Abstract
Background and Purpose
We tested the hypothesis that posterior brain arteries differ pathologically from anterior brain arteries and that this difference varies with age.
Methods
Brain large arteries from 194 autopsied individuals (mean age 56 ± 17 years, 63% men, 25% non-white, 17% with brain infarcts) were analyzed to obtain the areas of arterial layers and lumen as well as the relative content of elastin, collagen and amyloid. Visual rating was used to determine the prevalence of atheroma, calcification, vasa vasorum, pattern of intima thickening and internal elastic lamina gaps. We used multilevel models adjusting for age, sex, ethnicity, vascular risk factors, artery type and location, and multiple comparisons.
Results
Out of 1362 large artery segments, 5% had vasa vasorum, 5% had calcifications, 15% had concentric intimal thickening, and 11% had atheromas. Posterior brain arteries had thinner walls, less elastin, and more concentric intima thickening than anterior brain arteries. Compared to anterior brain arteries, the basilar artery had higher arterial area encircled by the internal elastic lamina, while the vertebral arteries had higher prevalence of elastin loss, concentric intima thickening and non-atherosclerotic stenosis. In younger individuals, vertebral artery calcifications were more likely than calcification in anterior brain arteries, but this difference attenuated with age.
Conclusions
Posterior brain arteries differ pathologically from anterior brain arteries in the degree of wall thickening, elastin loss and concentric intimal thickening.
BACKGROUND
Intracranial large artery atherosclerosis (ILAA) is a well-known cause of stroke and may account for up to 15% of ischemic strokes.1-3 Although traditional vascular risk factors are associated with ILAA as well as with extracranial atherosclerosis, diabetes and metabolic syndrome seem to be important determinants of ILAA. 1, 4 Furthermore, differential associations with vascular risk factors and stroke mechanisms have been cited between the posterior and anterior intracranial vasculatures5, and pathological evidence exists that more advanced brain arterial aging is noted in the posterior circulation compared with the anterior circulation6. American and Asian studies have shown higher incidence of radiographic ILAA in the internal carotid and middle cerebral arteries (ICA and MCA) than in the basilar and vertebral arteries (BA and VA)5, 7. Possible explanations for the pathological differences between the anterior and posterior flow systems include differences in flow dynamics due to geometrical disparities8, divergent embryological origins9, or perhaps even differences in arterial wall nourishment as conferred by the relative paucity of vasa vasorum reported in posterior circulation arteries10, 11.
Large prospective cohort data is equivocal, however, regarding differential mechanisms of anterior and posterior circulation stroke12, 13. Additionally, focal luminal narrowing of arteries is often used as a radiographic surrogate for ILAA14-16, a method that underestimates the extent of atherosclerosis and cannot distinguish atheromas from non-atherosclerotic intima thickening seen with aging6, 16. Consequently, the study of the differences between anterior and posterior circulation radiographically- and histologically-defined intracranial atherosclerosis is problematic due to the preponderance of lumen-based studies, samples restricted to patients with stroke, and limited sample size11, 17, 18. Identifying whether the arterial remodeling responses to aging and vascular risk factors vary in the anterior versus the posterior circulation may allow individualized therapies beyond the recommended aggressive control of vascular risk factors.
In a large sample of autopsied brains with carefully characterized large brain arteries, we aim to test the hypothesis that posterior circulation arteries differ in their histopathological characteristics compared to anterior circulation arteries and that this difference varies with age.
METHODS
Specimens for this study were collected as part of the Brain Arterial Remodeling Study (BARS), a collection of large brain arteries from 336 subjects with and without HIV. We included in this analysis 194 subjects without HIV, whose brains were collected from four brain banks: The New York Psychiatric Institute/ Macedonia Tissue Collection (N=104), the Manhattan HIV Brain Bank (N=52), New York Brain Bank at Columbia University (N=25) and the Brain Endowment Bank at University of Miami (N=13). Demographics and vascular risk factor information were obtained from chart review, self-reported by subjects while alive or by family proxy report during post-autopsy interview. Each case underwent neuropathological assessment which included the identification of brain ischemic infarcts.
