Abstract
Endothelial progenitor cells (EPCs) have reparative potential in overcoming the endothelial dysfunction and reducing cardiovascular risk. EPC depletion has been demonstrated in the setting of established atherosclerotic diseases. We evaluated whether reduced EPCs population are associated with endothelial dysfunction, subclinical atherosclerosis, and inflammatory markers in ankylosing spondylitis (AS) patients without any known traditional cardiovascular risk factor. We performed a cross-sectional study of 30 consecutive AS patients and 25 age- and sex-matched healthy controls. Patients with traditional cardiovascular risk factors were excluded. Circulating EPCs (CD34+/CD133+) were quantified by flow cytometry. The assessment of endothelial function by brachial artery flow-mediated dilatation (FMD) and ultrasound assessment of carotid intima-media thickness (CIMT) was measured in both the groups. EPCs cells were significantly (0.020 ± 0.001 vs. 0.040 ± 0.010%, p < 0.001) reduced in patients with AS compared with healthy controls. Endothelial function (7.35 ± 2.54 vs. 10.27 ± 1.73, p = 0.002), CIMT (0.63 ± 0.01 vs. 0.35 ± 0.02, p < 0.001), and inflammatory markers were also significantly (p < 0.01) altered as compared with controls. EPCs inversely correlated with tumor necrosis factor (TNF)-α and C-reactive protein (CRP) and positively correlated with endothelial function. Present study results demonstrate depleted EPC population in AS patients compared with controls. Increased level of CRP and TNF-α appears to play a key role in EPC depletion and the latter contributes to endothelial dysfunction and atherosclerosis in AS. EPC population would, therefore, represent an attractive measure of endothelial dysfunction and accelerated atherosclerosis disease associated with AS.
Keywords: ankylosing spondylitis, endothelial progenitor cells, endothelial function, carotid intima-media thickness, inflammation
Ankylosing spondylitis (AS) is a chronic inflammatory disease associated with an increased cardiovascular risk.1 The causes underlying this increased incidence of cardiovascular risk are not entirely understood. It is primarily due to the early development of advanced atherosclerotic vascular changes in AS.1 Vascular endothelial dysfunction, depleted endothelial progenitor cells (EPCs), and increased carotid intima-media thickness (CIMT) and inflammation are surrogates of atherosclerosis with a significant prognostic role in high-risk populations.2 3 4 5 EPCs are bone-marrow–derived stem cells that were first described in 1997.6 EPCs contribute to vascular homeostasis and may serve as a circulating pool of cells to improve endothelial dysfunction.7 The mechanisms underlying the association of AS with vascular dysfunction are complex. Traditional coronary risk factors such as hyperlipidemia, smoking, hypertension, diabetes, and alcohol have been implicated, but cannot fully explain the increased risk of cardiovascular disease (CVD) in this population.8 9 AS-related parameters such as disease severity and disease duration, and other associated emerging risk factors such as circulating endothelial cells, inflammation, endothelial dysfunction, subclinical atherosclerosis may also contribute to the impairment of arterial function in AS. The relative importance of these risk factors in the induction of vascular dysfunction in AS patients has not been well studied previously.
The aim of our study was to investigate EPC population and its association with disease-related parameters and proven markers of atherosclerosis, without any known traditional cardiovascular risk factor in AS patients.
Materials and Methods
Subjects
A total of 30 patients (age range: 26–55 years) with previously diagnosed AS were consecutively recruited from the Rheumatology Outpatient Clinic and 25 (age range: 23–52 years) age- and sex-matched healthy controls were recruited. Diagnosis of AS was defined according to the 1984 modified New York diagnostic criteria.10 All patients were on a combination of nonsteroidal anti-inflammatory drugs (mostly etoricoxib or piroxicam) and a stable dose of sulfasalazine 1 to 3 g/day for at least 3 months before study enrollment.
Patients were excluded from the study if they had a history of hypertension, hypercholesterolemia, diabetes, smoking, alcoholic, chronic liver and renal insufficiency, coronary artery diseases, stroke, thyroid disorder, multiple sclerosis, human immunodeficiency virus, and psychiatric disorders. Patients taking medication likely to affect endothelial function (anti-TNF-α inhibitors, β - blockers, an angiotensin-converting enzyme inhibitor, angiotensin-receptor blocker, statins and aldosterone antagonist, peroxisome proliferator-activated receptor, and steroids) were also excluded from the study. Patients were also excluded if they had rheumatic disorders other than AS. The study was performed in accordance with the Declaration of Helsinki, and approval was obtained from the Institutional Clinical Ethics Committee (ICEC) (ICEC/16/2012). All participants gave written informed consent for participation in the study.
Risk Factor Assessment
All participants underwent a medical history concerning disease, cardiovascular risk factors and general use of medications. A physical examination was then performed including measurement of blood pressure (sustained elevation of ≥ 140 mm Hg in the systolic pressure or ≥ 90 mm Hg in the diastolic pressure was considered as hypertension), height, and weight. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Patients who had stopped smoking during the last 12 months before the study were defined as nonsmokers.
