This review summarizes the neurotoxicity data for combining systemic therapy (chemotherapeutics, molecular targeted agents, or monoclonal antibodies) with concurrent radiotherapy of brain metastases. For most systemic therapies, the currently available literature does not show an increase in neurotoxicity when these therapies are used concurrently with cranial radiotherapy.
Keywords: Brain metastases, Radiotherapy, Systemic therapy, Molecular targeted agents, Monoclonal antibodies, Toxicity
Abstract
The incidence of brain metastases of solid tumors is increasing. Local treatment of brain metastases is generally straightforward: cranial radiotherapy (e.g., whole‐brain radiotherapy or stereotactic radiosurgery) or resection when feasible. However, treatment becomes more complex when brain metastases occur while other metastases, outside of the central nervous system, are being controlled with systemic therapy (chemotherapeutics, molecular targeted agents, or monoclonal antibodies). It is known that some anticancer agents can increase the risk for neurotoxicity when used concurrently with radiotherapy. Increased neurotoxicity decreases quality of life, which is undesirable in this predominantly palliative patient group. Therefore, it is of utmost importance to identify the compounds that should be temporarily discontinued when cranial radiotherapy is needed.
This review summarizes the (neuro)toxicity data for combining systemic therapy (chemotherapeutics, molecular targeted agents, or monoclonal antibodies) with concurrent radiotherapy of brain metastases. Because only a limited amount of high‐level data has been published, a risk assessment of each agent was done, taking into account the characteristics of each compound (e.g., lipophilicity) and the microenvironment of brain metastasis. The available trials suggest that only gemcitabine, erlotinib, and vemurafenib induce significant neurotoxicity when used concurrently with cranial radiotherapy. We conclude that for most systemic therapies, the currently available literature does not show an increase in neurotoxicity when these therapies are used concurrently with cranial radiotherapy. However, further studies are needed to confirm safety because there is no high‐level evidence to permit definitive conclusions.
Implications for Practice.
The treatment of symptomatic brain metastases diagnosed while patients are receiving systemic therapy continues to pose a dilemma to clinicians. Will concurrent treatment with cranial radiotherapy and systemic therapy (chemotherapeutics, molecular targeted agents, and monoclonal antibodies), used to control intra‐ and extracranial tumor load, increase the risk for neurotoxicity? This review addresses this clinically relevant question and evaluates the toxicity of combining systemic therapies with cranial radiotherapy, based on currently available literature, in order to determine the need to and interval to interrupt systemic treatment.
Introduction
Systemic treatment options for metastasized solid tumors have increased [1], [2], [3], [4], [5], [6]. Thus, long‐term progression‐free survival can sometimes be achieved, especially when a driver oncogene in the tumorigenesis of a specific tumor can be targeted, such as BRAF V600E in melanoma. Moreover, radical treatment for patients with oligometastatic disease has become an accepted treatment modality [7]. With a growing number of long‐term cancer survivors, brain metastases are frequently observed, especially in patients with non‐small cell lung cancer (NSCLC), breast cancer, melanoma, and renal cell carcinoma (RCC) [8].
Some systemic treatment schedules (chemotherapeutics, targeted agents, and monoclonal antibodies) show intracranial response and/or stabilization of brain metastases [9], [10]. Contrary to traditional chemotherapeutical agents, recently developed targeted agents show the potential to cross the blood‐brain barrier (BBB). These targeted agents may be able to control brain metastases, especially when the brain metastases are relatively small and asymptomatic [1]. However, despite initial responses, brain metastases often progress during systemic treatment. Traditionally, whole‐brain radiotherapy (WBRT) is the cornerstone of the treatment of symptomatic multiple (four or more) brain metastases. The use of partial‐brain radiation therapy techniques, including but not limited to stereotactic radiosurgery (SRS) and stereotactic radiotherapy, has increased and is the preferred initial treatment option in patients with a limited number of (one to three) brain metastases smaller than 4 cm [2]. Depending on national guidelines, number, size, and leptomeningeal spread of the brain metastases, cranial radiotherapy is the treatment modality of choice.
Some chemotherapeutics and targeted agents have shown to be radiosensitizing for healthy brain tissue, which may increase the risk for complications. These complications have mainly been described in the extracranial setting (e.g., bleeding, bowel perforation, radionecrosis) and resulted in severe morbidity and impaired quality of life [11], [12], [13].
To minimize the risk for neurotoxicity, systemic therapies are often discontinued during cranial radiotherapy. However, the disadvantage of discontinuation is a potential tumor flare of extracranial disease. Ninety‐seven percent of systemically administered oncolytic drugs is eliminated from the blood after 5 times the half‐life (t½). Because of the long t½ of most targeted agents (or their active metabolites) and monoclonal antibodies, this would mean a long period (weeks to months) of discontinuation when this level of elimination is pursued, which is undesirable in the control of extracranial tumor load. Additionally, 97% of drug excretion might not be sufficient to abolish the metabolic activity of these agents in brain metastases.
The aim of this review is to determine which of the commonly used systemic therapies can be safely continued during cranial radiotherapy and which should be discontinued, based on the currently available literature. We discuss the chemotherapeutics, targeted agents, and monoclonal antibodies that are most commonly used in the subset of solid tumors that most frequently metastasize to the brain.
Materials and Methods
Systemic therapies that are commonly used in the treatment of tumors that metastasize to the brain were selected for inclusion. The search was conducted on papers describing the combination of these systemic agents and cranial radiotherapy of cerebral metastases of solid tumors. Additional literature describing the ability of the systemic therapy to penetrate the BBB and pharmacological characteristics was gathered as supportive information. With these data, a narrative review was executed, as this is the best review format that can be obtained considering the available information.
Inclusion and Exclusion Criteria
Papers describing the combination of cranial radiotherapy and one of the selected systemic therapies and having (neuro)toxicity as an outcome measure were eligible for inclusion. All types of articles (including reviews; case reports; and phase I, II, and III studies) were included.
Papers describing the treatment of primary brain tumors were excluded. All non‐English‐language articles were excluded. Additional literature was searched on combined chemotherapeutic schedules because literature on separately used chemotherapeutics was very scarce. However, this was not part of the main search strategy.
Identification of Studies
The literature search was conducted independently by two researchers (M.V. and H.M.) up to June 2015 using the databases PubMed, MEDLINE, Cochrane, and Web of Science. References from the included studies were also reviewed for eligible literature. The complete search strategy can be found in the supplemental online data (Appendix A).
Study Selection
Studies were initially selected on the basis of title; further selection took place according to the abstracts. For articles found to be eligible, the whole article was read. Eligibility was assessed by two reviewers (M.V. and H.M.). Disagreements were resolved by consensus.
Data Extraction
From the included papers, data were extracted on (a) trial characteristics; (b) treatment schedule used; and (c) the described neurotoxicity, methods of evaluating toxicity, assessment of quality of life (QoL) and neurocognition, and follow‐up characteristics.
