Abstract
End-stage renal disease (ESRD) presents a complex syndrome in which inflammatory and metabolic processes contribute to disease progression and development of comorbid conditions. Over $1 trillion is spent globally on ESRD care.
Plasma samples collected from 83 ESRD patients prior to hemodialysis were profiled for metabolic and inflammatory biomarker concentrations. Concentrations were compared between groups with and without history of stroke, acute coronary syndrome (ACS), congestive heart failure (CHF), and coronary artery disease (CAD).
The 25 patients (30.1%) with history of stroke demonstrated decreased plasma interferon-γ levels (p = 0.042) and elevated plasma resistin, interleukin (IL)-1α, and leptin levels (p = 0.008, 0.021, 0.026, respectively) when compared with ESRD patients without history of stroke. The 14 patients (16.9%) with history of ACS demonstrated elevated plasma IL-6 levels (p = 0.040) when compared with ESRD patients without history of ACS. The 30 patients (36.1%) with history of CHF demonstrated decreased plasma leptin levels (p = 0.031) and elevated plasma IL-1β levels (p = 0.042) when compared with ESRD patients without history of CHF. Finally, the 39 patients (47.0%) with history of CAD demonstrated elevated plasma IL-1α levels (p = 0.049) when compared with ESRD patients without history of CAD.
Plasma biomarker concentration disturbances were observed in ESRD patients with history of stroke, ACS, CHF, and CAD when compared with ESRD patients without such history. Proinflammatory biomarker elevations were seen in stroke, ACS, CHF and CAD, while adipocytokine aberrations were observed in both stroke and CHF. These studies demonstrate that biomarker profiling of vascular comorbidities in ESRD may provide useful diagnostic and prognostic information in the management of ESRD patients.
Keywords: acute coronary syndrome, cardiovascular disease, hemodialysis, myocardial infarction, renal failure, stroke, tumor necrosis factor-α
Renal insufficiency has been shown to be correlated with risk of death, cardiovascular events, and hospitalization in a graded fashion.1 End-stage renal disease (ESRD) presents a complex illness associated with severely reduced renal function and profoundly increased risk of cardiovascular disease.2 As the final stage in the progression of chronic kidney disease (CKD), ESRD illustrates advanced features of diverse metabolic and hemodynamic dysfunction (impaired fluid homeostasis, insulin resistance, dyslipidemia, hypertension).3 These mechanisms contribute to the increased incidence of coronary artery calcification in CKD patients and to the observation that CKD exists as an independent risk factor for incidence of stroke.4 5 Multiple hormonal, inflammatory, and nutritional factors may be implicated in the pathogenesis of the complex disease-state seen in ESRD.6 These alterations include activation of proinflammatory cytokines, altered hepatic acute phase reactants, over activation of the renin–angiotensin–aldosterone system, and adipocytokine dysfunction.6 Adipocytokines like leptin and resistin have been previously shown to have independent association with risk and severity of chronic kidney disease.7 Secreted in quantities proportional to fat mass, leptin has impaired clearance in CKD and is considered a uremic toxin that “plays a key role in the pathogenesis of complications associated with CKD such as cachexia, protein energy wasting, chronic inflammation, insulin resistance, cardiovascular damages, and bone complications.”8 9 Leptin plays a significant role in regulating diet and energy expenditure. Furthermore, when chronically elevated, it also plays a role in the etiology of hypertension.10 Although the mechanism of inducing elevated blood pressure has not been well-defined, one can infer that chronic hyperleptinemia may contribute to the various deleterious end-organ effects observed in hypertensive patients, such as left-ventricular hypertrophy, nephropathy, and retinopathy.10 Prior studies have demonstrated that ESRD patients have elevated plasma resistin levels compared with normal—elevated resistin has recently been shown to be correlated with all-cause mortality in a systematic review and meta-analysis.11 Inflammatory processes in humans such as obesity and insulin resistance have been shown to be correlated with elevated levels of adipocyte-derived resistin.12 In the pediatric population, resistin is also known to play a role in the inflammatory milieu, displaying increased serum levels with decreased glomerular filtration rate.13 14