All patients underwent a clinical and biochemical assessment at the time of recruitment. All clinical, biochemical, and vascular assessment (FMD, CIMT, and EPC) were performed after overnight fasting on the same day of recruitment from the same blood sample.
Clinical and Biochemical Assessment
Clinical assessment included disease severity which was evaluated by use of the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). By definition, a BASDAI score ≥ 4 was considered an active disease. Functional ability was monitored by using Bath Ankylosing Spondylitis Functional Index (BASFI). The biochemical analysis included a complete blood count, liver function tests, renal function test, vitamin B12, thyroid stimulating hormone, serum fasting glucose, lipid profile, including serum total cholesterol levels, serum triglyceride levels, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol were analyzed. Inflammatory markers, that is, the erythrocyte sedimentation rate (ESR), were measured using the Westergren method and C-reactive protein (CRP) measured using turbidimetry method and urine analysis to detect proteinuria, hematuria, and cellular casts were determined.
Vascular Measurements
All vascular studies were performed early in the morning with the subjects fasting for at least 12 hours before the study. They were kept in the supine position in a quiet, air-conditioned room (constant temperature 22–25°C) throughout the study. All studies were performed by the same operator in blinded conditions as to presence/absence of rheumatologic disease and ongoing treatment schedules.
Assessment of Endothelial Function
Subjects were studied in the morning, after overnight fasting, in a quiet room with controlled temperature. With the subjects lying supine and their arms in a comfortable position, we assessed endothelial function by brachial artery flow-mediated dilatation (FMD) by using, AngioDefender (Everist Genomics, Ann Arbor, MI). The AngioDefender device uses a novel, proprietary software algorithm to analyze pulse wave data collected before and after brachial artery (BA) occlusion by an upper arm sphygmomanometric cuff. At the end, of the testing procedure (∼15 minutes), the maximal relative postocclusion change in the diameter of the BA relative to baseline is calculated and expressed as a percentage of FMD (%FMD). AngioDefender test results are not dependent on user technique or operator proficiency.11 The mean of the two measures was considered for analysis. The intra- and interobserver variability had the coefficient of variations as 1.50 and 2.25%, respectively, for the measurements of FMD.
Assessment of Carotid Intima-Media Thickness
All subjects were examined using a high-resolution Doppler ultrasound (HD 11 XE ultrasound machine, Philips Medical System, Sorrento, Italy) using a 13 to 5-MHz linear array transducer in the supine position. The common carotid arteries (CCAs) intima-media thickness (IMT) was defined as the average of the maximum IMT of the near and far wall measurements in the distal CCA (1 cm proximal to the carotid bulb). IMT was measured at three points on the far walls of both the left and right CCAs. The three locations were then averaged to produce the mean IMT for each side. All images of the CCAs were recorded on the hard disk of the ultrasound system for subsequent analysis and evaluated by a well-experienced radiologist who was blinded to the clinical characteristics of the participants.12 The subjects had fasted overnight, and they were studied in the morning between 9 and 11 am. The intra- and interobserver variability had the coefficient of variations as 2.05 and 2.65%, respectively, for measurements of CIMT.
Assessment of EPC Population through Flow Cytometry Analysis
After patients and control subjects fasted overnight, peripheral blood was taken at rest, in the morning, at the forearm, together with routine analysis. EPCs were quantified by fluorescence-activated cell sorting (FACS) calibur flow cytometer (Canto II; BD Biosciences, San Jose, CA).
FACS analysis was performed with utilization of the following three markers:
Fluorescein isothiocyanate anti-CD45 (BD Biosciences)
Phycoerythrin anti-CD34 (BD Biosciences)
Allophycocyanin) anti-CD133 (Miltenyi Biotec, Bergisch Gladbach, Germany)
Peripheral blood in EDTA (200 μL) was labeled with a panel of above-mentioned antibodies and incubated for 1 hour at room temperature. After conjugation, red blood cells were lysed with ammonium chloride for 15 minutes at room temperature. Thereafter, cells were washed and resuspended in 500 μL phosphate buffered saline. The appropriate sequential analysis was used to enumerate total EPCs and to exclude debris. Putative EPCs were defined as positive for anti-CD34 and anti-CD133. At least 250,000 cells per sample were acquired. Data were analyzed with Cell Quest software (Becton Dickinson, San Jose, CA). Results are expressed as a percentage of cells gated.13 14
Statistical Analysis
Data are expressed as (arithmetic) means ± standard deviation. Comparison between the two groups (patients with AS vs. controls) was performed using unpaired Student t-test. The Mann-Whitney U test was performed for comparison of not normally distributed parameters and chi-square tests, as applicable. Pearson correlation coefficients were calculated in the AS group to study the relationship between EPC population and other disease parameters. Multivariate linear regression analyses were performed where the univariate p value was < 0.005. Statistical significance was assumed when a null hypothesis could be rejected at p < 0.05. Statistical analysis was performed using Sigmastat 5.5 for Windows 7 (Systat Software, San Jose, CA).
