Sorafenib and sunitinib significantly increased the risk for clinically relevant hypothyroidism; the risk was at least 4 times greater with SUN than with SOR. Patients receiving sunitinib and sorafenib had a similar elevated risk for clinically relevant HTN.
Keywords: sorafenib, sunitinib, hypertension, hypothyroidism, toxicity
Abstract
Background.
Thyroid dysfunction and hypertension (HTN) have been sporadically reported with sunitinib (SUN) and sorafenib (SOR). Determination of the side effect incidence will enhance monitoring and management recommendations.
Methods.
An observational cohort study was performed using deidentified pharmacy claims data from a 3‐year period to evaluate patients prescribed SUN, SOR, or capecitabine (CAP; comparison group). The primary outcome was time to first prescription for thyroid replacement or HTN treatment. Hazard ratios (HRs) with 95% confidence intervals (CIs) were estimated by Cox proportional hazards models.
Results.
A total of 20,061 patients were eligible for evaluation of thyroid replacement therapy, which was initiated in 11.6% of those receiving SUN (HR, 16.77; 95% CI, 13.54–20.76), 2.6% of those receiving SOR (HR, 3.47; 95% CI, 2.46–4.98), and 1% of those receiving CAP, with median time to initiation of 4 months (range, 1–35 months). A total of 14,468 patients were eligible for evaluation of HTN therapy, which was initiated in 21% of SUN recipients (HR, 4.91; 95% CI, 4.19–5.74), 14% of SOR recipients (HR, 3.25; 95% CI, 2.69–3.91), and 5% of CAP recipients, with median time to initiation of 1 month (range, 1–18 months) for SOR and 2 months (range, 1–25 months) for SUN.
Conclusion.
SUN and SOR significantly increased the risk for clinically relevant hypothyroidism; the risk was at least 4 times greater with SUN than with SOR. Patients receiving SUN and SOR had a similar elevated risk for clinically relevant HTN. These data provide robust measures of the incidence and time to onset of these clinically actionable adverse events.
Implications for Practice.
The side effect profiles for novel therapies are typically used to create monitoring and management recommendations using clinical trial data from patient populations that may not represent those seen in standard clinical practice. This analysis using a large pharmacy claims database better reflects typical patients treated with sorafenib or sunitinib outside of a clinical trial. The findings of increased need for thyroid replacement in patients receiving sunitinib compared with sorafenib and a similar increase in need for hypertension therapy with both agents can be used to form clinically relevant monitoring recommendations for these agents.
Introduction
Sunitinib and sorafenib are orally bioavailable small molecule receptor multi‐tyrosine kinase inhibitors. Receptor tyrosine kinases (RTKs) are transmembrane glycoproteins that, when activated by ligand binding to the extracellular domain, trigger intracellular signal transduction pathways, which ultimately result in the transcription of genes involved in cell proliferation, survival, angiogenesis, adhesion, and motility [1]. The antitumor activity of sunitinib and sorafenib is believed to be mediated by inhibition of multiple RTKs, such as vascular endothelial growth factor receptor, platelet‐derived growth factor receptor, and stem cell factor receptor, which leads to apoptotic and antiangiogenic effects [2], [3]. Despite similarity between the two agents in terms of their targets, each agent also has additional RTKs it inhibits to varying degrees; this may explain similarities and differences in efficacy and toxicity. The U.S. Food and Drug Administration (FDA) approved sunitinib for the treatment of advanced renal cell carcinoma (RCC) in 2006; imatinib‐resistant/‐intolerant gastrointestinal stromal tumors (GISTs) in 2006; and certain patients with progressive, well‐differentiated pancreatic neuroendocrine tumors in 2011. The FDA approved sorafenib for the treatment of advanced RCC in 2005; unresectable hepatocellular carcinoma (HCC) in 2007; and certain patients with progressive, differentiated thyroid carcinoma that is refractory to radioactive iodine therapy in 2013.
