Abstract
Background and Purpose
Multipotent mesenchymal stromal cell (MSC) harvested exosomes are hypothesized as the major paracrine effectors of MSCs. In vitro, the miR-17-92 cluster promotes oligodendrogenesis, neurogenesis and axonal outgrowth. We therefore investigated whether the miR-17-92 cluster enriched exosomes (Exo-miR-17-92+) harvested from MSCs transfected with a miR-17-92 cluster plasmid enhance neurological recovery compared to control MSC derived exosomes (Exo-Con).
Methods
Rats subjected to 2 hours of transient middle cerebral artery occlusion (MCAO) were intravenously administered Exo-miR-17-92+, Exo-Con, or liposomes, and were sacrificed 28 days post MCAO. Histochemistry, immunohistochemistry and Golgi-Cox staining were used to assess dendritic, axonal, synaptic and myelin remodeling. Expression of phosphatase and tensin homolog (PTEN) and activation of its downstream proteins, protein kinase B (PKB or Akt), mechanistic target of rapamycin (mTOR), and glycogen synthase kinase 3 beta (GSK-3β) in the peri-infarct region were measured by means of Western blots.
Results
Compared with the liposome treatment, both exosome treatment groups exhibited significant improvement of functional recovery, but Ex-miR-17-92+ treatment had significantly more robust effects on improvement of neurological function, and enhancements of oligodendrogenesis, neurogenesis and neurite remodeling/neuronal dendrite plasticity in the ischemic boundary zone (IBZ) than the Ex-Con treatment. Moreover, Ex-miR-17-92+ treatment substantially inhibited PTEN, a validated miR-17-92 cluster target gene, and subsequently increased the phosphorylation of PTEN downstream proteins, Akt, mTOR and GSK-3β compared to Ex-Con treatment.
Conclusions
Our data suggest that treatment of stroke with tailored exosomes enriched with the miR-17-92 cluster increases neural plasticity and functional recovery after stroke, possibly via targeting PTEN to activate the PI3K/Akt/mTOR/GSK-3β signaling pathway.
Keywords: miR-17-92 cluster, exosome, stroke, neurogenesis, oligodendrogenesis, neural plasticity, functional recovery
Introduction
Multipotent mesenchymal stromal cells (MSCs) are self-renewing, multipotent progenitor cells, which robustly release exosomes1–3. MSCs improve neurological outcome after stroke3–5, and may exert their therapeutic effects through exosomes6–9. MSC harvested exosomes are involved in cell-to-cell communication, and are hypothesized as the paracrine effectors of MSCs, by encapsulating and transferring a large number of functional factors including regulatory RNAs, proteins or lipids6, 10, 11. In our previous studies, we have found that MSC generated exosomes mediate therapeutic benefits of MSCs for stroke recovery7. Intravenous administration of cell-free MSC exosomes post stroke improves functional recovery and enhances neurite remodeling, neurogenesis and angiogenesis. Thus, exosome therapy provides a novel treatment for stroke, and has the potential to replace cell-based therapy7.
The miR-17-92 cluster gene is primarily transcripted into an 800bp long polycistron and is subsequently processed into six individual miRNAs (miR-17, miR-18a, miR-19a, miR-19b, miR-20a and miR-92a)12. The miR-17-92 cluster is broadly expressed at every stage of development, with the mature miRNAs detectable in almost all tissues at variable levels13, 14. Our previous studies showed the miR-17-92 cluster plays an important role in mediating neural progenitor cell function, by increasing cell proliferation and inhibiting cell death15. Axonal alteration of the miR-17-92 cluster expression contributes to axonal outgrowth of embryonic cortical neurons by locally modulating PTEN protein levels16. In vitro, the tailored MSC exosomes containing elevated miR-17-92 cluster further enhance axonal growth compared to control MSC exosomes17. Collectively, these studies suggest that tailored MSC exosomes carrying the elevated miR-17-92 cluster enhance stroke recovery.