Results
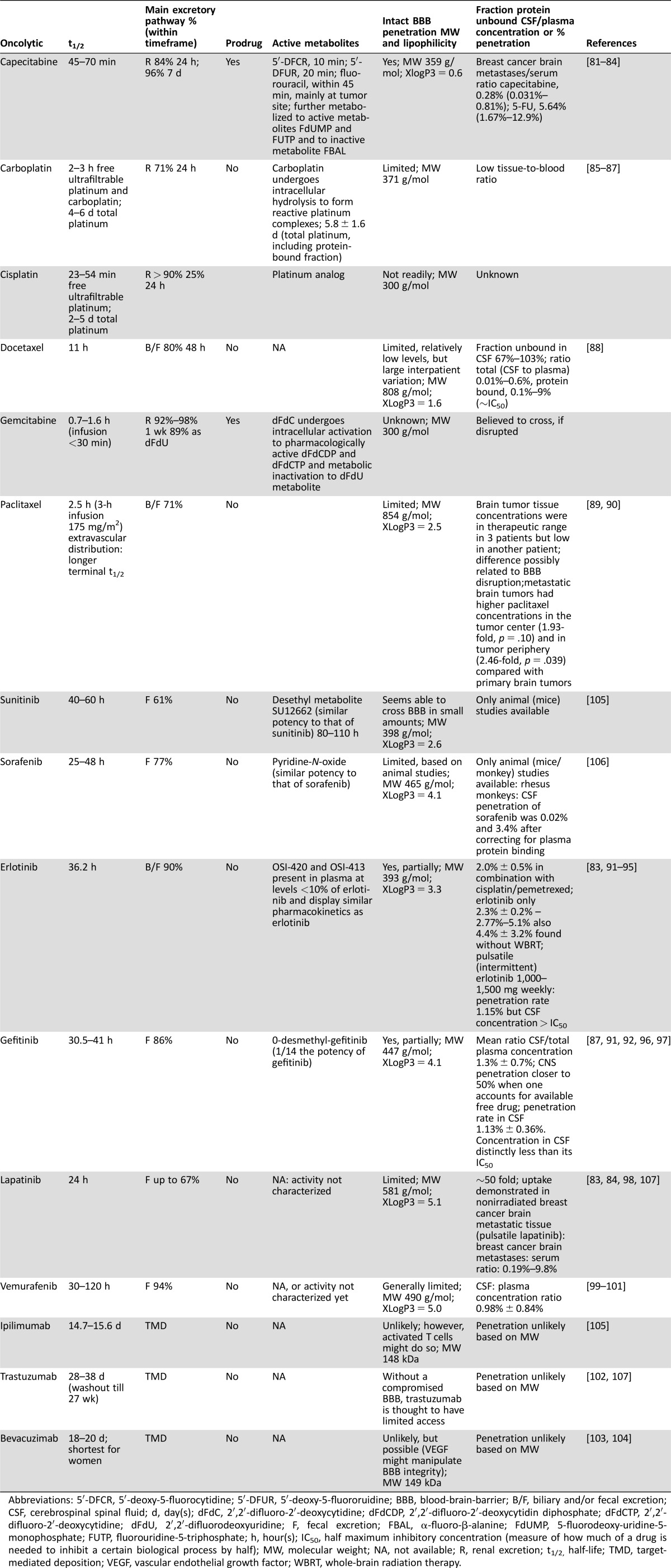
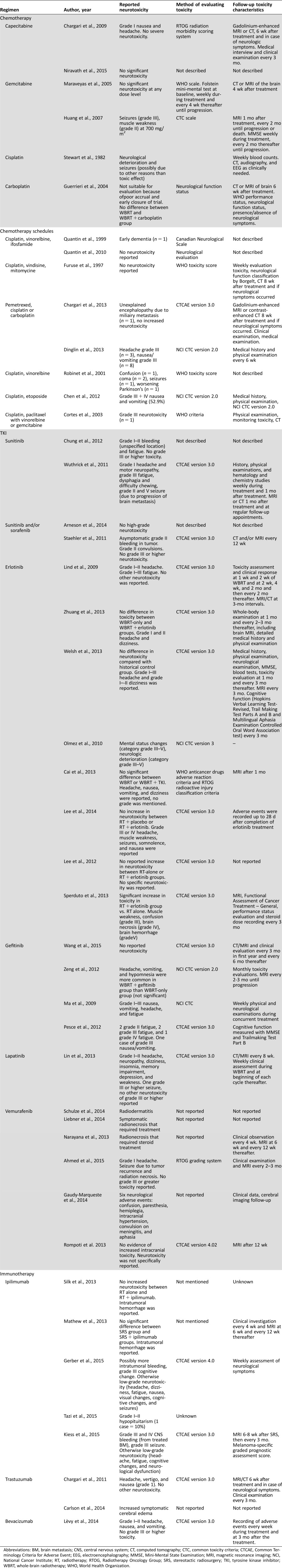
An overview of the trial characteristics can be found in the supplemental online data (Appendix B). Working mechanisms of the discussed systemic therapies are reported in Appendix C of the supplemental online data. Table 1 describes pharmacological characteristics of the systemic agents discussed in this review. Table 2 describes all reported neurotoxicity.
Table 1. Pharmacological characteristics of systemic therapies.
Abbreviations: 5′‐DFCR, 5′‐deoxy‐5‐fluorocytidine; 5′‐DFUR, 5′‐deoxy‐5‐fluororuidine; BBB, blood‐brain‐barrier; B/F, biliary and/or fecal excretion; CSF, cerebrospinal spinal fluid; d, day(s); dFdC, 2′,2′‐difluoro‐2′‐deoxycytidine; dFdCDP, 2′,2′‐difluoro‐2′‐deoxycytidin diphosphate; dFdCTP, 2′,2′‐difluoro‐2′‐deoxycytidine; dFdU, 2′,2′‐difluorodeoxyuridine; F, fecal excretion; FBAL, α‐fluoro‐β‐alanine; FdUMP, 5‐fluorodeoxy‐uridine‐5‐monophosphate; FUTP, fluorouridine‐5‐triphosphate; h, hour(s); IC50, half maximum inhibitory concentration (measure of how much of a drug is needed to inhibit a certain biological process by half); MW, molecular weight; NA, not available; R, renal excretion; t1/2, half‐life; TMD, target‐mediated deposition; VEGF, vascular endothelial growth factor; WBRT, whole‐brain radiation therapy.
Table 2. Overview of reported toxicity with concurrent use of systemic therapies and cerebral radiotherapy.
Abbreviations: BM, brain metastasis; CNS, central nervous system; CT, computed tomography; CTC, common toxicity criteria; CTCAE, Common Terminology Criteria for Adverse Event; EEG, electroencephalography; MMSE, Mini‐Mental State Examination; MRI, magnetic resonance imaging; NCI, National Cancer Institute; RT, radiotherapy; RTOG, Radiotherapy Oncology Group; SRS, stereotactic radiosurgery; TKI, tyrosine kinase inhibitor; WBRT, whole‐brain radiotherapy; WHO, World Health Organization.
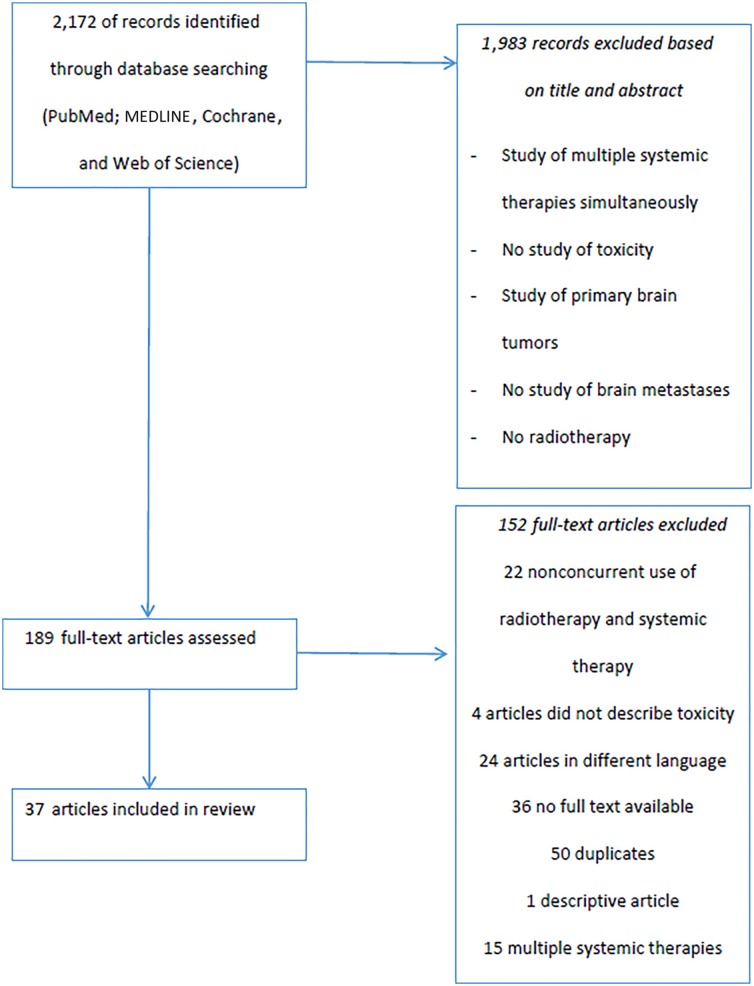
A total of 2,172 records were identified by using the search strategy (Appendix A). In the end, 37 articles were included in the review. Figure 1 provides an overview of the complete selection process.