Both sunitinib and sorafenib are relatively well tolerated; the most common side effects are gastrointestinal distress, hypertension, skin toxicity, and fatigue. In a meta‐analysis of 4,999 patients with multiple malignancies treated with sunitinib, the incidence of all‐grade hypertension (HTN) was 21.6% (95% confidence interval [CI], 18.7%–24.8%) and 6.8% (95% CI, 5.3%–8.8%) for high‐grade HTN; it varied between malignancies as well as dosing schedule (continuous vs. intermittent) [4]. The incidence of all‐grade and high‐grade sorafenib‐induced HTN in trials across multiple malignancies has ranged widely. A meta‐analysis including data from 13,555 patients reported an incidence of 19.1% (95% CI, 15.8%–22.4%) for all‐grade and 4.3% (95% CI, 3%–5.5%) for high‐grade HTN, with RCC patients having a higher incidence than patients with non‐RCC malignancies (24.9% compared with 15.7%; p < .05 for all‐grade HTN) [5]. With these agents, HTN onset can occur at any time during therapy, although it is often reported 3–4 weeks after drug initiation; HTN has been documented to respond to traditional antihypertensive therapy with diuretics, β‐blockers, angiotensin‐converting enzyme (ACE) inhibitors, and calcium‐channel blockers [5], [6], [7]. However, these data are limited to clinical trial participants, and estimates of HTN incidence in the general (nontrial) patient population are lacking.
The rates of sunitinib‐ and sorafenib‐induced hypothyroidism reported by the manufacturers were 1%–4%; however, the incidence reported in the published literature was much higher, ranging from 10% to 85% for sunitinib and 6.3% to 27% for sorafenib [8], [9], [10], [11], [12], [13], [14], [15]. This wide range is partially due to the different definitions of “hypothyroidism” in the trials, which varied from alterations in thyroid stimulation hormone to subclinical or clinical hypothyroidism requiring thyroid hormone replacement. The onset of hypothyroidism occurred between 2 weeks to 36 months after initiation of therapy, and severity ranged from subclinical (asymptomatic) thyroid dysfunction to overt hypothyroidism necessitating hospitalization and treatment. The severity of hypothyroidism is suggested to depend on the dose and duration of sunitinib or sorafenib treatment [8], [9], [10], [11], [12], [13]. The current literature on sunitinib‐ and sorafenib‐induced thyroid disease is sparse and mostly retrospective in nature. In addition, it is difficult to adequately assess the incidence and nature of sunitinib‐ or sorafenib‐induced thyroid dysfunction because the studied populations have been extremely small.
Both hypertension and hypothyroidism can have life‐threatening consequences if left untreated. However, the medically manageable nature of these adverse effects associated with sunitinib and sorafenib therapy underscores the importance of accurately elucidating their true incidence and prevalence. This will be critical in the development of appropriate monitoring and treatment recommendations in this patient population. This may also improve clinical outcomes and patient quality of life because the high incidence of fatigue, which can be a dose‐limiting side effect, may also represent unrecognized hypothyroidism. Appropriate management of hypothyroidism may improve the tolerability of sunitinib and sorafenib and may enable more patients to continue therapy with these agents. The primary objective of this analysis was to determine the incidence and time to onset of clinically actionable hypothyroidism and HTN in a large heterogeneous patient population.
Materials and Methods
Patient Selection
This observational cohort study used data from a deidentified pharmacy claims database for more than 60 million individuals. The study population comprised participants in prescription benefit plans managed by Medco Health Solutions Inc. who were prescribed sunitinib, sorafenib, or capecitabine during the study time frame from January 1, 2006, through September 30, 2009. Capecitabine was used as a comparison agent for both drug‐induced hypothyroidism and hypertension because it is an oral chemotherapy known to not induce these adverse events. Patients were included in the analysis if they had at least 2 consecutive prescriptions or 45 days of consecutive oral anticancer therapy. Exclusion criteria included the presence of a prescription for one of the targeted drugs (sunitinib, sorafenib, or capecitabine) before the study period or an active prescription for thyroid replacement or antihypertensive therapy prior to the initiation of oral anticancer therapy.
