Abstract
Lessons Learned.
Ideally, patients should have access to an oral formulation of paclitaxel, as well as an intravenous formulation, to allow development of regimens exploring alternate schedules and to avoid reactions to Cremophor EL (BASF Corp., Ludwigshafen, Germany, https://www.basf.com).
DHP107 is a novel oral paclitaxel formulation that is a tolerable and feasible regimen for patients with gastric cancer, with data suggesting efficacy similar to that of intravenous paclitaxel.
Background.
We evaluated the maximum tolerated dose (MTD) of DHP107, a novel oral paclitaxel formulation, and the efficacy and safety of the agent in patients with advanced solid tumors.
Patients and Methods.
Phase I study: cohorts of 3–6 patients with advanced solid tumors received escalating DHP107 doses. Phase IIa study: patients with measurable advanced gastric cancer received DHP107, 200 mg/m2 b.i.d., on days 1, 8, and 15 every 4 weeks. Pharmacokinetics, safety, and efficacy were analyzed.
Results.
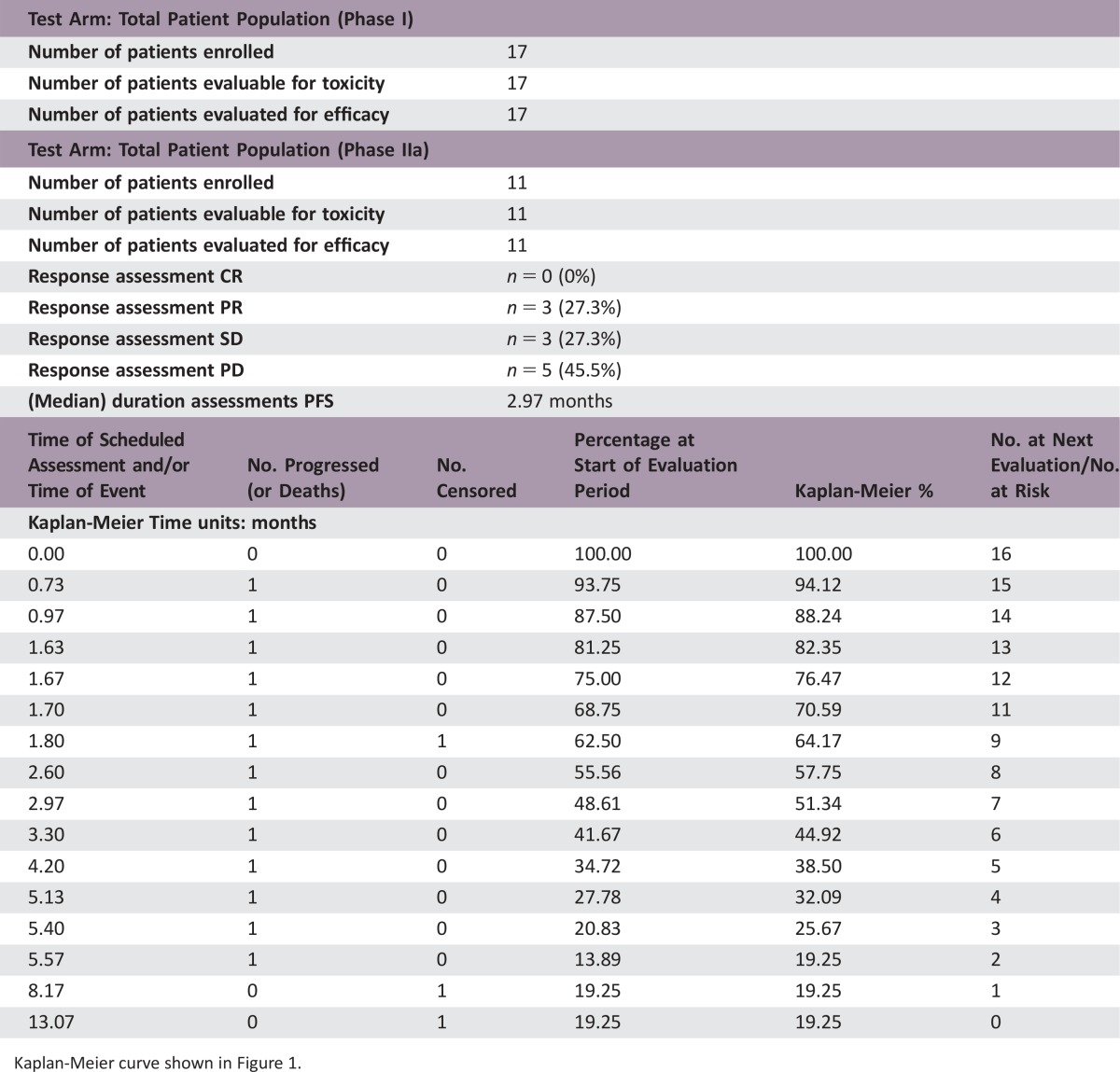
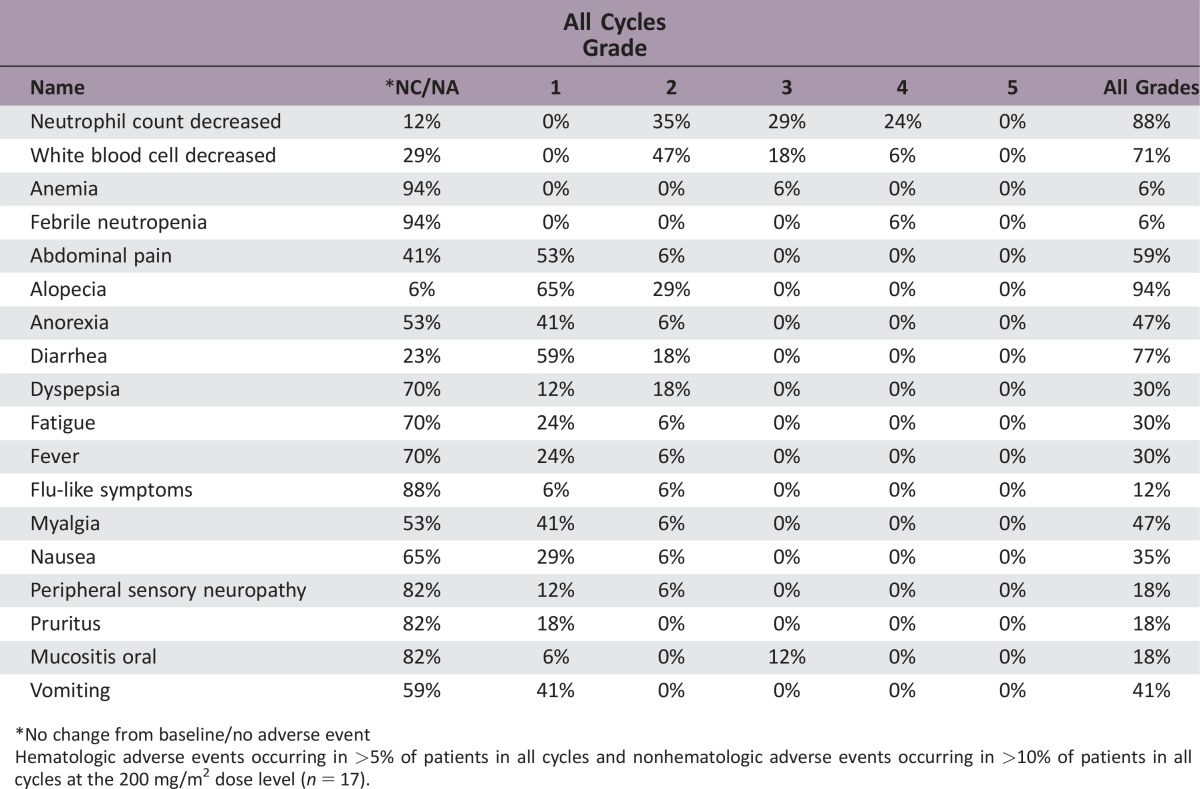
Phase I: 17 patients received a dose‐escalating regimen of DHP107, 150–250 mg/m2 b.i.d. Dose‐limiting toxicities were neutropenia and febrile neutropenia. The MTD (recommended dose) for phase IIa was 200 mg/m2 b.i.d. Phase IIa: 11 patients with measurable advanced gastric cancer in whom first‐line therapy failed received DHP107 (MTD). Three confirmed partial responses were observed. Median progression‐free survival of gastric cancer patients (n = 16) treated at the MTD was 2.97 (95% confidence interval, 1.67–5.40) months (Fig. 1). The most frequent grade 3/4 adverse events were neutropenia (35.3%) and leukopenia (17.6%) at the MTD (phase I and IIa combined; n = 17).