Figure 1.
Selection process of literature search.
Combination of Radiotherapy and Chemotherapy
Antimetabolites: Capecitabine/5‐Fluorouracil and Gemcitabine.
Two studies describe toxicity (Table 2) of capecitabine used concurrently with cranial radiotherapy. One study reported overall mild toxicity and no high‐grade neurotoxicity [14]. A second study, a phase II trial, in which capecitabine was combined with WBRT followed by sunitinib, closed early because of low efficacy and excessive toxicity, although no high‐grade neurotoxicity was mentioned [15]. Neurocognitive impairment and QoL, both salient endpoints, were not separately scored in these studies.
Two trials investigated toxicity (Table 2) for the combination of gemcitabine and cranial radiotherapy. A phase I trial reported dose‐limiting toxicity at the highest dose level (70 mg/m2 twice weekly) to be grade IV neutropenia. No significant nonhematological toxicities were observed at any dose level. Moreover, no deterioration in mental function was observed by the Folstein test [16]. In another phase I trial, dose‐limiting neurotoxicity was observed, at a dose of 700 mg/m2, consisting of grade III seizures and grade II muscle weakness [17]. Neither study scored for QoL. Additionally, gemcitabine‐induced radiation recall, a serious inflammatory reaction often leading to necrosis, has been described. Radiation recall reactions were seen in the central nervous system, skin, gastrointestinal tract, and lymphatic and musculoskeletal systems. The time between initiation of radiation and recall of the radiation phenomenon ranged from 3 weeks to 8 months from the time gemcitabine was initiated. The usual dosage of gemcitabine in these cases was 1,000 mg/m2 given weekly [18], [19], [20].
Platinum Analogs: Cisplatin and Carboplatin.
One study described the toxicity (Table 2) of cisplatin combined with cranial radiotherapy. In this study, 4 of 14 patients had possible central nervous system toxicity, categorized as neurological deterioration and seizure. However, in all 4 patients other possible reasons for the transient neurologic deterioration were present, such as tapering of corticosteroids [21].
Cisplatin combined with other chemotherapeutic agents did not cause significant neurotoxicity when used concurrently with radiotherapy. Several studies describe cerebral radiotherapy in combination with cisplatin and other chemotherapeutics, such as vinorelbine and ifosfamide [22], [23] vindesine and mitomycin [24], pemetrexed [25], [26], vinorelbine [27], and etoposide [28]. None of these trials reported high‐grade neurotoxicity (Table 2).
One study described (Table 2) the concurrent use of carboplatin and cranial radiotherapy. This randomized phase III trial showed no significant increase in toxicity, whether carboplatin was added to WBRT or not. The pitfall of this trial was its early closing due to poor accrual. Because 43% of patients in the WBRT‐alone arm and 14% in the combined‐modality arm were not assessed for symptom control, no conclusion can be made concerning differences in neurotoxicity [29].
Taxanes: Paclitaxel/Docetaxel.
Paclitaxel concurrently administered with WBRT and combined with cisplatin every 3 weeks is a feasible treatment schedule for patients with brain‐metastasized NSCLC, without evidence for inducing significant neurotoxicity. This study did not score for QoL [30].
To our knowledge, no published clinical trials have administered docetaxel, alone or in a multiagent chemotherapy schedule, concurrently with cranial radiotherapy for the treatment of brain metastases.
Combination of Radiotherapy and Targeted Agents
Sunitinib and Sorafenib.
Four studies described the toxicity (Table 2) of sunitinib (25 mg once daily or 37.5 mg once daily or 50 mg once daily for 4 weeks on, 2 weeks off) and/or sorafenib (400 mg once or twice daily) combined with cranial radiotherapy. Three studies described no grade III or higher neurotoxicity. Neurocognition and QoL were not assessed [31], [32], [33]. One study showed grade III fatigue and grade III dysphagia and drooling/difficulty chewing. One patient died of grade V seizures, but this was attributed to progression of brain metastasis. No testing was done regarding neurocognition or QoL [34].
Erlotinib.
Eight studies with concurrent use of erlotinib (100 mg or 150 mg once daily) and cranial radiotherapy were identified (Table 2). One study reported no high‐grade neurotoxicity [35]. Another study reported 1 case (out of 50) with grade III headache This study also evaluated neurocognition and found no statistical difference between the treatment group and a historical control group [36]. Four studies compared radiotherapy alone with radiotherapy combined with erlotinib. None of these trials reported a significant difference in neurotoxicity between the treatment arms [37], [38], [39], [40]. QoL was assessed in one study (EuroQol EQ‐5D questionnaire), and no significant difference was observed between the treatment groups [37]. One retrospective study found grade III–V mental status changes in three cases without clear etiology. No other neurotoxicity was reported. Neurocognition and QoL were not assessed [41]. A phase III study compared the combination of WBRT and SRS with or without erlotinib. In the erlotinib group, grade III neurotoxicity, including fatigue, muscle weakness, confusion, and ataxia; grade IV brain necrosis; and one grade V hemorrhagic stroke were found. A significant increase in overall toxicity was found when erlotinib was added to cranial radiotherapy. QoL (by performance status) was also negatively affected by the addition of erlotinib to cranial radiotherapy [42].
Gefitinib.
Five studies described toxicity (Table 2) when gefitinib (250 mg once daily) was combined with cranial radiotherapy. One study reported no neurotoxicity [43]; however, another study reported few cases of grade III neurotoxicity [44]. Both reported an improvement in QoL (activities of daily living scoring or Functional Assessment of Cancer Therapy‐Brain [FACT‐Br]) after treatment with gefitinib and cranial radiotherapy. One retrospective study reported slightly more headache, vomiting, and hypomnesia, although not significantly so, in the concurrent‐use group compared with the gefitinib‐only group. The performance score did not differ between the groups before or after treatment [45]. Another retrospective study compared toxicity between radiotherapy with or without gefitinib and found no difference in adverse events. Neurocognition and QoL were not assessed [39]. A phase II study reported different levels of fatigue. Grade III nausea and vomiting were also reported. Neurocognition and QoL (Folstein test, Trail‐making Test part B, and Quality of Life Questionnaire‐Core 36 score) were stable before and after the treatment period [46].
Lapatinib.
One study described the toxicity (Table 2) of lapatinib (maximum, 1,500 mg once daily) combined with cranial radiotherapy. This trial reported a seizure in one patient. No other grade III or higher neurotoxicity was reported. QoL was scored by using FACT‐Br and showed a general decline of QoL after the treatment. However, only a limited number of patients could be tested both at baseline and after finishing treatment [47].