Because incidental, subclinical hypothyroidism or HTN can occur, the focus was on “clinically actionable” events for which medication intervention was required. The primary endpoint was the occurrence of prescriptions for thyroid replacement or hypertension therapy for 45 days or more after beginning treatment with sunitinib, sorafenib, or capecitabine. For each analysis year, prescription claims were identified for thyroid replacement and hypertension therapy. Thyroid replacement therapy was defined as levothyroxine and liothyronine. Hypertension therapy was defined as an ACE inhibitor, angiotensin‐receptor blocker, dihydropyridine calcium‐channel blocker, hydrochlorothiazide, or β‐blocker. Additionally, time to first prescription for thyroid replacement or hypertension therapy (measured from the date of the first sunitinib, sorafenib, or capecitabine prescription) was also assessed.
Statistical Analysis
Kaplan‐Meier event‐free survival curves were constructed for the sunitinib versus capecitabine and sorafenib versus capecitabine cohorts to examine time to first event rates. Cox proportional hazards models were used to estimate unadjusted and adjusted hazard ratios (HRs) with 95% CIs. The date of the index prescription that defines each of the cohorts was time zero for each analysis, and observations were censored on the day of lost eligibility; if there was not a hypertension or thyroid replacement event, censoring occurred on September 30, 2009. Models were adjusted for the baseline variables age, sex, and Chronic Disease Score (CDS) at the time of drug initiation. The CDS is a commonly used comorbidity severity indicator that is determined for patients receiving their pharmacy benefits from Medco Health Solutions. It is derived by using methods similar to those described by others and correlates with physician ratings of physical disease severity. It was also shown to predict the need for hospitalization and mortality in the following year [16], [17]. All statistical analyses were conducted by using SAS software, version 9.1 (SAS Institute, Inc., Cary, NC, http://www.sas.com)
