Abstract
Background
Studies demonstrated that visit-to-visit variability in low-density lipoprotein cholesterol (LDLC) is an independent predictor of cardiovascular events in subjects with coronary artery disease. Whether visit-to-visit variability in LDLC levels affects subclinical atherosclerosis is unknown. This study sought to evaluate the role of visit-to-visit variability in LDLC levels on subclinical atherosclerosis.
Methods
We evaluated 162 type 2 diabetic patients with measurement of carotid intima-media thickness (IMT). Intrapersonal mean and standard deviation (SD) of six measurements of LDLC during 12 months were calculated. Multivariate linear regressions assessed the independent correlates of carotid IMT.
Results
The mean and SD of LDLC were 112 ± 22 and 15 ± 10 mg/dL, respectively, and 43.2% of patients were on hypolipidemic drugs. Age (standardized β = 0.355, P < 0.001), male sex (standardized β = 0.234, P = 0.002) and SD-LDLC (standardized β = 0.201, P = 0.009) emerged as independent determinants of carotid maximum IMT independently of mean LDLC levels, body mass index (BMI), waist circumference, duration and treatment of diabetes, means and SDs of glycemic and other lipid variables, and uses of hypolipidemic and anti-hypertensive medications (R2 = 0.15). Results did not change when mean IMT was used instead of maximum IMT. After controlling for age and sex, maximum IMT was thicker in patients with the highest compared to those with other three quartiles of SD-LDLC combined (1.14 ± 0.04 (SE) vs. 1.01 ± 0.02 mm, P = 0.01). Independent determinants of SD-LDLC were mean LDLC, use of hypolipidemic drugs, fasting triglyceride and visit-to-visit variability in HbA1c.
Conclusions
Consistency of LDLC levels may be important to subclinical atherosclerosis in real-world patients with type 2 diabetes. It may be important for patients on lipid-lowering drugs to prevent non-compliance.
Keywords: LDLC, Annual variability, Carotid intima-media thickness, Type 2 diabetes
Introduction
Variability in heart rate and blood pressure has emerged as a novel prognostic marker [1-4]. Recently, visit-to-visit variability in low-density lipoprotein cholesterol (LDLC) has been shown to be an independent predictor of adverse long-term cardiovascular outcomes in patients with coronary artery disease on statin treatment [5, 6]. We have recently shown a direct association between visit-to-visit HbA1c variability and kidney function decline in patients with type 2 diabetes [7]. Whether visit-to-visit variability in LDLC affects subclinical atherosclerosis remains unclear. Our objective was to evaluate the cross-sectional relationship between visit-to-visit variability in LDLC and carotid intima-media thickness (IMT), a marker of subclinical atherosclerosis, in type 2 diabetic patients.
Patients and Methods
We here show results of 162 patients, in whom carotid IMT was measured during the first 12 months after enrollment, out of 168 patients with type 2 diabetes whose details have been reported elsewhere [7]. Patients with hepatitis B surface antigen or antibodies against hepatitis C virus were excluded. Those who had aspartate aminotransferase and alanine aminotransferase of 100 U/L or greater and serum creatinine ≥ 2.0 mg/dL were excluded as well. Study protocol was consistent with the Japanese Government’s Ethical Guidelines Regarding Epidemiological Studies in accordance with the Declaration of Helsinki.
For each subject on each monthly visit, waist circumference, weight and blood pressure (BP) were measured by registered nurses. As previously reported in details [7], blood was withdrawn on two occasions: at 2 h after breakfast taken at home and after an overnight fasting. This was done every other month in the majority of patients (94%). Plasma glucose, serum cholesterol, triglycerides (TG), high-density lipoprotein cholesterol (HDLC), creatinine and other blood tests were measured by standard methods using an autoanalyzer. LDLC was calculated using Friedewald’s formula [8] using serum cholesterol, TG and HDLC measured in samples taken after an overnight fasting. Complete blood cell count was analyzed using an automated blood cell counter. HbA1c values were determined by high performance liquid chromatography.
Of 162 patients, 153 patients (94%) had 12 measurements of anthropometric variables, systolic BP and HbA1c, and six measurements of LDLC, fasting and post-breakfast concentrations of plasma glucose (PG) and serum TG. Intrapersonal mean and SD of these variables taken during the first 12 months after enrollment were calculated.