In the present study, for the first time, we employ exosomes engineered with a specific miRNA cluster gene to treat stroke, and demonstrate that these tailored exosomes provide an increased therapeutic effect on neurological recovery compared to the functional benefits derived from treatment with naive exosomes. Intravenously (IV) administered tailored MSC exosomes containing elevated miR-17-92 cluster were administered to rats subjected to middle cerebral artery occlusion (MCAO). We investigated the effects of these exosomes on the differential fate of the neural progenitor cells, as well as on the promotion of neurite remodeling, neural plasticity, and subsequently, functional recovery post stroke. We demonstrate that MSC exosomes containing elevated miR-17-92 cluster increase neural differentiation and plasticity, and neurological recovery post stroke compared with the control MSC exosomes. The miR-17-92 cluster regulates PTEN expression and its downstream signaling pathway, which affects phosphorylation of protein kinase B (Akt), mechanistic target of rapamycin (mTOR), and glycogen synthase kinase 3 beta (GSK-3β), and may underlie the differential therapeutic benefit of miR-17-92 augmented exosomes for stroke.
Material and methods
All experimental procedures were carried out in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Henry Ford Health System. All persons who performed the experiments, collected data, and assessed outcome were blinded to the treatment allocation throughout the course of the experiments.
Tailored MSC exosomes containing elevated miR-17-92 cluster
To generate tailored MSC-exosomes containing elevated miR-17-92 cluster, a miR-17-92 cluster contained plasmid (pCAG-GFP-miR-17-92, constructed according to our published protocol16) was transfected by electroporation into primary cultured MSCs isolated from rat bone marrow, with empty pcDNA3.1 expression plasmids (GenScript, Piscataway, NJ) transfection as control. Accordingly, the exosomes generated from the cultured media of those MSCs are referred to as, miR-17-92 cluster elevated MSC exosome (Exo-miR-17-92+) and control MSC exosome (Exo-Con), respectively. See supplemental methods.
Exosome isolation and quantification
Exosome isolation from the cell cultured media was performed at 4°C via multi-step centrifugation, as previously described7, 8. See supplemental methods.
MCAO model, exosome treatment and behavioral tests
Adult male Wistar rats (weighing 270–300 g) purchased from Charles River (Wilmington, MA) were subjected to 2h MCAO, using a method of intraluminal vascular occlusion, as modified in our laboratory4, 18. Exo-miR-17-92+ or Exo-Con dissolved into 0.5 ml phosphate-buffered solution (PBS) were IV administered to the rats (100 µg total exosome protein, per rats, respectively) at 24h after induction of stroke. Rats subjected to MCAO treated with 0.5 ml PBS diluted synthetic liposomes (3×1011 particles, which is comparable to the particle number of 100 µg total protein exosomes, as determined in our laboratory.) were employed as vehicle control (n=8/group, respectively). In order to mimic the MSC exosomal lipid layer, we prepared liposomes consisting of the three primary fatty acids that we identified in MSC exosomal lipid analysis via the thin-film hydration technique (See supplemental methods)19. To label cell proliferation, rats received intraperitoneal injections of the 5-bromodeoxyuridine (BrdU, 50 mg/kg) daily starting 24h after MCAO for 14 days.
A modified neurological severity score (mNSS) and Foot-fault tests were performed by a blinded investigator before MCAO and at 1, 3, 7, 14, 21 and 28 days after MCAO, as previously described20. All rats were sacrificed at 28 days post MCAO. Randomly selected five rat brains per group were snap frozen in liquid nitrogen after saline perfusion, and the frozen coronal sections were cut (40µm thickness for molecular studies, e.g., Western blot, 8µm thickness for immunohistochemical staining), and stored in −80°C for later analysis. The remaining three rat brains per group were removed and rinsed with distilled water after saline perfusion, followed by Golgi-Cox staining.