Vemurafenib.
Six papers described toxicity (Table 2) with the concurrent use of vemurafenib (960 mg twice daily) and cranial radiotherapy. Two case reports of patients with brain metastases of melanoma reported radiation dermatitis on the radiated site [48]. Two other case reports of patients with brain metastases of melanoma reported symptomatic radionecrosis; however, neurotoxicity was not scored [49]. A retrospective study also identified symptoms due to radionecrosis in 1 of 12 patients [50]. Another retrospective study reported 1 melanoma patient with brain metastases who developed a seizure. However, it was not clear whether the seizure was caused by tumor progression or by radiation necrosis. No (other) significant toxicity was reported [51]. A third retrospective study reported the following symptoms in 6 melanoma patients with brain metastases: confusion, paresthesia, hemiplegia, intracranial hypertension, convulsion on meningitis, and aphasia. No grading system was used to assess the severity. All these patients had highly metastatic (4–20 brain metastases) cerebral disease [52]. Additionally, 4 case reports in patients with brain metastases from melanoma described no evidence of increased intracranial toxicity with concurrent use of WBRT and vemurafenib [53]. None of these studies assessed neurocognition or QoL.
Combination of Radiotherapy and Monoclonal Antibodies
Immune Checkpoint Inhibitors: Ipilimumab.
Five articles describe toxicity (Table 2) when ipilimumab (3 or 10 mg/kg every 3 weeks) was combined with cranial radiotherapy. One study reported no high‐grade neurotoxicity, and QoL (performance status) did not change for 8 of 10 patients [54]. Two studies compared a radiotherapy‐only group with a radiotherapy plus ipilimumab group and reported no difference in toxicity [55], [56]. One retrospective study described possibly more intratumoral bleeding with administration of ipilimumab concurrently with radiotherapy. However, this did not require any intervention. This trial also described one patient with grade III cognitive impairment during WBRT. Other reported neurotoxicity was only low grade. QoL was not assessed [57]. Another retrospective study described grade III–IV toxicity in 20% of melanoma patients treated with radiation therapy combined with ipilimumab, including both neurological and nonneurological toxicity. Neurological toxicity included bleeding from treated brain metastasis (grade III and IV) and seizures (grade III). QoL was not assessed [58].
Trastuzumab.
Two studies reported the toxicity (Table 2) of the combination of trastuzumab (2 mg/kg weekly or 6 mg/kg every 3 weeks) and cranial radiotherapy. One retrospective study reported no high‐grade neurotoxicity. QoL was not scored [59]. Another article reported four case reports in which multiple neurological symptoms (headache, nausea/vomiting, speech impairment, short‐term memory deficits, imbalance, gait disturbance, and visual deficits) were observed, all of which were attributed to brain edema due to concurrent use of trastuzumab and cranial radiotherapy. The level of neurotoxicity was not scored [60].
Bevacizumab.
One study evaluated toxicity (Table 2) for the concurrent use of bevacizumab (15 mg/kg three times a month) and cranial radiotherapy for brain metastases. This phase I study reported no high‐grade neurotoxicity and did not assess neurocognition and QoL [61].
Discussion
Approximately 10%–20% of all solid tumors will eventually metastasize to the brain. NSCLC, small cell lung cancer, breast cancer, melanoma, and RCC are the most common primary tumors that develop brain metastases [8]. Cranial radiotherapy is still the standard treatment of symptomatic brain metastases. There is growing evidence that some systemic anticancer agents may also control brain metastases in selected patients, especially when brain metastases are asymptomatic and relatively small [2]. When brain metastases progress and become symptomatic and unresponsive to the systemic anticancer treatment that is administered to control the extracerebral metastases, a repeated question is whether the systemic treatment should be discontinued at the time of cranial radiotherapy of brain metastases in order to prevent significant neurotoxicity. Instead of long‐term discontinuation, a more pragmatic approach can be defended, for example, discontinuation 1 week before to 1 week after the cranial radiotherapy. This approach seemed to be feasible on the basis of the data from a recently published retrospective study, which combined SRS with multiple types of systemic anticancer therapies, without increasing neurotoxicity. However, no information on issues such as treatment schedule and drug dosage was provided [62].
It is obvious that multiple factors should be taken into account in addressing whether to discontinue systemic therapies during cranial radiotherapy. One of these factors is a significant interpatient and intrapatient variation in the intratumoral concentration of specific oncolytics in brain metastases due to differences in permeability of the blood‐tumor‐barrier (BTB) for different anticancer agents and also in the expression of drug efflux pumps, such as P‐glycoprotein [63], [64], [65], [66]. Table 1 shows these pharmacological characteristics for the different anticancer agents.
The trials described in this article do not show increased neurotoxicity for capecitabine, 5‐fluoroucil (5‐FU), cisplatin and carboplatin, or taxanes when administered concurrently with cranial radiotherapy. This is the case for both monotherapy and also combined in multidrug schedules, commonly used in the palliative treatment of metastasized solid tumors (Table 2). However, it should be taken into account that high‐level evidence is lacking. Moreover, data regarding neurocognitive impairment and decreases in QoL are extremely sparse because most studies are retrospective or did not include these data. Also, the methods used to assess neurocognition (e.g., Folstein test) are insensitive and therefore cannot secure a lack of neurocognitive toxicity [67]. On the basis of the available information the chemotherapy regimens containing 5‐FU or capecitabine could be continued, when symptomatic brain metastases require treatment with cranial radiotherapy, and only when extracerebral tumor load necessitates protracted systemic treatment.
Combining gemcitabine, at a dose of 700 mg/m2, with cranial radiotherapy is not feasible because of a significant increase in neurotoxicity [17]. It is expected that the commonly used schedule of administrating gemcitabine at a dose of 1,000 mg/m2 will result in clinically significant neurotoxicity when used concurrently with WBRT. The t½ of gemcitabine is short: 0.7–1.6 hours (Table 1). It is expected that 97% of this drug is eliminated after 8 hours (5 times t½). Because gemcitabine is also metabolized into active metabolites intra‐tumorally, neurotoxicity can still occur when cranial radiotherapy is started after complete drug elimination is reached. There are no data available that define when cranial radiotherapy can be safely started after discontinuing gemcitabine; gemcitabine‐induced radiation recall has been described from 3 weeks to 8 months from the time gemcitabine was initiated. According to the available literature, which is sparse and might underestimate the risk for increased neurotoxicity, the concurrent and sequential use of gemcitabine and cranial radiotherapy should be discouraged.
Inhibitors of angiogenesis (e.g., sunitinib and sorafenib) are routinely used to treat metastasized RCC [68]. For patients with symptomatic brain metastases of RCC, combination with cranial radiotherapy did not result in clinically significant neurotoxicity. However, clinical trials included only a small number of patients, and both neurocognitive testing and scoring of QoL were limited. Again, high‐level evidence is lacking. Because these drugs are known to be radiosensitizing, one should be reluctant to combine these multi‐tyrosine kinase inhibitors with cranial radiotherapy.
It is not clear how long these targeted agents should be discontinued before cranial radiotherapy begins in order to sufficiently abolish their metabolic activity. On the basis of t½ of these molecules and their active metabolites, sunitinib should be stopped at least for 550 hours (5 times t½) and sorafenib for 240 hours (5 times t½) to achieve 97% excretion before radiation therapy is started (Table 1) [69], [70]. During this long period of discontinuation, flare of tumor activity of extracerebral metastases might occur. In addition, it is important to realize that even after complete excretion, the metabolic activity of these agents in brain metastases might still be ongoing; it has not been established that 5 times t½ or 97% excretion is directly correlated to the metabolic activity of these drugs.