Results
Demographics
The demographic characteristics of the study population are shown in Table 1. The total number of patients eligible for evaluation of hypertension therapy was 14,468; 1,207 received sunitinib, 1,102 received sorafenib, and 12,159 received capecitabine. The total number of patients eligible for evaluation of thyroid replacement therapy was 20,061; 1,965 received sunitinib, 1,790 received sorafenib, and 16,306 received capecitabine. Patients in all cohorts were similar in terms of age and CDS, but more patients in the capecitabine cohort were female, as would be expected because this agent is a common oral therapy for treatment of advanced breast cancer.
Table 1. Demographic characteristics.
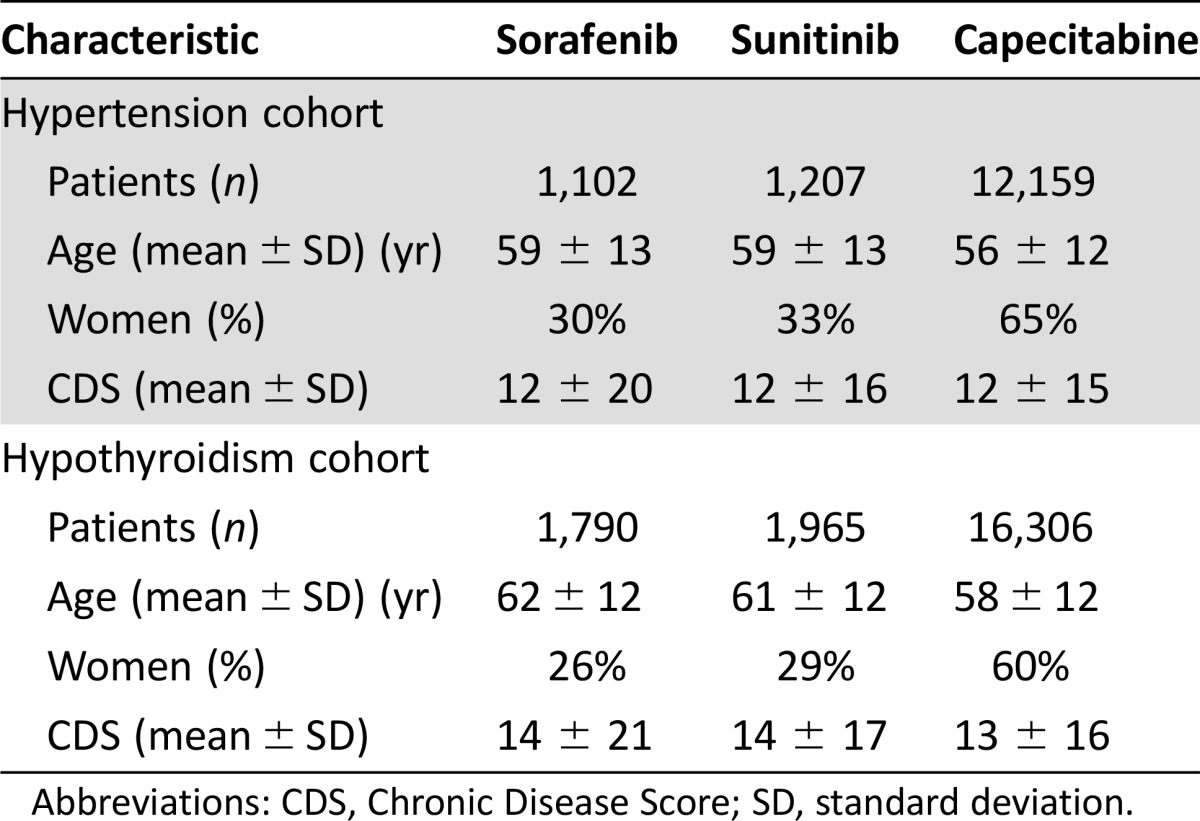
Abbreviations: CDS, Chronic Disease Score; SD, standard deviation.
Hypertension
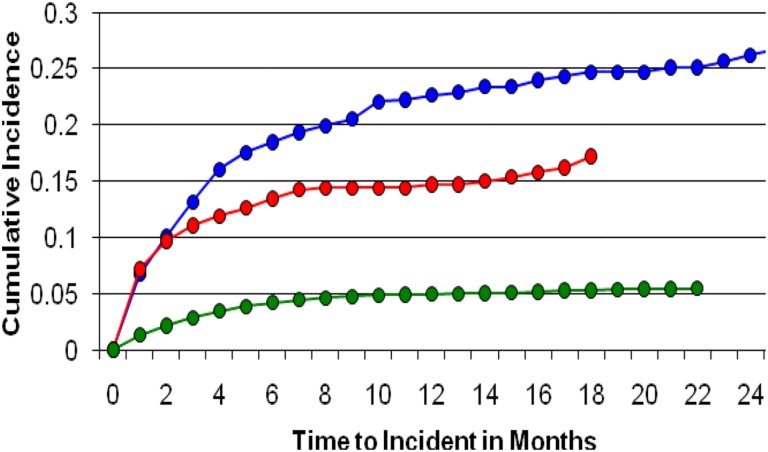
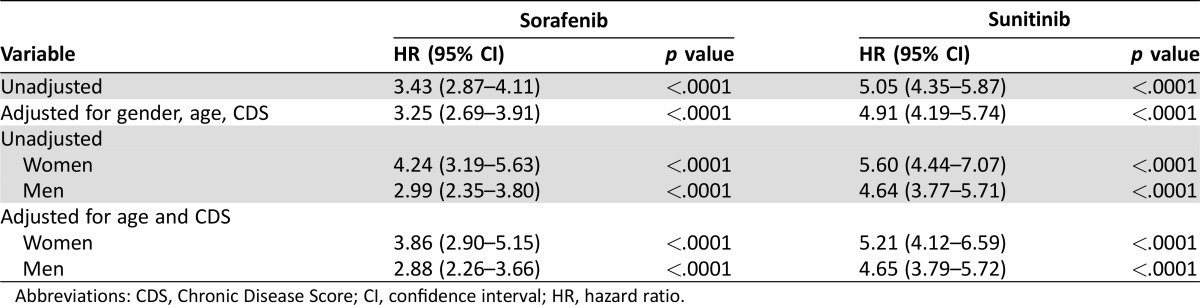
Hypertension therapy was initiated in 20.5% of patients receiving sunitinib, 14% receiving sorafenib, and 5% receiving capecitabine. The median time to initiation of hypertension therapy was 63 days in patients receiving sunitinib, 28 days for sorafenib recipients, and 69 days for capecitabine recipients (Fig. 1). Compared with patients receiving capecitabine, patients receiving sunitinib had an approximately 5‐fold greater risk of requiring antihypertensive therapy (unadjusted HR, 5.05; 95% CI, 4.35–5.87; p < .0001). This risk was similar when controlled for age, sex, and CDS (HR, 4.91; 95% CI, 4.19–5.74; p < .0001). Men and women receiving sunitinib had a similar risk of requiring antihypertensive therapy (Table 2). Patients receiving sorafenib had