Urinary albumin was measured in random urine samples using a turbidimetric immunoassay and expressed as albumin/creatinine ratio (ACR). Normoalbuminuria, microalbuminuria and macroalbuminuria were defined as an ACR < 30 mg/g, ACR between 30 and 299 mg/g and ACR ≥ 300 mg/g, respectively [9]. Serum and urinary creatinine were measured enzymatically and estimated glomerular filtration rate (eGFR) was determined using the equation recommended by the Japanese Society for Nephrology [10]. Chronic kidney disease was defined as eGFR < 60 mL/min/1.73 m2 and/or ACR ≥ 30 mg/g [11].
IMT was measured by a well-trained medical technologist of Sadamitsu Hospital using ultrasonic diagnosis equipment (Shimadzu SDU-2200, Shimadzu, Tokyo, Japan) that was programmed with IMT software (Intimascope; Media Cross Co. Ltd, Tokyo, Japan) as previously described [12]. Carotid artery ultrasonography was performed using a 10-MHz scanning frequency in B mode with the participant in the supine position. Computer-based IMT was evaluated by two methods: maximum and average evaluations. Maximum evaluation was obtained by the IMT value at a maximal point of the region. Mean IMT is the average value of 250 computer-based points in the region. Mean values of the right and left maximum IMT and mean IMT were used for statistical analysis. The intra-observer coefficient of variation (CV) for IMT measurements was 5.6±0.8% and inter-observer CV ranged from 2.5% to 10.9% with an average of 5.9% [12].
Statistical analysis
Data were presented as mean ± SD unless otherwise stated. Differences between two groups were analyzed by t-test and frequencies of conditions by Chi-square tests. Correlations of carotid IMT and SD-LDLC were evaluated by Pearson correlation analyses. Stepwise multiple linear regression analyses were performed to further identify the most significant variables contributing to carotid IMT and SD-LDLC. Potential confounders were forced into the model and standardized β coefficients were calculated. The explanatory power of the model was expressed as adjusted R2 values. A two-tailed P < 0.05 was considered statistically significant. All calculations were performed with SPSS system 15.0 (SPSS Inc., Chicago, IL, USA).
Results
As previously reported [7], patients studied had relatively good glycemic, lipid and BP control (Table 1). The mean and SD of LDLC were 112 ± 22 and 15 ± 10 mg/dL, respectively, and 70 patients (43.2%) were on hypolipidemic drugs (59 on statins, eight on fibrates and three on both). Maximum and mean IMT averaged 1.04 ± 0.30 and 0.83 ± 0.18 mm, respectively.
Table 1. Characteristics of 162 Patients With Type 2 Diabetes and Correlation Coefficients of Maximum Carotid IMT (Age- and Sex-Adjusted) and SD-LDLC.
| Overall (n = 162) | Correlation coefficients |
|||
|---|---|---|---|---|
| Maximum IMT | SD-LDLC | |||
| Male sex (n, %) | 89 (54.9) | -0.17* | Adjusted | 0.159* |
| Age (years) | 62 ± 10 | 0.29*** | Adjusted | -0.14 |
| Smokers (n, %) | 53 (32.7) | 0.06 | -0.01 | 0.04 |
| BMI (kg/m2) | 24.2 ± 3.7 | -0.02 | 0.05 | 0.06 |
| Waist circumference (cm) | 87.0 ± 10.0 | 0.07 | 0.12 | 0.2** |
| Duration of diabetes (years) | 9.7 ± 7.2 | 0.10 | -0.01 | 0.01 |
| Treatment of diabetes | ||||
| Diet/OHA/insulin (n, %) | 53/81/28 (33/50/17) | -0.01 | -0.02 | -0.03 |
| Users of CCB/RASi/diuretics (n, %) | 56/68/7 (35/42/4) | 0.15 | 0.10 | -0.06 |
