This study evaluates, for the first time, the role of doxorubicin and cyclophosphamide followed by paclitaxel and dual anti‐HER2 therapy with trastuzumab and pertuzumab in patients with early stage HER2‐positive breast cancer.
Keywords: HER2‐positive, Neoadjuvant, Breast cancer, Pertuzumab, Anthracycline
Abstract
Objectives.
Trastuzumab (H) and pertuzumab (P) with standard chemotherapy is approved for use in the neoadjuvant setting for human epidermal growth receptor 2 ‐positive patients. A retrospective analysis was performed of patients treated with dose‐dense (dd) doxorubicin and cyclophosphamide (AC) followed by paclitaxel (T), trastuzumab, and pertuzumab (THP) in the neoadjuvant setting. Here, the pathologic complete response (pCR) rates are reported.
Methods.
An electronic medical record review was conducted of patients treated with HP‐based therapy in the neoadjuvant setting from September 1, 2013, to March 1, 2015. Data on patient demographics, stage of breast cancer, pathology reports, surgical data, and information on systemic therapy were collected. The pCR was defined as total (tpCR, ypT0/is ypN0), German Breast Group (GBG) pCR (ypT0 ypN0), breast pCR (bpCR) with in situ disease (ypT0/is) and without in situ disease (ypT0), and explored axillary pCR (ypN0).
Results.
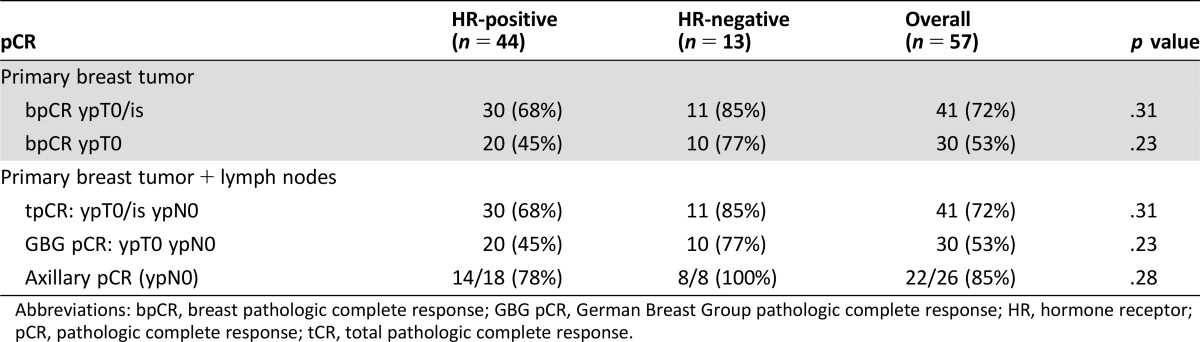
Charts from 66 patients were reviewed, and 57 patients were evaluable for pCR. Median age was 46 years (range 26–68 years). Median tumor size was 4 cm. Of 57 patients, 53 (93%) had operable breast cancer (T1‐3, N0‐1, M0). Three patients (5.3%) had locally advanced disease (T2‐3, N2‐3, M0 or T4a‐c, any N, M0), and 1 (1.7%) had inflammatory breast cancer (T4d, any N, M0). Overall, 44 (77%) and 13 (23%) had hormone receptor (HR)‐positive and negative diseases, respectively. Median numbers of cycles of neoadjuvant treatment were as follows: AC (4, range 1–4), T (4, range 1–4), trastuzumab (6, range 3–8), and pertuzumab (6, range 2–8). In these 57 patients, the rates of tpCR and bpCR with in situ disease were demonstrated in 41/57 (72%) patients, and the rates of GBG pCR and bpCR without in situ disease were found in 30/57 (53%) patients. Of 26 patients with biopsy‐proven lymph nodal involvement, axillary pCR occurred in 22 (85%) patients.
Conclusion.
At a single center, the tpCR and GBG pCR rates of dd AC followed by THP are high at 72% and 53%, respectively.
Implications for Practice.
This is the first study describing the role of doxorubicin and cyclophosphamide followed by paclitaxel and dual anti‐HER2 therapy with trastuzumab and pertuzumab (ACTHP) in patients with early stage HER2‐positive breast cancer. Total (breast + lymph node) pathological complete remission (pCR) remission (ypT0/is ypN0) and German Breast Group pCR rates (ypT0/ ypN0) were high at 72% and 53%, respectively, with the ACTHP regimen. Rate of axillary clearance in patients with known axillary involvement was high at 85%, which may translate into less extensive axillary surgeries in this subset in the future.