an approximately 3.5‐fold greater risk of requiring antihypertensive therapy (unadjusted HR, 3.43; 95% CI, 2.87–4.11; p < .0001). This risk was also similar when controlled for age, sex, and CDS (HR, 3.25; 95% CI, 2.69–3.91; p < .0001). Women receiving sorafenib had a higher risk of requiring antihypertensive therapy than men (Table 2).
Figure 1.
Incidence of treatment for hypertension with sunitinib (blue), sorafenib (red), and capecitabine (green).
Table 2. Incidence of hypertension therapy compared with capecitabine recipients.
Abbreviations: CDS, Chronic Disease Score; CI, confidence interval; HR, hazard ratio.
Hypothyroidism
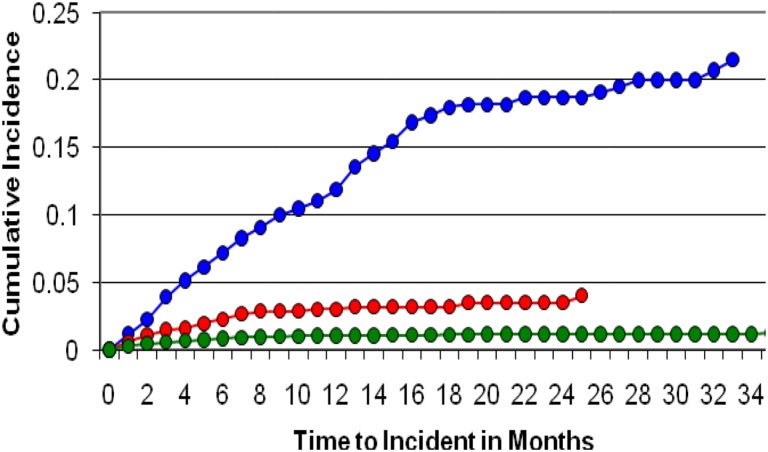
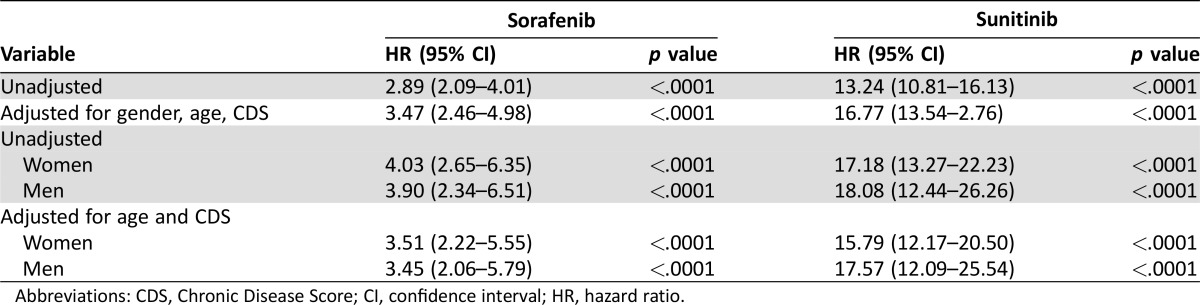
Thyroid replacement therapy was initiated in 11.6% of patients receiving sunitinib, 2.6% receiving sorafenib, and 1% receiving capecitabine. The median time to need for thyroid replacement was 152 days in patients receiving sunitinib, 79 days for sorafenib recipients, and 62 days for capecitabine recipients (Fig. 2). Compared with patients receiving capecitabine, patients receiving sunitinib had an approximately 13‐fold greater risk of requiring thyroid replacement therapy (unadjusted HR, 13.24; 95% CI, 10.81–16.13; p < .0001). This increased to an approximately 17‐fold greater risk when controlled for age, sex, and CDS (HR, 16.77; 95% CI, 13.54–20.76; p < .0001). Men and women receiving sunitinib had a similar risk of requiring thyroid replacement therapy (Table 2). Patients receiving sorafenib had an approximately 3‐fold greater risk of requiring thyroid replacement therapy (unadjusted HR, 2.89; 95% CI, 2.09–4.01; p < .0001). This value was slightly increased when controlled for age, sex, and CDS (HR, 3.47; 95% CI, 2.46–4.98; p < .0001). As with sunitinib, men and women receiving sorafenib had a similar risk of requiring thyroid replacement therapy (Table 3).