Five-millimeter long cross-sectional cuts were obtained from large arteries of the circle of Willis in formalin-fixed brains, including the intracranial portion of the internal carotid artery (ICA), anterior, middle and posterior cerebral arteries (ACA, MCA, PCA), vertebral and basilar arteries (VA, BA). From each segment, when available, two segments were obtained, one in the most proximal aspects of the artery and one before its bifurcation or coalescence into another arterial segment (for example, as in the VA). Each segment was labeled accordingly and embedded in paraffin. Six-micron-thick cuts were obtained from each arterial block for staining (one section per staining) with hematoxylin & eosin for structural evaluation, elastic Van Gieson (EVG), Masson's trichrome, and Congo red to approximate content of elastin, collagen and amyloid, respectively. Each slide was photographed using Olympus Soft Imaging Solutions software and microscope (Münster, Germany), at 10× magnification and resolution of 0·643 μm/pixel. Each artery was segmented into individual files. Debris artifact was corrected by systematically removing impurities from the background.16 Folding artifact was addressed by using the outer perimeter of the artery as the referent the potential arterial size.19 Shrinkage artifact was corrected by multiplying the perimeter by a factor of 1.25.20, 21
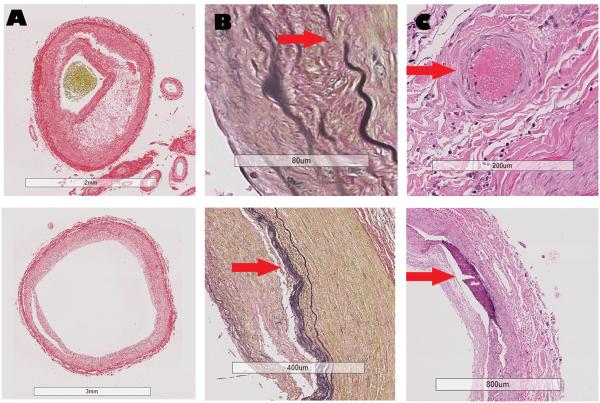
We collected 8 semi-automated and 4 visually-rated characteristics from each artery. Percentage of luminal stenosis, arterial area encircled by the internal elastic lamina (IEL proportion), and the thickness of the arterial wall and the media were derived from the areas of the lumen, intima, media and adventitia obtained through color segmentation with good to excellent reliability22. Elastin, collagen and amyloid approximate content were defined by automated pixel color intensity measurement carried out using Visiopharm Integrator System ® version 4.6.3.857 (Hoersholm, Denmark) as reported elsewhere. The pixel intensity was adjusted by background color intensity in order to be able to more fairly use the samples stained in different batches, which may introduce method-related artifact in color intensity (online figure e1). Additionally, we discarded unevenly stained slides and repeated the staining until the coloration appeared even across arteries. Large artery amyloidosis and high collagen deposition were ascertained if the background-adjusted pixel intensity fell in the upper tertile of the pixel distribution, and elastin loss was defined if the pixel adjusted intensity for EVG fell in the lowest tertile of the pixel distribution. Concentric intimal thickening, IEL gaps, presence of calcifications, and vasa vasorum were rated visually as present or not, with good reliability (Figure 1)23. The presence of atherosclerosis as well as measurements related to atheroma and fibrous cap were carried out using the revised AHA atherosclerosis classification system24.
Figure 1. Examples of the arterial characteristics evaluated in this study.
Legend: Column A, Red Sirius staining. Top: Evidence of necrotic core separated from the lumen by a thin fibrous cap in the context of eccentric intima thickening. Bottom: Concentric intima hyperplasia without evidence of cholesterol deposition. Column B, Van Gieson staining. Top: Evidence of gap in the internal elastic lamina (arrow). Bottom: Duplication of the internal elastic lamina (arrow). Column C, H&E staining. Top: evidence of vasa vasorum (arrow) in the adventitia of an artery. Bottom: Evidence of confluent arterial calcification in absence of cholesterol deposition or atheroma.