Concerning the risk for intratumoral bleeding, a database search of clinical trials that included patients with or without central nervous system (CNS) metastases and treated with sunitinib, sorafenib, or bevacizumab did not show an increased risk for cerebral hemorrhage. The safety of bevacizumab with regard to cerebral hemorrhage in patients with CNS metastasis of breast cancer, NSCLC, RCC, and colorectal cancer was also described and confirmed in a large retrospective analysis [71], [72].
Gefitinib was not shown to cause increased neurotoxicity or a deterioration of neurocognition and QoL when combined with cranial radiotherapy, although the tests used to measure neurocognition and QoL were insensitive and therefore not conclusive. Concurrent use of erlotinib, another tyrosine‐kinase inhibitor of endothelial growth factor receptor, was associated with significant neurotoxicity when both WBRT and SRS were used [42]. Although high‐level evidence is lacking, this combination should be discouraged. The safety of cranial radiotherapy combined with TKIs, used in NSCLC, was recently reviewed by Hendriks et al. [73].
Gefitinib was not shown to cause increased neurotoxicity or a deterioration of neurocognition and QoL when combined with cranial radiotherapy, although the tests used to measure neurocognition and QoL were insensitive and therefore not conclusive.
An incidental increase in neurotoxicity has also been reported when other tyrosine kinase inhibitors, such as vemurafenib, are combined with cranial radiotherapy. This is supported by a statement from the manufacturer, in agreement with the European Medicines Agency, which advises caution when vemurafenib is used before, during, or after cranial radiotherapy [74]. A recently published literature review on the combination of BRAF inhibitors and cranial radiotherapy recommends holding BRAF inhibitors for at least 3 days before and after fractional radiotherapy and at least 1 day before and after stereotactic radiosurgery [75].
However, the duration of discontinuation of systemic oncolytics remains a matter of debate.
Ninety‐seven percent plasma elimination of these drugs is achieved after 5 times t½. However, this does not include any remaining metabolic activities of these tyrosine kinase inhibitors intratumorally, as mentioned earlier. Thus, increased neurotoxicity with neurocognitive deterioration and decreased QoL can never be completely prevented, especially because it is not known whether there is a dose‐dependent effect for radiosensitization. Therefore, the risks for extracerebral tumor flare after stopping TKI should be weighed against the risks for increased neurotoxicity due to concurrent treatment.
Immune checkpoint modulators, such as ipilimumab, a large monoclonal antibody, are unable to penetrate the BBB in patients without brain metastases (Table 1) [76]. However, in brain metastases the BBB is mostly disrupted, which may facilitate ipilimumab to cross the perivascular space and activate peripherally recruited T cells. Alternatively, ipilimumab‐activated T cells in the peripheral circulation may enter the brain metastases through the BBB/BTB. Combining radiation therapy for melanoma brain metastases with ipilimumab appears to be safe and well tolerated. However, a mostly asymptomatic, transient increase of lesion size, suggesting subacute inflammatory response or intratumoral bleeding, was seen in several studies in which SRS was combined with ipilimumab. This pseudoprogression was not present when SRS was given after completion of ipilimumab treatment [58].
Further studies are needed to investigate the ideal sequence of ipilimumab and SRS in order to minimize side effects while maximizing efficacy. Research is also ongoing on whether SRS before or during ipilimumab and potentially other immune checkpoint modulators might increase the immune response by increasing the release of tumor antigens. In addition, one could anticipate an effect on nonirradiated lesions and ultimately the prevention of new metastatic events, the so‐called abscopal effect [77], [78].
Other monoclonal antibodies, such as trastuzumab, are also very limited in passing the intact BBB because of their large molecular weight (Table 1). However, trastuzumab can prolong median overall survival in patients with brain metastasized HER2‐expressing breast tumors, suggesting its permeation through the BTB [79], [80]. When brain metastases develop during trastuzumab treatment, combination with radiation therapy seems to be safe and feasible.
Conclusion
Brain metastases may develop during systemic treatment schedules that successfully control extracerebral tumor metastases. Cranial radiotherapy is traditionally used to control symptomatic brain metastases. Among all the systemic cancer treatments reviewed, an elevated risk for neurotoxicity was described with gemcitabine, erlotinib, and vemurafenib. However, caution should be used in interpreting these results because most studies are retrospective studies or small phase I trials that performed only limited screening for neurotoxicity. Therefore, no definite conclusions can be made regarding the concurrent use of these oncolytics with cranial radiotherapy on the basis of the data currently available. However, there is growing evidence that not all systemic therapies need to be discontinued during cranial radiotherapy. Prospective randomized trials with overall survival as well as neurocognition and QoL as endpoints are needed in this palliative patient subset. Immune checkpoint modulators and treatments that activate the immune system are promising. They are being investigated with regard to whether their combination with cranial radiotherapy may improve treatment outcome in the setting of brain metastases to induce an abscopal effect.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
We thank Alicia Lucardie for proofreading the manuscript.
Author Contributions
Conception/Design: Maikel Verduin, Jaap D. Zindler, Hanneke M.A. Martinussen, Rob L.H. Jansen, Sander Croes, Lizza E.L. Hendriks, Danielle B.P Eekers, Ann Hoeben
Provision of study material or patients: Maikel Verduin, Jaap D. Zindler, Hanneke M.A. Martinussen, Rob L.H. Jansen, Sander Croes, Lizza E.L. Hendriks, Danielle B.P Eekers, Ann Hoeben
Collection and/or assembly of data: Maikel Verduin, Hanneke M.A. Martinussen,
Data analysis and interpretation: Maikel Verduin, Jaap D. Zindler, Hanneke M.A. Martinussen, Rob L.H. Jansen, Sander Croes, Lizza E.L. Hendriks, Danielle B.P Eekers, Ann Hoeben
Manuscript writing: Maikel Verduin, Hanneke M.A. Martinussen, Ann Hoeben
Final approval of manuscript: Maikel Verduin, Jaap D. Zindler, Hanneke M.A. Martinussen, Rob L.H. Jansen, Sander Croes, Lizza E.L. Hendriks, Danielle B.P Eekers, Ann Hoeben
Disclosures
The authors indicated no financial relationships.