Conclusion.
DHP107 showed good antitumor efficacy and was tolerable. The MTD (200 mg/m2 b.i.d.) is recommended for use in further studies comparing DHP107 with standard intravenous paclitaxel therapy.
Abstract
经验总结
在理想情况下, 患者应能同时使用紫杉醇口服制剂和静脉注射制剂, 以便开发替代给药方案并规避聚氧乙烯蓖麻油(巴斯夫公司, 德国路德维希港, https://www.basf.com)的不良反应
DHP107是一种新型紫杉醇口服制剂, 对于胃癌患者而言是一种可耐受的可行治疗方案, 数据显示其疗效与紫杉醇静脉注射制剂相似。
摘要
背景. 本研究评价了新型紫杉醇口服制剂DHP107的最大耐受剂量(MTD)及其在晚期实体瘤患者中的疗效和安全性。
患者和方法. I期研究:多个队列中的晚期实体瘤患者(3‐6例/队列)接受剂量递增的DHP107给药。IIa期研究:可测量晚期胃癌患者在第1、8、15天接受DHP107 200mg/m2 b.i.d.给药, 每4周为一个周期。分析了药代动力学、安全性和疗效。
结果. I期:17例患者接受DHP107 150–250mg/m2 b.i.d.剂量递增方案。剂量限制性毒性为中性粒细胞减少症和发热性中性粒细胞减少症。IIa期研究的MTD(推荐剂量)为200mg/m2 b.i.d.。IIa期:11例一线治疗失败的可测量晚期胃癌患者接受DHP107(MTD)给药。观测到3例经证实的部分缓解。胃癌患者(16例)接受MTD治疗时的中位无进展生存期为2.97个月(95%置信区间:1.67‐5.40个月)(图1)。MTD下最常见的3/4级不良事件为中性粒细胞减少症(35.3%)和白细胞减少症(17.6%)(I期和IIa期合并;17例)。
结论. DHP107具有良好的抗肿瘤疗效且可耐受。建议在DHP107与静脉注射用紫杉醇标准治疗的进一步比较研究中采用MTD(200mg/m2 b.i.d.)。
Discussion
DHP107, developed by Daehwa Pharmaceutical Co. Ltd., is a lipid‐based single‐agent oral paclitaxel formulation that is systemically absorbed without the need for P‐glycoprotein inhibitors or Cremophor EL (BASF Corp., Ludwigshafen, Germany, https://www.basf.com). We carried out a phase I/IIa study using a weekly regimen (days 1, 8, and 15) in which DHP107 was given b.i.d. to increase patients’ exposure to the drug to determine toxicities and the maximum tolerated dose. By using a b.i.d. regimen in this study, exposure at or above the therapeutic threshold (8.5 ng/mL) was maintained for approximately 24 hours.