Statistical analysis
As stated above, the outcome for this study was 12 arterial characteristics and the main independent variable of interest was the posterior circulation as a whole, and also the vertebral and basilar arteries separately. We did not carry out a stratified analysis for the PCA due to the small number of available samples. We used multilevel models to adjust for the expected covariance between arteries from within the same individual. Mixed models were used for continuous variables and generalized linear models for categorical variables. We first analyzed whether the arterial characteristics varied by anterior vs. posterior. We carried out Bonferroni correction for multiple comparisons as stated in each table. To test whether the differences in pathological characteristics vary by age, we tested for interactions with age (continuously), and only if the interaction reached a significance of 0.004 or less (after adjusting for multiple comparisons), we stratified the model by age groups. Also, we were interested in determining the correlation between anterior brain arteries pathological features with posterior brain arteries. In this case, we used the same arterial characteristic noted in the anterior circulation as predictors of the same characteristics in posterior brain arteries. In each model, we adjusted by the arterial size, arterial segment (ACA, MCA, etc), and whether the given segment was proximal or distal within the artery to account for expected differences in dimensions and pathology attributable to size and location. The statistical analysis was carried out with SAS software, version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
A) Sample description
The sample mean age was 56 ± 17 years; the majority were men (63%), non-Hispanic white (75%) and smokers (52%, Table 1). The prevalence of brain infarcts was 17%, and in only a few of these cases the infarct was related to the cause of death. Of 1362 large artery segments, 5% had vasa vasorum, 5% had calcifications, 15% had concentric intimal thickening, and 19% had IEL gaps. Atherosclerosis (defined by the presence of an atheroma) was found in 11% of the arteries.
Table 1.
Characteristics of the sample studied
| Age, years (Mean ± SD, range) |
56 ± 17, 21-81 | |
| Male sex (%) | 63 | |
| Ethnicity (%) | ||
| Non-Hispanic white |
75 | |
| Black or African American |
12 | |
| Hispanic | 13 | |
| Hypertension (%) | 39 | |
| Diabetes (%) | 17 | |
| Dyslipidemia (%) | 21 | |
| Smoking (%) | 52 | |
| Prior cardiac disease (%) | 39 | |
| Evidence of brain infarct at autopsy (%) |
17 | |
Abbreviations: SD, standard deviation.
B) Posterior brain arteries characteristics
In a model adjusted for arterial location (i.e. proximal and distal), arterial size, arterial segment (i.e., MCA, ICA or ACA), and multiple comparisons, posterior brain arteries had larger IEL proportion, less elastin, and more concentric intimal thickening compared with anterior circulation arteries (table 2). Adjusting further for demographics and vascular risk factors did not change the significance of these associations. In this same fully adjusted model, both the BA and the VA had more elastin loss and concentric intimal thickening than arteries in the anterior circulation (table 2). The BA and the VA differed in other features, however, with respect to arteries in the anterior circulation. While the BA had a higher IEL proportion (indicative of arterial dilatation) than arteries in the anterior circulation (B=1.7 ± 0.5%, P<0.005), the VA had a higher degree of stenosis (B=2.5 ± 0.7 %, P<0.005) and the VA also had higher prevalence of vasa vasorum (B=2.2 ± 0.5, P<0.005) than arteries in the anterior circulation or in the basilar.
Table 2.
Posterior circulation arteries compared with anterior circulation arteries
| Posterior circulation arteries | Basilar Artery | Vertebral arteries | ||
|---|---|---|---|---|
| Model 0 | Model 1 | Model 1 | Model 1 | |
| Estimate ± SE | Estimate ± SE | Estimate ± SE | Estimate ± SE | |
| Continuous variables | ||||
| Arterial wall thickness (microns) |
−22 ± 9* | −0.27 ± 9† | −27 ± 9† | 15 ± 7* |
| Media thickness (microns) |
−5 ± 3 | −5 ± 3 | −5 ± 3 | −7 ± 3* |
| Arterial area encircled by the IEL (%) |
1.2 ± 0.4† | 1.0 ± 0.4* | 1.7 ± 0.5† | 0.1 ± 0.5 |
| Lumen stenosis (%) | 1.5 ± 0.6* | 1.0 ± 0.6 | −1.0 ± 0.8 | 2.5 ± 0.7† |
| Categorical variables | ||||
| Elastin loss |
0.7 ± 0.2† | 0.6 ± 0.2† | 0.8 ± 0.2† | 0.8 ± 0.2† |
| Increased collagen deposition |
−0.1 ± 0.2 | −0.1 ± 0.2 | −0.2 ± 0.2 | −0.1 ± 0.2 |
| Large artery amyloidosis |
−0.1 ± 0.1 | −0.1 ± 0.1 | −0.1 ± 0.1 | −0.1 ± 0.1 |
| Concentric intima thickening |
2.3 ± 0.2 † | 2.4 ± 0.3† | 2.6 ± 0.4 † | 2.8 ± 0.3 † |
| IEL gaps |
0.2 ± 0.2 | 0.1 ± 0.2 | −0.3 ± 0.2 | 0.2 ± 0.2 |
| Any calcification |
0.7 ± 0.3* | 0.6 ± 0.4 | 0.1 ± 0.4 | 1.3 ± 0.8 |
|
Vasa
vasorum |
0.7 ± 0.2 | 0.6 ± 0.2 | −0.9 ± 0.5 | 2.2 ± 0.5† |
Abbreviations: IEL, internal elastic lamina
Model 0: Adjusted for artery location (proximal versus distal), interadventitial diameter and type (anterior cerebral artery, internal carotid artery, middle cerebral artery, etc.).