Histochemistry and Immunohistochemistry
Bielschowsky silver combined with Luxol fast blue histochemistry staining as well as immunostaining with antibodies against the phosphorylated epitope of neurofilament heavy polypeptide (NF-H) and synaptophysin were employed, respectively. To detect neurogenesis and oligodendrogenesis in the IBZ, we double stained the specific differentiation markers of neurons (NeuN), and progenitor (NG2) and mature oligodendrocytes (MBP) with bromodeoxyuridine (BrdU). See supplemental methods.
Golgi-Cox staining
To investigate the changes of neuronal dendrites and dendritic spines in the ischemic brain after treatment, a Golgi-Cox impregnation based FD Rapid GolgiStain™ Kit (PK401, FD Neuro-Technologies, Inc., Columbia, MD 21046) was employed to stain the neurons and glia, following the manufacture`s protocol with modifications in our lab21. See supplemental methods.
Western blot assay
To detect PTEN expression and subsequent activation of PTEN downstream proteins, Akt, and mTOR, as well as inhibition of GSK-3β activity, the total protein extracted from the IBZ area of frozen brain section was used for Western blot assay following the standard protocol (Molecular Clone, Edition II). See supplemental methods.
Statistical Analysis
Data are summarized and presented using mean±standard error (SE). The differences between mean values were evaluated with the one-way Analysis of Variance (ANOVA) and Dunnett’s post-hoc test. The Global test using the Generalized Estimating Equation (GEE) was employed to evaluate the MSC exosome treatment effects influenced by miR17-92 cluster enrichment on functional recovery22. All statistical analyses were conducted using SAS software (version 9.2; SAS Institute, Cary, NC).
Results
MiR-17-92 cluster elevated MSC exosomes improve neurological outcome after stroke