In addition, ESRD has displayed correlation with metabolic syndrome (MetS), a condition identified by a cluster of cardiovascular risk factors (elevated blood pressure, dyslipidemia, elevated fasting glucose, central obesity), which even further indicates the presence of a complex systemic microenvironment in these patients.15 16
It is clear that care for ESRD patients proposes a considerable global economic burden with estimates reaching greater than $1 trillion annually.17 Small advancements in early identification of ESRD and its comorbid conditions may make a large impact on the natural disease progression and its economic burden. Despite emerging understanding of biomarker contribution to the progression of CKD, there is a current paucity data to support sensitive and specific biomarkers as predictors of CKD progression. Microarray technology presents a novel method to rapidly profile patients for plasma biomarker concentrations. These levels offer a snapshot into the systemic circulating factors that may contribute to the pathogenesis of vascular comorbid conditions like stroke, coronary artery disease (CAD), acute coronary syndrome (ACS), and congestive heart failure (CHF). Insight into these aggregated aberrancies may provide a framework for future advances in risk stratification and predictive modelling.
Our study provides a cross-sectional observation of this microenvironment in 83 ESRD patients by comparing plasma metabolic and inflammatory biomarker concentrations between groups with and without a history of the aforementioned vascular comorbidities.
Methods
Our study represents a cross-sectional observational study of sequential ESRD patients currently on hemodialysis (HD) and a 10-year retrospective review of their history of comorbid vascular conditions.
Biomarker Evaluation
Under the approval of the institutional review board, plasma samples were collected from 83 patients with ESRD prior to hemodialysis from November 1 to 2, 2013. The patients displayed a representative population to the national demographics of ESRD patients (Table 1).18 Samples were stored at -80°C. Inflammatory and metabolic biochips were purchased from RANDOX (Co. Antrim, Northern Ireland) to profile concentrations of C peptide, ferritin, insulin, leptin, resistin, tumor necrosis factor-α (TNF-α), plasminogen activator inhibitor-1 (PAI-1), interleukin-1α (IL-1α), IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, vascular endothelial growth factor (VEGF), endothelial growth factor (EGF), interferon-γ (IFN-γ), and monocyte chemoattractant protein-1 (MCP-1).
Table 1. Sample patient demographics.
| n = 83 | |
|---|---|
| Age (y) | 65 ± 14 |
| Male (%) | 46.1 |
| Body mass index | 29.4 ± 7.9 |
| Hypertension (%) | 96.6 |
| Diabetes mellitus type 2 (%) | 58.4 |
| Metabolic syndrome15 (%) | 83.5 |
Note: Baseline demographic data on 83 hemodialysis patients
Retrospective Medical Record Review
The electronic medical records for 83 patients were accessed and searched for evidence of current or prior diagnoses in the following conditions: cerebrovascular accident, transient ischemic attack, or stroke (encompassed under our label as “stroke”); unstable angina, ST-segment elevation myocardial infarction, non-ST-segment elevation myocardial infarction (encompassed under our label as “ACS”); heart failure, systolic heart failure, diastolic heart failure, heart failure with reduced ejection fraction, heart failure with preserved ejection fraction (encompassed under our label as “CHF”; coronary artery disease (CAD). Patients were selected sequentially. Patient's history of the aforementioned comorbidities was assigned in a binary fashion by detailed review of ICD-9 diagnosis codes, and physician notes (history and physicals, discharge summaries, nephrology progress notes, cardiology progress notes, and neurology progress notes) over a retrospective period of 10 years from the time of sample collection.
Statistical Analysis
All data will be analyzed using GraphPad Prism Software (San Diego, CA). Unpaired, nonparametric t-tests were conducted to determine the significance of patterns among variables. Statistical significance was defined by p ≤ 0.05.