Results
Clinical Characteristics
The AS and healthy control groups were well matched with respect to age, sex, arterial blood pressure, lipid levels, and similar nonobese BMI (Table 1). Accordingly, there were no differences in measures associated with obesity and, systolic blood pressure or serum lipid profile. The majority of AS patients had the long-standing disease (9.17 ± 6.87 years) and the BASDAI score was high (4.57 ± 1.91 [0.6–5.8]). Patients with AS also have high functional disability measured by BASFI (Table 1).
Table 1. Characteristics of ankylosing spondylitis and healthy control subjects.
| Variables | Ankylosing spondylitis | Healthy controls | p Value |
|---|---|---|---|
| n | 30 | 25 | |
| Age (y) | 33.41 ± 10.25 | 29.36 ± 8.64 | 0.165 |
| Sex (men/women) | 19/11 | 16/9 | – |
| Disease duration (y) | 9.17 ± 6.87 | – | – |
| Height (cm) | 158 ± 8.2 | 162 ± 7.3 | 0.33 |
| Body weight (kg) | 67.04 ± 11.28 | 66.31 ± 12.3 | 0.08 |
| BMI (kg/m2) | 23.45 ± 5.03 | 22.85 ± 3.42 | 0.54 |
| Systolic BP (mm Hg) | 118.2 ± 11.6 | 112.6 ± 10.4 | 0.64 |
| Diastolic BP (mm Hg) | 78.5 ± 10.1 | 76.4 ± 9.6 | 0.78 |
| HbA1c (%) | 5.7 ± 0.64 | 5.4 ± 0.60 | 0.11 |
| Fasting serum glucose (mg/dL) | 101.42 ± 13.4 | 96.1 ± 9.2 | 0.09 |
| BASDAI | 4.57 ± 1.91 | – | – |
| BASFI | 3.10 ± 2.49 | – | – |
| ESR (mm first h) | 24.64 ± 6.78 | 16.68 ± 4.54 | 0.001 |
| CRP (mg/dl) | 10.79 ± 7.96 | 3.93 ± 2.10 | 0.003 |
| Total cholesterol (mg/dL) | 178.2 ± 32.7 | 162.7 ± 29.2 | 0.364 |
| HDL cholesterol (mg/dL) | 45.38 ± 5.64 | 52.70 ± 10.2 | 0.096 |
| LDL cholesterol (mg/dL) | 96.47 ± 28.6 | 87.22 ± 24.5 | 0.423 |
| Serum creatinine (μmol/L) | 0.91 ± 13 | 0.86 ± 10 | 0.527 |
Abbreviations: BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Note: Values are mean ± SD. Values in bold indicate, p-value < 0.05.
Comparison of Ankylosing Spondylitis and Healthy Controls Subjects
The EPC population (CD34+/CD133+) was statistically significantly lower in patients with AS compared with the healthy controls (p < 0.001) (Table 2). Endothelial function assessed by FMD was significantly reduced in patients with AS versus healthy controls (7.35 ± 2.54% vs. controls 10.27 ± 1.73%, p = 0.002), (Table 2). CIMT was significantly different in patients with AS compared with that seen in healthy control subjects (0.63 ± 0.01 vs. 0.35 ± 0.02, p < 0.001) (Table 2). There were significantly higher levels of ESR (p = 0.001), CRP (p = 0.003), TNF-α (p = 0.001), interleukin (IL)-6 (p = 0.004), and IL-1 (p = 0.001) in AS patients than in healthy controls (Table 2).
Table 2. Comparison of clinical characteristics in ankylosing spondylitis and healthy controls.
| Variables | Ankylosing spondylitis | Healthy controls | p Value |
|---|---|---|---|
| n | 30 | 25 | |
| EPC % | 0.020 ± 0.001 | 0.040 ± 0.010 | < 0.001 |
| FMD % | 7.35 ± 2.54 | 10.27 ± 1.73 | 0.002 |
| CIMT (cm) | 0.63 ± 0.01 | 0.35 ± 0.02 | < 0.001 |
| TNF-α (pg/mL) | 5.07 ± 2.04 | 3.6 ± 1.5 | 0.001 |
| IL-6 (pg/mL) | 8.77 ± 3.67 | 4.2 ± 2.83 | 0.004 |
| IL-1 (pg/mL) | 179.0 ± 74.8 | 102.4 ± 36.3 | 0.001 |
Abbreviations: CIMT, carotid intima-media thickness; EPC, endothelial progenitor cells; FMD, flow-mediated dilatation; IL-1, interleukin-1; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
Note: Values are mean ± SD. Values in bold indicate, p-value < 0.05.