Model 1: Model 0 plus age, sex, ethnicity, hypertension, diabetes, dyslipidemia, smoking, country of origin.
P value < 0.05
P value < 0.005 (Bonferroni correction 0.05/11).
We found that the differences between anterior and posterior brain arteries collagen deposition and arterial calcification differed by age (p for interaction =<0.05, supplemental table 1). For both the VA and BA, the collagen content was higher than in arteries in the anterior circulation, but in older age categories, there was less collagen deposition in arteries in posterior brain arteries compared with anterior brain arteries. We also found evidence that vertebral calcification appeared prematurely in younger individuals compared to arteries in the anterior circulation, and this difference attenuated in older age groups.
C) Atherosclerosis and posterior brain arteries
Basilar artery segments had a lower AHA atherosclerosis score (−0.35 ± 0.09, p<0.005) than anterior circulation arterial segments; this difference was not seen with VA segments or with posterior brain arteries as a whole (Table 3). More specifically, BA segments had significantly less pathological intimal thickening (B=−1.07 ± 0.49, p<0.05) and thin fibrous cap atheroma (M=−1.80 ± 0.51, p<0.005) than anterior circulation arteries. Vertebral artery segments had significantly more intimal thickening (0.87 ± 0.30, p<0.05) but were otherwise not significantly different than anterior circulation arteries.
Table 3.
Differences in atherosclerosis by anterior versus posterior brain arteries
| All posterior brain arteries |
Basilar Artery | Vertebral arteries | |
|---|---|---|---|
| Model 1 | Model 1 | Model 1 | |
| Estimate ± SE | Estimate ± SE | Estimate ± SE | |
| AHA Athero score | −0.07 ± 0.07 | −0.35 ± 0.09† | 0.11 ± 0.08 |
| Intima xanthoma | 0.22 ± 0.28 | 0.24 ± 0.45 | 0.38 ± 0.35 |
| Intima thickening | 0.37 ± 0.23 | 0.12 ± 0.34 | 0.87 ± 0.30† |
| Pathological intima thickening |
−0.15 ± 0.32 | −1.06 ± 0.49* | 0.56 ± 0.37 |
| Fibrous cap atheroma |
−0.14 ± 0.39 | −0.81 ± 0.59 | 0.32 ± 0.52 |
| Thin fibrous cap atheroma |
−0.64 ± 0.45 | −1.80 ± 0.51† | 0.32 ± 0.54 |
| Fibrocalcific plaque |
0.20 ± 1.13 | NA | 1.78 ± 1.04 |
| Percentage of plaque occupied by necrotic core |
−3.1 ± 2.5 | −5.3 ± 3.6 | −2.9 ± 3.3 |
| Fibrous cap thickness (microns) |
13.0 ± 31.0 | −11.0 ± 47.0 | −20.0 ± 43.0 |
| Area of atheroma (mm3) |
0.1 ± 0.1 | 0.2 ± 0.2 | 0.1 ± 0.2 |
Abbreviations: IEL, internal elastic lamina; NA, not available.
Model 1: artery location (proximal versus distal), arterial size, arterial type (anterior cerebral artery, internal carotid artery, middle cerebral artery, etc.), age, sex, ethnicity, hypertension, diabetes, dyslipidemia, smoking, country of origin.
P value < 0.05
P value < 0.005 (Bonferroni correction 0.05/10).
D) Association between posterior brain arteries with anterior brain arteries pathology
Posterior brain arteries media thickness (0.12 ± 0.04, p<0.003), stenosis (0.13 ± 0.04, p<0.003), elastin loss (2.20 ± 0.49, p<0.003), and large artery amyloidosis (2.36 ± 0.58, p<0.003) were significant predictors of the same features in the anterior circulation. Posterior brain arteries atherosclerosis score (0.11 ± 0.02, p<0.003) used continuously, or categorically as thin fibrous cap atheromas (2.19 ± 0.35, p<0.003) were associated with the presence of the same features in the anterior circulation, irrespective of whether the BA or VA were analyzed separately.