Results
Of the 83 ESRD patients undergoing HD, 25 (30.1%) had history of stroke, 14 (16.9%) had history of ACS, 30 (36.1%) had history of CHF, and 39 (47.0%) had history of CAD. Table 2 provides a representative biomarker profile comparison between our cohort with ESRD and our cohort with ESRD and stroke history. Significant concentration aberrations were observed in each of the comorbid conditions examined (Table 3). ESRD patients with +Stroke history demonstrated decreased plasma IFN-γ levels (p = 0.042) and elevated plasma resistin, IL-1α, and leptin levels (p = 0.008, 0.021, 0.026, respectively) when compared with ESRD-stroke patients (Fig. 1). ESRD patients with +ACS history demonstrated elevated plasma IL-6 levels (p = 0.040) when compared with ESRD-ACS patients. ESRD patients with +CHF history demonstrated decreased plasma leptin levels (p = 0.031) and elevated plasma IL-1β levels (p = 0.042) in comparison to ESRD-CHF patients. Finally, ESRD patients with +CAD history demonstrated elevated plasma IL-1α levels (p = 0.049) in comparison to ESRD-CAD patients (Fig. 2).
Table 2. Plasma biomarker concentrations in ESRD patients ± history of stroke.
| Biomarker | + Stroke | – Stroke | p-value |
|---|---|---|---|
| C peptide (ng/mL) | 16.19 | 12.79 | 0.0659 |
| Insulin (μLU/mL) | 19.88 | 14.75 | 0.1842 |
| Ferritin (ng/mL) | 254.2 | 323.3 | 0.4938 |
| Leptin (ng/mL) | 40.71 | 10.76 | 0.0264* |
| Resistin (ng/mL) | 18.04 | 14.29 | 0.0077* |
| PAI-1 (ng/mL) | 5.6 | 3.23 | 0.1775 |
| IL-1α (pg/mL) | 1.22 | 0.78 | 0.0211* |
| IL-1β (pg/mL) | 0.895 | 0.725 | 0.8090 |
| IL-2 (pg/mL) | 6.9 | 8.98 | 0.5333 |
| IL-4 (pg/mL) | 1.36 | 2.205 | 0.2970 |
| IL-6 (pg/mL) | 4.37 | 4.86 | 0.9611 |
| IL-8 (pg/mL) | 22.97 | 15.8 | 0.6401 |
| IL-10 (pg/mL) | 1.225 | 1.06 | 0.8371 |
| TNF-α (pg/mL) | 33.8 | 21.61 | 0.2541 |
| IFN-γ (pg/mL) | 1.08 | 1.56 | 0.0421* |
| VEGF (pg/mL) | 35.61 | 34.94 | 0.5904 |
| EGF (pg/mL) | 2.865 | 1.83 | 0.3556 |
| MCP-1 (pg/mL) | 208.4 | 182.6 | 0.2456 |
Abbreviations: ESRD, end-stage renal disease; IFN, interferon; IL, interleukin; MCP, monocyte chemoattractant protein; PAI, plasminogen activator inhibitor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Note: Biomarker concentrations in 83 end-stage renal disease patient serum samples with and without history of stroke (± Stroke).
p values ≤0.05 were deemed significant.
Table 3. Summary of plasma biomarker concentration differences in ESRD patients ± comorbidity.
| p-value | % difference | |
|---|---|---|
| Stroke | ||
| IFN-γ | 0.0421 | –192 |
| Resistin | 0.0077 | 26.2 |
| IL-1α | 0.0211 | 30.2 |
| Leptin | 0.0264 | 52.0 |
| ACS | ||
| IL-6 | 0.0399 | 79.4 |
| CHF | ||
| Leptin | 0.0311 | –35.9 |
| IL-1β | 0.0422 | –15.3 |
| CAD | ||
| IL-1a | 0.0492 | 937 |
Abbreviations: ACS, acute coronary syndrome; CAD, coronary artery disease; CHF, congestive heart failure; ESRD, end-stage renal disease; IFN, interferon; IL, interleukin.