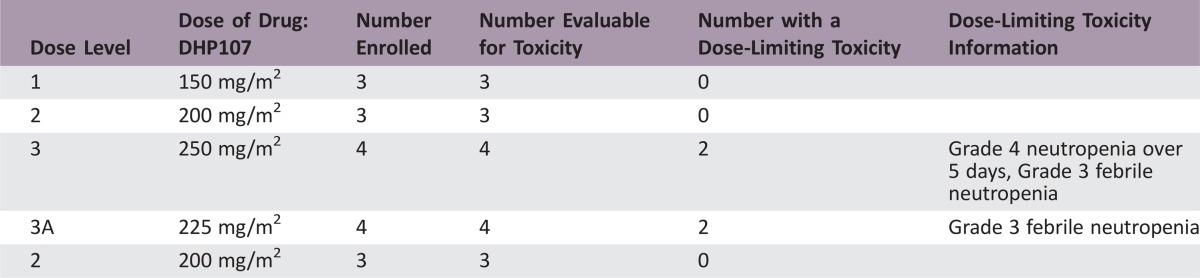
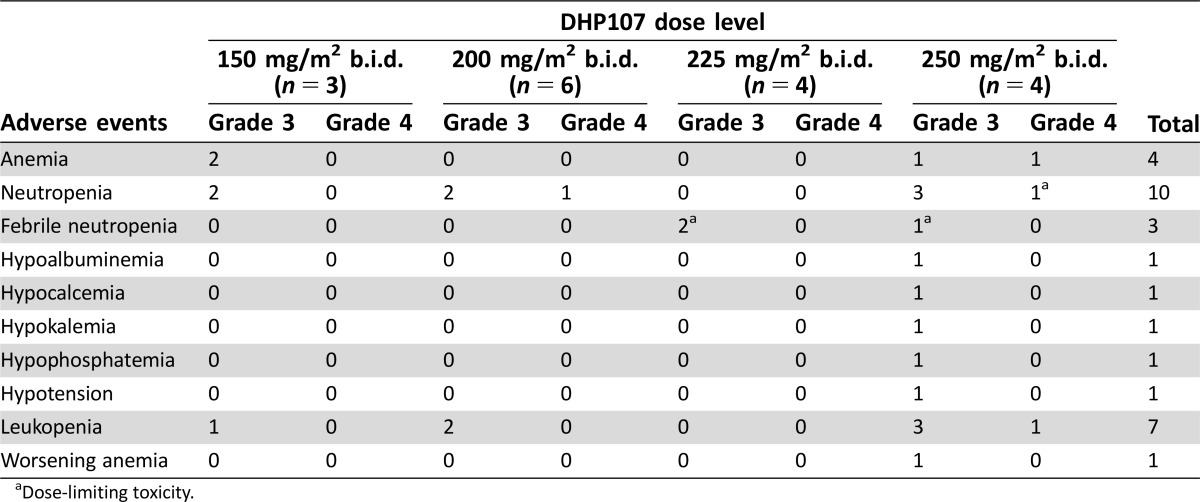
In the phase I (dose‐escalation) portion, 2 of 4 patients experienced dose‐limiting toxicities (DLTs; febrile neutropenia) with DHP107, 225 mg/m2 b.i.d.; 2 of 4 patients had DLTs (neutropenia and febrile neutropenia) with DHP107, 250 mg/m2 b.i.d. No DLTs occurred among the 6 patients who received DHP107 200 mg/m2 b.i.d.; hence, this was considered the MTD. Overall, 200 mg/m2 was tolerable in the day 1, 8, and 15 schedule, with neutropenia as the main side effect; only 77% of patients had grade 1 or 2 diarrhea and 35% had grade 1 or 2 nausea. In the phase IIa study, 11 patients with measurable advanced gastric cancer were enrolled at the MTD for a total of 17 patients who received DHP107, 200 mg/m2 b.i.d., to allow preliminary evaluation of efficacy. On the basis of the optimal two‐stage design, depending on patients’ responses, we planned to enroll up to 17 gastric cancer patients in the phase IIa study. When 11 patients had been recruited, 3 showed confirmed PRs, providing an overall response rate of 27.3% (95% confidence interval [CI], 0.0%–54.9%). As a result, additional enrollment was discontinued, and the efficacy was considered adequate to support further phase III study.
The development of an oral formulation of paclitaxel is an important goal for patient convenience and lessening side effects; it could allow the development of novel regimens, for low‐dose, long exposure to paclitaxel. If oral paclitaxel is proven to deliver equally effective therapy, it could also replace intravenous paclitaxel in some regimens, thereby preventing infusion reactions due to Cremophor EL diluent. The current study indicates that DHP107 is active and safe enough for continued development.
Trial Information
- Disease
Advanced cancer/solid tumor
- Stage of disease/treatment
Metastatic/advanced
- Prior Therapy
No designated number of regimens
- Type of study
Phase I/IIa
- Primary Endpoint
-
Phase I: Maximum tolerated dose (MTD)
Phase IIa: Response rate (RR)
- Secondary Endpoint
Safety
- Secondary Endpoint
Efficacy
- Additional Details of Endpoints or Study Design
The aims of the current phase I/IIa study were to determine the MTD for repeated administration of DHP107 by weekly schedule in patients with metastatic solid tumors and to evaluate DHP107 efficacy in patients with advanced gastric cancer
- Investigator's Analysis
Active and should be pursued further
Drug Information
- Generic/Working name
DHP107 (Oral paclitaxel)
- Company name
Daehwa Pharmaceutical Co. Ltd.
- Drug class
Tubulin/microtubules targeting agent
- Dose
200 mg/m2
- Route
p.o.
- Schedule of Administration
DHP107 was administered b.i.d. on days 1, 8, and 15 of a 28‐day cycle. The dose identified as the MTD was selected for the phase IIa portion of the study
Patient Characteristics (Phase I)
- Number of patients, male
10
- Number of patients, female
7
- Stage
IV
- Age
Median (range): 55 (30 – 67)
- Number of prior systemic therapies
Median (range): not collected
- Performance Status: ECOG
-
0 — 2
1 — 15
2 — 0
3 — 0
unknown — 0
- Cancer Types or Histologic Subtypes
-
Gastric 13
Colorectal 2
Parotid gland 1
Salivary gland 1
Patient Characteristics (Phase IIa)
- Number of patients, male
5
- Number of patients, female
6
- Age
52 (33 – 70)
- Performance Status: ECOG
-
0 — 1
1 — 10
2 — 0
3 — 0
unknown — 0
- Cancer Types or Histologic Subtypes
Gastric 11
Primary Assessment Method
- Test Arm: Total Patient Population (Phase I)
- Number of patients enrolled
17
- Number of patients evaluable for toxicity
17
- Number of patients evaluated for efficacy
17
- Test Arm: Total Patient Population (Phase IIa)
- Number of patients enrolled
11
- Number of patients evaluable for toxicity
11
- Number of patients evaluated for efficacy
11
- Response assessment CR
n = 0 (0%)
- Response assessment PR
n = 3 (27.3%)
- Response assessment SD
n = 3 (27.3%)
- Response assessment PD
n = 5 (45.5%)
- (Median) duration assessments PFS
2.97 months
Kaplan‐Meier curve shown in Figure 1.