| Users of hypolipidemic drugs (n, %) | 70 (43.2) | 0.17* | 0.22** | 0.26*** |
| HbA1c (%) | 7.0 ± 0.8 | 0.13 | 0.10 | 0.01 |
| Fasting PG (mg/dL) | 125 ± 23 | 0.03 | 0.00 | 0.02 |
| Post-breakfast PG (mg/dL) | 154 ± 48 | 0.06 | 0.01 | -0.11 |
| CV-HbA1c (%) | 6.7 ± 5.7 | -0.04 | 0.01 | 0.25*** |
| Total cholesterol (mg/dL) | 188 ± 21 | 0.02 | 0.11 | 0.27*** |
| LDLC (mg/dL) | 112 ± 22 | 0.12 | 0.19* | 0.28*** |
| SD-LDLC (mg/dL) | 15 ± 10 | 0.10 | 0.21* | 1 |
| HDLC (mg/dL) | 55 ± 15 | -0.18* | -0.13 | -0.07 |
| Fasting TG (mg/dL) | 114 ± 51 | 0.07 | 0.07 | 0.21** |
| Post-breakfast TG (mg/dL) | 145 ± 62 | 0.01 | -0.05 | 0.13 |
| Serum creatinine (mg/dL) | 0.8 ± 0.2 | 0.18* | 0.03 | -0.16* |
| eGFR (mL/min/1.73 m2) | 76 ± 16 | -0.13 | 0.01 | 0.18* |
| Uric acid (mg/dL) | 5.2 ± 1.3 | 0.10 | 0.03 | -0.12 |
| Systolic BP (mm Hg) | 128 ± 12 | 0.13 | 0.09 | 0.06 |
| SD-systolic BP (mm Hg) | 8.1 ± 2.2 | 0.20* | 0.15 | -0.01 |
| Diastolic BP (mm Hg) | 72 ± 6 | 0.00 | 0.08 | 0.12 |
| Urinary ACR (mg/g) | 86 ± 327 | 0.01 | -0.01 | 0.06 |
| Log ACR | 1.3 ± 0.6 | 0.23** | 0.18* | 0.06 |
| Leucocyte count (103/μL) | 5.8 ± 1.5 | 0.05 | 0.03 | 0.14 |
| Maximum IMT (mm) | 1.04 ± 0.30 | 1 | 1 | 0.10 |
| Mean IMT (mm) | 0.83 ± 0.18 | 0.93*** | 0.92*** | 0.05 |
| ACR ≥ 30 mg/g (n, %) | 53 (32.7) | 0.20* | 0.15 | 0.034 |
| eGFR < 60 mL/min/1.73 m2) | 25 (15.4) | 0.11 | 0.03 | -0.12 |
| Chronic kidney disease (n, %) | 66 (40.7) | 0.18* | 0.10 | 0.00 |
Mean ± SD or n, %. SD-LDLC: standard deviation of low-density lipoprotein cholesterol; OHA: oral hypoglycemic agents; CCB: calcium channel blockers; RASi: renin-angiotensin system inhibitors; PG: plasma glucose; CV: coefficient of variation; eGFR: estimated glomerular filtration rate; BP: blood pressure; ACR: albumin/creatinine ratio; IMT: intima-media thickness. *P < 0.05. **P < 0.01. ***P < 0.001.
Maximum IMT was associated with male sex, age and use of lipid-lowering drugs (Table 1). It showed positive associations with chronic kidney disease and log ACR, although there was no significant association with albuminuria (ACR ≥ 30 mg/g) and reduced kidney function (eGFR < 60 mL/min/1.73 m2). Further, maximum IMT was associated positively with SD-systolic BP and red blood cell count. Although HDLC showed inverse association with maximum IMT, there was no significant association with other lipid and glycemic variables studied. After controlling for sex and age (Table 1), associations with uses of lipid-lowering drugs, log ACR and red blood cell count remained significant. Associations with LDLC and SD-LDLC, which were not significant in simple regression analyses, became significant after adjustment for sex and age. However, associations with SD-systolic BP and HDLC turned to be non-significant.
Multiple stepwise linear regression analysis (Table 2) revealed that age (standardized β, 0.355, P < 0.001), male sex (standardized β, 0.234, P = 0.002) and SD-LDLC (standardized β, 0.201, P = 0.009) emerged as independent determinants of maximum IMT. These three variables explained 15% of variability of maximum IMT. The associations were independently of mean LDLC levels, BMI, waist circumference, duration and treatment of diabetes, means and SDs of systolic BP, and glycemic and other lipid variables, and uses of hypolipidemic and anti-hypertensive medications. Results did not change when mean IMT was used as a dependent variable instead of maximum IMT (data not shown).
