared risk factors, prothrombotic state, and exposure to cardiotoxic chemotherapeutic agents, as well as mediastinal radiotherapy; the latter predisposes them to accelerated atherosclerosis [4], [5].
Aspirin therapy is the mainstay of medical treatment in ACS. Results of the ISIS‐2 trial and several others have shown that in patients suspected of having acute myocardial infarction (AMI), treatment with aspirin can significantly improve survival [6]. Therefore, in the absence of contraindications, clinical guidelines strongly recommend immediate administration of aspirin in AMI [7], [8]. It is widely recognized that platelets play a crucial role in the pathogenesis of AMI by participating in the formation of thrombotic vascular occlusions at ruptured coronary atherosclerotic plaques [9]. However, thrombocytopenia does not protect against AMI. Roughly 4%–11% of patients with AMI have baseline thrombocytopenia [10], [11], [12]. Importantly, baseline thrombocytopenia (platelet count < 100,000 cells per µL) is strongly associated with early and late major adverse cardiovascular events and predicts increased overall mortality in AMI [11]. Bleeding and thrombocytopenia are both regarded as contraindications to aspirin therapy.
The safety of aspirin in cancer patients with AMI and severe thrombocytopenia (sTP) (platelet count <50,000 cells µL) is largely unknown because of lack of data in this high‐risk population. Aspirin is underused in the treatment of AMI in the cancer population. In a prior study, only 46% of cancer patients received aspirin as part of their AMI treatment; the most common contraindication for aspirin therapy was thrombocytopenia [13]. Another study suggested that aspirin may be beneficial in this setting; however, this study used a platelet count of <100,000 cells per µL as a definition for thrombocytopenia, so it did not address the population at highest risk for bleeding (i.e., severely thrombocytopenic patients) [14]. We hypothesized that, in patients with hematologic malignancies and AMI, the benefit of aspirin would outweigh the risk for bleeding, even in the presence of sTP.
Materials and Methods
Medical records of all patients with hematologic malignancies diagnosed with AMI from 2005 to 2014 at Memorial Sloan Kettering Cancer Center, New York, New York, USA, were reviewed. The diagnosis of AMI was based on the third universal definition of AMI [15]. AMI was diagnosed by the presence of elevated cardiac troponin I with at least one value above the 99th percentile of the upper reference limit (0.06 ng/mL) and with at least one of the following: (a) symptoms of myocardial ischemia; (b) electrocardiographic changes, particularly development of pathologic Q waves, new significant ST‐segment/T‐wave changes or new left bundle‐branch block; (c) evidence of new loss of viable myocardium or new regional wall motion abnormality on imaging; and (d) identification of an intracoronary thrombus by angiography or autopsy. Alternatively, AMI was diagnosed in the setting of cardiac death that was preceded by symptoms suggestive of myocardial ischemia and presumed new ischemic electrocardiographic changes but that occurred before troponin was obtained or before troponin would have been increased. ST‐segment elevation myocardial infarction (STEMI) was defined as ST‐segment elevation in two contiguous leads of ≥1 mm in limb leads or ≥2 mm in precordial leads. The current study was exempt from the institutional review board approval because existing anonymized data were used.
Patient characteristics, including age, sex, height, weight, type of hematologic malignancy, time between disease diagnosis and MI, previous chemotherapy, hematopoietic stem cell transplantation, previous chest radiation therapy, cardiac comorbidities, smoking status, family history of premature coronary artery disease, cardiovascular medications, laboratory values, presenting symptoms, and vital signs at the time of AMI were collected. Electrocardiography and echocardiography findings were reviewed and recorded.
We collected data on the status of the patient's disease stage and disease risk index according to the work done by Armand et al. [16]. Briefly, low‐risk stage was defined by complete or partial remission or untreated disease, whereas high‐risk stage was defined as induction failure, relapse, or accelerated/blast phase chronic myelogenous leukemia. Disease risk index classified patients to low‐, intermediate‐, high‐, and very‐high‐risk groups by using a combination of a ternary breakdown for disease risk by type and a binary breakdown for remission status, as described by Armand et al. [16]. Management details, including cardiovascular medication administration profiles, cardiac catheterization and intervention, coronary artery bypass surgery reports, and platelet and packed red blood cell transfusion events during index hospitalizations, were also reviewed and collected.
Additional data were collected to identify the cause of death for the included patients. Because autopsies were not commonly performed in the studied population, the cause of death was identified by chart review in most cases. In a small proportion of patients (18/118 patients) in whom the cause of death was unknown, we assumed, on the basis of recently published guidelines [17], that the cause of death was cardiovascular.
Patients were divided into two groups based on platelet counts within 1 week of index AMI: patients with platelet count >50,000 cells per µL and severely thrombocytopenic patients with platelet count ≤50,000 cells per µL. Patients were further categorized according to aspirin administration profiles. The primary endpoints of this study were (a) overall and cardiovascular death‐free survival at 1 month and 1 year after AMI in patients with sTP versus those without sTP and (b) overall and cardiovascular death‐free survival at 1 month and 1 year in sTP patients who received aspirin for AMI versus those who did not. The secondary endpoint of this study was the rate of bleeding complications. Survival was measured from the date of index AMI to the date of death or last available follow‐up. Follow‐up data after discharge were collected with clinical visits and/or phone calls.
Bleeding events were classified by using the Bleeding Academic Research Consortium (BARC) definitions [18]. BARC type 3a bleeding was defined as overt bleeding plus a decrease in hemoglobin of 3–5 g/dL (provided hemoglobin decrease was related to bleeding) and transfusion with overt bleeding. BARC type 3b bleeding was identified as overt bleeding plus a hemoglobin decrease of ≥5 g/dL (provided hemoglobin decrease was related to bleeding), or cardiac tamponade or bleeding necessitating surgical intervention for control or bleeding necessitating vasoactive agents. BARC type 3c bleeding was defined as intracranial hemorrhage confirmed by autopsy, imaging, or lumbar puncture or intraocular bleeding compromising vision. BARC type 5 bleeding was defined as probable or definite fatal bleeding. Only information on BARC types 3 and 5 were collected in this study. For the purpose of this study, we arbitrarily used all BARC type 3 events as major bleeding events.