Supplementary Information
References
- 1. Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol 2005;23:6207–6219. [DOI] [PubMed] [Google Scholar]
- 2. Lin X, DeAngelis LM. Treatment of brain metastases. J Clin Oncol 2015;33:3475–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dy GK, Adjei AA. Systemic cancer therapy: Evolution over the last 60 years. Cancer 2008;113:1857–1887. [DOI] [PubMed] [Google Scholar]
- 4. Jordan VC, Brodie AM. Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids 2007;72:7–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Segal NH, Saltz LB. Evolving treatment of advanced colon cancer. Annu Rev Med 2009;60:207–219. [DOI] [PubMed] [Google Scholar]
- 6. Colozza M, de Azambuja E, Cardoso F et al. Breast cancer: Achievements in adjuvant systemic therapies in the pre‐genomic era. The Oncologist 2006;11:111–125. [DOI] [PubMed] [Google Scholar]
- 7. Salama JK, Milano MT. Radical irradiation of extracranial oligometastases. J Clin Oncol 2014;32:2902–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nussbaum ES, Djalilian HR, Cho KH et al. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer 1996;78:1781–1788. [PubMed] [Google Scholar]
- 9. Dzienis MR, Atkinson VG. Response rate to vemurafenib in patients with B-RAF-positive melanoma brain metastases: A retrospective review. Melanoma Res 2014;24:349–353. [DOI] [PubMed] [Google Scholar]
- 10. Fan Y, Xu X, Xie C. EGFR‐TKI therapy for patients with brain metastases from non‐small‐cell lung cancer: A pooled analysis of published data. Onco Targets Ther 2014;7:2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peters NA, Richel DJ, Verhoeff JJ et al. Bowel perforation after radiotherapy in a patient receiving sorafenib. J Clin Oncol 2008;26:2405–2406. [DOI] [PubMed] [Google Scholar]
- 12. Inoue T, Kinoshita H, Komai Y et al. Two cases of gastrointestinal perforation after radiotherapy in patients receiving tyrosine kinase inhibitor for advanced renal cell carcinoma. World J Surg Oncol 2012;10:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhuang H, Yuan Z, Chang JY et al. Radiation pneumonitis in patients with non–small‐cell lung cancer treated with erlotinib concurrent with thoracic radiotherapy. J Thorac Oncol 2014;9:882–885. [DOI] [PubMed] [Google Scholar]
- 14. Chargari C, Kirova YM, Dieras V et al. Concurrent capecitabine and whole‐brain radiotherapy for treatment of brain metastases in breast cancer patients. J Neurooncol 2009;93:379–384. [DOI] [PubMed] [Google Scholar]
- 15. Niravath P, Tham YL, Wang T et al. A phase II trial of capecitabine concomitantly with whole‐brain radiotherapy followed by capecitabine and sunitinib for brain metastases from breast cancer. The Oncologist 2015;20:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maraveyas A, Sgouros J, Upadhyay S et al. Gemcitabine twice weekly as a radiosensitiser for the treatment of brain metastases in patients with carcinoma: A phase I study. Br J Cancer 2005;92:815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang YJ, Wu YL, Xie SX, et al. Weekly gemcitabine as a radiosensitiser for the treatment of brain metastases in patients with non‐small cell lung cancer: Phase I trial. Chin Med J (Engl) 2007;120:458–462. [PubMed] [Google Scholar]
- 18. Schwarte S, Wagner K, Karstens JH et al. Radiation recall pneumonitis induced by gemcitabine. Strahlenther Onkol 2007;183:215–217. [DOI] [PubMed] [Google Scholar]
- 19. Schwartz BM, Khuntia D, Kennedy AW et al. Gemcitabine‐induced radiation recall dermatitis following whole pelvic radiation therapy. Gynecol Oncol 2003;91:421–422. [DOI] [PubMed] [Google Scholar]
- 20. Jeter MD, Janne PA, Brooks S et al. Gemcitabine‐induced radiation recall. Int J Radiat Oncol Biol Phys 2002;53:394–400. [DOI] [PubMed] [Google Scholar]
- 21. Stewart DJ, Leavens M, Maor M et al. Human central nervous system distribution of cis‐diamminedichloroplatinum and use as a radiosensitizer in malignant brain tumors. Cancer Res 1982;42:2474–2479. [PubMed] [Google Scholar]
- 22. Quantin X, Khial F, Reme‐Saumon M et al. Concomitant brain radiotherapy and vinorelbine‐ifosfamide‐cisplatin chemotherapy in brain metastases of non‐small cell lung cancer. Lung Cancer 1999;26:35–39. [DOI] [PubMed] [Google Scholar]
- 23. Quantin X, Bozonnat MC, Pujol JL. Recursive partitioning analysis groups II‐III brain metastases of non‐small cell lung cancer: A phase II randomized study comparing two concurrent chemoradiotherapy regimens. J Thorac Oncol 2010;5:846–851. [DOI] [PubMed] [Google Scholar]
- 24. Furuse K, Kamimori T, Kawahara M et al. A pilot study of concurrent whole‐brain radiotherapy and chemotherapy combined with cisplatin, vindesine and mitomycin in non‐small‐cell lung cancer with brain metastasis. Br J Cancer 1997;75:614–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dinglin XX, Huang Y, Liu H et al. Pemetrexed and cisplatin combination with concurrent whole brain radiotherapy in patients with brain metastases of lung adenocarcinoma: A single‐arm phase II clinical trial. J Neurooncol 2013;112:461–466. [DOI] [PubMed] [Google Scholar]
- 26. Chargari C, Pacaut C, Le Moulec S et al. First assessment of whole‐brain radiation therapy combined with pemetrexed‐based chemotherapy in non‐small‐cell lung carcinoma: Data on safety and efficacy. Anticancer Drugs 2013;24:736–742. [DOI] [PubMed] [Google Scholar]
- 27. Robinet G, Thomas P, Breton JL et al. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non‐small‐cell lung cancer: Groupe Francais de Pneumo‐cancerologie (GFPC) protocol 95–1. Ann Oncol 2001;12:59–67. [DOI] [PubMed] [Google Scholar]
- 28. Chen LK, Huang H, Liao H et al. Chemotherapy with concurrent brain and thoracic radiotherapy in brain‐only metastases of treatment naive small‐cell lung cancer: A phase II study. Med Oncol 2012;29:1687–1692. [DOI] [PubMed] [Google Scholar]
- 29. Guerrieri M, Wong K, Ryan G et al. A randomised phase III study of palliative radiation with concomitant carboplatin for brain metastases from non‐small cell carcinoma of the lung. Lung Cancer 2004;46:107–111. [DOI] [PubMed] [Google Scholar]
- 30. Arrieta O, Villarreal‐Garza C, Zamora J et al. Long‐term survival in patients with non‐small cell lung cancer and synchronous brain metastasis treated with whole‐brain radiotherapy and thoracic chemoradiation. Radiat Oncol 2011;6:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Staehler M, Haseke N, Nuhn P et al. Simultaneous anti‐angiogenic therapy and single‐fraction radiosurgery in clinically relevant metastases from renal cell carcinoma. BJU Int 2011;108:673–678. [DOI] [PubMed] [Google Scholar]
- 32. Arneson K, Mondshein J, Cmelak AJ et al. A phase 1 trial of concurrent sorafenib and stereotactic radiosurgery for patients with 1–4 brain metastases. Int J Radiat Oncol Biol Phys 2014;87:S271. [DOI] [PubMed] [Google Scholar]
- 33. Chung C, Menard C, Stevens C et al. Phase I dose escalation study of sunitinib and radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 2012;84(Suppl 12):S77. [Google Scholar]
- 34. Wuthrick EJ, Kamrava M, Curran WJ Jr. et al. A phase 1b trial of the combination of the antiangiogenic agent sunitinib and radiation therapy for patients with primary and metastatic central nervous system malignancies. Cancer 2011;117:5548–5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lind JS, Lagerwaard FJ, Smit EF et al. Phase I study of concurrent whole brain radiotherapy and erlotinib for multiple brain metastases from non‐small‐cell lung cancer. Int J Radiat Oncol Biol Phys 2009;74:1391–1396. [DOI] [PubMed] [Google Scholar]