We used a single harvest of miR-17-92 exosomes for the treatment of all animals. Real-time RT-PCR analysis with TaqMan miRNA assay kit revealed that levels of individual members of the miR-17-92 cluster in exosomes harvested from MSCs transfected by the miR-17-92 vector were increased compared to miRNAs in exosomes harvested from MSCs transfected by an empty vector (7.4 folds of miR-17, 1.3 folds of miR-18a, 3.6 folds of miR-19a, 4.8 folds of miR-19b, 29.2 folds of miR-20a and 3.0 folds of miR-92, respectively).
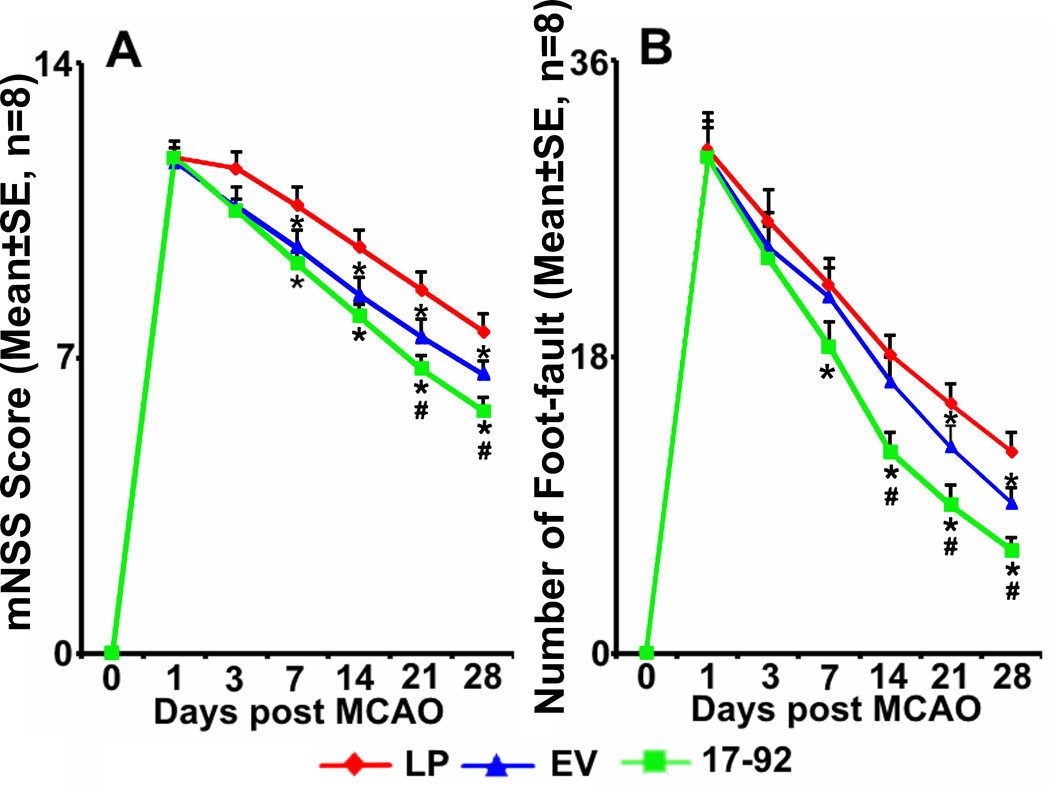
Compared with the liposome treatment, both exosome treatment groups exhibited significant reduction in neurological deficits in the mNSS test (Fig 1A) and Foot-fault test (Fig 1B). Significant improvement in neurological function was detected 7 days after MCAO in the Ex-miR-17-92+ and in the Ex-Con treatment groups on the mNSS test; however, for the foot-fault test, significant improvement was apparent 7 days and 21 days after MCAO in the Ex-miR-17-92+ and the Ex-Con treatment groups, respectively. Compared with the Ex-Con, the Ex-miR-17-92+ treatment significantly increased functional improvement, indicated by mNSS test (P<0.05 after day 21, Fig 1A) and Foot-fault test results (P<0.05 after day 14, Fig 1B).
Fig 1.
MiR-17-92 cluster elevated MSC exosomes augments the improvement of neurological outcome post MCAO. mNSS score (A) and Foot-fault test (B) data show that compared with the liposome treatment, both MSC exosome treatment groups exhibited significant improvement of functional recovery. Compared with the Ex-Con, Ex-miR-17-92+ significantly increased functional improvement. LP: MCAO rats treated with liposomes; EV: MCAO rats treated with Ex-Con; 17-92: MCAO rats treated with Ex-miR-17-92+. *P<0.05, compared to LP; # P<0.05 compared to EV, respectively. Mean±SE, n=8/group.
MiR-17-92 cluster elevated MSC exosomes increase neurite remodeling in the IBZ
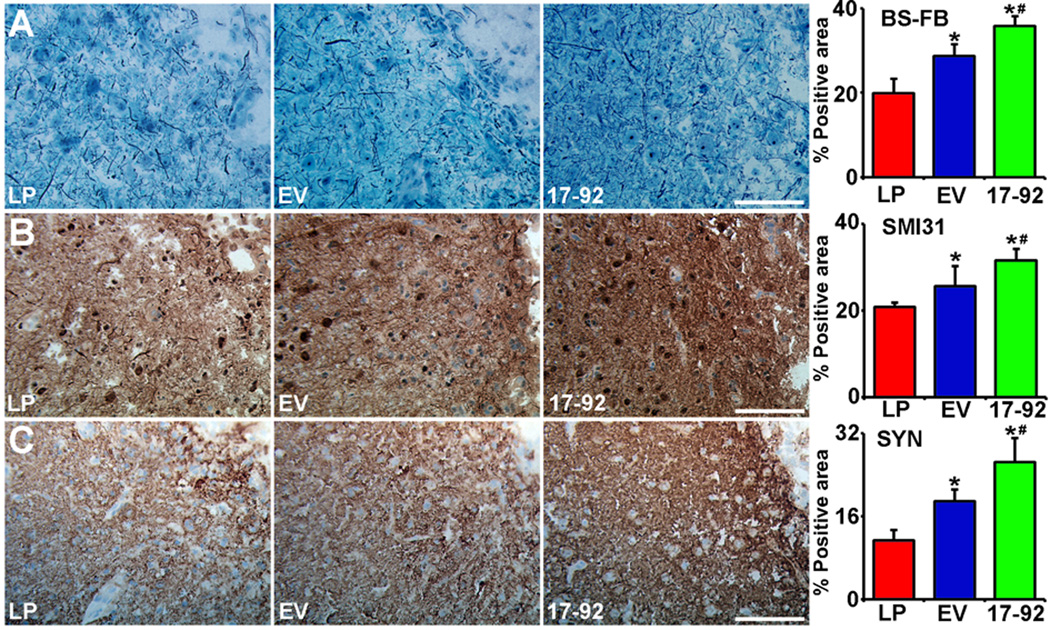