Figure 2.
Incidence of treatment for hypothyroidism with sunitinib (blue), sorafenib (red), and capecitabine (green).
Table 3. Incidence of hypothyroidism therapy compared with capecitabine recipients.
Abbreviations: CDS, Chronic Disease Score; CI, confidence interval; HR, hazard ratio.
Discussion
It is well characterized that patients receiving sunitinib and sorafenib have an elevated risk for hypertension. However, there are relatively few data comparing the incidence between these medications. Patients receiving sunitinib generally required antihypertensive therapy approximately 2 months after RTK initiation, whereas patients receiving sorafenib required antihypertensive drugs after approximately 1 month of therapy. This highlights the importance of routine blood pressure monitoring early in the course of therapy and continuing throughout. Approximately 20% of patients will need treatment, indicating a need for medical providers to anticipate this adverse event and support research directed toward determining agents for optimal management of vascular endothelial growth factor‐induced hypertension. There were also differences in sorafenib‐associated HTN between men and women, with the latter experiencing a slightly elevated risk compared with the former. The mechanisms behind these differences are not clear but may offer additional insights into the pharmacology of these RTKs and the pathophysiology of HTN.
Our findings on sorafenib‐associated HTN are similar to other published reports. A large meta‐analysis of 13,555 trial patients receiving sorafenib for a variety of malignancies demonstrated a relative risk for all‐grade HTN of 3.07 (95% CI, 2.05–4.60; p < .01) and high‐grade HTN of 3.31 (95% CI, 2.21–4.95; p < .01) [5]. Our findings of an adjusted hazard ratio of 3.86 (95% CI, 2.90–5.15) are similar, suggesting that the results represent the typical population receiving sorafenib. In contrast, our findings for sunitinib‐associated HTN suggest a higher incidence than previously published. A meta‐analysis that included 13 trials using sunitinib alone or in combination with other therapy demonstrated a relative risk for treatment emergent HTN of 3.48 (95% CI, 1.83–6.62), with no difference between the single‐agent and combination trials [18]. This value is slightly lower than our adjusted hazard ratio of 5.21 (95% CI, 4.12–6.59), indicating a slight underestimation from clinical trial data.
Hypothyroidism is often a neglected but clinically relevant problem in the oncology population; lack of treatment can affect patient quality of life. The need for thyroid replacement therapy was more than 4 times greater in patients receiving sunitinib compared with those receiving sorafenib. This also represented a more than 16‐fold increase in the need for thyroid replacement therapy compared with patients receiving capecitabine. Patients receiving sunitinib generally required thyroid replacement therapy between 1 and 7 months after sunitinib initiation, suggesting that thyroid monitoring should be initiated early and continue throughout the first year of therapy. Both sunitinib and sorafenib significantly increased the risk for hypothyroidism, indicating mechanistic commonality among these agents; however, the incidence was higher with sunitinib than sorafenib, a finding that may be secondary to differences in receptor‐binding affinity between the two RTKs.