We used t‐tests and Fisher exact tests to compare continuous and categorical variables, respectively, between groups. Survival curves were derived by using the Kaplan‐Meier product limit method with 95% confidence intervals (CIs) by groups, and the log‐rank statistic was used to compare survival among different groups. Univariate and multivariate logistic regression models were used to identify predictors of 30‐day survival. Predictors of 30‐day survival with p < .10 were included in the multivariate analysis. The final model was derived by using a forward selection procedure. A p value <.05 was considered to indicate a statistically significant difference. Analyses were performed with the GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, http://www.graphpad.com/) and the SPSS software, version 21.0 (IBM, Chicago, IL, https://www.ibm.com).
Results
A total of 118 patients with hematologic malignancies who had AMI while hospitalized at Memorial Sloan Kettering Cancer Center from 2005 to 2014 were studied. Fifty‐eight of 118 (49%) patients had sTP (mean platelet count ± SD, 31,000 ± 12,000 cells per µL); the remaining 60 of 118 (51%) had platelet counts ≥50,000 cells per µL (mean platelet count, 227,000 ± 136,000 cells per µL). Platelet counts ranged from 8,000 to 49,000 cells per µL in the sTP group and from 55,000 to 627,000 cells per µL in the non‐sTP group.
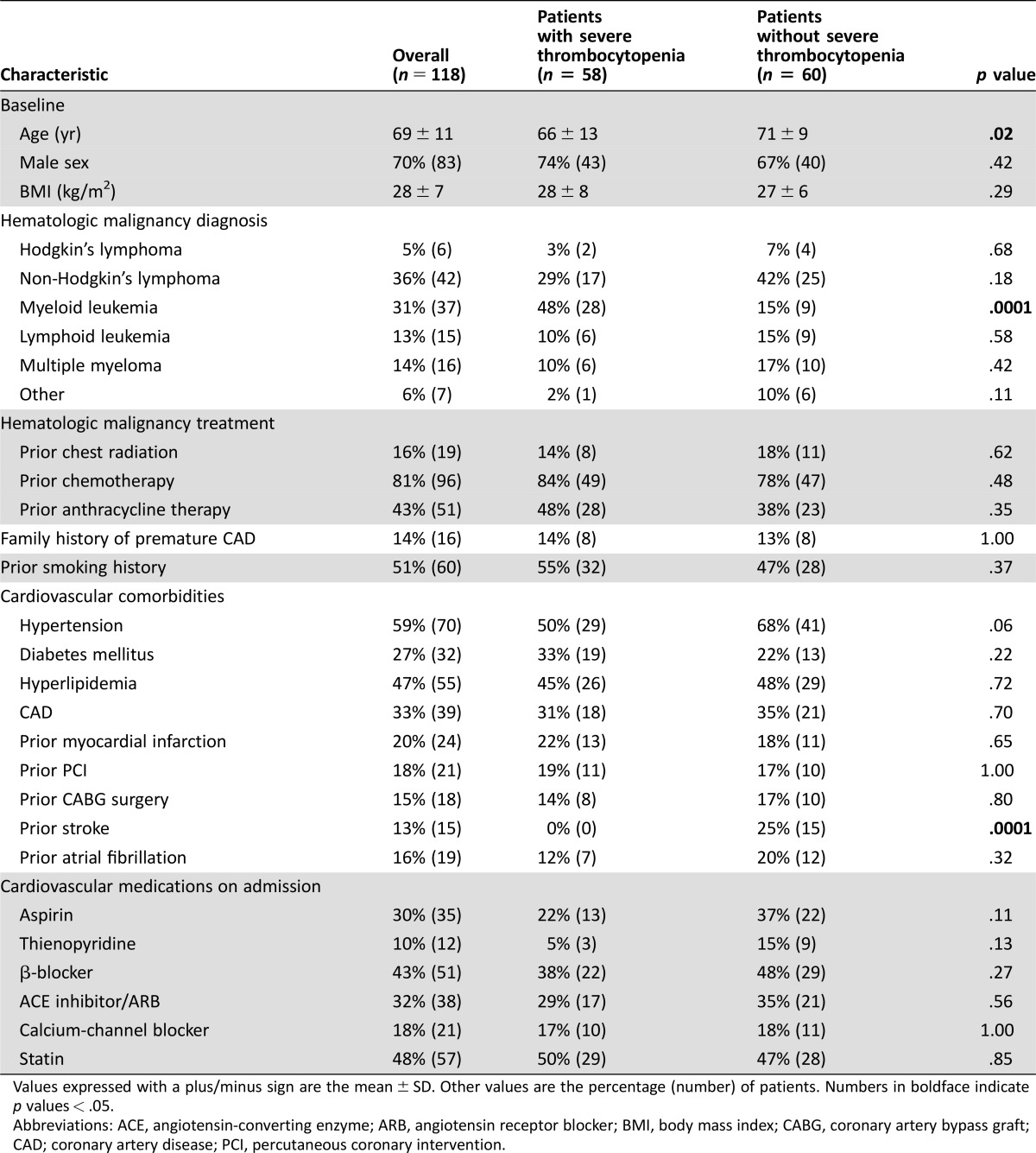
Patients with sTP were younger and more commonly had myeloid leukemia. Cardiovascular risk factor and cardiovascular medication profiles were similar between the two groups, with the exception of decreased incidence of prior stroke in patients with sTP. Prior chemotherapy, anthracycline therapy, and history of previous chest radiation did not significantly differ between the two groups. Prior coronary artery disease was present in one third of the overall patient population. Baseline patient characteristics are summarized in Table 1.
Table 1. Population characteristics.
Values expressed with a plus/minus sign are the mean ± SD. Other values are the percentage (number) of patients. Numbers in boldface indicate p values < .05.
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass graft; CAD; coronary artery disease; PCI, percutaneous coronary intervention.