Table 2. Multiple Stepwise Linear Regression Analysis for Maximum Carotid Intima-Media Thickness as a Dependent Variable.
| Independent variables | Standardized β | Cumulative R2 | P values |
|---|---|---|---|
| Sex | 0.355 | 0.083 | < 0.001 |
| Age | 0.234 | 0.117 | 0.002 |
| SD-LDLC | 0.201 | 0.151 | 0.009 |
Other independent variables included mean LDLC, BMI, waist circumference, duration and treatment of diabetes, means and SDs of systolic blood pressure, glycemic and other lipid variables, and uses of hypolipidemic and anti-hypertensive medications.
In order to confirm association between maximum IMT and SD-LDLC, patients were grouped according to quartiles of SD-LDLC (Table 3). The association between the two was not linear. As shown in Figure 1, maximum carotid IMT was greater in patients with the top quartile compared to the other three quartiles combined after adjustment for age and sex. Patients with the top SD-LDLC quartile were characterized by higher prevalence of lipid-lowering medications and higher CV-HbA1c, although mean HbA1c did not differ (Table 3). They also had higher fasting and post-meal TG, whereas there was no difference in HDLC. Urinary ACR was elevated and leukocyte count tended to be elevated in patients with the top as compared to the other three quartile groups combined.
Table 3. Characteristics of Type 2 Diabetic Patients With the Highest Compared to Other Three Quartiles of SD-LDLC Combined.
| Quartiles of SD-LDLC |
P values | ||
|---|---|---|---|
| Other three (n = 122) | Highest (n = 40) | ||
| Male sex (n, %) | 69 (57) | 20 (50) | 0.47 |
| Age (years) | 63 ± 10 | 60 ± 11 | 0.15 |
| Smokers (n, %) | 39 (32) | 14 (35) | 0.75 |
| BMI (kg/m2) | 24.1 ± 3.7 | 24.6 ± 3.8 | 0.49 |
| Waist circumference (cm) | 86.2 ± 8.4 | 89.4 ± 13.6 | 0.08 |
| Duration of diabetes (years) | 9.4 ± 7.1 | 10.5 ± 7.8 | 0.40 |
| Treatment of diabetes | |||
| Diet/OHA/insulin (n, %) | 41/58/23 (34/48/19) | 12/23/5 (30/58/13) | 0.49 |
| Users of CCB/RASi/diuretics (n, %) | 46/53/6 (38/43/5) | 10/15/1 (25/38/3) | 0.32 |
| Users of hypolipidemic drugs (n, %) | 38 (31) | 23 (58) | 0.003 |
| HbA1c (%) | 7.0 ± 0.9 | 7.0 ± 0.8 | 0.94 |
| Fasting PG (mg/dL) | 125 ± 22 | 126 ± 24 | 0.79 |
| Post-breakfast PG (mg/dL) | 157 ± 49 | 142 ± 46 | 0.11 |
| CV-HbA1c (%) | 5.9 ± 5.2 | 9.1 ± 6.7 | 0.002 |
| Total cholesterol (mg/dL) | 185 ± 21 | 197 ± 20 | 0.003 |
| LDLC (mg/dL) | 109 ± 22 | 121 ± 21 | 0.002 |
| SD-LDLC (mg/dL) | 11 ± 4 | 30 ± 8 | 0.000 |
| HDLC (mg/dL) | 56 ± 16 | 54 ± 14 | 0.47 |
| Fasting TG (mg/dL) | 109 ± 49 | 132 ± 52 | 0.01 |
| Post-breakfast TG (mg/dL) | 140 ± 61 | 164 ± 65 | 0.05 |
| Serum creatinine (mg/dL) | 0.8 ± 0.2 | 0.7 ± 0.2 | 0.34 |
| eGFR (mL/min/1.73 m2) | 75 ± 16 | 79 ± 18 | 0.10 |
| Uric acid (mg/dL) | 5.2 ± 1.4 | 5.2 ± 1.0 | 0.85 |
| Systolic BP (mm Hg) | 128 ± 12 | 129 ± 11 | 0.50 |
| SD-systolic BP (mm Hg) | 10.3 ± 3.0 | 10.2 ± 3.0 | 0.78 |
| Diastolic BP (mm Hg) | 72 ± 7 | 74 ± 6 | 0.15 |
| Urinary ACR (mg/g) | 47 ± 128 | 203 ± 608 | 0.01 |
| Log ACR | 1.3 ± 0.5 | 1.4 ± 0.7 | 0.10 |
| Leucocyte count (103/μL) | 5.7 ± 1.4 | 6.2 ± 1.6 | 0.06 |
| Maximum IMT (mm) | 1.03 ± 0.27 | 1.10 ± 0.39 | 0.17 |
| Mean IMT (mm) | 0.83 ± 0.18 | 0.85 ± 0.19 | 0.49 |
| ACR ≥ 30 mg/g (n, %) | 37 (30) | 16 (40) | 0.26 |
Mean ± SD or n, %. SD-LDLC: standard deviation of low-density lipoprotein cholesterol; OHA: oral hypoglycemic agents; CCB: calcium channel blockers; RASi: renin-angiotensin system inhibitors; PG: plasma glucose; CV: coefficient of variation; eGFR: estimated glomerular filtration rate; BP: blood pressure; ACR: albumin/creatinine ratio; IMT: intima-media thickness.