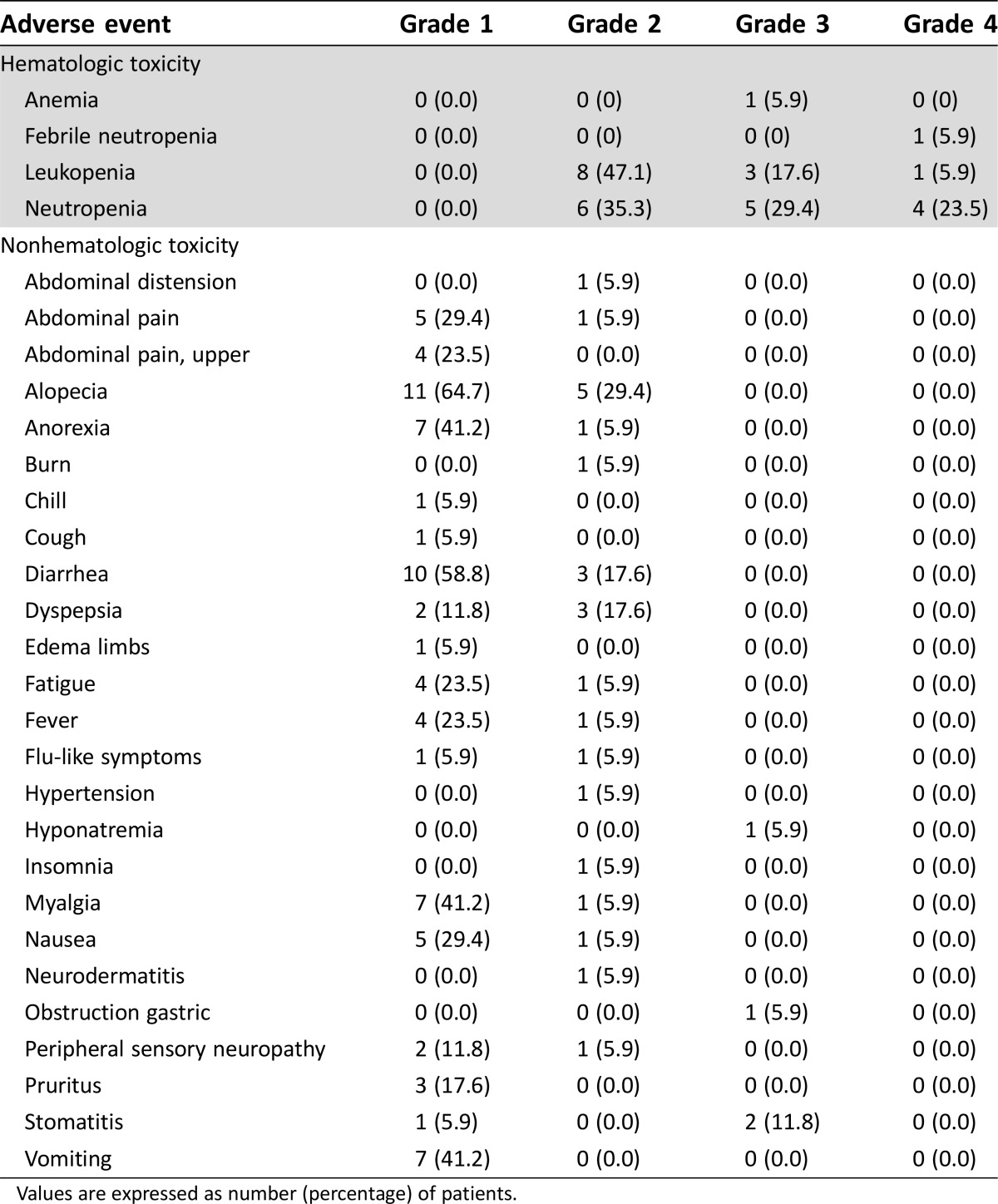
Adverse Events
*No change from baseline/no adverse event
Hematologic adverse events occurring in >5% of patients in all cycles and nonhematologic adverse events occurring in >10% of patients in all cycles at the 200 mg/m2 dose level (n = 17).
Dose‐Limiting Toxicities
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Active and should be pursued further
Paclitaxel has proven efficacy in treating a variety of cancers and is widely used to treat ovarian, gastric, breast, and non‐small cell lung cancers [1], [2], [3], [4]. Because paclitaxel has poor solubility in water, pharmaceutical agents, such as Cremophor EL (BASF Corp., Ludwigshafen, Germany), are used as a vehicle to aid intravenous administration [5], [6], [7], [8]. However, Cremophor EL can have biological implications, including hypersensitivity reactions [9]. Furthermore, it alters the pharmacokinetics of paclitaxel, causing it to have a nonlinear profile [10], [11].
A number of attempts have been made to reformulate paclitaxel to make it a more convenient and safer medication. Oral administration of paclitaxel is problematic because of low bioavailability related to P‐glycoprotein (P‐gp) and other membrane proteins in the gastrointestinal mucosa, which inhibit absorption. Moreover, cytochrome P450 isoenzymes in gastrointestinal tract and liver rapidly metabolize the drug [12], [13]. Development of an oral formulation has focused on improving the solubility and oral bioavailability of paclitaxel. To increase systemic exposure of oral paclitaxel, it has been coadministered with an orally applicable P‐gp blocker, such as cyclosporine A [12], [13]. However, the oral formulation of a cytotoxic agent combined with a P‐gp blocker has disadvantages because of potential interactions with concomitant medications, including substrates for P‐gp and/or with cytochrome P450 3A [14].