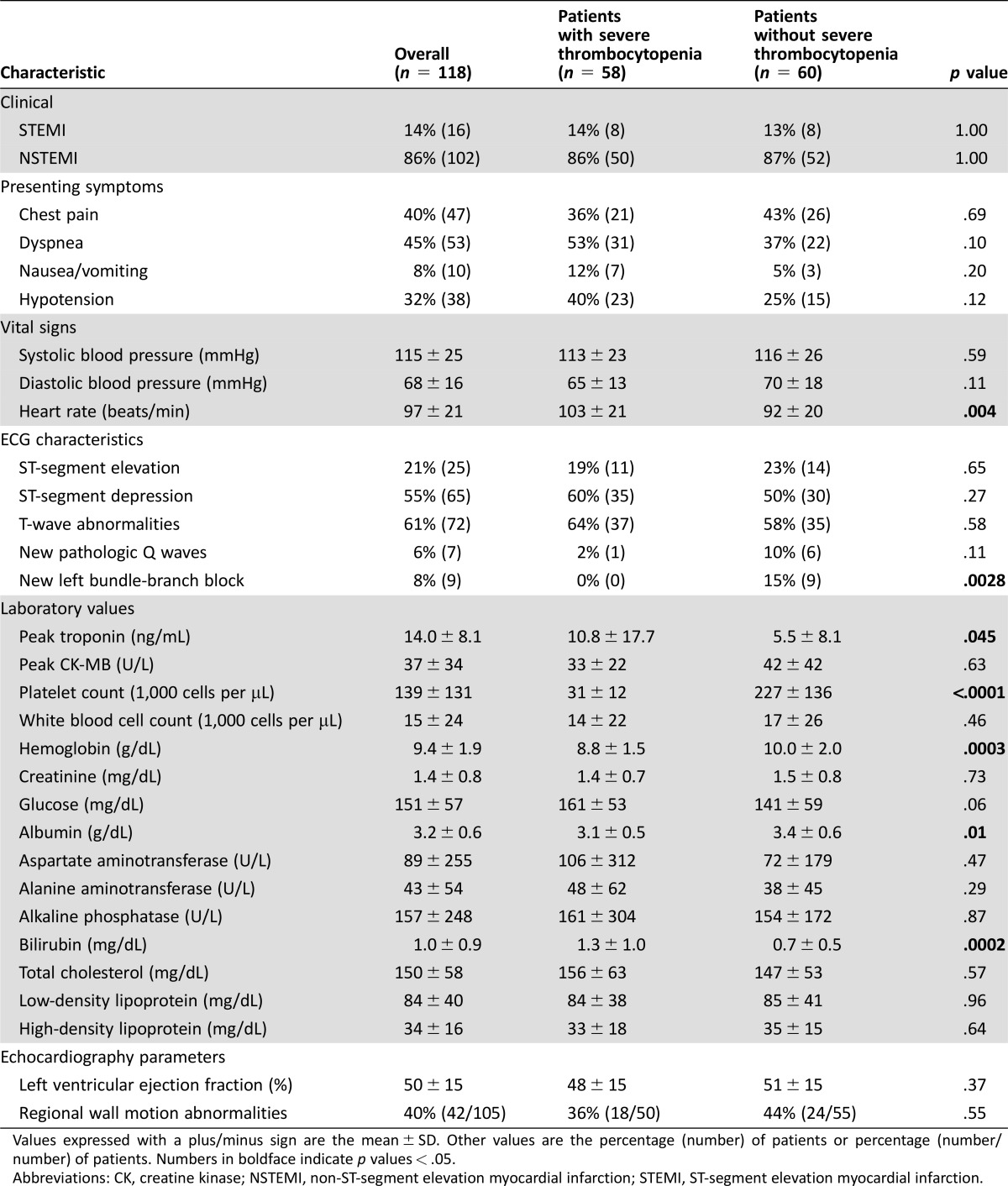
The presenting symptoms of AMI were similar in both groups; the most common symptom was dyspnea (45%), followed by chest pain (40%) and hypotension (32%) (Table 2). Patients with sTP were tachycardic, with significantly lower hemoglobin and higher troponin levels. The rate of STEMI was similar in the two groups. Echocardiographic left ventricular ejection fraction and the frequency of regional wall motion abnormalities were also similar in the two groups.
Table 2. Clinical characteristics.
Values expressed with a plus/minus sign are the mean ± SD. Other values are the percentage (number) of patients or percentage (number/number) of patients. Numbers in boldface indicate p values < .05.
Abbreviations: CK, creatine kinase; NSTEMI, non‐ST‐segment elevation myocardial infarction; STEMI, ST‐segment elevation myocardial infarction.
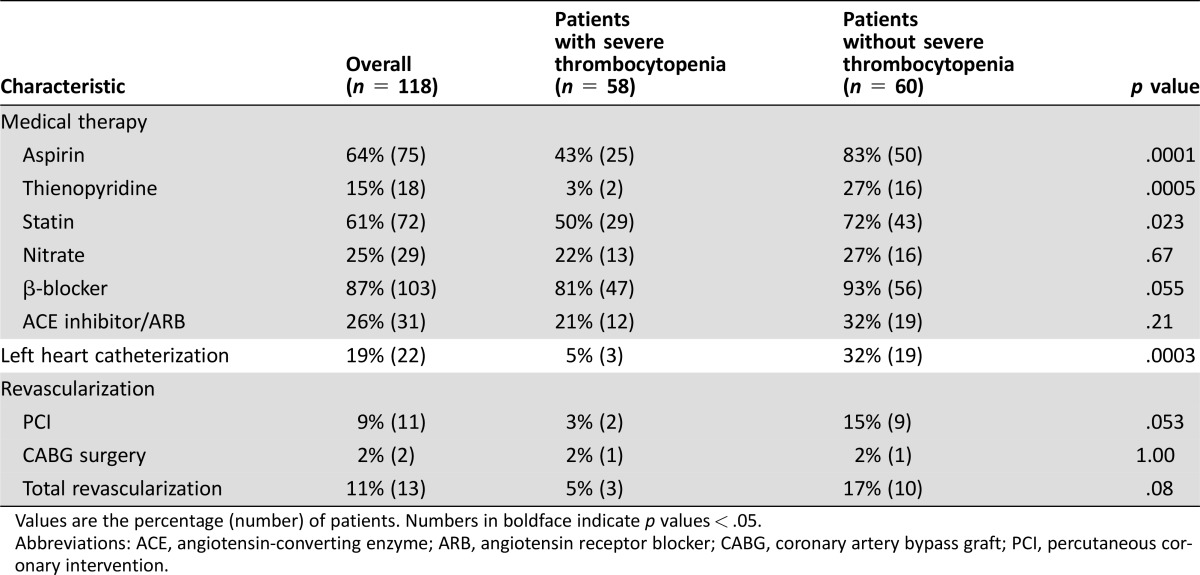
The medical management of AMI significantly differed between the two groups (Table 3). Patients with sTP were less likely to receive aspirin (25 of 58 vs. 50 of 60; p < .001), thienopyridine (2 of 58 vs. 16 of 60; p < .001), and statin therapy (29 of 58 vs. 43 of 60; p = .02). On the same admission as the index AMI, a significantly higher proportion of sTP patients received platelet transfusion compared with patients without sTP (46 of 58 vs. 8 of 60; p = .0001). Patients with sTP were also more likely to receive blood transfusion during the same admission compared with patients without sTP (46 of 58 vs. 31 of 60; p = .002).