To identify the histological basis underlying the enhanced functional recovery of tailored MSC-exosomes containing elevated miR-17-92 cluster, we employed a set of methods to assess dendritic, axonal, synaptic and myelin remodeling. To evaluate the axon-myelin bundles in the white matter in the IBZ, Bielshowsky silver and Luxol fast blue were employed for detecting axons and myelin, respectively. Our data show that the exosome treatment significantly increased axon-myelin bundle density along the IBZ compared with the liposome control 28 days after MCAO (Fig 2, row A, P<0.05, respectively), whereas Ex-miR-17-92+ further increased the axonal density compared with the Ex-Con (Fig 2, row A, P<0.05).
Fig 2.
MiR-17-92 cluster elevated MSC exosomes increase neurite remodeling in the IBZ. Bielshowsky silver and Luxol fast blue double-staining (A row), SMI-31 immunostaining (B row) and synaptophysin immunostaining (C row) show that based on the increase of neurite remodeling and synaptic plasticity in the IBZ by the Ex-Con treatment, the Ex-miR-17-92+ treatment further increased neurite remodeling and synaptic plasticity in the IBZ. LP: MCAO rats treated with liposome; EV: MCAO rats treated with Ex-Con; 17-92: MCAO rats treated with Ex-miR-17-92+. *P<0.05, compared to LP; # P<0.05 compared to EV, respectively. Mean±SE, n=5/group. Scale bar=50 µm.
Immunohistochemistry staining with anti-phosphorylated NF-H (p-NF-H) antibody identifies the accumulation of p-NF-H in axons and dendrites after stroke and reflects the axonal plasticity in the peri-infarct region. Our data show that the exosome treatment significantly increased the p-NF-H immunoreactive area in the IBZ compared with the liposome treatment 28 days after MCAO (Fig 2, row B, P<0.05, respectively), and similarly, Ex-miR-17-92+ further increased the p-NF-H immunoreactivity compared with the Ex-Con (Fig 2, row B, P<0.05).
To measure synaptic plasticity, synaptophysin was stained by immunohistochemistry. Immunostaining data show that compared with the liposome treatment group, the MSC exosome treatment significantly increased synaptophysin immunoreactivity in the IBZ 28 days after MCAO (Fig 2, row C, P<0.05, respectively), while Ex-miR-17-92+ further increased the synaptophysin immunoreactivity compared with the Ex-Con (Fig 2, row C, P<0.05)..