DHP107, developed by Daehwa Pharmaceutical Co. Ltd., is a lipid‐based single‐agent oral paclitaxel formulation that is systemically absorbed without the need for P‐gp inhibitors or Cremophor EL [15]. An animal study of DHP107 showed it has a similar antitumor effect compared with intravenous paclitaxel in human gastric cancer xenografts [16]. A previous phase I study in patients with advanced solid tumors refractory to all standard treatments showed no dose‐limiting toxicities (DLTs) with a single dose of DHP107 ranging from 60 to 600 mg/m2. DHP107 pharmacokinetics did not increase proportionally, and pharmacokinetic profiles, including area under the plasma concentration–time curve (AUC) and maximum plasma concentration (Cmax), plateaued at doses above 250 mg/m2 [17].
Intravenous paclitaxel has been one of the most commonly used salvage chemotherapies in gastric cancer. Although there has been no phase III comparative study of weekly paclitaxel versus every‐3‐weeks paclitaxel in gastric cancer, a phase II study of weekly paclitaxel showed antitumor effects similar to historical data for a every‐3‐weeks regimen as salvage chemotherapy [18]. With frequent use of weekly intravenous paclitaxel in gastric cancer and with the lower AUC and Cmax of a single dose of DHP107 compared with every‐3‐weeks intravenous paclitaxel [17], a weekly schedule of DHP107 was adopted in the current study.
The aims of the current phase I/IIa study were to determine the maximum tolerated dose (MTD) for repeated administration of DHP107 by weekly schedule in patients with metastatic solid tumors and to evaluate DHP107 efficacy in patients with advanced gastric cancer. The results of this study will guide phase III studies to compare the safety and efficacy of DHP107 versus intravenous paclitaxel.
Therefore, we carried out this phase I/IIa study using a weekly regimen (days 1, 8, and 15) in which DHP107 was given as a divided dose on the treatment day to increase patients’ exposure to the drug to allow determination of DLTs and the MTD.
In the phase I (dose‐escalation) portion of our study, 2 of 4 patients experienced DLTs (febrile neutropenia) with DHP107, 225 mg/m2 b.i.d., and 2 of 4 patients had DLTs (neutropenia and febrile neutropenia) with DHP107, 250 mg/m2 b.i.d. No DLTs occurred among the 6 patients who received DHP107, 200 mg/m2 b.i.d.; hence, this was determined as the MTD. After enrollment of additional patients, a total of 17 patients received DHP107, 200 mg/m2 b.i.d. At this dose level, 1 patient experienced febrile neutropenia and only 3 experienced grade 3/4 neutropenia in whole cycles. As a result, the dose was deemed tolerable. The most frequent non‐hematologic toxicities at the MTD were alopecia, diarrhea, and anorexia, which were generally of mild severity (grade 1/2). In the previously reported phase I study, 4 of 21 patients (19.0%) receiving a single administration of DHP107 at doses above 300 mg/m2 experienced grade 3 diarrhea; the grade of diarrhea seemed to increase with dose [17]. It is likely that the lipid‐based formulation of DHP107 leads to the increased incidence of diarrhea.
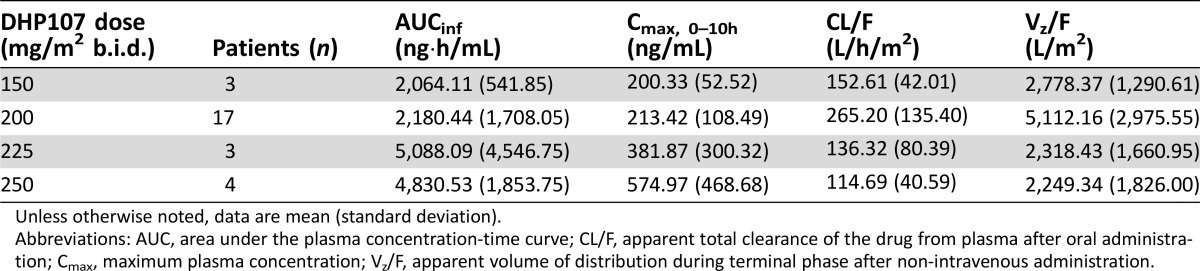
The pharmacokinetic parameters of DHP107, such as Cmax and AUCinf, were not linear in the dose range of 150–250 mg/m2 b.i.d. However, when the values of AUCinf in the phase IIa study were standardized with administered dose and compared with the data from the previous phase I study [17], there was no significant difference between dose‐normalized AUCinf values (Student's t test, p = 0.954). Mean Tmax was 2.7 hours; hence, the first dose will not interfere with the pharmacokinetics—in particular Cmax—of a second dose given after a 10‐hour interval. These pharmacokinetic characteristics are thought to be related to the specific absorption mechanism of DHP107 based on the lipid drug‐delivery system. The muco‐adhesiveness of the formulation in the gastrointestinal tract—especially in the stomach and upper intestine—inhibits absorption of the second dose of paclitaxel [20]. However, by using a b.i.d. regimen in this study, exposure at or above the therapeutic threshold (8.5 ng/mL) was maintained for approximately 24 hours [20]. In addition, no patients in the current study experienced severe diarrhea (grade 3/4) with DHP107, 200 mg/m2, a dose which is far below the dose that induced severe diarrhea in the previous phase I study. Therefore, this divided regimen of DHP107 is recommended in terms of efficacy and safety.
Gastrectomy is widely used as a standard therapy for patients with gastric cancer, and many patients with advanced gastric cancer will have undergone partial or total gastrectomy. The pharmacokinetic parameters of DHP107 were compared between gastrectomy and nongastrectomy patients with gastric cancer who received the 200 mg/m2 b.i.d. dose. Tmax values in the gastrectomy group were significantly lower (i.e., the drug was absorbed more rapidly) than in the nongastrectomy group; however, AUCinf and Cmax showed no significant difference by gastrectomy status. Therefore, the bioavailability of DHP107 is considered not to be affected by gastrectomy.