Correlation with EPC Population and Endothelial Function, CIMT, Inflammatory Markers and Clinical Variables in AS Patients
In the current study, there was a significant positive relationship between EPC (CD34+/CD133+) population and FMD (r = 0.870, p = 0.001) and a significant inverse correlation between CD34+/CD133+ population and CIMT (r = − 0.767, p < 0.001), TNF-α (r = − 0.847, p = 0.001), IL-6 (r = − 0.818, p = 0.001), BASDAI (r = − 0.438, p = 0.01), CRP (r = − 0.540, p = 0.003), and disease duration (r = − 0.580, p = 0.001) (Table 3). The associations between the vascular indices and other studied parameters are shown in Table 3. In multivariate linear regression analysis (Table 3), altered endothelial function (p = 0.001) (Fig. 1), TNF-α (p = 0.003) (Fig. 2), and increased level of CRP (p = 0.028) (Fig. 3) were significantly correlated (Table 3).
Table 3. The univariate and multivariate analysis between EPC population and endothelial function, CIMT, inflammatory markers, and clinical characteristics in the patients with AS.
| Variables | Univariate analysis | Multiple regression analysis | ||
|---|---|---|---|---|
| r | p Value | Correlation β coefficient | p Value | |
| EPC | ||||
| Age | −0.220 | 0.258 | ||
| Duration of AS | −0.580 | 0.001 | −1.611 | 0.124 |
| BASDAI | −0.438 | 0.01 | 0.522 | 0.608 |
| BASFAI | −0.458 | 0.01 | 0.150 | 0.150 |
| ESR | −0.060 | −0.730 | ||
| CRP | −0.540 | 0.003 | −2.379 | 0.028 |
| FMD % | 0.870 | 0.001 | 3.858 | 0.001 |
| CIMT | −0.767 | < 0.001 | −1.851 | 0.042 |
| TNF-α | −0.847 | 0.001 | −3.458 | 0.003 |
| IL-6 | −0.818 | 0.001 | −1.151 | 0.264 |
| IL-1 | −0.214 | 0.273 | ||
Abbreviations: BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; CIMT, carotid intima-media thickness; CRP, C-reactive protein; EPC, endothelial progenitor cells; ESR, erythrocyte sedimentation rate; FMD, flow-mediated dilatation; IL-1, interleukin-1; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
Note: Multivariate analysis was only performed for univariate result at p-value < 0.01.
Values in bold indicate, p-value < 0.05.
Fig. 1.
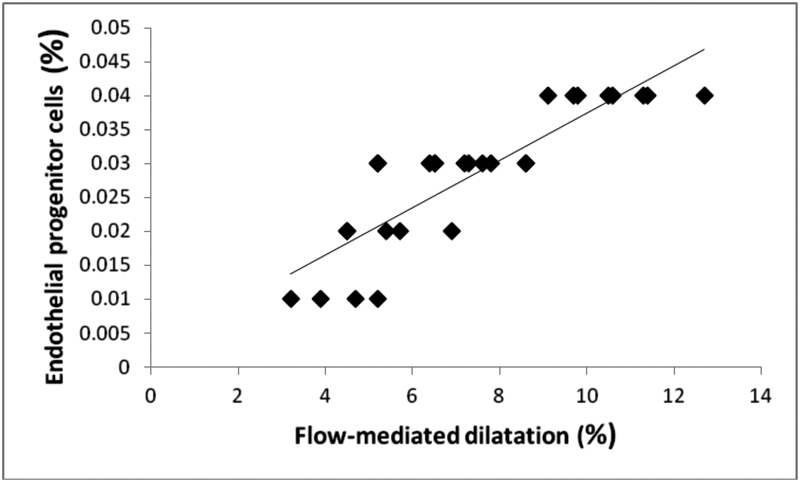
The relationship between EPC and FMD in AS patients. The best fit multiple linear regression line between the variables is plotted (β coefficients = 0.385, p = 0.001). AS, ankylosing spondylitis; EPC, endothelial progenitor cells; FMD, flow-mediated dilatation.
Fig. 2.
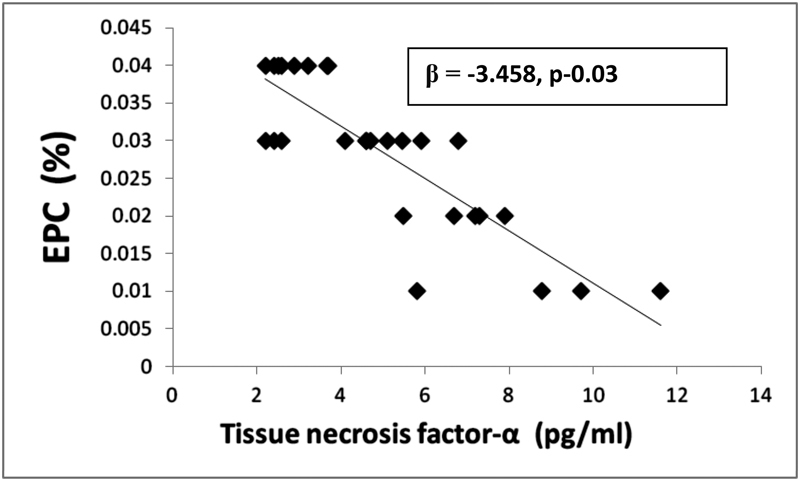
Multiple linear regression analysis between EPC% and CRP (mg/dL) in AS. AS, ankylosing spondylitis; EPC, endothelial progenitor cells; CRP, C-reactive protein.