Figure 1.
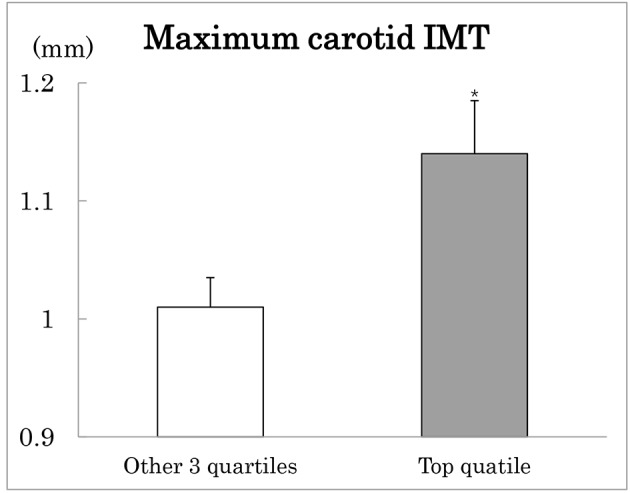
Maximum carotid intima-media thickness (IMT) in type 2 diabetes patients with the highest (black column) compared to the other three quartiles (white column) of SD-LDLC. Mean ± SE after adjustment for age and sex. *P < 0.05.
SD-LDLC was associated with mean LDLC, male sex, waist circumference and use of hypolipidemic drugs (Table 1). It showed positive associations with CV-HbA1c, fasting TG and eGFR. These seven variables were included as independent variables in multiple stepwise linear regression analysis for SD-LDLC as a dependent variable (Table 4). Mean LDLC, CV-HbA1c, uses of hypolipidemic drugs, fasting TG and eGFR emerged as independent determinants of SD-LDLC. These variables explained 21% of variability of SD-LDLC.
Table 4. Multiple Stepwise Linear Regression Analysis for SD-LDLC as a Dependent Variable.
| Independent variables | Standardized β | Cumulative R2 | P values |
|---|---|---|---|
| LDLC | 0.232 | 0.069 | 0.001 |
| CV-HbA1c | 0.189 | 0.124 | 0.011 |
| Users of hypolipidemic drugs | 0.247 | 0.180 | 0.001 |
| Fasting TG | 0.172 | 0.197 | 0.018 |
| eGFR | 0.151 | 0.213 | 0.039 |
Independent variables included waist circumference, users of hypolipidemic drugs, CV-HbA1c, mean LDLC, fasting TG, and eGFR. SD-LDLC: standard deviation of low-density lipoprotein cholesterol; OHA: oral hypoglycemic agents; CCB: calcium channel blockers; RASi: renin-angiotensin system inhibitors; PG: plasma glucose; CV: coefficient of variation; eGFR: estimated glomerular filtration rate; BP: blood pressure; ACR: albumin/creatinine ratio; IMT: intima-media thickness.
Discussion
To the best of our knowledge, this is the first report of a relationship between visit-to-visit LDLC variability and a marker of early atherosclerosis, carotid IMT. The results of this study indicate that visit-to-visit LDLC variability is a predictor of early atherosclerosis in type 2 diabetes patients who had relatively good glycemic, lipid and BP control. It was independent of mean HDLC and LDLC levels, BMI, waist circumference, duration and treatment of diabetes, means and SDs of systolic BP, glycemic and other lipid variables, and uses of statin and anti-hypertensive medications. The present study confirms independent roles of age and male sex on carotid IMT [13].