The efficacy results suggested that DHP107 is comparable to intravenous paclitaxel as a second‐line treatment in patients with advanced gastric cancer. Generally, the objective of a phase IIa study is to establish whether an intervention has sufficient efficacy against the disease to ensure further research [21]. Although the population of this study was small, based on an optimal two‐stage design, this regimen showed encouraging efficacy in poor‐prognosis patients. The overall response rate was 27.3% in the 11 patients with measurable disease; median progression‐free survival (PFS) was 2.97 months in the 16 patients with gastric cancer who received DHP107, 200 mg/m2 b.i.d. These results are in line with previous studies of intravenous weekly paclitaxel, in which response rates of 16%–24% and median PFS of 2.1–2.6 months were reported [18], [22].
Paclitaxel is a cell cycle‐specific agent. Accordingly, it is expected that paclitaxel is more effective with increasing exposure time than with increasing maximum concentration. Indeed, cell line experiments demonstrated that paclitaxel is more effective with increasing exposure time [23], [24]. In future studies, it is anticipated that oral administration of paclitaxel may make the development of a continuous low dose regimen possible. Such regimen would allow plasma concentration to be maintained above the therapeutic threshold over extended period without the need for a break during the therapy [5], [12]. Positive results have also been reported for low‐dose continuous chemotherapy regimens in patients with recurrent ovarian cancer and advanced cancers of various tumor types [25], [26], [27]. It is hoped that oral DHP107 will allow the development of a low‐dose continuous schedule that will reduce toxicity and increase antitumor effects.
In conclusion, DHP107 is a novel oral paclitaxel formulation that is mixed with edible oils without the need for absorption enhancers, such as P‐gp inhibitors. DHP107 is a potent and convenient chemotherapeutic agent for patients. In this study, DHP107 was a tolerable and feasible regimen for patients with gastric cancer, with efficacy that would seem to be similar to that of intravenous paclitaxel. On the basis of the results from this study, a phase III trial to assess the efficacy and safety of DHP107 compared with intravenous paclitaxel was conducted in patients with previously treated advanced gastric cancers (clinicaltrials.gov NCT01839773).
Figures and Tables
Figure 1.
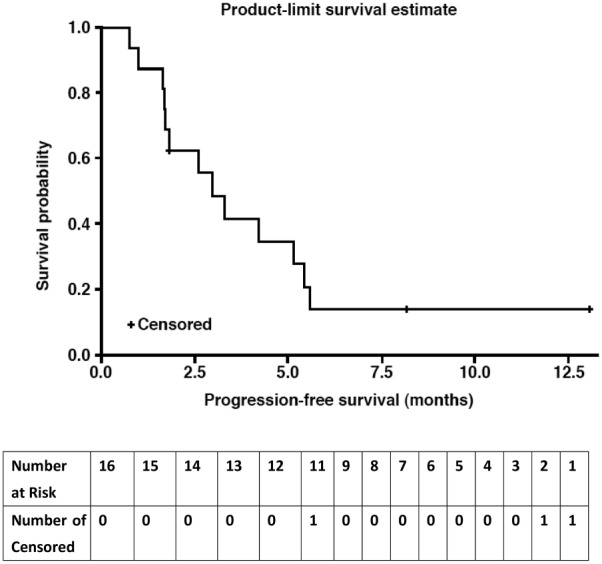
Kaplan‐Meier curve for progression‐free survival in patients with gastric cancer in the efficacy‐evaluable population (n = 16). These were patients with gastric cancer.
Figure 2.
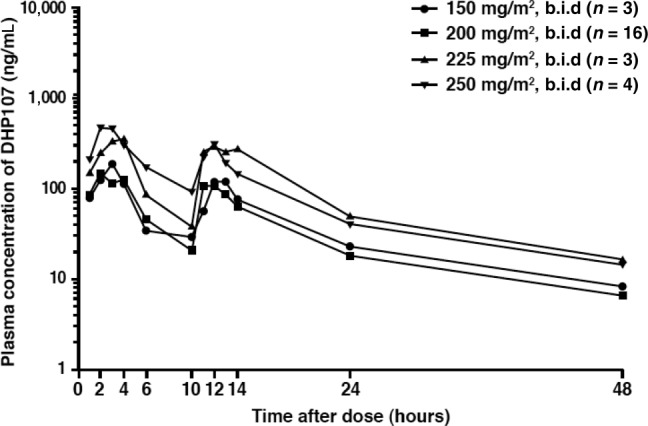
Plasma concentration of paclitaxel after oral administration of DHP107.
Table 1. Baseline patient characteristics.