Introduction
Neoadjuvant chemotherapy (NAC) is increasingly being administered, and the traditional approach to NAC is to render inoperable tumors operable and to facilitate breast‐conserving surgery. In recent years, NAC has been commonly recommended to patients with operable tumors. In 2012, Prowell and Pazdur published perspectives on the draft guidance from the U.S. Food and Drug Administration's (FDA) guidelines for granting accelerated drug approval based on a surrogate endpoint that would predict a clinical benefit. In the neoadjuvant setting, the surrogate endpoint is pathologic complete response (pCR) [1]. The FDA also reported the pooled analysis on 11,955 patients in 12 neoadjuvant trials and concluded that, in terms of pCR, the absence of invasive disease in the breast and axilla correlated the best with improved event‐free survival (EFS) or overall survival (OS) on a patient level. Notably, there was no difference in outcomes if in situ disease was left in the breast [2]. The FDA concluded that both the German Breast Group pCR (GBG pCR, ypT0 ypN0) and total pCR (tpCR, ypT0/is ypN0) were better associated with improved EFS (ypT0 ypN0 hazard ratio 0.44, 95% confidence interval [CI] 0.9–0.51; ypT0/is ypN0: 0.48, 0.43–0.54) and OS (0.36 [0.30–0.44]; 0.36 [0.31–0.42]) compared with breast pCR (bpCR) alone. The definition of tpCR was used for all subsequent analyses, and they found that the association between tpCR and improved long‐term outcomes was strongest in patients with triple negative breast cancer and human epidermal growth factor receptor 2 (HER2)‐positive/hormone receptor (HR)‐negative disease treated with trastuzumab (H) [2].
In 2013, the FDA granted accelerated approval of pertuzumab (P) with H combined with standard chemotherapy in the neoadjuvant treatment of patients with stage II–III human epidermal growth receptor (HER)‐positive disease [3], [4], [5]. This accelerated approval was based on the data from two important trials, namely the NEOSPHERE and TRYPHAENA studies, demonstrating high pCR rates in the breast with absence of invasive disease (ypT0/is) with dual anti‐HER2 therapy with H and P (HP) when combined with chemotherapy [4], [5]. However, the final approval is contingent on the confirmatory phase III adjuvant APHINITY trial (NCT01358877) in which the primary endpoint is invasive disease‐free survival when comparing H versus HP, all combined with standard chemotherapy.
In the NEOSPHERE and TRYPHAENA studies, the NAC regimens were docetaxel‐ and epribubicin‐based. However, the National Comprehensive Cancer Network (NCCN) also endorsed other standard anthracycline‐taxane‐based regimens, such as doxorubicin and cyclophosphamide (AC) followed by paclitaxel (T), to be combined with HP [6]. Of note, at the request of the FDA to evaluate the cardiac safety of HP following a doxorubicin‐based regimen, the BERENICE study (NCT02132949) was conducted, in which one cohort of patients was treated with AC followed by weekly T with HP. At Memorial Sloan Kettering Cancer Center, the regimen of dose‐dense (dd) AC followed by weekly T with HP (THP) given every 3 weeks is commonly used. Here, from a single center, we report a retrospective analysis of pCR rates in patients with HER2‐positive breast cancer who underwent neoadjuvant treatment with dd AC followed by THP.
Materials and Methods
Medical records of patients with HER2‐positive breast cancer being treated in the neoadjuvant setting were abstracted with a waiver approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center. Search criteria consisted of patients who had HER2‐positive breast cancer being treated with HP in the neoadjuvant setting from September 1, 2013, to March 1, 2015. HER2 positivity was defined as immunohistochemical (IHC) score of 3+ or fluorescent in situ hybridization (FISH) ratio of ≥ 2.0. Charts were analyzed for patient demographics, stage of breast cancer, pathology reports, surgical data, and data on systemic therapy. Clinical stage was determined by the surgeons’ assessment at the initial visit, based on clinical exam and available imaging.
Patients received dd AC (60/600 mg/m2) every 2 weeks for 4 cycles with pegylated granulocyte colony‐stimulating factor support followed by weekly T (80 mg/m2) for 12 weeks with H (8 mg/kg loading dose followed by 6 mg/kg) and P (840 mg loading dose followed by 420 mg) every 3 weeks from the start of T.