- 36. Welsh JW, Komaki R, Amini A et al. Phase II trial of erlotinib plus concurrent whole‐brain radiation therapy for patients with brain metastases from non‐small‐cell lung cancer. J Clin Oncol 2013;31:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee SM, Lewanski CR, Counsell N et al. Randomized trial of erlotinib plus whole‐brain radiotherapy for NSCLC patients with multiple brain metastases. J Natl Cancer Inst 2014;106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee HL, Chung TS, Ting LL et al. EGFR mutations are associated with favorable intracranial response and progression‐free survival following brain irradiation in non‐small cell lung cancer patients with brain metastases. Radiat Oncol 2012;7:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cai Y, Wang JY, Liu H. Clinical observation of whole brain radiotherapy concomitant with targeted therapy for brain metastasis in non‐small cell lung cancer patients with chemotherapy failure. Asian Pac J Cancer Prev 2013;14:5699–5703. [DOI] [PubMed] [Google Scholar]
- 40. Zhuang H, Yuan Z, Wang J et al. Phase II study of whole brain radiotherapy with or without erlotinib in patients with multiple brain metastases from lung adenocarcinoma. Drug Des Devel Ther 2013;7:1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Olmez I, Donahue BR, Butler JS et al. Clinical outcomes in extracranial tumor sites and unusual toxicities with concurrent whole brain radiation (WBRT) and erlotinib treatment in patients with non‐small cell lung cancer (NSCLC) with brain metastasis. Lung Cancer 2010;70:174–179. [DOI] [PubMed] [Google Scholar]
- 42. Sperduto PW, Wang M, Robins HI et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non‐small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int J Radiat Oncol Biol Phys 2013;85:1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang F, Ning F, Liu C et al. Comparison of gefitinib versus VMP in the combination with radiotherapy for multiple brain metastases from non‐small cell lung cancer. Cell Biochem Biophys 2015;71:1261–1265. [DOI] [PubMed] [Google Scholar]
- 44. Ma S, Xu Y, Deng Q et al. Treatment of brain metastasis from non‐small cell lung cancer with whole brain radiotherapy and gefitinib in a Chinese population. Lung Cancer 2009;65:198–203. [DOI] [PubMed] [Google Scholar]
- 45. Zeng YD, Zhang L, Liao H et al. Gefitinib alone or with concomitant whole brain radiotherapy for patients with brain metastasis from non‐small‐cell lung cancer: A retrospective study. Asian Pacif J Cancer Prev 2012;13:909–914. [DOI] [PubMed] [Google Scholar]
- 46. Pesce GA, Klingbiel D, Ribi K et al. Outcome, quality of life and cognitive function of patients with brain metastases from non‐small cell lung cancer treated with whole brain radiotherapy combined with gefitinib or temozolomide. A randomised phase II trial of the Swiss Group for Clinical Cancer Research (SAKK 70/03). Eur J Cancer 2012;48:377–384. [DOI] [PubMed] [Google Scholar]
- 47. Lin NU, Freedman RA, Ramakrishna N et al. A phase I study of lapatinib with whole brain radiotherapy in patients with human epidermal growth factor receptor 2 (HER2)‐positive breast cancer brain metastases. Breast Cancer Res Treat 2013;142:405–414. [DOI] [PubMed] [Google Scholar]
- 48. Schulze B, Meissner M, Wolter M et al. Unusual acute and delayed skin reactions during and after whole‐brain radiotherapy in combination with the braf inhibitor vemurafenib. Two case reports. Strahlenther Onkol 2014;190:229–232. [DOI] [PubMed] [Google Scholar]
- 49. Liebner DA, Walston SA, Cavaliere R et al. Radiation necrosis mimicking rapid intracranial progression of melanoma metastasis in two patients treated with vemurafenib. Melanoma Res 2014;24:172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Narayana A, Mathew M, Tam M et al. Vemurafenib and radiation therapy in melanoma brain metastases. J Neurooncol 2013;113:411–416. [DOI] [PubMed] [Google Scholar]
- 51. Ahmed KA, Freilich JM, Sloot S et al. Linac‐based stereotactic radiosurgery to the brain with concurrent vemurafenib for melanoma metastases. J Neurooncol 2015;122:121–126. [DOI] [PubMed] [Google Scholar]
- 52. Gaudy‐Marqueste C, Carron R, Delsanti C et al. On demand gamma‐knife strategy can be safely combined with BRAF inhibitors for the treatment of melanoma brain metastases. Ann Oncol 2014;25:2086–2091. [DOI] [PubMed] [Google Scholar]
- 53. Rompoti N, Schilling B, Livingstone E et al. Combination of BRAF inhibitors and brain radiotherapy in patients with metastatic melanoma shows minimal acute toxicity. J Clin Oncol 2013;31:3844–3845. [DOI] [PubMed] [Google Scholar]
- 54. Tazi K, Hathaway A, Chiuzan C et al. Survival of melanoma patients with brain metastases treated with ipilimumab and stereotactic radiosurgery. Cancer Med 2015;4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mathew M, Tam M, Ott PA et al. Ipilimumab in melanoma with limited brain metastases treated with stereotactic radiosurgery. Melanoma Res 2013;23:191–195. [DOI] [PubMed] [Google Scholar]
- 56. Silk AW, Bassetti MF, West BT et al. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2013;2:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gerber NK, Young RJ, Barker CA et al. Ipilimumab and whole brain radiation therapy for melanoma brain metastases. J Neurooncol 2015;121:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kiess AP, Wolchok JD, Barker CA et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: Safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys 2015;92:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chargari C, Idrissi HR, Pierga JY et al. Preliminary results of whole brain radiotherapy with concurrent trastuzumab for treatment of brain metastases in breast cancer patients. Int J Radiat Oncol Biol Phys 2011;81:631–636. [DOI] [PubMed] [Google Scholar]
- 60. Carlson JA, Nooruddin Z, Rusthoven C et al. Trastuzumab emtansine and stereotactic radiosurgery: An unexpected increase in clinically significant brain edema. Neurooncology 2014;16:1006–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lèvy C, Allouache D, Lacroix J et al. REBECA: A phase I study of bevacizumab and whole‐brain radiation therapy for the treatment of brain metastasis from solid tumours. Ann Oncol 2014;25:2351–2356. [DOI] [PubMed] [Google Scholar]
- 62. Shen CJ, Kummerlowe MN, Redmond KJ et al. Stereotactic radiosurgery: Treatment of brain metastasis without interruption of systemic therapy. Int J Radiat Oncol Biol Phys 2016;95:735–742. [DOI] [PubMed] [Google Scholar]
- 63. Cordon‐Cardo C, O'Brien JP, Boccia J et al. Expression of the multidrug resistance gene product (p‐glycoprotein) in human normal and tumor tissues. J Histochem Cytochem 1990;38:1277–1287. [DOI] [PubMed] [Google Scholar]
- 64. Demeule M, Shedid D, Beaulieu E et al. Expression of multidrug‐resistance p‐glycoprotein (MDR1) in human brain tumors. Int J Cancer 2001;93:62–66. [DOI] [PubMed] [Google Scholar]
- 65. Regina A, Demeule M, Laplante A et al. Multidrug resistance in brain tumors: Roles of the blood‐brain barrier. Cancer Metastasis Rev 2001;20:13–25. [DOI] [PubMed] [Google Scholar]
- 66. Lockman PR, Mittapalli RK, Taskar KS et al. Heterogeneous blood‐tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res 2010;16:5664–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Meyers CA, Wefel JS. The use of the Mini‐Mental State Examination to assess cognitive functioning in cancer trials: No ifs, ands, buts, or sensitivity. J Clin Oncol 2003;21:3557–3558. [DOI] [PubMed] [Google Scholar]
- 68. Berghoff AS, Ilhan‐Mutlu A, Dinhof C et al. Differential role of angiogenesis and tumour cell proliferation in brain metastases according to primary tumour type: Analysis of 639 cases. Neuropathol Appl Neurobiol 2015;41:e41–55. [DOI] [PubMed] [Google Scholar]