Variability in glycemia and systolic BP has been recognized as independent risk factors for macrovascular and microvascular complications in type 2 diabetes mellitus [14-18]. We have shown a direct association between CV-HbA1c variability and kidney function decline in patients with type 2 diabetes mellitus [7]. Recently, visit-to-visit variability in LDLC has been shown to be an independent predictor of adverse cardiovascular events in patients with coronary artery disease on statin treatment [5, 6]. In the present study, we measured carotid IMT, a marker of subclinical atherosclerosis, and variability in HbA1c, systolic BP and LDLC levels in type 2 diabetes patients, 43% of whom were on hypolipidemic drugs. The results of the present study indicate an independent effect of visit-to-visit variability in LDLC on subclinical atherosclerosis in patients with type 2 diabetes, population at high risk for cardiovascular disease. This was independent of variability in HbA1c and systolic BP, and achieved LDLC levels, which suggests that a more uniform and less variable visit-to-visit LDLC may be important.
As with most biological systems, serum lipoprotein levels are dynamic, reflecting a complex homeostatic integration of cholesterol synthesis, intestinal absorption, hepatic clearance, and fecal excretion [19]. The dose of statin and statin therapy itself may be associated with visit-to-visit LDLC variability in patients on statin [5, 6, 19]. In our analyses in type 2 diabetes patients, 57% of whom were not on hypolipidemic drugs, use of hypolipidemic drugs was an independent predictor of LDLC variability. Non-adherence to hypolipidemic drugs seems to be related to variability in LDLC [20], although we did not examine adherence to drugs. We speculated that patients with higher variability in LDLC are those in whom the rest of their diabetes management might be suboptimal [7]. This may be supported by independent association between visit-to-visit variations of HbA1c and LDLC in the present study.
Genetic polymorphisms in alleles that regulate the functions of LDL receptor (LDLR) and apolipoprotein E also may account for variability in LDLC [21]. Very recently, Gordts et al [22] have shown fasting TG levels to be affected through a mechanism that is dependent on LDLR and LDLR-related protein 1 (LRP1) in mice. They showed that turnover of TG-rich lipoproteins was inhibited by apolipoprotein C-III not by inhibiting lipoprotein lipase activity but by primarily through a hepatic clearance mechanism mediated by the LDLR/LRP1 axis. These findings may support independent association between fasting TG and SD-LDLC in the present study. We have no explanation for associations of SD-LDLC with eGFR.
The mechanism linking increased LDLC variability to an early subclinical atherosclerosis, carotid IMT, is unknown. As suggested by Bangalore et al [5], LDLC variability may be an epiphenomenon of other systemic conditions that increase cardiovascular risk. It is possible that patients with systemic conditions leading to generalized frailty might have higher variability of multiple biological parameters and increased risk caused by several pathologic mechanisms. In the present study, despite higher prevalence of lipid-lowering medications type 2 diabetes patients with the top SD-LDLC quartile had higher levels of LDLC, fasting and post-meal TG and visit-to-visit HbA1c variability, traditional, non-traditional and emerging cardiovascular disease risk factors, respectively. We speculate that suboptimal diabetes management might be a link between SD-LDLC and increased carotid IMT in the present study.
The strength of the current study is that we used a 1-year period when mean and SD of LDLC were calculated from six measurements in more than 90% of participants. In addition, BP control and variability and postprandial TG also have been taken into account. LDLC levels were measured in a fasting state and their variability was measured in real-world patients, not in patients from randomized trials [5, 6]. Major limitations are that study participants were small in number and from a single clinic in Japan. However, the characteristics of our study participants are similar to those reported in a previous large-scale study in Japan [23]. Another is that LDLC was calculated using fasting TG and HDLC. As in general fasting TG levels are elevated and HDLC levels are decreased in patients with type 2 diabetes, dyslipidemia may impact calculated LDLC levels. In our type 2 diabetes patients, however, effects of dyslipidemia were minimum because management of dyslipidemia was good: fasting TG and HDLC averaged 114 and 55 mg/dL, respectively.