Table 3. Treatment of acute myocardial infarction.
Values are the percentage (number) of patients. Numbers in boldface indicate p values < .05.
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention.
Left heart catheterization was performed in 19% (22 of 118) of the overall patient cohort but only in 5% (3 of 58) of patients with sTP (p = .0003). All patients with sTP who underwent left heart catheterization had significant coronary obstruction. Of these patients, 2 underwent percutaneous coronary intervention, whereas the third patient underwent coronary bypass surgery. Although the numbers are small, the rate of revascularization did not differ significantly among patients from the two groups who underwent left heart catheterization (3 of 58 vs. 10 of 60; p = .08).
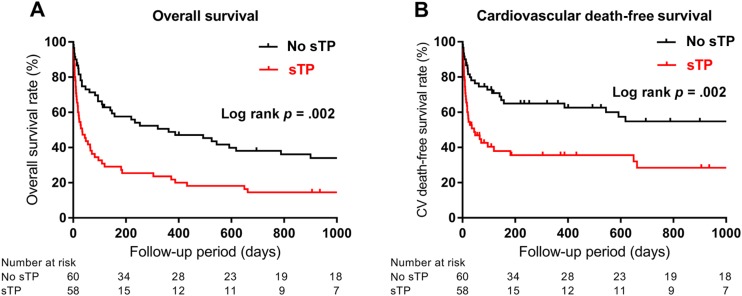
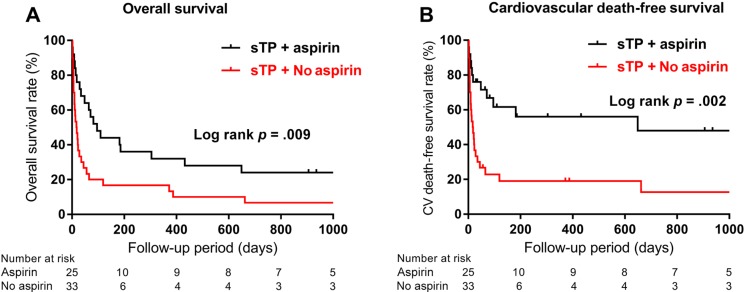
During a median follow‐up of 3.7 years, all‐cause mortality (log rank p = .002; Fig. 1A) and cardiovascular mortality (log rank p = .002; Fig. 1B) were significantly higher in cancer patients with AMI and sTP than in patients without sTP, according to Kaplan‐Meier analysis. The calculated median survival was 34 days in sTP patients compared with 362 days in those without sTP (hazard ratio [HR], 1.96; 95% CI, 1.27–3.05). Patients with sTP and AMI who received aspirin had significantly improved overall survival compared with sTP and AMI patients who did not receive aspirin (Fig. 2A). Survival rates were 95% vs. 72% at 1‐week, 74% vs. 39% at 30‐day, 47% vs. 19% at 6‐month, and 32% vs. 12% at 1‐year follow‐up, respectively (log rank p = .009) (Fig. 2A.). Patients with sTP who received aspirin had a median survival of 96 days, compared with 17.5 days in patients who did not receive aspirin (HR, 0.44; 95% CI, 0.24–0.81). In addition, sTP patients treated with aspirin had significantly higher cardiovascular death‐free survival when compared with patients not treated with aspirin (log rank p = .002; Fig. 2B). The rate of recurrent AMI did not differ in patients with sTP and patients without sTP (4 of 58 vs. 5 of 60; p = N.S.). Half of the patients with sTP who sustained reinfarction received aspirin at the time of index AMI (n = 2), with no difference in the rate of recurrent AMI between those who did and those who did not receive aspirin therapy (2 of 25 vs. 2 of 33; p = N.S.). Two patients with no thrombocytopenia at the time of index AMI required revascularization during follow‐up, with no difference in revascularization between the sTP and no sTP groups (0 of 58 vs. 2 of 60; p = N.S.). The rate of new stroke during follow‐up was also not significantly different between the sTP and no sTP groups (1 of 58 vs. 1 of 60; p = N.S.).
Figure 1.
Kaplan‐Meier estimates of overall (Panel A) and cardiovascular death‐free (Panel B) survival according to baseline platelet counts in patients with hematologic malignancies and acute myocardial infarction.
Abbreviation: sTP, severe thrombocytopenia.
Figure 2.
Kaplan‐Meier estimates of overall (Panel A) and cardiovascular death‐free (Panel B) survival in patients with hematologic malignancies with acute myocardial infarction and severe thrombocytopenia according to aspirin administration profiles.
Abbreviation: sTP, severe thrombocytopenia.