Our findings with RTK‐associated hypothyroidism are consistent with those from a similar German cohort trial that assessed the incidence of thyroid hormone replacement in sorafenib‐ or sunitinib‐treated patients using claims data for prescriptions covering 80% of pharmacies in Germany. This trial assessed 1,214 patients receiving sorafenib; thyroid hormone replacement was started in 6.3% of the sorafenib recipients compared with the 13.7% of 1295 patients receiving sunitinib [15]. These incidences were similar to those in our population, in whom thyroid hormone replacement was started in 2.6% and 11.6% of patients receiving sorafenib and sunitinib, respectively.
Limitations that must be considered in interpreting these data include the fact that the need for hypertension and thyroid replacement therapy was used as a surrogate for the occurrence of clinically relevant hypertension and hypothyroidism. Given the nature of the study design, a causative relationship cannot be determined, nor can confounding factors. Additionally, this study does not capture hypertension or hypothyroidism that did not require treatment. However, this analysis may provide a more clinically relevant assessment of hypertension and hypothyroidism because the outcome was focused on the initiation of a therapeutic intervention. The use of capecitabine to serve as a control may not have been optimal because this agent is used predominantly in patients with colorectal and advanced breast cancer, compared with RCC, GIST, and HCC for the RTKs. If the susceptibility to hypertension and hypothyroidism differed between these populations, the nature of the study design did not permit consideration of primary malignancy or other patient factors to the development of these conditions. The time frame of data collection strongly suggests a high percentage of patients having RCC. The data collection time was from January 1, 2006, until September 30, 2009; during this time period, sorafenib was approved for RCC, followed in 2007 by HCC, while sunitinib was approved for RCC and GIST. It is important to consider the unique patient risk factors for the RCC population, especially in terms of development of hypertension. Ideally, the control group should match the interventional cohort(s) as closely as possible in terms of risk factors that may influence the primary outcome. Capecitabine was chosen because it is an oral chemotherapeutic agent not known to cause hypertension and because of the limited number of other agents available at the time of the data collection, especially in a cancer population that would match the one receiving sorafenib or sunitinib.
Regardless of the underlying causes and mechanisms, our findings clearly support an increased risk for HTN and hypothyroidism in patients receiving RTKs. A strength of our study was the large sample size and the use of a pharmacy claims database that provides a heterogeneous, nonprospective clinical trial patient population that may better estimate the actual occurrence of clinically relevant HTN and/or hypothyroidism in standard clinical practice. Although sorafenib and sunitinib are still frequently used in clinical practice, several novel multikinase‐targeted inhibitors are now available, including pazopanib, regorafenib, and axitinib. This type of data analysis is valuable for providing a real‐world view of toxicity profiles and can be used to inform and augment monitoring guidelines, especially in terms of timeline to certain events.
Conclusion
Patients receiving sorafenib or sunitinib have an elevated risk of developing clinically relevant HTN and/or hypothyroidism. Given that both side effects are amenable to successful therapeutic intervention, the results of this study can assist with directing monitoring recommendations for these agents in an effort to initiate therapy in a timely manner and improve patient‐related outcomes. Comparison between the two agents in terms of these adverse effects also supports similarities as well as differences in their mechanisms of action and off target effects, which will require further research to fully elucidate.
Acknowledgments
This work was supported in part by the University of North Carolina Carolina Partnership. The results of this study were presented at the general poster session in Patient and Survivor Care at the 2010 American Society of Clinical Oncology Annual Meeting (abstract #9149).