Conclusions
Visit-to-visit LDLC variability was a predictor of early atherosclerosis in type 2 diabetes patients who had relatively good glycemic, lipid and BP control. It was independent of mean HDLC and LDLC levels and known cardiovascular risk factors. Although yet to be confirmed in future studies, our results are important, given the increased variability in LDLC in recent clinical trials that used monoclonal antibodies to PCSK-9 with every 4 weeks dosing versus every 2 weeks dosing [24] and with intermittent statin dosing strategies [25], as suggested by Bangalore et al [5].
Acknowledgments
The authors are indebted to all the participants for their dedicated and conscientious collaboration. We also thank Mr. Ken-ichi Sakamoto, Sadamitsu Hospital, for measurement of carotid IMT, Dr. Takeo Sadamitsu, Ms. Ayako Kameyama and Ms. Atsuko Muranaka, Sadamitsu Hospital, Mayu Terazawa-Watanabe, for their help.
Conflicts of Interest
We declare that we have no conflicts of interest.
Author Contributions
AKT, AYT, KK, SK and MK have made substantial contributions to acquisition, analysis and interpretation of data. KF has been involved in drafting the manuscript. TK has been involved in revising it critically for important intellectual content, have given final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Abbreviations
- LDLC
low-density lipoprotein cholesterol
- IMT
intima-media thickness
- BP
blood pressure
- TG
triglycerides
- PG
plasma glucose
- ACR
albumin/creatinine ratio
- eGFR
estimated glomerular filtration rate
- SD
standard deviation
References
- 1.Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59(4):256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 2.Malik M, Camm AJ, Janse MJ, Julian DG, Frangin GA, Schwartz PJ. Depressed heart rate variability identifies postinfarction patients who might benefit from prophylactic treatment with amiodarone: a substudy of EMIAT (The European Myocardial Infarct Amiodarone Trial) J Am Coll Cardiol. 2000;35(5):1263–1275. doi: 10.1016/S0735-1097(00)00571-4. [DOI] [PubMed] [Google Scholar]
- 3.Rossignol P, Cridlig J, Lehert P, Kessler M, Zannad F. Visit-to-visit blood pressure variability is a strong predictor of cardiovascular events in hemodialysis: insights from FOSIDIAL. Hypertension. 2012;60(2):339–346. doi: 10.1161/HYPERTENSIONAHA.111.190397. [DOI] [PubMed] [Google Scholar]
- 4.Diaz KM, Tanner RM, Falzon L, Levitan EB, Reynolds K, Shimbo D, Muntner P. Visit-to-visit variability of blood pressure and cardiovascular disease and all-cause mortality: a systematic review and meta-analysis. Hypertension. 2014;64(5):965–982. doi: 10.1161/HYPERTENSIONAHA.114.03903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bangalore S, Breazna A, DeMicco DA, Wun CC, Messerli FH. Visit-to-visit low-density lipoprotein cholesterol variability and risk of cardiovascular outcomes: insights from the TNT trial. J Am Coll Cardiol. 2015;65(15):1539–1548. doi: 10.1016/j.jacc.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Boey E, Gay GM, Poh KK, Yeo TC, Tan HC, Lee CH. Visit-to-visit variability in LDL- and HDL-cholesterol is associated with adverse events after ST-segment elevation myocardial infarction: A 5-year follow-up study. Atherosclerosis. 2016;244:86–92. doi: 10.1016/j.atherosclerosis.2015.10.110. [DOI] [PubMed] [Google Scholar]
- 7.Takenouchi A, Tsuboi A, Terazawa-Watanabe M, Kurata M, Fukuo K, Kazumi T. Direct association of visit-to-visit HbA1c variation with annual decline in estimated glomerular filtration rate in patients with type 2 diabetes. J Diabetes Metab Disord. 2015;14:69. doi: 10.1186/s40200-015-0201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 9.(9) Microvascular complications and foot care. Diabetes Care. 2015;38(Suppl):S58–66. doi: 10.2337/dc15-S012. [DOI] [PubMed] [Google Scholar]