Univariate analysis of the entire patient cohort showed that treatment of AMI with aspirin, angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB), and statin was associated with decreased all‐cause mortality 30 days after AMI (supplemental online Table 1). Other predictors of reduced all‐cause mortality were low‐risk stage, low/moderate overall disease risk, lack of severe thrombocytopenia, and lack of platelet transfusion. In univariate analysis, all these factors were also predictors of reduced 30‐day cardiovascular mortality in addition to β‐blocker therapy; on the other hand elevated body mass index and low ejection were predictors of increased cardiovascular mortality. In multivariate analysis, aspirin and ACE inhibitors were independent predictors of both reduced all‐cause mortality (odds ratio [OR], 0.26 [95% CI, 0.09–0.55; p = .001]; OR, 0.20 [95% CI, 0.06–0.66; p = .008], respectively) and reduced cardiovascular mortality (OR, 0.24 [95% CI, 0.08–0.69; p = .008]; OR, 0.15 [95% CI, 0.03–0.77; p = .024], respectively). Additional independent predictors were low‐risk stage for reduced all‐cause mortality (OR, 0.28; 95% CI, 0.11–0.7; p = .006) and low/moderate overall disease risk (OR, 0.29; 95% CI, 0.10–0.85; p = .024) for reduced cardiovascular mortality in the overall patient cohort.
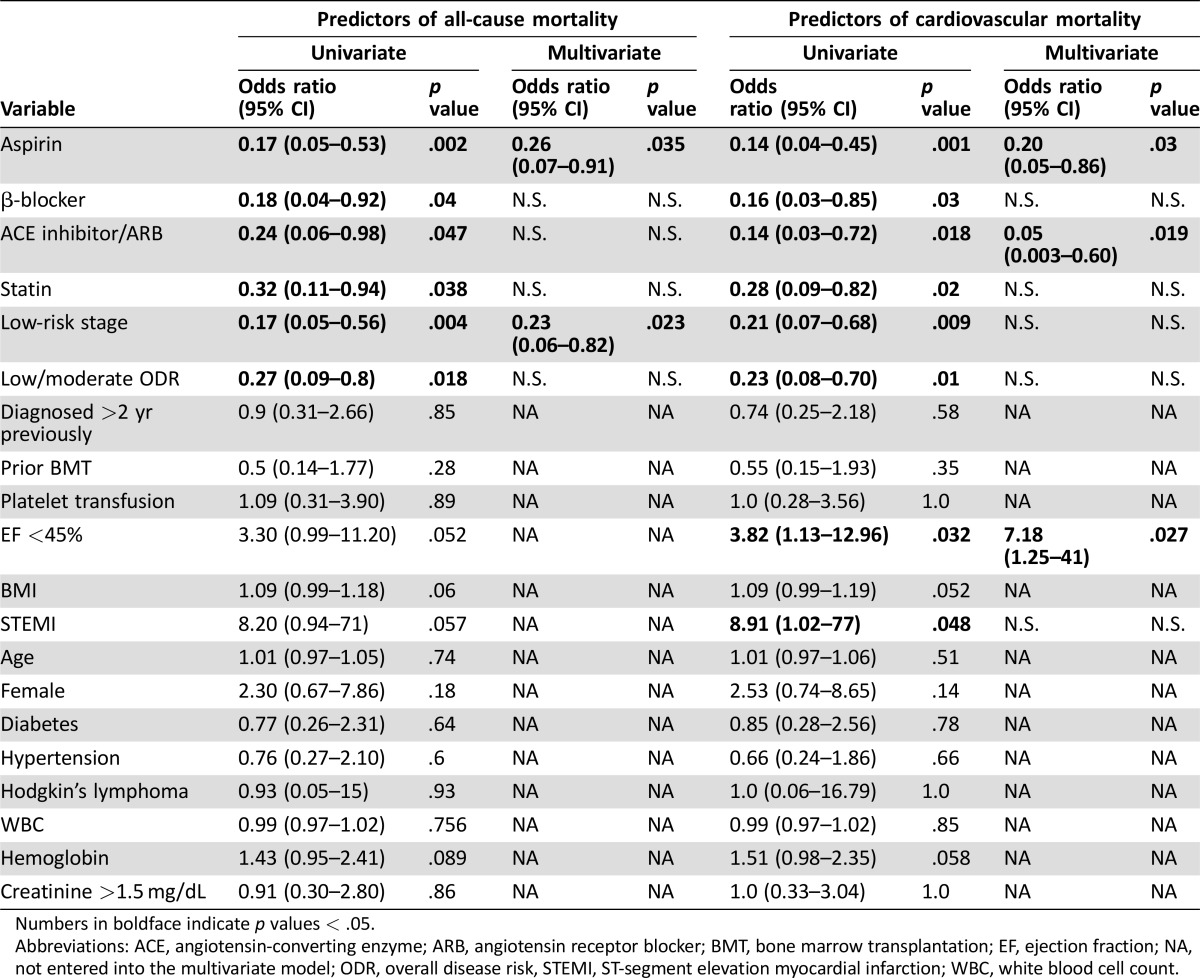
In univariate analysis of patients with sTP, treatment of AMI with aspirin, β‐blocker, ACE inhibitor or ARB, and statin along with low‐risk stage and low/moderate overall disease risk were associated with decreased 30‐day all‐cause and cardiovascular mortality (Table 4). In addition, reduced ejection fraction and STEMI were associated with increased 30‐day all‐cause mortality. Multivariate analysis identified aspirin (OR, 0.26; 95% CI, 0.07–0.91; p = .035) and low‐risk stage (OR, 0.23; 95% CI, 0.06–0.82; p = .023) as independent predictors of reduced 30‐day all‐cause mortality. Multivariate analysis also identified aspirin (OR, 0.24; 95% CI, 0.05–0.86; p = .03) and ACE inhibitor therapy (OR, 0.05; 95% CI, 0.003–0.60; p = .019) as independent predictors of reduced 30‐day cardiovascular mortality and reduced ejection function (OR, 7.18; 95% CI, 1.25–41; p = .027) as independent predictors of increased cardiovascular mortality.
Table 4. Regression analysis for 30 days: restricted to severe thrombocytopenia.
Numbers in boldface indicate p values < .05.
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMT, bone marrow transplantation; EF, ejection fraction; NA, not entered into the multivariate model; ODR, overall disease risk, STEMI, ST‐segment elevation myocardial infarction; WBC, white blood cell count.