Following NAC, all patients had definitive breast and axillary surgery. All clinically node‐negative patients at presentation had sentinel node biopsy (SNB) after NAC. Initially, node‐positive patients, who converted to clinically node negative after NAC, had SNB with dual tracer mapping using technetium‐99m sulfur colloid and isosulfan blue dye and retrieval of at least three sentinel nodes (SNs). Axillary lymph node dissection (ALND) was indicated for failed SN mapping, retrieval of fewer than three SNs, and for any positive SNs. pCR was calculated with the standard definitions per the FDA as tpCR (ypT0/is ypN0) and GBG pCR (ypT0 ypN0). Additionally, bpCR rates with (ypT0/is) and without in situ disease (ypT0) were also analyzed. For patients who had biopsy‐proven axillary nodal involvement prior starting NAC, we explored axillary pCR (ypN0).
Descriptive statistics are used to summarize the data. The chi‐square test was used to compare response rates by HR status.
Results
From September 1, 2013, to March 1, 2015, 66 patients were treated with neoadjuvant HP‐based therapy. Of these 66 patients, 57 were evaluable for pCR and 9 were not (3 patients: no anthracycline regimen used, 1 patient: incomplete chart, 1 patient: pursued care elsewhere, 1 patient: diagnosed with metastatic during chemotherapy, 1 patient: received AC postoperatively, 2 patients: did not receive weekly T). Demographics of the patient population are summarized in Table 1. Median age was 46 years (range 26–68 years). Median tumor size was 4 cm. Of