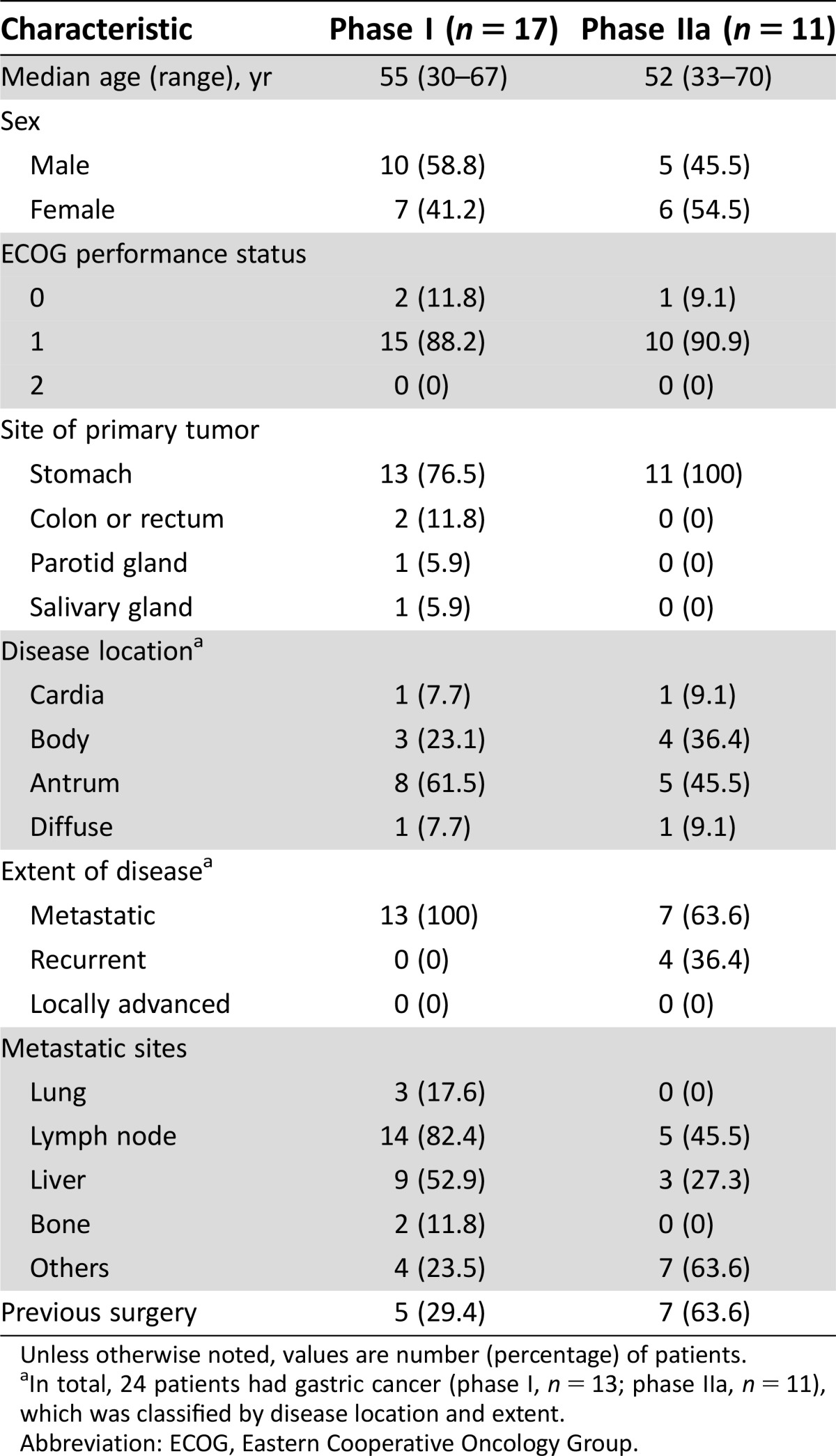
Unless otherwise noted, values are number (percentage) of patients.
In total, 24 patients had gastric cancer (phase I, n = 13; phase IIa, n = 11), which was classified by disease location and extent.
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Table 2. Grade 3/4 adverse events in the first cycle of the phase I portion (n = 17).
Dose‐limiting toxicity.
Table 3. Antitumor efficacy in patients with measurable lesions in the phase IIa study (n = 11).
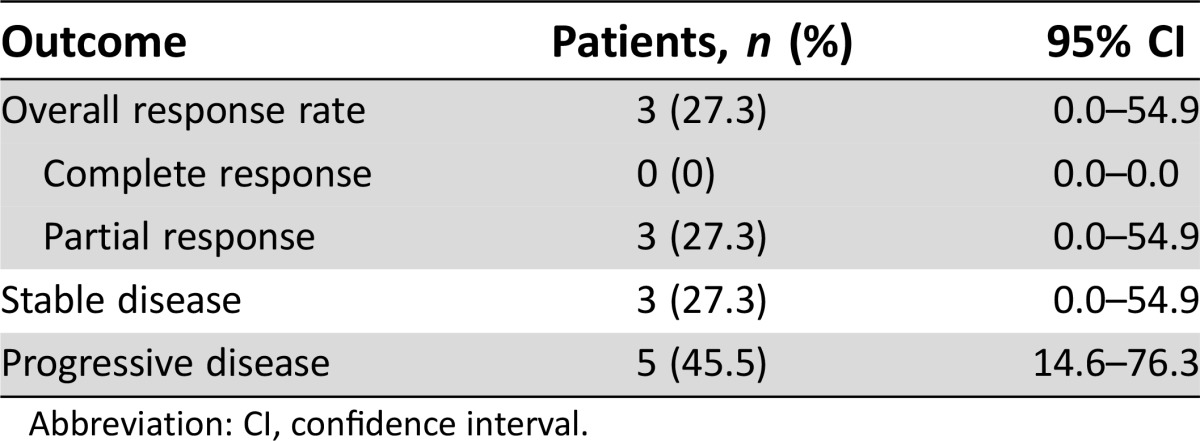
Abbreviation: CI, confidence interval.
Table 4. Adverse events across all cycles in dose level 2 (200 mg/m2) group (n = 17).
Values are expressed as number (percentage) of patients.
Table 5. Plasma pharmacokinetics of paclitaxel after oral administration of DHP107.
Unless otherwise noted, data are mean (standard deviation).
Abbreviations: AUC, area under the plasma concentration‐time curve; CL/F, apparent total clearance of the drug from plasma after oral administration; Cmax, maximum plasma concentration; Vz/F, apparent volume of distribution during terminal phase after non‐intravenous administration.
Acknowledgment
This research was supported by a grant from Daehwa Pharmaceutical Co. Ltd., and Gangwon Institute for Regional Program Evaluation by Korean government. Medical editing support was provided by Lee Miller from Miller Medical Communications Ltd. Funding for medical editing work was provided by Daehwa Pharmaceutical Co. Ltd.
Footnotes
ClinicalTrials.gov Identifier: NCT02890511
Sponsor: Daehwa Pharmaceutical Co. Ltd.