Fig. 3.
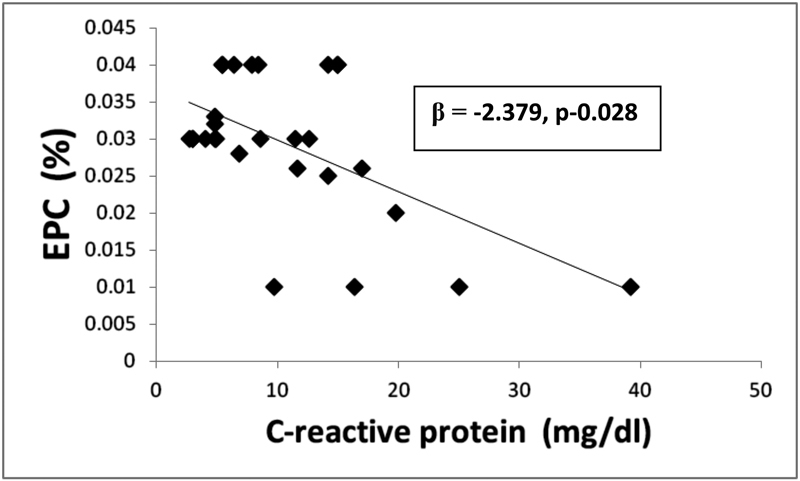
Multiple linear regression analysis between EPC% and TNF-α (pg/mL) in AS patients. AS, ankylosing spondylitis; EPC, endothelial progenitor cells; TNF-α, tumor necrosis factor-α.
Discussion
The present study aimed to identify the determinant of vascular function and atherosclerosis in patients with AS in the absence of conventional cardiovascular risk factors (obesity, hypertension, hyperlipidemia, diabetes, and smokers). We found statistically significant differences in EPC population, endothelial function, CIMT, and inflammatory markers (ESR, CRP, TNF-α, IL-6, and IL-1) in AS patients compared with the respective healthy control group. We also found a significant association between EPC and biomarkers of inflammation, vascular determinants (FMD and CIMT) and disease activity. To our knowledge, this is the first study to report a plausible association between EPC population and both endothelial function, subclinical atherosclerosis, and the interaction between various surrogate markers of increased cardiovascular risk in AS.
AS is characterized by chronic inflammatory disease associated with enhanced morbidity and mortality attributable to accelerated atherosclerosis.15 Atherosclerosis is the leading cause of death in autoimmune rheumatic diseases.16 EPCs are a unique population of bone-marrow–derived stem cells which have reparative potential in overcoming the endothelial damage and protect against atherosclerotic vascular disease.17 Impairment of EPC population is considered to have negative effects on the cardiovascular system and patients with a reduced number of EPCs are at an increased risk of endothelial injury and arteriosclerotic plaque development.18 Recently, we have demonstrated depleted EPC population in AS.19 Risk factors or surrogate markers recognized today in the pathogenesis of atherosclerosis and cardiovascular disorders are endothelial dysfunction, CIMT, and biomarkers of inflammation (CRP, IL-6, TNF-α, IL-1).5 14 20 21 22 23 An altered EPC level and association between EPC and susceptibility to cardiovascular events has been discovered in various conditions associated with vascular disease such as diabetes, rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), systemic sclerosis, antineutrophil cytoplasmic antibody-associated vasculitis (AAV), coronary artery disease, and hypertension.24 25 26 27
In the current study, we determined EPC (CD34+/CD133+) population and found a significant reduction in EPC population in AS patients in absence of traditional cardiovascular risk factor. This finding is consistent with the idea that traditional risks by themselves, including smoking, hypertension, diabetes, obesity, and elevated lipid levels described in RA, were not sufficient to explain the increased CV risk.28
Here, we also extend the findings of altered EPC population by assessment in relation to functional measures of endothelial dysfunction and biomarkers of atherosclerosis. Several studies have shown that the occurrence of endothelial dysfunction in AS may be associated with the clinical expressions of atherosclerosis.20 21 Endothelial dysfunction, in turn, is a key early event in atherogenesis and an independent predictor of CVDs appearing long before the formation of structural atherosclerotic changes.29 Measurement of IMT has been established as a clinically useful index for identifying early-stage atherosclerosis.30 The common carotid artery IMT strongly correlates with the presence of coronary artery disease.31 In our study, we found an underlying trend of increased IMT in the AS group as compared with matched controls. This finding is consistent with the increased incidence of subclinical atherosclerosis seen in AS patients.5 32
FMD measurement positively correlated with CD34+/CD133+ population, a correlation which persisted after multivariate regression analysis suggesting that depleted EPC population may be an early determinant of endothelial injury in AS. We also found a univariate relationship between CIMT and EPCs in the AS group. A previous study in middle-aged general population did find a significant correlation between EPC number and CIMT.33 However, FMD and CIMT may be looking at different parts of the same pathway of arterial wall damage, the former perhaps abnormalities of function and the latter early structural change, indicating that depleted EPC population represents at the same time a pathogenic step of atherogenesis and a biomarker of cardiovascular risk.