the 57 patients, 53 (93%) had operable breast cancer (T1‐3, N0‐1, M0). Three patients (5.3%) had locally advanced disease (T2‐3, N2‐3, M0 or T4a‐c, any N, M0), and one (1.7%) had inflammatory breast cancer (T4d, any N, M0). Forty‐four (77%) and 13 (23%) had HR‐positive and HR‐negative disease, respectively. Median time from initiation of AC to breast surgery was 5.4 months (range 3.8–7.3 months). Overall, 29 patients (51%) underwent lumpectomy and 28 (49%) underwent mastectomy. Of the 26 patients with biopsy‐proven axillary nodal metastasis, 12 (46%) had a SNB and 14 (54%) had ALND post NAC. The median number of nodes removed in SNB patients was 4 (range 1–13) compared with 14 (range 3–34) in ALND patients (Table 2). Notably, one patient had only one SN removed instead of at least three SNs as per institutional policy. She was recommended to have ALND but she declined.
Table 1. Patient demographics (n = 57).
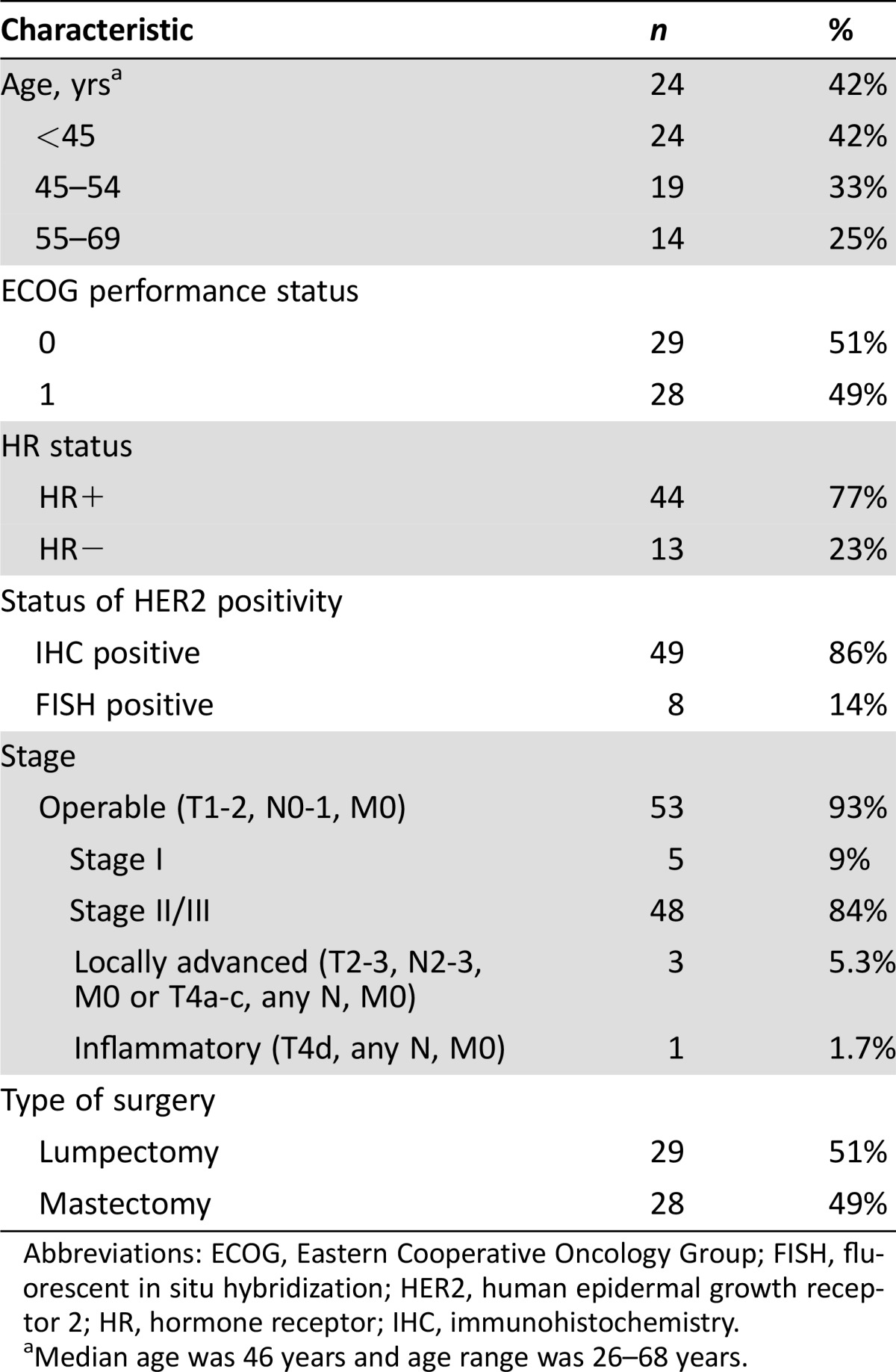
Abbreviations: ECOG, Eastern Cooperative Oncology Group; FISH, fluorescent in situ hybridization; HER2, human epidermal growth receptor 2; HR, hormone receptor; IHC, immunohistochemistry.
Median age was 46 years and age range was 26–68 years.
Table 2. Type of axillary surgery among patients with biopsy‐proven node‐positive disease before neoadjuvant therapy.
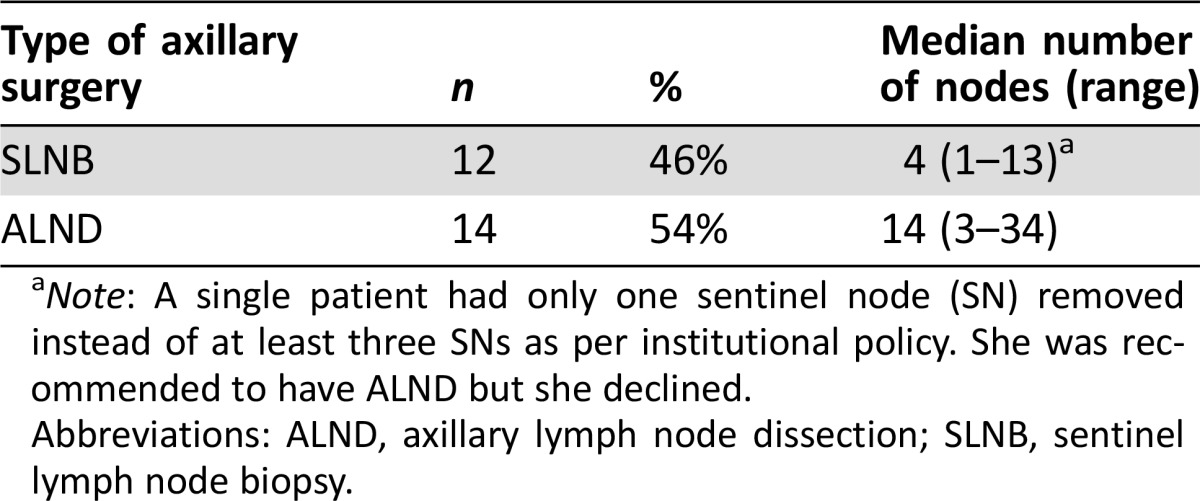
Note: A single patient had only one sentinel node (SN) removed instead of at least three SNs as per institutional policy. She was recommended to have ALND but she declined.