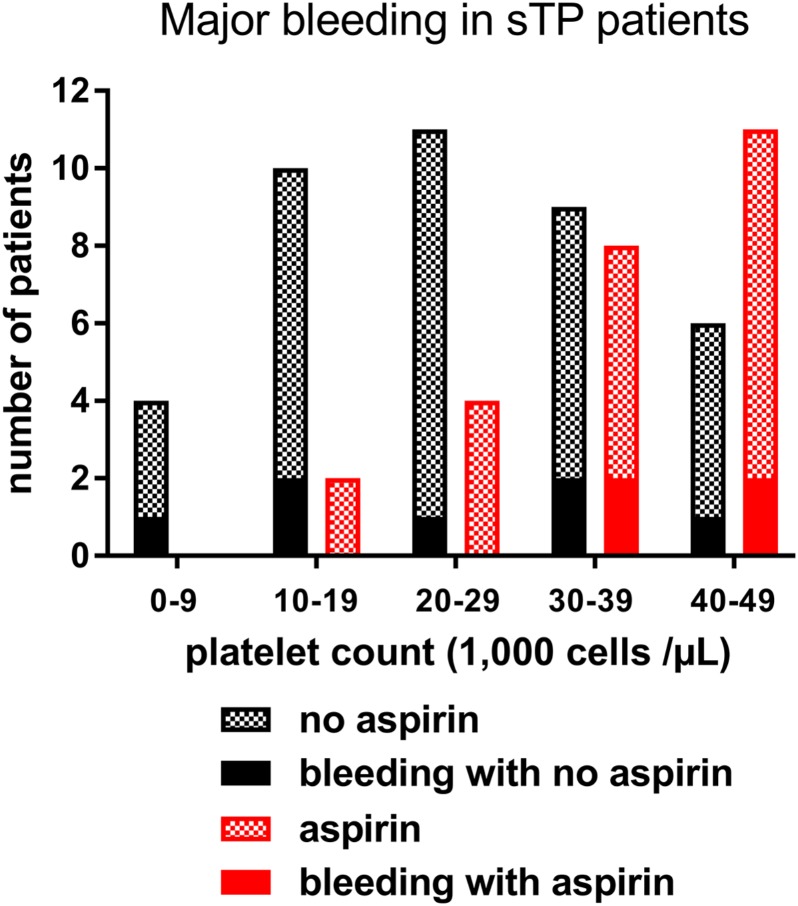
No fatal bleeding complications were observed in the patient cohort during follow‐up. Major bleeding (BARC type 3a or type 3b) occurred in 16 patients. The bleeding was most often of gastrointestinal origin (n = 10). Among these patients, hypotension prompting intravenous vasopressor therapy occurred in 3, and 1 patient with long‐standing gastrointestinal bleeding required sigmoidectomy. The remaining patients with major bleeding had epistaxis (n = 4), hemoptysis (n = 2), and hematuria (n = 1, in a patient who had simultaneous gastrointestinal bleeding), all of which were severe enough to prompt the initiation of blood transfusion. Bleeding complications were not significantly associated with sTP or aspirin administration (p = .10 and .11, respectively). Figure 3 depicts the number of major bleeding complications based on platelet counts and aspirin administration profiles in severely thrombocytopenic patients. In the sTP group, major bleeding occurred in 19% of patients (n = 11 of 58) without a difference between patients who received aspirin and those who did not (n = 4 of 25 [16%] vs. n = 7 of 33 [21%], respectively; p = N.S.).
Figure 3.
Number of major bleeding complications in patients with hematologic malignancies with acute myocardial infarction and severe thrombocytopenia grouped based on platelet counts and aspirin administration profiles.
Discussion
The major finding of the current study is that in patients with hematologic malignancies and AMI with severe thrombocytopenia, aspirin therapy is associated with improved survival without an increase in major bleeding. To our knowledge, our study is the first to investigate the role of aspirin therapy in AMI in severely thrombocytopenic cancer patients.
Recent studies have shown that thrombosis can be a presenting sign in as many as 10% of leukemia cases [19]. In certain subtypes of leukemia, such as acute promyelocytic leukemia [20], [21], acute arterial thrombosis has been reported in the setting of antileukemic therapy [22]. In addition, thrombocytopenic patients are also known to have a higher proportion of reticulated platelets, which are more reactive and ready to participate in thrombotic events [23]. Autopsy studies in patients with hematologic malignancy who had AMI identified leukemic infiltration of the coronary walls [24], [25], severe atherosclerotic disease [26], and platelet‐fibrin‐rich thrombus formation [27]. Although autopsy data were not available in our patient population, left heart catheterization performed in three severely thrombocytopenic patients confirmed the presence of obstructive coronary artery disease necessitating revascularization.
In the current patient cohort, sTP patients had worse clinical outcomes than patients without sTP, similar to previous observations [11], [13], [14]. Patients with sTP also demonstrated a greater rise in cardiac enzyme levels, suggesting increased myocardial damage in the sTP group. Of note, sTP patients were less likely to receive standard medical therapy with proven cardiovascular benefit in AMI, including aspirin, thienopyridine, and statins. These findings are consistent with prior observations that thrombocytopenic cancer patients diagnosed with cardiovascular comorbidities often receive suboptimal treatment for their cardiovascular illness, and this might partially explain the increased myocardial damage and worse survival rates. Severe thrombocytopenia, even without the presence of AMI, has been associated with reduced overall survival in hematologic malignancy patients [28], [29].
Previously, disease remission status and disease risk index have been reported to be strongly prognostic in patients with hematologic malignancies [16], [30]. On the basis of these findings, it was not unexpected that our study found low‐risk stage, which reflects disease remission status, to be a predictor of reduced 30‐day mortality both in the overall patient cohort and in sTP patients. In contrast to aspirin, which was an independent predictor of reduced all‐cause and cardiovascular mortality, low‐risk stage was not a predictor of decreased cardiovascular mortality. This finding suggests that disease status modifies survival unrelated to cardiovascular health.
Although our data do not explain the current practice pattern, potential contributors to the decision to withhold aspirin therapy may include (a) fear of major bleeding and (b) the lack of applicability of the findings of major cardiovascular trials to this patient population (these trials commonly excluded patients with cancer or thrombocytopenia).