Note: Summary of biomarker concentration significant differences in end-stage renal disease patients ± vascular comorbidity. Percent difference values use a negative history of the comorbidity as the reference value.
Fig. 1.
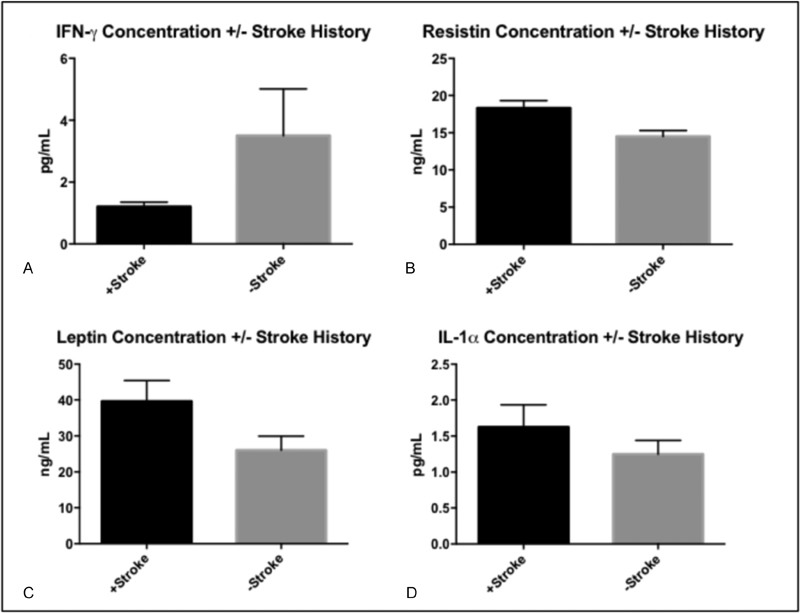
Summary of significant biomarker concentration differences amongst end-stage renal disease (ESRD) patients with history of stroke (+ Stroke) and ESRD patients without history of stroke (- Stroke). Histograms reflect mean values and error bars reflect standard error of the mean. (A) IFN-γ; (B) Resistin; (C) Leptin; (D) IL-1α.
Fig. 2.
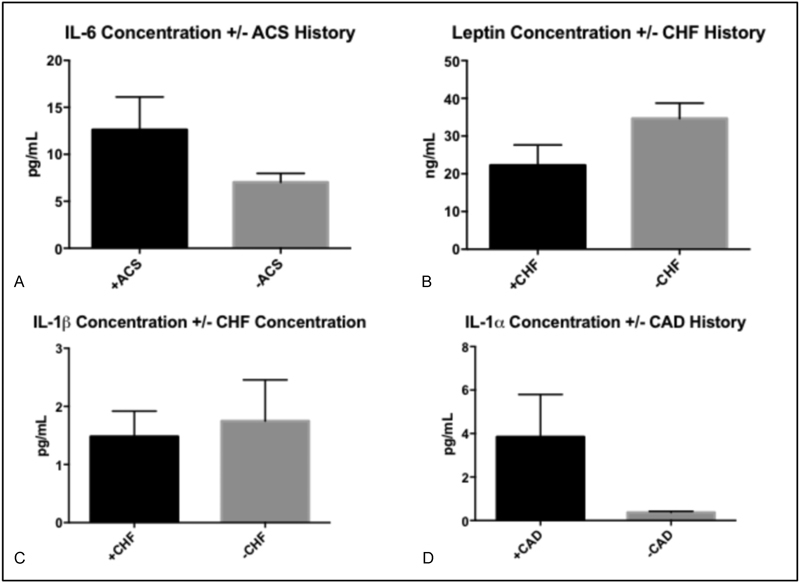
Summary of significant biomarker concentration differences amongst end-stage renal disease (ESRD) patients with history acute coronary syndrome (ACS), congestive heart failure (CHF), or coronary artery disease (CAD) (+ACS, +CHF, or +CAD) and ESRD patients without history of ACS, CHF, or CAD (-ACS, -CHF, or -CAD) Histograms reflect mean values and error bars reflect standard error of the mean. (A) IL-6; (B) Leptin; (C) IL-1β; (D) IL-1α.