Abbreviations: ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy.
Treatment Exposure
The median number of cycles of AC received was 4 (range 1–4), and the number for T was 4 (range 1–4); one cycle of T consisted of 3 weekly doses. The median number of cycles of H was 6 (range 3–8) and P was 6 (range 2–8). One patient developed Stevens–Johnson syndrome after the first cycle of AC and was not subsequently rechallenged. One patient developed desquamation and swelling of the digits of her hands after 3 cycles, leading to discontinuation of AC. T was discontinued in six patients for the following reasons: pneumonitis (n = 2), allergic reaction (n = 2), recurrent cellulitis of the hand after extravasation from prior anthracycline (n = 1), and patient preference (n = 1).
pCR
In terms of the pCR in the breast and axilla, tpCR (ypT0/is ypN0) occurred in 41/57 (72%) patients overall and in 11/13 (85%) and 30/44 (68%) patients with HR‐negative and HR‐positive disease (p = .31), respectively. The GBG pCR (ypT0 yp N0) occurred in 30/57 (53%) patients overall and in 10/13 (77%) and 20/44 (45%) patients with HR‐negative and HR‐positive disease (p = .23), respectively. Pertaining to the pCR in the breast only, bpCR with in situ disease (ypT0/is) occurred in 41/57 (72%) patients overall and in 11/13 (85%) and 30/44 (68%) patients with HR‐negative and HR‐positive disease (p = .31), respectively. The bpCR without in situ disease (ypT0) occurred in 30/57 (53%) patients overall and in 10/13 (77%) and 20/44 (45%) patients with HR‐negative and HR‐positive diseases (p = .23), respectively. In the 26 patients with biopsy‐proven nodal metastasis, axillary pCR (ypN0) occurred in 22/26 (85%) patients overall and in 8/8 (100%) and 14/18 (78%) patients with HR‐negative and HR‐positive disease (p = .28), respectively (Table 3).
Table 3. Pathologic complete response rates.
Abbreviations: bpCR, breast pathologic complete response; GBG pCR, German Breast Group pathologic complete response; HR, hormone receptor; pCR, pathologic complete response; tCR, total pathologic complete response.
Discussion
At a single center, the rates of tpCR and GBG pCR were high at 72% and 53%, respectively, with neoadjuvant dd AC followed by THP. Additionally, the bpCR rates with and without in situ cancer were 72% and 53%, respectively. An exploratory analysis of axillary pCR demonstrated a high rate of 85%. Regardless of the definitions used, these rates were higher in patients with HR‐negative than HR‐positive disease, which would be consistent with other studies, including the NEOSPHERE and TRYPHAENA studies [4], [5]. In the NEOSPHERE study of 417 patients, the combination of HP with docetaxel showed a higher bpCR (ypT0/is) rate (45.8%, 95% CI 36.1–55.7) than when compared with either H and docetaxel (29%, 95% CI 20.6–38.5), P and docetaxel (24%, 95% CI 15.8–33.7), or dual anti‐HER2 antibody therapy alone (16.8%, 95% CI 10.3–25.3). In the TRYPHAENA study, 220 patients were randomized to 5‐fluorouracil, epirubicin and cyclophosphamide (FEC) with HP followed by docetaxel/HP (Arm A), FEC followed by docetaxel/HP (Arm B), or to docetaxel/carboplatin/HP (Arm C). All three arms showed high bpCR (yp0/is) rates of 61% (Arm A), 57% (Arm B), and 66% (Arm C) and GBG pCR rates of 50.7% (Arm A), 45.3% (Arm B), and 51.9% (Arm C) [4], [5]. The bpCR (ypT0/is) and GBG pCR rates in our study were comparable to the rates reported in the TRYPHAENA study. With these high pCR rates, in the future, a retrospective analysis could be performed to evaluate for biomarkers that may correlate with pCR, EFS, and OS.