Bleeding is a devastating complication of thrombocytopenia. However, the concerns for bleeding at these platelet counts are not supported by scientific evidence. In one large retrospective series spanning 10 years, multivariate analyses did not show a relationship between the first morning platelet count or the lowest platelet count of the day and the risk for hemorrhage [31]. Indeed, current standard of care calls for prophylactic platelet transfusion strategy only when platelet counts fall below 10,000 cells per µL. In the setting of venous thrombosis necessitating anticoagulant therapy, expert opinion consensus and data have shown that anticoagulant therapy with low‐molecular‐weight heparin (LMWH) can be continued even below platelet counts of 50,000 cells per µL and recommend half‐dose LMWH for platelet counts between 20,000 and 50,000 cells per µL [32]. Limited evidence from case series suggests that use of prophylactic doses of LMWH can be tolerated in patients with platelet counts <20,000 cells per µL, with associated resolution of thrombosis symptoms [33].
In the present study, aspirin was safely administered without excessive bleeding under platelet counts of 50,000 cells per µL. Of the 25 patients with sTP who received aspirin, only 4 had significant bleeding (16%), which is not significantly different from the number of such patients among those sTP patients who did not receive aspirin (7 of 33 [21%]).
Major clinical guidelines recommend immediate administration of aspirin in AMI when no contraindication is present [7], [8]. In the current study, 64% of cancer patients received aspirin therapy; this rate correlates with prior observations showing that antiplatelet therapy is highly underused in cancer population [13]. Multivariate analysis in the sTP group showed that administration of aspirin was associated with improved 30‐day overall survival and, importantly, with improved cardiovascular death‐free survival; these findings suggest that the benefit of aspirin is via cardiovascular protection. It has been demonstrated in the second International Study of Infarct Survival (ISIS‐2) trial that the survival benefit of early aspirin administration is restricted to the first month, with little further benefit or loss during long‐term follow‐up, indicating the importance of early intervention with aspirin therapy [34]. Sarkiss et al. previously reported that the administration of aspirin was associated with better 7‐day survival in thrombocytopenic (platelet count <100,000 cells per µL) cancer patients with ACS [14]. In that study, similar to the current findings, the lack of aspirin administration was an independent predictor of 7‐day mortality. Of note, this observation was not accompanied by any increase in major bleeding complications.
Thus, the risks for arterial events are underestimated and the risks for bleeding are overestimated in patients with hematologic neoplasms and thrombocytopenia, such that patients who can benefit from aspirin therapy in the event of AMI do not receive the benefit of antiplatelet therapy. A larger prospective study is warranted but will be difficult to perform. Most patients in the current study who received aspirin had platelet counts >30,000 cells per µL. These data add further evidence to the existing literature that aspirin therapy can be life‐saving and should be considered in cancer patients with AMI and thrombocytopenia with platelets counts as low as 30,000 cells per µL.
Limitations
The major limitations of this study are those inherent to a retrospective study design. The number of variables that could be included in the multivariate analysis limited the calculation of precise risk estimates. However, the data were collected in a systematic fashion, with close to a 100% 1‐year follow‐up rate. The overall treatment profile and survival rates were similar to previously published studies on myocardial infarction in cancer patients [13], [14]. In our study, most patients receiving aspirin had platelet counts >30,000 cells per µL; therefore, our results should be interpreted accordingly.
Conclusion
Patients with hematologic malignancies are at risk for AMI and severe thrombocytopenia. Guideline‐recommended medical therapy for ACS is often withheld because of the fear of major bleeding in this high‐risk cohort. Our study shows that the benefit of aspirin in AMI outweighs the risk for major bleeding in severely thrombocytopenic patients with hematologic malignancies.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
The results of this study were presented as an oral presentation at the American Heart Association's Scientific Sessions 2015 on November 9, 2015, in Orlando, Florida.
Author Contributions
Conception/Design: Attila Feher, Polydoros Kampaktsis, Rekha Parameswaran, Eytan M. Stein, Richard Steingart, Dipti Gupta
Provision of study material or patients: Attila Feher
Collection and/or assembly of data: Attila Feher, Polydoros Kampaktsis
Data analysis and interpretation: Attila Feher, Polydoros Kampaktsis, Dipti Gupta
Manuscript writing: Attila Feher, Rekha Parameswaran, Eytan M. Stein, Richard Steingart, Dipti Gupta
Final approval of manuscript: Attila Feher, Polydoros Kampaktsis, Rekha Parameswaran, Eytan M. Stein, Richard Steingart, Dipti Gupta
Disclosures
The authors indicated no financial relationships.