Author Contributions
Conception/Design: Christine M. Walko, Ronald E. Aubert, Vivian Herrera, Robert S. Epstein, Howard L. McLeod
Collection and/or assembly of data: Christine M. Walko, Ronald E. Aubert, Gosia Clore, Vivian Herrera
Data analysis and interpretation: Christine M. Walko, Ronald E. Aubert, Ninh M. La‐Beck, Vivian Herrera, Helen Kourlas, Howard L. McLeod
Manuscript writing: Christine M. Walko
Final approval of manuscript: Christine M. Walko, Gosia Clore, Howard L. McLeod
Disclosures
Howard L. McLeod: Kew Group, Saladax (C/A), Cancer Genetics (OI); Robert Epstein: Illuminia, Fate Therapeutics, Veractye, Proteus Digital (OI). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther 2005;315:971–979. [DOI] [PubMed] [Google Scholar]
- 2. Mendel DB, Laird AD, Xin X et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet‐derived growth factor receptors: Determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 2003;9:327–337. [PubMed] [Google Scholar]
- 3. Wilhelm SM, Carter C, Tang L et al. BAY 43‐9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099–7109. [DOI] [PubMed] [Google Scholar]
- 4. Zhu X, Stergiopoulos K, Wu S. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: Systematic review and meta‐analysis. Acta Oncol 2009;48:9–17. [DOI] [PubMed] [Google Scholar]
- 5. Li Y, Li S, Zhu Y et al. Incidence and risk of sorafenib‐induced hypertension: A systematic review and meta‐analysis. J Clin Hypertens (Greenwich) 2014;16:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Izzedine H, Ederhy S, Goldwasser F et al. Management of hypertension in angiogenesis inhibitor‐treated patients. Ann Oncol 2009;20:807–815. [DOI] [PubMed] [Google Scholar]
- 7. Wu S, Chen JJ, Kudelka A et al. Incidence and risk of hypertension with sorafenib in patients with cancer: A systematic review and meta‐analysis. Lancet Oncol 2008;9:117–123. [DOI] [PubMed] [Google Scholar]
- 8. Desai J, Yassa L, Marqusee E et al. Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Ann Intern Med 2006;145:660–664. [DOI] [PubMed] [Google Scholar]
- 9. Wong E, Rosen LS, Mulay M et al. Sunitinib induces hypothyroidism in advanced cancer patients and may inhibit thyroid peroxidase activity. Thyroid 2007;17:351–355. [DOI] [PubMed] [Google Scholar]
- 10. Mannavola D, Coco P, Vannucchi G et al. A novel tyrosine‐kinase selective inhibitor, sunitinib, induces transient hypothyroidism by blocking iodine uptake. J Clin Endocrinol Metab 2007;92:3531–3534. [DOI] [PubMed] [Google Scholar]
- 11. Rini BI, Tamaskar I, Shaheen P et al. Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 2007;99:81–83. [DOI] [PubMed] [Google Scholar]
- 12. Wolter P, Stefan C, Decallonne B et al. The clinical implications of sunitinib‐induced hypothyroidism: A prospective evaluation. Br J Cancer 2008;99:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tamaskar I, Bukowski R, Elson P et al. Thyroid function test abnormalities in patients with metastatic renal cell carcinoma treated with sorafenib. Ann Oncol 2008;19:265–268. [DOI] [PubMed] [Google Scholar]
- 14. Clemons J, Gao D, Naam M et al. Thyroid dysfunction in patients treated with sunitinib or sorafenib. Clin Genitourin Cancer 2012;10:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feldt S, Schussel K, Quinzler R et al. Incidence of thyroid hormone therapy in patients treated with sunitinib or sorafenib: a cohort study. Eur J Cancer 2012;48:974–981. [DOI] [PubMed] [Google Scholar]
- 16. Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol 1992;45:197–203. [DOI] [PubMed] [Google Scholar]
- 17. Fishman PA, Goodman MJ, Hornbrook MC et al. Risk adjustment using automated ambulatory pharmacy data: The RxRisk model. Med Care 2003;41:84–99. [DOI] [PubMed] [Google Scholar]
- 18. Abdel‐Rahman O, Fouad M. Risk of cardiovascular toxicities in patients with solid tumors treated with sunitinib, axitinib, cediranib or regorafenib: An updated systematic review and comparative meta‐analysis. Crit Rev Oncol Hematol 2014;92:194–207. [DOI] [PubMed] [Google Scholar]