Effective anti‐HER2 therapies (including H, P, ado‐H emtansine, and lapatinib) have revolutionized the treatment and changed the history of this aggressive disease [7], [8]. Pertaining to dual anti‐HER2 antibody therapy with HP in the metastatic setting, the CLEOPATRA trial demonstrated significant improvements in progression‐free survival (PFS) and overall OS with the addition of P to H and docetaxel when compared with control in the first‐line setting [9], [10]. Based on the work of Seidman et al. on weekly T and H [11], [12] at Memorial Sloan Kettering Cancer Center, the next logical step was to conduct a trial evaluating the efficacy of P added to T and H in the metastatic setting; this study reported a median PFS of 24.2 and 16.4 months for those being treated in first‐ and second‐line settings, respectively; these results were recently updated, and outcomes remained the same [13], [14]. With the benefits of dual anti‐HER2 antibody therapy in the metastatic and neoadjuvant settings, the results of dual antibody therapy in the adjuvant setting are eagerly awaited (APHINITY trial, NCT01358877). Furthermore, the results of the neoadjuvant BERENICE trial (NCT02132949), in which one arm of treatment included dd AC followed by THP, will be reported.
Conclusion
To our knowledge, this is the first trial providing the pCR results of neoadjuvant dd AC followed by T with dual HER2 blockade using HP. Notably, in the 26 patients with biopsy‐proven axillary nodal metastasis, the rate of axillary pCR was high at 85% with neoadjuvant dual HER2 therapy, which may potentially translate into less extensive axillary surgery and avoidance of complete ALND in select patients eligible for downstaging. Notably, the false negative rates of SNB in node‐positive patients treated with neoadjuvant therapy are <10%, provided that three or more negative SNs are obtained [15], [16]. In this patient cohort, longer follow‐up is needed to determine regional recurrence rates in patients treated with SNB alone.
These patients will be followed during adjuvant anti‐HER2 therapy to complete one year's duration and will be monitored for long‐term outcomes. Additionally, the cardiac toxicity profile of AC followed by THP will be reported in detail in a separate manuscript. In closing, patients with residual invasive disease in the breast and axilla have worse EFS and OS than those without [2]; thus, these findings suggest that future trials are needed to improve the outcomes in this population.
Footnotes
For Further Reading: Anthony F. Yu, Carlos Manrique, Shawn Pun et al. Cardiac Safety of Paclitaxel Plus Trastuzumab and Pertuzumab in Patients With HER2‐PositiveMetastatic Breast Cancer. The Oncologist 2016;21:418–424.
Implications for Practice: Dual anti‐HER2 therapy with trastuzumab and pertuzumab in combination with taxane‐based chemotherapy improves overall survival in patients with metastatic HER2‐positive breast cancer. There is a critical need to investigate the potential cardiotoxicity of dual anti‐HER2 blockade, given the importance of HER2 signaling in cardiac homeostasis and stress response. Global longitudinal strain and cardiac biomarkers have been proposed as adjuncts to left ventricular ejection fraction for the early detection of cardiotoxicity. In this phase II study of combination trastuzumab and pertuzumab with paclitaxel, no clinically significant change was observed in global longitudinal strain or cardiac biomarkers. These results further support the cardiac safety of dual anti‐HER2 blockade previously reported in the CLEOPATRA study. The findings in the current study also call into question the role of intensive cardiac monitoring among patients treated with anti‐HER2 therapy in the absence of anthracyclines. Less frequent cardiac assessments could lead to a reduction in unnecessary treatment interruption and is an important consideration given the rise in medical expenditures, but this requires further investigation.