DISCUSSION
In our study, posterior brain arteries had greater concentric intimal thickening, more elastin loss and increase in IEL proportion, suggesting outward remodeling compared to the anterior circulation arteries. When the BA and VA were analyzed separately, the BA had greater IEL proportion, elastin loss and thinner arterial wall, all of which point towards a predisposition to dilate. This fits well with the previous notion that among all the intracranial arteries, the BA has the highest prevalence of dolichoectasia.25 The VA had higher prevalence of concentric intimal thickening, stenosis and vasa vasorum, but not of markers specific of atherosclerosis. This latter finding suggests a special susceptibility of the VA to non-atherosclerotic aging. This susceptibility is further supported by the premature VA non-atherosclerotic calcification in respect to arteries in the anterior circulation among 20-39 years old individuals. The lack of association between calcification with atherosclerosis in cerebral arteries is not novel,26 and recent data provides evidence that calcification of intracranial arteries does not predict stroke.27
Interestingly, BA proximal or distal segments did demonstrate less atherosclerosis compared to anterior circulation segments. Prior autopsy studies have suggested that the distal BA is among the most commonly affected behind only the ICA bifurcation segments28. This discrepancy in findings may be related to patient demographics, as the present study included a high proportion of Hispanic and African-American individuals (Table 1). Prior studies have shown increased incidence of strokes related to intracranial atherosclerosis in these communities1, 29, though comparisons between posterior and anterior circulation were not performed. Additionally, prior studies in Korean and Japanese communities have shown relative increase in atherosclerotic changes in the anterior compared to posterior circulation arteries, though these communities were not represented in the present study5, 7. Moreover, asymptomatic infarcts are underrepresented in the posterior circulation,30 which may suggest a lower clinical threshold for brainstem infarcts.
From the clinical and epidemiological perspectives, the results presented here should raise awareness that intracranial arterial disease in the form of atherosclerosis or aging is rarely restricted to a single artery but it rather diffuse. The best predictor of anterior circulation atherosclerosis, media thickness, stenosis, elastin loss and large artery amyloidosis are the presence of these same findings in the posterior brain arteries. This fact suggests that with aging and exposure to vascular risk factors, intracranial arteries undergo remodeling changes that are not limited to a given branch. When focal stenosis is noted in lumen-based studies, it is likely that atherosclerosis is widespread and at an advanced stage of arterial disease.16 The finding of focal stenosis fits well with the high risk of stroke recurrence among patients with high-degree stenosis in intracranial arteries,15, 31 and should alert clinicians to aggressively control vascular risk factors early on before the disease progresses to this late stage. The mechanisms favoring the phenotype of high-degree focal stenosis from a background of widespread non-stenotic atherosclerosis versus longitudinal non-focal high-degree stenosis remain unknown.
Divergent embryological origins of the posterior circulation may account for some of the differences observed in our study. The primitive posterior fossa structures are supplied almost exclusively by the fetal anterior circulation via carotid-vertebrobasilar anastomoses.9 By the 12 mm stage of growth, however, posterior fossa structures are supplied independently by the nascent BA and VA, and the antero-posterior anastomoses are typically obliterated with the exception of the posterior communicating arteries. The VA throughout its extra- and intracranial course forms from fusion of vascular cells islands, while the BA forms from fusion of the neural arteries. These different origins contrasts to the more typical angiogenesis by sprouting described in the anterior circulation.32-34 Differential flow dynamics in the posterior circulation, given its unique anatomy, may affect the distribution of blood flow, shear stress, vessel morphology and geometry of intracranial vessels that may explain partially some of the noted discrepancies compared with arteries in the anterior circulation. 35, 36 For example, the obtuse angle of confluence at the vertebrobasilar junction has been proposed as contributing to the susceptibility of the posterior circulation to long-standing stress related to flow dynamics37.
Strengths and limitations
The strengths of this study include the relatively large sample size; to date this is among the largest reported histopathological surveys of the intracranial vasculature. Additionally, the majority of the measurements of each artery were automated, which increases the reliability of our estimates. Data was also derived from a non-stroke population; hence, the data is more representative of the prevalence of pathological correlates of arterial aging and atherosclerosis in an unselected population. The prevalence of vascular risk factors in the sample population did correspond fairly to broader epidemiological estimates, but categorization of vascular risk may lead to loss of information relating to atherosclerosis.19 Hispanics and African-Americans are well-represented in our sample, which is important given the different rates of atherosclerosis in these communities. Our study also has limitations. First, Asian populations were not represented in our data set, limiting generalizability to these populations. The lack of immune-based stainings decreases the specificity of some of the measure reported here, which should be taken into account when interpreting these data. An additional limitation involves sampling of the most proximal and distal portions of the vessels, which omits the central portion of vessels, which limits the sensitivity of identifying structural vascular changes in this part of the vessel.