Biomarkers of inflammation such as ESR, CRP, TNF-α, IL-6, and IL-1 are increased in people at risk of CVD.34 Previous studies have suggested that inflammatory biomarkers are increased in people with rheumatic diseases.5 14 Here, those observations have been extended by finding increased levels of ESR, CRP, TNF-α, IL-6, and IL-1 in AS patients than healthy controls. In the present study, negative univariate correlations were found between EPC population and CRP, TNF-α, IL-6, disease activity as measured by BASDAI, and disease duration suggesting inflammation promotes EPC depletion in AS and might therefore be involved in increased vascular dysfunction and atherosclerosis in AS. Correlation between EPC alteration and disease activity appears to be complex. While EPCs in RA negatively correlate with disease activity, EPC depletion also occurs in young RA patients with low disease activity and proven endothelial dysfunction.14 35 In addition, a negative correlation of EPCs with systemic lupus erythematous disease activity (SLEDAI) has been documented in SLE patients.36 However, research regarding EPC biology in AS is evolving and such a relationship has not yet been reported in AS.
In the present study, in multivariate analysis, CRP, TNF-α, and FMD were significantly correlated found to be the independent predictor of decreased EPC. Wang et al demonstrated a high level of CRP negatively correlated with reduced number of EPC and suggested that CRP plays an important role in reducing the number of EPCs, angiogenic function, and accelerating atherosclerosis.37 It has been shown that CRP has the potential to contribute to vascular damage and is a predictor of future CVD events by acting on EPC apoptosis, proliferation, and differentiation.22 38 CRP has emerged as one of the most important predictors of myocardial infarction, stroke, and vascular death in several settings.34 CRP, however, is not only a marker but also a mediator of atherogenesis.39 Amongst the relevant cytokines, TNF-α is considered a key player and has been shown to significantly affect EPC biology. TNF-α and its relation with EPCs has also been demonstrated in RA and this proinflammatory cytokine effects mobilization and differentiation of EPC.35 Treatment with 25 to 50 mg prednisolone over 7 days and TNF-α inhibitor, infliximab, increased the EPC number in RA patients with concomitant improvement in disease activity.23 Similarly, immunosuppressant treatment of AAV resulted in increased level of EPCs.40
Preventing premature CVD remains an important challenge in the management of patients with AS. There is no targeted therapy to reduce CVD in AS patients. There is great potential to utilize the homeostatic endothelial repair mechanisms to prevent the development of endothelial dysfunction, at a time point long before the development of the clinical disease or even appearance of atherosclerotic plaque. EPCs are a robust biomarker of vascular dysfunction, based on their direct interaction and influence on endothelial function and EPCs also provide a novel therapeutic and biomarker of response to treatment. To develop and implement these novel approaches, we must explore the EPC biology in greater detail to treat/cure CVD associated with rheumatic disorders.
Conclusions
We observed depleted EPC population in patients with AS compared with matched controls. The finding of decreased EPC population and their relationship with abnormal FMD, CIMT, and biomarkers of inflammation suggests that abnormalities of endothelial repair mechanisms could have an important role in the initiation or exacerbation of early vascular injury. Also worthy of further attention is the use of decreased EPC population as a potential novel approach to identifying those people with AS who are at risk of CVD and novel therapeutic target for preventing cardiovascular risk associated with endothelial dysfunction in AS.
Acknowledgment
We are very grateful to the University Grant Commission, New Delhi (Govt. of India) for providing the research fellowship (grant no. F.10–15/2007 [SA-I]).
Conflict of Interest None.
Disclosure
None.