- 10.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K. et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT. et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 12.Yanase T, Nasu S, Mukuta Y, Shimizu Y, Nishihara T, Okabe T, Nomura M. et al. Evaluation of a new carotid intima-media thickness measurement by B-mode ultrasonography using an innovative measurement software, intimascope. Am J Hypertens. 2006;19(12):1206–1212. doi: 10.1016/j.amjhyper.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Baldassarre D, Amato M, Bondioli A, Sirtori CR, Tremoli E. Carotid artery intima-media thickness measured by ultrasonography in normal clinical practice correlates well with atherosclerosis risk factors. Stroke. 2000;31(10):2426–2430. doi: 10.1161/01.STR.31.10.2426. [DOI] [PubMed] [Google Scholar]
- 14.Okada H, Fukui M, Tanaka M, Inada S, Mineoka Y, Nakanishi N, Senmaru T. et al. Visit-to-visit variability in systolic blood pressure is correlated with diabetic nephropathy and atherosclerosis in patients with type 2 diabetes. Atherosclerosis. 2012;220(1):155–159. doi: 10.1016/j.atherosclerosis.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 15.McMullan CJ, Lambers Heerspink HJ, Parving HH, Dwyer JP, Forman JP, de Zeeuw D. Visit-to-visit variability in blood pressure and kidney and cardiovascular outcomes in patients with type 2 diabetes and nephropathy: a post hoc analysis from the RENAAL study and the Irbesartan Diabetic Nephropathy Trial. Am J Kidney Dis. 2014;64(5):714–722. doi: 10.1053/j.ajkd.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Hirakawa Y, Arima H, Zoungas S, Ninomiya T, Cooper M, Hamet P, Mancia G. et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: the ADVANCE trial. Diabetes Care. 2014;37(8):2359–2365. doi: 10.2337/dc14-0199. [DOI] [PubMed] [Google Scholar]
- 17.Hata J, Arima H, Rothwell PM, Woodward M, Zoungas S, Anderson C, Patel A. et al. Effects of visit-to-visit variability in systolic blood pressure on macrovascular and microvascular complications in patients with type 2 diabetes mellitus: the ADVANCE trial. Circulation. 2013;128(12):1325–1334. doi: 10.1161/CIRCULATIONAHA.113.002717. [DOI] [PubMed] [Google Scholar]
- 18.Okada H, Fukui M, Tanaka M, Matsumoto S, Mineoka Y, Nakanishi N, Asano M. et al. Visit-to-visit blood pressure variability is a novel risk factor for the development and progression of diabetic nephropathy in patients with type 2 diabetes. Diabetes Care. 2013;36(7):1908–1912. doi: 10.2337/dc12-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baber U, Halperin JL. Variability in low-density lipoprotein cholesterol and cardiovascular risk: should consistency be a new target? J Am Coll Cardiol. 2015;65(15):1549–1551. doi: 10.1016/j.jacc.2015.02.048. [DOI] [PubMed] [Google Scholar]
- 20.Mann DM, Glazer NL, Winter M, Paasche-Orlow MK, Muntner P, Shimbo D, Adams WG. et al. A pilot study identifying statin nonadherence with visit-to-visit variability of low-density lipoprotein cholesterol. Am J Cardiol. 2013;111(10):1437–1442. doi: 10.1016/j.amjcard.2013.01.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lussier-Cacan S, Xhignesse M, Kessling AM, Davignon J, Sing CF. Sources of variation in plasma lipid and lipoprotein traits in a sample selected for health. Am J Epidemiol. 1999;150(11):1229–1237. doi: 10.1093/oxfordjournals.aje.a009950. [DOI] [PubMed] [Google Scholar]
- 22.Gordts PL, Nock R, Son NH, Ramms B, Lew I, Gonzales JC, Thacker BE. et al. ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J Clin Invest. 2016;126(8):2855–2866. doi: 10.1172/JCI86610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sone H, Tanaka S, Iimuro S, Oida K, Yamasaki Y, Oikawa S, Ishibashi S. et al. Long-term lifestyle intervention lowers the incidence of stroke in Japanese patients with type 2 diabetes: a nationwide multicentre randomised controlled trial (the Japan Diabetes Complications Study) Diabetologia. 2010;53(3):419–428. doi: 10.1007/s00125-009-1622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F, Liu T. et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380(9858):2007–2017. doi: 10.1016/S0140-6736(12)61770-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mancini GB, Baker S, Bergeron J, Fitchett D, Frohlich J, Genest J, Gupta M. et al. Diagnosis, Prevention, and Management of Statin Adverse Effects and Intolerance: Canadian Consensus Working Group Update (2016) Can J Cardiol. 2016;32(7 Suppl):S35–65. doi: 10.1016/j.cjca.2016.01.003. [DOI] [PubMed] [Google Scholar]