Supplementary Information
References
- 1. Bennett D, Suppapanya N, Grotzinger K. Thrombocytopenia in hematologic malignancy and solid tumors in the United States. J Clin Oncol 2012;30:e12001. [Google Scholar]
- 2. Falanga A, Marchetti M. Venous thromboembolism in the hematologic malignancies. J Clin Oncol 2009;27:4848–4857. [DOI] [PubMed] [Google Scholar]
- 3. Castelli R, Ferrari B, Cortelezzi A, et al. Thromboembolic complications in malignant haematological disorders. Curr Vasc Pharmacol 2010;8:482–494. [DOI] [PubMed] [Google Scholar]
- 4. Carver JR, Shapiro CL, Ng A, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: Cardiac and pulmonary late effects. J Clin Oncol 2007;25:3991–4008. [DOI] [PubMed] [Google Scholar]
- 5. Gupta D, Pun SC, Verma S et al. Radiation‐induced coronary artery disease: A second survivorship challenge? Future Oncol 2015;11:2017–2020. [DOI] [PubMed] [Google Scholar]
- 6.Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: Isis‐2. Isis‐2 (second international study of infarct survival) collaborative group. Lancet 1988;2:349–360. [PubMed] [Google Scholar]
- 7. O'Gara PT, Kushner FG, Ascheim DD et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:e78–140. [DOI] [PubMed] [Google Scholar]
- 8. Amsterdam EA, Wenger NK, Brindis RG et al. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139–228. [DOI] [PubMed] [Google Scholar]
- 9. Fitzgerald DJ, Roy L, Catella F et al. Platelet activation in unstable coronary disease. N Engl J Med 1986;315:983–989. [DOI] [PubMed] [Google Scholar]
- 10. Caixeta A, Dangas GD, Mehran R et al. Incidence and clinical consequences of acquired thrombocytopenia after antithrombotic therapies in patients with acute coronary syndromes: Results from the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial. Am Heart J 2011;161:298–306. [DOI] [PubMed] [Google Scholar]
- 11. Hakim DA, Dangas GD, Caixeta A et al. Impact of baseline thrombocytopenia on the early and late outcomes after ST‐elevation myocardial infarction treated with primary angioplasty: Analysis from the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS‐AMI) trial. Am Heart J 2011;161:391–396. [DOI] [PubMed] [Google Scholar]
- 12. Wang TY, Ou FS, Roe MT et al. Incidence and prognostic significance of thrombocytopenia developed during acute coronary syndrome in contemporary clinical practice. Circulation 2009;119:2454–2462. [DOI] [PubMed] [Google Scholar]
- 13. Yusuf SW, Daraban N, Abbasi N et al. Treatment and outcomes of acute coronary syndrome in the cancer population. Clin Cardiol 2012;35:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sarkiss MG, Yusuf SW, Warneke CL et al. Impact of aspirin therapy in cancer patients with thrombocytopenia and acute coronary syndromes. Cancer 2007;109:621–627. [DOI] [PubMed] [Google Scholar]
- 15. Thygesen K, Alpert JS, Jaffe AS et al. Third universal definition of myocardial infarction. Circulation 2012;126:2020–2035. [DOI] [PubMed] [Google Scholar]
- 16. Armand P, Gibson CJ, Cutler C, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood 2012;120:905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hicks KA, Tcheng JE, Bozkurt B et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol 2015;66:403–469. [DOI] [PubMed] [Google Scholar]
- 18. Mehran R, Rao SV, Bhatt DL et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 19. De Stefano V, Sora F, Rossi E et al. The risk of thrombosis in patients with acute leukemia: Occurrence of thrombosis at diagnosis and during treatment. J Thromb Haemost 2005;3:1985–1992. [DOI] [PubMed] [Google Scholar]
- 20. Cahill TJ, Chowdhury O, Myerson SG et al. Myocardial infarction with intracardiac thrombosis as the presentation of acute promyelocytic leukemia: Diagnosis and follow‐up by cardiac magnetic resonance imaging. Circulation 2011;123:e370–372. [DOI] [PubMed] [Google Scholar]
- 21. Sargsyan Z, Higgins C, Alexandrescu S et al. Acute promyelocytic leukemia as a cause of intracoronary drug‐eluting‐stent thrombosis. Tex Heart Inst J 2012;39:416–419. [PMC free article] [PubMed] [Google Scholar]
- 22. Tachibana T, Tanaka M, Ishigatsubo Y et al. Thrombosis at ascending aorta following chemotherapy in a patient with acute myeloid leukemia. Int J Hematol 2012;96:293–294. [DOI] [PubMed] [Google Scholar]
- 23. Macchi I, Chamlian V, Sadoun A et al. Comparison of reticulated platelet count and mean platelet volume determination in the evaluation of bone marrow recovery after aplastic chemotherapy. Eur J Haematol 2002;69:152–157. [DOI] [PubMed] [Google Scholar]
- 24. Assiri AH, Lamba M, Veinot JP. Chronic lymphocytic leukemia involving the coronary arteries with accompanying acute myocardial infarction. Cardiovasc Pathol 2005;14:324–326. [DOI] [PubMed] [Google Scholar]
- 25. Cheng H, Feldman T, Butt Y et al. T‐cell prolymphocytic leukemia with extensive cardiovascular infiltrate leading to multiple myocardial infarctions and cardiac death. Tex Heart Inst J 2014;41:626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cohen Y, Amir G, Da'as N et al. Acute myocardial infarction as the presenting symptom of acute myeloblastic leukemia with extreme hyperleukocytosis. Am J Hematol 2002;71:47–49. [DOI] [PubMed] [Google Scholar]
- 27. Solomons HD, Stanley A, King PC et al. Acute promyelocytic leukaemia associated with acute myocardial infarction. A case report. S Afr Med J 1986;70:117–118. [PubMed] [Google Scholar]
- 28. Neukirchen J, Blum S, Kuendgen A et al. Platelet counts and haemorrhagic diathesis in patients with myelodysplastic syndromes. Eur J Haematol 2009;83:477–482. [DOI] [PubMed] [Google Scholar]
- 29. Chen CC, Yang CF, Yang MH et al. Pretreatment prognostic factors and treatment outcome in elderly patients with de novo acute myeloid leukemia. Ann Oncol 2005;16:1366–1373. [DOI] [PubMed] [Google Scholar]
- 30. Armand P, Kim HT, Logan BR et al. Validation and refinement of the disease risk index for allogeneic stem cell transplantation. Blood 2014;123:3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Friedmann AM, Sengul H, Lehmann H et al. Do basic laboratory tests or clinical observations predict bleeding in thrombocytopenic oncology patients? A reevaluation of prophylactic platelet transfusions. Transfus Med Rev 2002;16:34–45. [DOI] [PubMed] [Google Scholar]
- 32. Lee AY, Levine MN, Baker RI et al. Low‐molecular‐weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146–153. [DOI] [PubMed] [Google Scholar]
- 33. Herishanu Y, Misgav M, Kirgner I et al. Enoxaparin can be used safely in patients with severe thrombocytopenia due to intensive chemotherapy regimens. Leuk Lymphoma 2004;45:1407–1411. [DOI] [PubMed] [Google Scholar]
- 34. Baigent C, Collins R, Appleby P et al. ISIS‐2: 10 year survival among patients with suspected acute myocardial infarction in randomised comparison of intravenous streptokinase, oral aspirin, both, or neither. The ISIS‐2 (Second International Study of Infarct Survival) collaborative group. BMJ 1998;316:1337–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.