References
- 1.Azevedo V F, Pecoits-Filho R. Atherosclerosis and endothelial dysfunction in patients with ankylosing spondylitis. Rheumatol Int. 2010;30(11):1411–1416. doi: 10.1007/s00296-010-1416-3. [DOI] [PubMed] [Google Scholar]
- 2.Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004;109(21) 01:II27–II33. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- 3.Willerson J T, Ridker P M. Inflammation as a cardiovascular risk factor. Circulation. 2004;109(21) 01:II2–II10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- 4.Goodson N J, Symmons D P, Scott D G, Bunn D, Lunt M, Silman A J. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: a ten-year followup study of a primary care-based inception cohort. Arthritis Rheum. 2005;52(8):2293–2299. doi: 10.1002/art.21204. [DOI] [PubMed] [Google Scholar]
- 5.Mathieu S, Joly H, Baron G. et al. Trend towards increased arterial stiffness or intima-media thickness in ankylosing spondylitis patients without clinically evident cardiovascular disease. Rheumatology (Oxford) 2008;47(8):1203–1207. doi: 10.1093/rheumatology/ken198. [DOI] [PubMed] [Google Scholar]
- 6.Asahara T Murohara T Sullivan A et al. Isolation of putative progenitor endothelial cells for angiogenesis Science 1997275(5302):964–967. [DOI] [PubMed] [Google Scholar]
- 7.Gill M, Dias S, Hattori K. et al. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001;88(2):167–174. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 8.del Rincón I D, Williams K, Stern M P, Freeman G L, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44(12):2737–2745. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 9.Asanuma Y, Oeser A, Shintani A K. et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349(25):2407–2415. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 10.van der Linden S, Valkenburg H A, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27(4):361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 11.Garg N, Krishan P, Syngle A. Rosuvastatin improves endothelial dysfunction in ankylosing spondylitis. Clin Rheumatol. 2015;34(6):1065–1071. doi: 10.1007/s10067-015-2912-3. [DOI] [PubMed] [Google Scholar]
- 12.Huang K, Zou C C, Yang X Z, Chen X Q, Liang L. Carotid intima-media thickness and serum endothelial marker levels in obese children with metabolic syndrome. Arch Pediatr Adolesc Med. 2010;164(9):846–851. doi: 10.1001/archpediatrics.2010.160. [DOI] [PubMed] [Google Scholar]
- 13.Allanore Y, Batteux F, Avouac J, Assous N, Weill B, Kahan A. Levels of circulating endothelial progenitor cells in systemic sclerosis. Clin Exp Rheumatol. 2007;25(1):60–66. [PubMed] [Google Scholar]
- 14.Herbrig K, Haensel S, Oelschlaegel U, Pistrosch F, Foerster S, Passauer J. Endothelial dysfunction in patients with rheumatoid arthritis is associated with a reduced number and impaired function of endothelial progenitor cells. Ann Rheum Dis. 2006;65(2):157–163. doi: 10.1136/ard.2005.035378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choe J Y, Lee M Y, Rheem I, Rhee M Y, Park S H, Kim S K. No differences of carotid intima-media thickness between young patients with ankylosing spondylitis and healthy controls. Joint Bone Spine. 2008;75(5):548–553. doi: 10.1016/j.jbspin.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Shoenfeld Y, Gerli R, Doria A. et al. Accelerated atherosclerosis in autoimmune rheumatic diseases. Circulation. 2005;112(21):3337–3347. doi: 10.1161/CIRCULATIONAHA.104.507996. [DOI] [PubMed] [Google Scholar]
- 17.Haque S, Alexander M Y, Bruce I N. Endothelial progenitor cells: a new player in lupus? Arthritis Res Ther. 2012;14(1):203–213. doi: 10.1186/ar3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill J M, Zalos G, Halcox J P. et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 19.Verma I, Syngle A, Krishan P. Endothelial progenitor cell biology in ankylosing spondylitis. Int J Rheum Dis. 2015;18(3):336–340. doi: 10.1111/1756-185X.12487. [DOI] [PubMed] [Google Scholar]
- 20.Sastry K V, Moudgal R P, Mohan J, Tyagi J S, Rao G S. Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal Biochem. 2002;306(1):79–82. doi: 10.1006/abio.2002.5676. [DOI] [PubMed] [Google Scholar]
- 21.van Ejick I C, Peters M JL, Seme E H. et al. Microvascular function is impaired in ankylosing spondylitis and improves after tumor necrosis factor αblockade. Ann Rheum Dis. 2009;68(3):362–366. doi: 10.1136/ard.2007.086777. [DOI] [PubMed] [Google Scholar]
- 22.Verma S, Kuliszewski M A, Li S H. et al. C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation. 2004;109(17):2058–2067. doi: 10.1161/01.CIR.0000127577.63323.24. [DOI] [PubMed] [Google Scholar]