Future directions
We reported that the histological features of the posterior circulation differ from the anterior circulation in the degree of wall thickening, elastin content and concentric intimal thickening. Understanding the drivers of posterior circulation remodeling with immunological staining, hemodynamic measurements and genetic testing may broaden our overall understanding of brain arterial remodeling and open new ways to reduce the burden of related to intracranial arterial disease.
Supplementary Material
Table 4.
Correlation between posterior circulation pathology with anterior circulation pathology
| All posterior brain arteries |
Basilar Artery | Vertebral arteries | |
|---|---|---|---|
| Model 1 | Model 1 | Model 1 | |
| Estimate ± SE | Estimate ± SE | Estimate ± SE | |
| Continuous variables | |||
| Arterial wall thickness (in microns) |
0.09 ± 0.03* | 0.09 ± 0.03* | 0.10 ± 0.03† |
| Media thickness (in microns) |
0.12 ± 0.04† | 0.12 ± 0.04† | 0.13 ± 0.04* |
| Arterial area encircled by the IEL |
0.04 ± 0.03 | 0.05 ± 0.03 | 0.06 ± 0.04 |
| Lumen stenosis (%) | 0.13 ± 0.04† | 0.10 ± 0.05* | 0.14 ± 0.04† |
| Categorical variables | |||
| Concentric intima thickening |
0.74 ± 0.50 | 1.06 ± 0.66 | 0.54 ± 0.51 |
| Elastin loss | 2.20 ± 0.49† | 2.47 ± 0.57† | 1.91 ± 0.55† |
| Increased collagen deposition |
0.68 ± 0.29* | 0.52 ± 0.41 | 0.78 ± 0.29* |
| Large artery amyloidosis |
2.36 ± 0.58† | 2.96 ± 0.51† | 1.92 ± 0.64* |
| IEL gaps | 0.51 ± 0.29 | 0.51 ± 0.36 | 0.51 ± 0.31 |
| Any calcification | 0.48 ± 0.55 | 0.71 ± 0.73 | 0.56 ± 0.78 |
| Vasa vasorum | 0.37 ± 0.64 | −20.06 | 0.51 |
| AHA atherosclerosis score |
0.11 ± 0.02† | 0.08 ± 0.03† | 0.12 ± 0.02† |
| Thin fibrous cap atheroma |
2.19 ± 0.35† | 2.14 ± 0.46† | 2.22 ± 0.43† |
Abbreviations: IEL, internal elastic lamina;
Model 1: Adjusted for artery location (proximal versus distal), arterial size, arterial type (aca, ica, mca etc), age, sex, ethnicity, hypertension, diabetes, dyslipidemia, smoking, country of origin.
P value < 0.05
P value < 0.003 (Bonferroni correction 0.05/13).
Acknowledgments
SOURCE OF FUNDING:
-American Heart Association 13CRP14800040 (PI Jose Gutierrez)
-NIH R01MH64168 (PI Andrew Dwork)
-NIH R25MH080663 and U24MH100931 (PI Susan Morgello)
-NIH P50AG08702 (PI Scott Small)
-NIH N271201300028C (PI Deborah Mash)
Footnotes
DISCLOSURES:
Drs. Gutierrez, Goldman, Mohr, Marshall and Morgello report no disclosures
Dr. Elkind receives compensation for providing consultative services for Biogen IDEC, Biotelemetry/Cardionet, BMS-Pfizer Partnership, Boehringer-Ingelheim, Daiichi-Sankyo, and Janssen Pharmaceuticals; receives research support from diaDexus, Inc., and the NIH/NINDS; has given expert legal opinions on behalf of Merck/Organon (NuvaRing® and stroke litigation); and serves on the National, Founders Affiliate, and New York City chapter boards of the American Heart Association/American Stroke Association. He receives royalties from UpToDate for chapters related to stroke.