Author Contributions
Conception/Design: Jasmeet C. Singh, Monica Morrow, Steven Sugarman, Lee W. Jones, Anthony F. Yu, Shanu Modi, Larry Norton, Jose Baselga, Clifford A. Hudis, Chau Dang
Provision of study material or patients: Jasmeet C. Singh, Anita Mamtani, Andrea Barrio, Monica Morrow, Steven Sugarman, Lee W. Jones, Anthony F. Yu, Daniel Argolo, Sarah Schweber, Larry Norton, Jose Baselga, Clifford A. Hudis, Chau Dang
Collection and/or assembly of data: Jasmeet C. Singh, Andrea Barrio, Monica Morrow, Daniel Argolo, Lilian M. Smyth, Shanu Modi, Sarah Schweber, C. Boafo, Sujata Patil, Larry Norton, Jose Baselga, Clifford A. Hudis, Chau Dang
Data analysis and interpretation: Jasmeet C. Singh, Anita Mamtani, Andrea Barrio, Anthony F. Yu, Daniel Argolo, Lilian M. Smyth, Shanu Modi, Sarah Schweber, Camilla Boafo, Sujata Patil, Larry Norton, Jose Baselga, Clifford A. Hudis, Chau Dang
Manuscript writing: Jasmeet C. Singh, Anita Mamtani, Andrea Barrio, Steven Sugarman, Lee W. Jones, Anthony F. Yu, Daniel Argolo, Lilian M. Smyth, Shanu Modi, Sarah Schweber, Camilla Boafo, Sujata Patil, Larry Norton, Jose Baselga, Clifford A. Hudis, Chau Dang
Final approval of manuscript: Jasmeet C. Singh, Anita Mamtani, Andrea Barrio, Monica Morrow, Steven Sugarman, Lee W. Jones, Anthony F. Yu, Daniel Argolo, Lilian M. Smyth, Shanu Modi, Sarah Schweber, Camilla Boafo, Sujata Patil, Larry Norton, Jose Baselga, Clifford A. Hudis, Chau Dang
Disclosures
Lilian M. Smyth: AstraZeneca (C/A), AstraZeneca, Roche/Genentech (RF); Shanu Modi: Genentech, Novartis, Seattle Genetics (RF); Clifford A. Hudis: Roche/Genentech, AstraZeneca, Bristol‐Myers Squibb, Pfizer, Lilly, Novartis (CA); Chau Dang: Genentech BioOncology (C/A), Genentech/Roche/GlaxoSmithKlien (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. New Engl J Med 2012;366:2438–2441. [DOI] [PubMed] [Google Scholar]
- 2. Cortazar P, Zhang L, Untch M et al. Pathological complete response and long‐term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014;384:164–172. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Food and Drug Administration . Available at http://www.fda.gov/. Accessed March 29, 2016.
- 4. Gianni L, Pienkowski T, Im YH et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2‐positive breast cancer (NeoSphere): A randomised multicentre, open‐label, phase 2 trial. Lancet Oncol 2012;13:25–32. [DOI] [PubMed] [Google Scholar]
- 5. Schneeweiss A, Chia S, Hickish T et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline‐containing and anthracycline‐free chemotherapy regimens in patients with HER2‐positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013;24:2278–2284. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network . Evidence‐Based Cancer Guidelines, Oncology Drug Compendium, Oncology Continuing. Available at: http://www.nccn.org/. Accessed March 29, 2016.
- 7. Singh JC, Jhaveri K, Esteva FJ. HER2‐positive advanced breast cancer: Optimizing patient outcomes and opportunities for drug development. Br J Cancer. 2014;111:1888–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu X, Verma S. Targeted therapy in HER2‐positive metastatic breast cancer: A review of the literature. Curr Oncol 2015;22(suppl 1):S19–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baselga J, Cortés J, Kim SB et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Swain SM, Baselga J, Kim SB et al. Pertuzumab, trastuzumab, and docetaxel in HER2‐positive metastatic breast cancer. N Engl J Med 2015;372:724–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seidman AD, Berry D, Cirrincione C et al. Randomized phase III trial of weekly compared with every‐3‐weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER‐2 overexpressors and random assignment to trastuzumab or not in HER‐2 nonoverexpressors: Final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol 2008;26:1642–1649. [DOI] [PubMed] [Google Scholar]
- 12. Seidman AD, Fornier MN, Esteva FJ et al. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol 2001;19:2587–2595. [DOI] [PubMed] [Google Scholar]
- 13. Smyth LM, Iyengar NM, Chen MF et al. Weekly paclitaxel with trastuzumab and pertuzumab in patients with HER2‐overexpressing metastatic breast cancer: Overall survival and updated progression‐free survival results from a phase II study. Breast Cancer Res Treat 2016;158:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dang C, Iyengar N, Datko F et al. Phase II study of paclitaxel given once per week along with trastuzumab and pertuzumab in patients with human epidermal growth factor receptor 2‐positive metastatic breast cancer. J Clin Oncol 2015;33:442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boughey JC, Suman VJ, Mittendorf EA et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node‐positive breast cancer: The ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013;310:1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuehn T, Bauerfeind I, Fehm T et al. Sentinel‐lymph‐node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): A prospective, multicentre cohort study. Lancet Oncol 2013;14:609–618. [DOI] [PubMed] [Google Scholar]