- 23.Grisar J, Aletaha D, Steiner C W. et al. Endothelial progenitor cells in active rheumatoid arthritis: effects of tumour necrosis factor and glucocorticoid therapy. Ann Rheum Dis. 2007;66(10):1284–1288. doi: 10.1136/ard.2006.066605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westerweel P E, Luijten R K, Hoefer I E, Koomans H A, Derksen R H, Verhaar M C. Haematopoietic and endothelial progenitor cells are deficient in quiescent systemic lupus erythematosus. Ann Rheum Dis. 2007;66(7):865–870. doi: 10.1136/ard.2006.065631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park Y J, Kim J Y, Park J, Choi J J, Kim W U, Cho C S. Bone erosion is associated with reduction of circulating endothelial progenitor cells and endothelial dysfunction in rheumatoid arthritis. Arthritis Rheumatol. 2014;66(6):1450–1460. doi: 10.1002/art.38352. [DOI] [PubMed] [Google Scholar]
- 26.Werner N, Wassmann S, Ahlers P. et al. Endothelial progenitor cells correlate with endothelial function in patients with coronary artery disease. Basic Res Cardiol. 2007;102(6):565–571. doi: 10.1007/s00395-007-0680-1. [DOI] [PubMed] [Google Scholar]
- 27.Imanishi T, Moriwaki C, Hano T, Nishio I. Endothelial progenitor cell senescence is accelerated in both experimental hypertensive rats and patients with essential hypertension. J Hypertens. 2005;23(10):1831–1837. doi: 10.1097/01.hjh.0000183524.73746.1b. [DOI] [PubMed] [Google Scholar]
- 28.Sibal L, Aldibbiat A, Agarwal S C. et al. Circulating endothelial progenitor cells, endothelial function, carotid intima-media thickness and circulating markers of endothelial dysfunction in people with type 1 diabetes without macrovascular disease or microalbuminuria. Diabetologia. 2009;52(8):1464–1473. doi: 10.1007/s00125-009-1401-0. [DOI] [PubMed] [Google Scholar]
- 29.Mudau M, Genis A, Lochner A, Strijdom H. Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovasc J Afr. 2012;23(4):222–231. doi: 10.5830/CVJA-2011-068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wofford J L, Kahl F R, Howard G R, McKinney W M, Toole J F, Crouse J R III. Relation of extent of extracranial carotid artery atherosclerosis as measured by B-mode ultrasound to the extent of coronary atherosclerosis. Arterioscler Thromb. 1991;11(6):1786–1794. doi: 10.1161/01.atv.11.6.1786. [DOI] [PubMed] [Google Scholar]
- 31.Lekakis J P, Papamichael C M, Cimponeriu A T. et al. Atherosclerotic changes of extracoronary arteries are associated with the extent of coronary atherosclerosis. Am J Cardiol. 2000;85(8):949–952. doi: 10.1016/s0002-9149(99)00907-8. [DOI] [PubMed] [Google Scholar]
- 32.Sharma S K, Prasad K T, Handa R, Sharma S K. Increased prevalence of subclinical atherosclerosis in ankylosing spondylitis. Indian J Rheumatol. 2015;10(2):53–57. [Google Scholar]
- 33.Fadini G P, Coracina A, Baesso I. et al. Peripheral blood CD34+KDR+ endothelial progenitor cells are determinants of subclinical atherosclerosis in a middle-aged general population. Stroke. 2006;37(9):2277–2282. doi: 10.1161/01.STR.0000236064.19293.79. [DOI] [PubMed] [Google Scholar]
- 34.Ridker P M Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: implications for longevity Nutr Rev 200765(12 Pt 2):S253–S259. [DOI] [PubMed] [Google Scholar]
- 35.Grisar J, Aletaha D, Steiner C W. et al. Depletion of endothelial progenitor cells in the peripheral blood of patients with rheumatoid arthritis. Circulation. 2005;111(2):204–211. doi: 10.1161/01.CIR.0000151875.21836.AE. [DOI] [PubMed] [Google Scholar]
- 36.Moonen J R, de Leeuw K, van Seijen X J. et al. Reduced number and impaired function of circulating progenitor cells in patients with systemic lupus erythematosus. Arthritis Res Ther. 2007;9(4):R84. doi: 10.1186/ar2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H Y, Gao P J, Ji K D. et al. Circulating endothelial progenitor cells, C-reactive protein and severity of coronary stenosis in Chinese patients with coronary artery disease. Hypertens Res. 2007;30(2):133–141. doi: 10.1291/hypres.30.133. [DOI] [PubMed] [Google Scholar]
- 38.Suh W, Kim K L, Choi J H. et al. C-reactive protein impairs angiogenic functions and decreases the secretion of arteriogenic chemo-cytokines in human endothelial progenitor cells. Biochem Biophys Res Commun. 2004;321(1):65–71. doi: 10.1016/j.bbrc.2004.06.107. [DOI] [PubMed] [Google Scholar]
- 39.Pasceri V, Willerson J T, Yeh E T. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102(18):2165–2168. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- 40.de Groot K, Goldberg C, Bahlmann F H. et al. Vascular endothelial damage and repair in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2007;56(11):3847–3853. doi: 10.1002/art.23070. [DOI] [PubMed] [Google Scholar]