Principal Investigator: Yoon-Koo Kang
IRB Approved: Yes
Disclosures
Tae Won Kim: Merck Serono, Bayer, Roche (RF), Amgen, Eli Lilly (H); Yeong-Woo Jo: Daehwa Pharmaceutical Co. Ltd. (E); Hyun Ju Cho: Daehwa Pharmaceutical Co. Ltd. (E); Yoon-Koo Kang: Novartis (RF), Novartis, Bayer, Lilly, Sanofi, Taiho, Pfizer (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Vergote I, Tropé CG, Amant F et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010;363:943–953. [DOI] [PubMed] [Google Scholar]
- 2. Kang HJ, Chang HM, Kim TW et al. A phase II study of paclitaxel and capecitabine as a first‐line combination chemotherapy for advanced gastric cancer. Br J Cancer 2008;98:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Di Leo A, Gomez HL, Aziz Z et al. Phase III, double‐blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first‐line treatment for metastatic breast cancer [published correction appears in J Clin Oncol 2009;27:1923]. J Clin Oncol 2008;26:5544–5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sandler A, Gray R, Perry MC et al. Paclitaxel‐carboplatin alone or with bevacizumab for non‐small‐cell lung cancer [published correction appears in N Engl J Med 2007;356:318]. N Engl J Med 2006;355:2542–2550. [DOI] [PubMed] [Google Scholar]
- 5. Sparreboom A, Van Asperen J, Mayer U et al. Limited oral bioavailability and active epithelial secretion of paclitaxel (Taxol) caused by p‐glycoprotein in the intestine. Proc Natl Acad Sci USA 1997;94:2031–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rowinsky EK, Wright M, Monsarrat B et al. Clinical pharmacology and metabolism of Taxol (paclitaxel): Update 1993. Ann Oncol 1994;5(suppl 6):S7–S16. [PubMed] [Google Scholar]
- 7. Sonnichsen DS, Liu Q, Schuetz EG et al. Variability in human cytochrome P450 paclitaxel metabolism. J Pharmacol Exp Ther 1995;275:566–575. [PubMed] [Google Scholar]
- 8. Walle T, Walle K, Kumar GN et al. Taxol metabolism and disposition in cancer patients. Drug Metab Dispos 1995;23:506–512. [PubMed] [Google Scholar]
- 9. Weiss RB, Donehower RC, Wiernik PH et al. Hypersensitivity reactions from taxol. J Clin Oncol 1990;8:1263–1268. [DOI] [PubMed] [Google Scholar]
- 10. Kearns CM, Gianni L, Egorin MJ. Paclitaxel pharmacokinetics and pharmacodynamics. Semin Oncol 1995;3:16–23. [PubMed] [Google Scholar]
- 11. Gianni L, Kearns CM, Giani A et al. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J Clin Oncol 1995;13:180–190. [DOI] [PubMed] [Google Scholar]
- 12. Malingré MM, Beijnen JH, Rosing H et al. A phase I and pharmacokinetic study of bi‐daily dosing of oral paclitaxel in combination with cyclosporin A. Cancer Chemother Pharmacol 2001;47:347–354. [DOI] [PubMed] [Google Scholar]
- 13. Malingré MM, Beijnen JH, Rosing H et al. Co‐administration of GF120918 significantly increases the systemic exposure to oral paclitaxel in cancer patients. Br J Cancer 2001;84:42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Binkhathlan Z, Lavasanifar A. p‐glycoprotein inhibition as a therapeutic approach for overcoming multidrug resistance in cancer: Current status and future perspectives. Curr Cancer Drug Targets 2013;13:326–346. [DOI] [PubMed] [Google Scholar]
- 15. Hong JW, Lee IH, Kwak YH et al. Efficacy and tissue distribution of DHP107, an oral paclitaxel formulation. Mol Cancer Ther 2007;6:3239–3247. [DOI] [PubMed] [Google Scholar]
- 16. Na YS, Jung KA, Yang SJ et al. Antitumor effects of oral paclitaxel DHP107 on gastric cancer xenografts. Cancer Res 2011;71(suppl 8):2539. [Google Scholar]
- 17. Hong YS, Kim KP, Lim HS et al. A phase I study of DHP107, a mucoadhesive lipid form of oral paclitaxel, in patients with advanced solid tumors: Crossover comparisons with intravenous paclitaxel. Invest New Drugs 2013;31:616–622. [DOI] [PubMed] [Google Scholar]
- 18. Hironaka Y, Zenda S, Boku N et al. Weekly paclitaxel as second‐line chemotherapy for advanced or recurrent gastric cancer. Gastric Cancer 2006;9:14–18. [DOI] [PubMed] [Google Scholar]
- 19. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 20. Jang Y, Jo YW, Lee H et al. Absorption profiles of an oral paclitaxel formulation, DHP107 with variable dosing intervals in mice. Abstract presented at the 40th Annual Meeting and Exposition of the Controlled Release Society; July 21–24, 2013; Hawaii Convention Center, Honolulu, Hawaii. Available at https://issuu.com/scisoc/docs/2013crsprogrambook. Accessed [DATE].
- 21. Simon R. Optimal two‐stage designs for clinical trials. Control Clin Trials 1989;10:1–10. [DOI] [PubMed] [Google Scholar]
- 22. Kodera Y, Ito S, Mochizuki Y et al. A phase II study of weekly paclitaxel as second‐line chemotherapy for advanced gastric cancer (CCOG0302 Study). Anticancer Res 2007;27:2667–2671. [PubMed] [Google Scholar]
- 23. Liebmann JE, Cook JA, Lipschultz C et al. Cytotoxic studies of paclitaxel (Taxol) in human tumour cell lines. Br J Cancer 1993;68:1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raymond E, Hanauske A, Faivre S et al. Effects of prolonged versus short‐term exposure paclitaxel (Taxol) on human tumor colony‐forming units. Anticancer Drugs 1997;8:379–385. [DOI] [PubMed] [Google Scholar]
- 25. Jurado JM, Sánchez A, Pajares B et al. Combined oral cyclophosphamide and bevacizumab in heavily pre‐treated ovarian cancer [published correction appears in Clin Transl Oncol 2008;10:772]. Clin Transl Oncol 2008;10:583–586. [DOI] [PubMed] [Google Scholar]
- 26. Garcia AA, Hirte H, Fleming G et al. A trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol 2008;26:76–82. [DOI] [PubMed] [Google Scholar]
- 27. Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: New rationale for new directions. Nat Rev Clin Oncol 2010;7:455–465. [DOI] [PubMed] [Google Scholar]