Conclusion
We hypothesized that our cross-sectional observational study would allow us to compare the plasma microenvironment of 83 ESRD patients and display metabolic and inflammatory biomarker concentration changes between groups with and without a history of stroke, ACS, CHF, and CAD. Such biomarker concentration disturbances were observed in ESRD patients with known history of vascular comorbid conditions when compared with ESRD patients without such history. Plasma IL-1α elevation was observed in ESRD patients with history of stroke or CAD. This finding is consistent with the biomarker's known involvement in systemic pathways involving a proinflammatory state.
Plasma leptin variations were found in both the stroke and CHF cohorts. A decreased plasma leptin concentration was seen in the +CHF cohort, compared with the –CHF cohort which is in line with the converse association of serum leptin concentration and cardiovascular disease-related mortality in stable maintenance hemodialysis patients.19 In the +Stroke cohort, however, an elevated plasma leptin concentration was observed which, we propose, may be primarily impacted by increased adiposity in these patients. ESRD patients with history of stroke were found to have significantly elevated resistin when compared with those without. This may indicate the biomarker's important role in the disease process following stroke.
Discussion
High levels of proinflammatory cytokines IL-1, IL-6, and TNF-α have been shown to be associated with decreased survival and worse outcomes in ESRD patients.20 Such markers of inflammatory activity, including IL-6, have demonstrated correlation with vascular microanatomic changes in CKD patients, which provides insight into the pathophysiologic systemic etiology of the vast array of comorbid vascular conditions that arise in these complex patients.21 These observations are corroborated by our observation of elevated IL-6 in ESRD patients with history of ACS. Future studies may be directed at elucidating details regarding the potential impact of IL-6 on the development of coronary microvascular pathology contributing to the anatomic disregulation seen clinically in ACS. Subsequent efforts may aggregate a substantially larger population and utilize a prospective design to evaluate ESRD patients throughout the progression of their respective disease processes. Such studies may provide much-needed diagnostic and prognostic data to be applied in predictive models in an effort to optimize the care of these patients. Clinically, plasma sampling of ESRD patients on hemodialysis may provide a future modality to risk stratify patients and provide justification for earlier intervention in risk populations.
Limitations
We note presence of limitations in our cross-sectional pilot study methods and design. Although many of our biomarkers are known to have rapid concentration changes in plasma, our primary aim was to illustrate a framework and initial observations in relation to the biomarker array's role in examining a snapshot of the complex ESRD patient's plasma microenvironment. We also acknowledge that our procedure of labeling patients in a binary fashion into groups with and without a given comorbidity is in many ways an over-simplification of the diversity seen in these conditions. In addition, larger sampling of this diverse population would further illuminate the presence or absence of metabolic and inflammatory derangements and therefore strengthen the correlations made.