REFERENCES
- 1.Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The northern manhattan stroke study. Stroke. 1995;26:14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- 2.Turan TN, Makki AA, Tsappidi S, Cotsonis G, Lynn MJ, Cloft HJ, et al. Risk factors associated with severity and location of intracranial arterial stenosis. Stroke. 2010;41:1636–1640. doi: 10.1161/STROKEAHA.110.584672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bang OY, Saver JL, Liebeskind DS, Lee PH, Sheen SS, Yoon SR, et al. Age-distinct predictors of symptomatic cervicocephalic atherosclerosis. Cerebrovasc Dis. 2009;27:13–21. doi: 10.1159/000172629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Silva DA, Woon FP, Lee MP, Chen CL, Chang HM, Wong MC. Metabolic syndrome is associated with intracranial large artery disease among ethnic chinese patients with stroke. J Stroke Cerebrovasc Dis. 2009;18:424–427. doi: 10.1016/j.jstrokecerebrovasdis.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Kim JS, Nah HW, Park SM, Kim SK, Cho KH, Lee J, et al. Risk factors and stroke mechanisms in atherosclerotic stroke: Intracranial compared with extracranial and anterior compared with posterior circulation disease. Stroke. 2012;43:3313–3318. doi: 10.1161/STROKEAHA.112.658500. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez J, Honig L, Elkind MS, Mohr JP, Goldman J, Dwork AJ, et al. Brain arterial aging and its relationship to alzheimer dementia. Neurology. 2016;86:1507–1515. doi: 10.1212/WNL.0000000000002590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Resch JA, Okabe N, Loewenson R, Kimoto K, Katsuki S, Baker AB. A comparative study of cerebral atherosclerosis in a japanese and minnesota population. Journal of atherosclerosis research. 1967;7:687–693. doi: 10.1016/s0368-1319(67)80044-9. [DOI] [PubMed] [Google Scholar]
- 8.Boyajian RA, Schwend RB, Wolfe MM, Bickerton RE, Otis SM. Measurement of anterior and posterior circulation flow contributions to cerebral blood flow. An ultrasound-derived volumetric flow analysis. J Neuroimaging. 1995;5:1–3. doi: 10.1111/jon1995511. [DOI] [PubMed] [Google Scholar]
- 9.Menshawi K, Mohr JP, Gutierrez J. A functional perspective on the embryology and anatomy of the cerebral blood supply. J Stroke. 2015;17:144–158. doi: 10.5853/jos.2015.17.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aydin F. Do human intracranial arteries lack vasa vasorum? A comparative immunohistochemical study of intracranial and systemic arteries. Acta Neuropathol. 1998;96:22–28. doi: 10.1007/s004010050856. [DOI] [PubMed] [Google Scholar]
- 11.Takaba M, Endo S, Kurimoto M, Kuwayama N, Nishijima M, Takaku A. Vasa vasorum of the intracranial arteries. Acta Neurochir (Wien) 1998;140:411–416. doi: 10.1007/s007010050118. [DOI] [PubMed] [Google Scholar]
- 12.Bogousslavsky J, Van Melle G, Regli F. The lausanne stroke registry: Analysis of 1,000 consecutive patients with first stroke. Stroke. 1988;19:1083–1092. doi: 10.1161/01.str.19.9.1083. [DOI] [PubMed] [Google Scholar]
- 13.Vemmos KN, Takis CE, Georgilis K, Zakopoulos NA, Lekakis JP, Papamichael CM, et al. The athens stroke registry: Results of a five-year hospital-based study. Cerebrovasc Dis. 2000;10:133–141. doi: 10.1159/000016042. [DOI] [PubMed] [Google Scholar]
- 14.Pasterkamp G, Schoneveld AH, van Wolferen W, Hillen B, Clarijs RJ, Haudenschild CC, et al. The impact of atherosclerotic arterial remodeling on percentage of luminal stenosis varies widely within the arterial system. A postmortem study. Arterioscler Thromb Vasc Biol. 1997;17:3057–3063. doi: 10.1161/01.atv.17.11.3057. [DOI] [PubMed] [Google Scholar]
- 15.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993–1003. doi: 10.1056/NEJMoa1105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutierrez J, Elkind MS, Virmani R, Goldman J, Honig L, Morgello S, et al. A pathological perspective on the natural history of cerebral atherosclerosis. Int J Stroke. 2015;10:1074–1080. doi: 10.1111/ijs.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritz K, Denswil NP, Stam OC, van Lieshout JJ, Daemen MJ. Cause and mechanisms of intracranial atherosclerosis. Circulation. 2014;130:1407–1414. doi: 10.1161/CIRCULATIONAHA.114.011147. [DOI] [PubMed] [Google Scholar]