- 69. Caffo M, Barresi V, Caruso G et al. Innovative therapeutic strategies in the treatment of brain metastases. Int J Mol Sci 2013;14:2135–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kim A, McCully C, Cruz R et al. The plasma and cerebrospinal fluid pharmacokinetics of sorafenib after intravenous administration in non‐human primates. Invest New Drugs 2012;30:524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Besse B, Lasserre SF, Compton P et al. Bevacizumab safety in patients with central nervous system metastases. Clin Cancer Res 2010;16:269–278. [DOI] [PubMed] [Google Scholar]
- 72. Carden CP, Larkin JM, Rosenthal MA. What is the risk of intracranial bleeding during anti‐vegf therapy? Neurooncology 2008;10:624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hendriks LE, Schoenmaekers J, Zindler JD et al. Safety of cranial radiotherapy concurrent with tyrosine kinase inhibitors in non‐small cell lung cancer patients: A systematic review. Cancer Treat Rev 2015;41:634–645. [DOI] [PubMed] [Google Scholar]
- 74.Roche Products Ltd . Potentiation of radiation toxicity associated with zelboraf (vemurafenib). Letter to Healthcare Workers. 2015. [Google Scholar]
- 75. Anker CJ, Grossmann KF, Atkins MB et al. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: Consensus guidelines from the Eastern Cooperative Oncology Group (ECOG). Int J Radiat Oncol Biol Phys 2016;95:632–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Carson MJ, Doose JM, Melchior B et al. CNS immune privilege: Hiding in plain sight. Immunol Rev 2006;213:48–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rodel F, Frey B, Multhoff G et al. Contribution of the immune system to bystander and non‐targeted effects of ionizing radiation. Cancer Lett 2015;356:105–113. [DOI] [PubMed] [Google Scholar]
- 78. Stamell EF, Wolchok JD, Gnjatic S et al. The abscopal effect associated with a systemic anti‐melanoma immune response. Int J Radiat Oncol Biol Phys 2013;85:293–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Park YH, Park MJ, Ji SH et al. Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2‐overexpressing breast cancer patients. Br J Cancer 2009;100:894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Park IH, Ro J, Lee KS et al. Trastuzumab treatment beyond brain progression in HER2‐positive metastatic breast cancer. Ann Oncol 2009;20:56–62. [DOI] [PubMed] [Google Scholar]
- 81. Twelves C, Glynne‐Jones R, Cassidy J et al. Effect of hepatic dysfunction due to liver metastases on the pharmacokinetics of capecitabine and its metabolites. Clin Cancer Res 1999;5:1696–1702. [PubMed] [Google Scholar]
- 82. Judson IR, Beale PJ, Trigo JM et al. A human capecitabine excretion balance and pharmacokinetic study after administration of a single oral dose of 14c‐labelled drug. Invest New Drugs 1999;17:49–56. [DOI] [PubMed] [Google Scholar]
- 83.European Medicines Agency . European public assessment reports 2015. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp&mid=WC0b01ac058001d124. Accessed May 29, 2015.
- 84. Morikawa A, Peereboom DM, Thorsheim HR et al. Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients: A prospective study. Neurooncology 2015;17:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. van der Vijgh WJ. Clinical pharmacokinetics of carboplatin. Clin Pharmacokinet 1991;21:242–261. [DOI] [PubMed] [Google Scholar]
- 86. Go RS, Adjei AA. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol 1999;17:409–422. [DOI] [PubMed] [Google Scholar]
- 87. Pitz MW, Desai A, Grossman SA et al. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J Neurooncol 2011;104:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. ten Tije AJ, Loos WJ, Zhao M et al. Limited cerebrospinal fluid penetration of docetaxel. Anticancer Drugs 2004;15:715–718. [DOI] [PubMed] [Google Scholar]
- 89. Heimans JJ, Vermorken JB, Wolbers JG et al. Paclitaxel (Taxol) concentrations in brain tumor tissue. Ann Oncol 1994;5:951–953. [DOI] [PubMed] [Google Scholar]
- 90. Fine RL, Chen J, Balmaceda C et al. Randomized study of paclitaxel and tamoxifen deposition into human brain tumors: Implications for the treatment of metastatic brain tumors. Clin Cancer Res 2006;12:5770–5776. [DOI] [PubMed] [Google Scholar]
- 91. Deng Y, Feng W, Wu J et al. The concentration of erlotinib in the cerebrospinal fluid of patients with brain metastasis from non‐small‐cell lung cancer. Mol Clin Oncol 2014;2:116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Togashi Y, Masago K, Masuda S et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non‐small cell lung cancer. Cancer Chemother Pharmacol 2012;70:399–405. [DOI] [PubMed] [Google Scholar]
- 93. Yang H, Yang X, Zhang Y et al. Erlotinib in combination with pemetrexed/cisplatin for leptomeningeal metastases and cerebrospinal fluid drug concentrations in lung adenocarcinoma patients after gefitinib failure. Target Oncol 2015;10:135–140. [DOI] [PubMed] [Google Scholar]
- 94. Clarke JL, Pao W, Wu N et al. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol 2010;99:283–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Raizer JJ, Abrey LE, Lassman AB et al. A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neurooncology 2010;12:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhao J, Chen M, Zhong W et al. Cerebrospinal fluid concentrations of gefitinib in patients with lung adenocarcinoma. Clin Lung Cancer 2013;14:188–193. [DOI] [PubMed] [Google Scholar]
- 97. Hofer S, Frei K. Gefitinib concentrations in human glioblastoma tissue. J Neurooncol 2007;82:175–176. [DOI] [PubMed] [Google Scholar]
- 98. Polli JW, Humphreys JE, Harmon KA et al. The role of efflux and uptake transporters in [n‐{3‐chloro‐4‐[(3‐fluorobenzyl)oxy]phenyl}‐6‐[5‐({[2‐(methylsulfonyl)ethyl]amino}methyl)‐2‐furyl]‐4‐quinazolinamine (gw572016, lapatinib) disposition and drug interactions. Drug Metab Dispos 2008;36:695–701. [DOI] [PubMed] [Google Scholar]
- 99. Sakji‐Dupre L, Le Rhun E, Templier C et al. Cerebrospinal fluid concentrations of vemurafenib in patients treated for brain metastatic braf‐v600 mutated melanoma. Melanoma Res 2015;25:302–305. [DOI] [PubMed] [Google Scholar]
- 100. Lee JM, Mehta UN, Dsouza LH et al. Long‐term stabilization of leptomeningeal disease with whole‐brain radiation therapy in a patient with metastatic melanoma treated with vemurafenib: A case report. Melanoma Res 2013;23:175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mittapalli RK, Vaidhyanathan S, Sane R et al. Impact of p‐glycoprotein (abcb1) and breast cancer resistance protein (abcg2) on the brain distribution of a novel BRAF inhibitor: Vemurafenib (plx4032). J Pharmacol Exp Ther 2012;342:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bartsch R, Rottenfusser A, Wenzel C et al. Trastuzumab prolongs overall survival in patients with brain metastases from her2 positive breast cancer. J Neurooncol 2007;85:311–317. [DOI] [PubMed] [Google Scholar]
- 103. Yoshida Y, Hoshino S, Aisu N et al. Efficacy of XELOX plus bevacizumab in brain metastasis from rectal cancer. Case Rep Oncol 2014;7:117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ay I, Francis JW, Brown RH, Jr. VEGF increases blood‐brain barrier permeability to evans blue dye and tetanus toxin fragment C but not adeno‐associated virus in ALS mice. Brain Res 2008;1234:198–205. [DOI] [PubMed] [Google Scholar]
- 105. Caffo M, Barresi V, Caruso G et al. Innovative therapeutic strategies in the treatment of brain metastases. Int J Molec Sci 2013;14:2135–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kim A, McCully C, Cruz R et al. The plasma and cerebrospinal fluid pharmacokinetics of sorafenib after intravenous administration in non‐human primates. Invest New Drugs 2012;30:524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mehta AI, Brufsky AM, Sampson JH. Therapeutic approaches for HER2‐positive brain metastases: Circumventing the blood‐brain barrier. Cancer Treat Rev 2013;39:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.