MiR-17-92 cluster elevated MSC exosomes increase neuronal dendritic plasticity
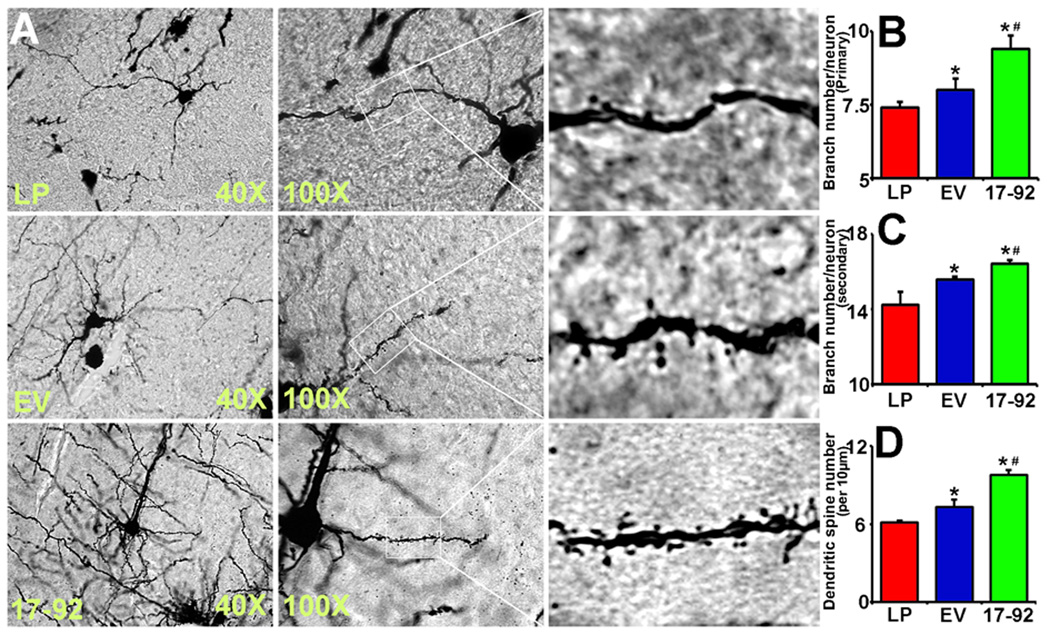
Dendrites and dendritic spines provide support for axonal outgrowth23, and axonal outgrowth accompanied with dendritic plasticity is present in the cortical peri-infarct area after experimental stroke21. We analyzed dendritic plasticity by means of Golgi silver impregnation and found that compared with liposome treatment, the exosome treatment significantly increased primary and secondary neurite branching as well as spine density, and Ex-miR-17-92+ further increased dendritic plasticity compared with the Ex-Con (Fig 3B, 3C and 3D, P<0.05, respectively).
Fig 3.
MiR-17-92 cluster elevated MSC exosomes increase neuronal dendritic plasticity. Panel A are representative optical microscopy images show the morphology of Golgi silver impregnation stained neurons and their dendrites (individual row represents for LP, EV and 17-92 treatment, respectively). The primary (panel B) and secondary (panel C) neurite branching as well as the spine density (panel D) were significantly increased after exosome treatment, and Ex-miR-17-92+ further increased dendritic plasticity compared with the Ex-Con (P<0.05, respectively). LP: MCAO rats treated with liposome; EV: MCAO rats treated with Ex-Con; 17-92: MCAO rats treated with Ex-miR-17-92+. *P<0.05, compared to LP. #P<0.05, compared to EV. Mean±SE, n=3/group.