References
- 1.Go A S, Chertow G M, Fan D, McCulloch C E, Hsu C Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Sarnak M J, Levey A S, Schoolwerth A C. et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42(5):1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi S, Maesato K, Moriya H, Ohtake T, Ikeda T. Insulin resistance in patients with chronic kidney disease. Am J Kidney Dis. 2005;45(2):275–280. doi: 10.1053/j.ajkd.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 4.Lamprea-Montealegre J A, McClelland R L, Astor B C. et al. Chronic kidney disease, plasma lipoproteins, and coronary artery calcium incidence: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;33(3):652–658. doi: 10.1161/ATVBAHA.112.300624. [DOI] [PubMed] [Google Scholar]
- 5.Hart R G Eikelboom J W Brimble K S McMurtry M S Ingram A J Stroke prevention in atrial fibrillation patients with chronic kidney disease Can J Cardiol 201329(7, Suppl):S71–S78. [DOI] [PubMed] [Google Scholar]
- 6.Slee A D. Exploring metabolic dysfunction in chronic kidney disease. Nutr Metab (Lond) 2012;9(1):36. doi: 10.1186/1743-7075-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mills K T, Hamm L L, Alper A B. et al. Circulating adipocytokines and chronic kidney disease. PLoS One. 2013;8(10):e76902. doi: 10.1371/journal.pone.0076902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alix P M, Guebre-Egziabher F, Soulage C O. Leptin as an uremic toxin: deleterious role of leptin in chronic kidney disease. Biochimie. 2014;105:12–21. doi: 10.1016/j.biochi.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Cumin F, Baum H P, Levens N. Leptin is cleared from the circulation primarily by the kidney. Int J Obes Relat Metab Disord. 1996;20(12):1120–1126. [PubMed] [Google Scholar]
- 10.Bełtowski J. Role of leptin in blood pressure regulation and arterial hypertension. J Hypertens. 2006;24(5):789–801. doi: 10.1097/01.hjh.0000222743.06584.66. [DOI] [PubMed] [Google Scholar]
- 11.Fontana A, Spadaro S, Copetti M. et al. Association between resistin levels and all-cause and cardiovascular mortality: a new study and a systematic review and meta-analysis. PLoS One. 2015;10(3):e0120419. doi: 10.1371/journal.pone.0120419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehrke M, Reilly M P, Millington S C, Iqbal N, Rader D J, Lazar M A. An inflammatory cascade leading to hyperresistinemia in humans. PLoS Med. 2004;1(2):e45. doi: 10.1371/journal.pmed.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nehus E, Furth S, Warady B, Mitsnefes M. Correlates of resistin in children with chronic kidney disease: the chronic kidney disease in children cohort. J Pediatr. 2012;161(2):276–280. doi: 10.1016/j.jpeds.2012.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Axelsson J, Bergsten A, Qureshi A R. et al. Elevated resistin levels in chronic kidney disease are associated with decreased glomerular filtration rate and inflammation, but not with insulin resistance. Kidney Int. 2006;69(3):596–604. doi: 10.1038/sj.ki.5000089. [DOI] [PubMed] [Google Scholar]
- 15.Alberti K G, Eckel R H, Grundy S M. et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 16.Saluk J, Bansal V, Hoppensteadt D, Syed D, Abro S, Fareed J. Prevalence of metabolic syndrome in patients with end stage renal disease and relevance of biomarkers. Int Angiol. 2016;35(1):47–56. [PubMed] [Google Scholar]
- 17.Wouters O J, O'Donoghue D J, Ritchie J, Kanavos P G, Narva A S. Early chronic kidney disease: diagnosis, management and models of care. Nat Rev Nephrol. 2015;11(8):491–502. doi: 10.1038/nrneph.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tonelli M, Karumanchi S A, Thadhani R. Epidemiology and mechanisms of uremia-related cardiovascular disease. Circulation. 2016;133(5):518–536. doi: 10.1161/CIRCULATIONAHA.115.018713. [DOI] [PubMed] [Google Scholar]
- 19.Bian X, Liu N, Bai Y. et al. Association of leptin with mortality in patients on maintenance hemodialysis: a prospective study. Iran J Kidney Dis. 2014;8(4):314–320. [PubMed] [Google Scholar]
- 20.Cohen S D, Phillips T M, Khetpal P, Kimmel P L. Cytokine patterns and survival in haemodialysis patients. Nephrol Dial Transplant. 2010;25(4):1239–1243. doi: 10.1093/ndt/gfp625. [DOI] [PubMed] [Google Scholar]
- 21.Ghanavatian S, Diep L M, Bárány P. et al. Subclinical atherosclerosis, endothelial function, and serum inflammatory markers in chronic kidney disease stages 3 to 4. Angiology. 2014;65(5):443–449. doi: 10.1177/0003319713483000. [DOI] [PubMed] [Google Scholar]