- 18.Mazighi M, Labreuche J, Gongora-Rivera F, Duyckaerts C, Hauw JJ, Amarenco P. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke. 2008;39:1142–1147. doi: 10.1161/STROKEAHA.107.496513. [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez J, Goldman J, Honig LS, Elkind MS, Morgello S, Marshall RS. Determinants of cerebrovascular remodeling: Do large brain arteries accommodate stenosis? Atherosclerosis. 2014;235:371–379. doi: 10.1016/j.atherosclerosis.2014.05.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loraine Lowder M, Li S, Carnell PH, Vito RP. Correction of distortion of histologic sections of arteries. J Biomech. 2007;40:445–450. doi: 10.1016/j.jbiomech.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Dobrin PB. Effect of histologic preparation on the cross-sectional area of arterial rings. J Surg Res. 1996;61:413–415. doi: 10.1006/jsre.1996.0138. [DOI] [PubMed] [Google Scholar]
- 22.Gutierrez J, Elkind MS, Petito C, Chung DY, Dwork AJ, Marshall RS. The contribution of hiv infection to intracranial arterial remodeling: A pilot study. Neuropathology. 2013;33:256–263. doi: 10.1111/j.1440-1789.2012.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutierrez J, Glenn M, Isaacson RS, Marr AD, Mash D, Petito C. Thinning of the arterial media layer as a possible preclinical stage in hiv vasculopathy: A pilot study. Stroke. 2012;43:1156–1158. doi: 10.1161/STROKEAHA.111.643387. [DOI] [PubMed] [Google Scholar]
- 24.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 25.Gutierrez J, Bagci A, Gardener H, Rundek T, Ekind MS, Alperin N, et al. Dolichoectasia diagnostic methods in a multi-ethnic, stroke-free cohort: Results from the northern manhattan study. J Neuroimaging. 2014;24:226–231. doi: 10.1111/j.1552-6569.2012.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cipolla MJ, Godfrey JA. Effect of hyperglycemia on brain penetrating arterioles and cerebral blood flow before and after ischemia/reperfusion. Translational stroke research. 2010;1:127–134. doi: 10.1007/s12975-010-0014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taoka T, Iwasaki S, Nakagawa H, Sakamoto M, Fukusumi A, Takayama K, et al. Evaluation of arteriosclerotic changes in the intracranial carotid artery using the calcium score obtained on plain cranial computed tomography scan: Correlation with angiographic changes and clinical outcome. J Comput Assist Tomogr. 2006;30:624–628. doi: 10.1097/00004728-200607000-00012. [DOI] [PubMed] [Google Scholar]
- 28.BAKER AB, IANNONE A. Cerebrovascular disease. I. The large arteries of the circle of willis. Neurology. 1959;9:321–332. doi: 10.1212/wnl.9.5.321. [DOI] [PubMed] [Google Scholar]
- 29.White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, et al. Ischemic stroke subtype incidence among whites, blacks, and hispanics: The northern manhattan study. Circulation. 2005;111:1327–1331. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- 30.Gutierrez J, Elkind MS, Cheung K, Rundek T, Sacco RL, Wright CB. Pulsatile and steady components of blood pressure and subclinical cerebrovascular disease: The northern manhattan study. J Hypertens. 2015;33:2115–2122. doi: 10.1097/HJH.0000000000000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 32.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 33.Padget DH. The circle of willis: Its embryology and anatomy. In: Dandy WE, editor. Intracranial arterial aneurysm. Comstock; New York: 1945. pp. 74–85. [Google Scholar]
- 34.PADGET DH. The development of the cranial arteries in the human embryo. Contributions to embryology. 1948;32:212. [Google Scholar]
- 35.Ravensbergen J, Ravensbergen JW, Krijger JK, Hillen B, Hoogstraten HW. Localizing role of hemodynamics in atherosclerosis in several human vertebrobasilar junction geometries. Arterioscler Thromb Vasc Biol. 1998;18:708–716. doi: 10.1161/01.atv.18.5.708. [DOI] [PubMed] [Google Scholar]
- 36.FB G. Hemodynamic theories of atherogenesis. Circ Res. 1973;33:259–266. [Google Scholar]
- 37.Ravensbergen J, Krijger JK, Hillen B, Hoogstraten HW. The influence of the angle of confluence on the flow in a vertebro-basilar junction model. J Biomech. 1996;29:281–299. doi: 10.1016/0021-9290(95)00064-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.