MiR-17-92 cluster elevated MSC exosomes increase neurogenesis and oligodendrogenesis in the IBZ
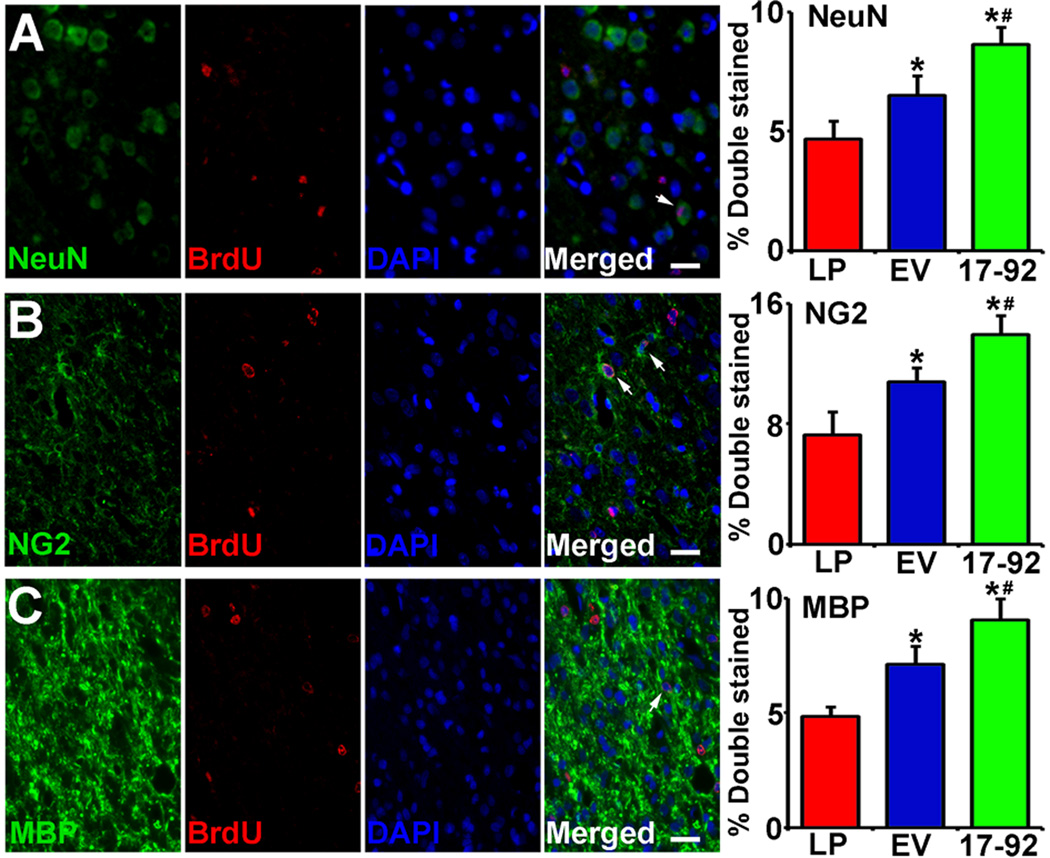
The miR-17-92 cluster increases neural progenitor cell proliferation and reduces neural progenitor cell death15, and regulates oligodendrogenesis during development24. Our present data show that newly generated neurons (identified by cells with BrdU and NeuN positive staining), oligodendrocyte progenitor cells (identified by cells with BrdU and NG2 positive staining) and mature oligodendrocytes (identified by cells with BrdU and MBP positive staining) were significantly increased after exosome treatment compared with liposome treatment. Treatment with Ex-miR-17-92+ significantly increased neurogenesis and oligodendrogenesis, respectively (Fig 4A, 4B and 4C, P<0.05, respectively), compared to the Ex-Con treatment.
Fig 4.
MiR-17-92 cluster elevated MSC exosomes increase neurogenesis and oligodendrogenesis in the IBZ. Representative micrographs show the double stained cells with NeuN and BrdU (A row), NG2 and BrdU (B row) or MBP and BrdU (C row). Compared with liposome treatment, exosome treatment significantly increased the percentage of BrdU-NeuN stained cells, BrdU-NG2 stained cells and BrdU-MBP stained cells in the IBZ in rats after stroke, and Ex-miR-17-92+ further increased these double stained cells compared with the Ex-Con (P<0.05, respectively). LP: MCAO rats treated with liposome; EV: MCAO rats treated with Ex-Con; 17-92: MCAO rats treated with Ex-miR-17-92+. *P<0.05, compared to LP. #P<0.05, compared to EV. Mean±SE, n=5/group. Scale bar=25 µm.
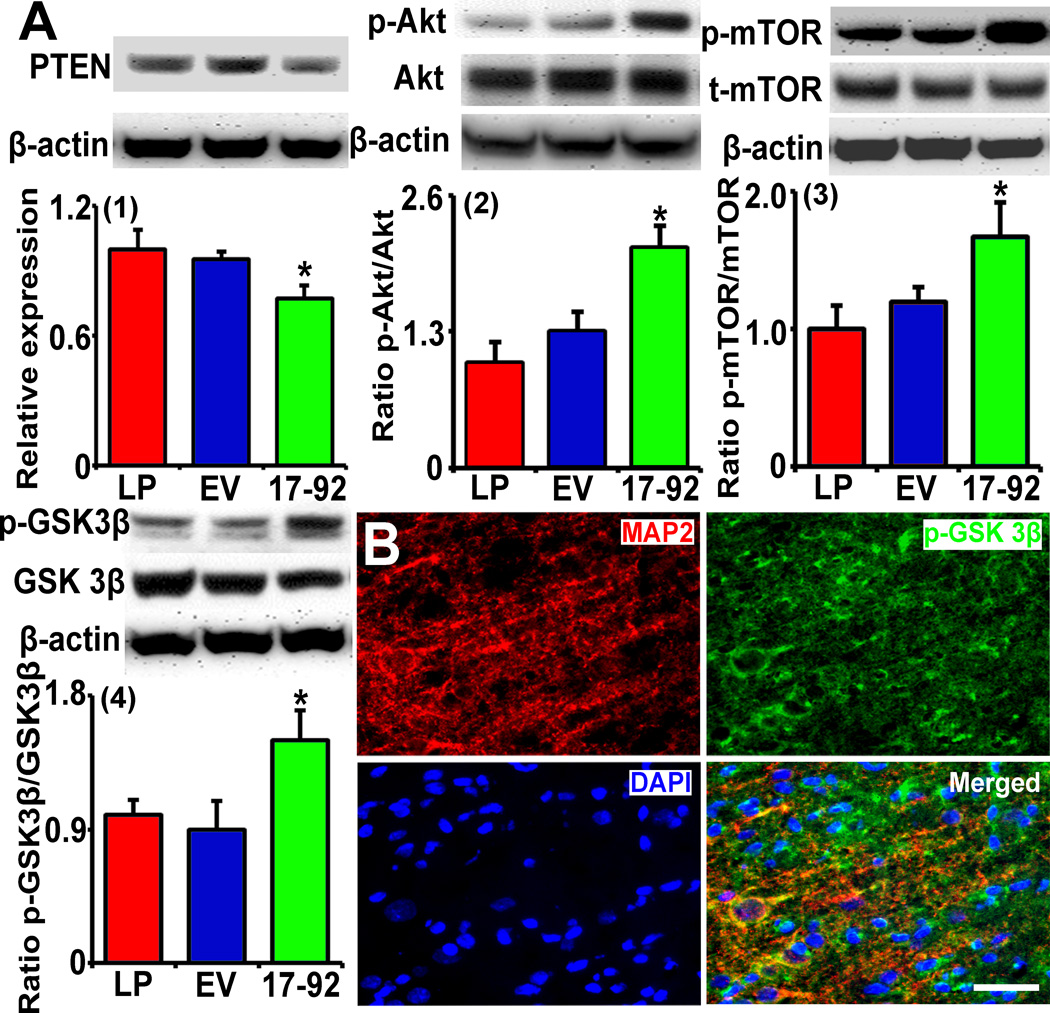
MiR-17-92 cluster elevated MSC exosomes down-regulate PTEN level and subsequently activate the PI3K/Akt/mTOR signaling pathway
PTEN is a validated target of the miR-17-92 cluster, and its downstream PI3K/Akt/mTOR signaling pathway controls neurite remodeling16, 25, cell proliferation and differentiation15, 26. By employing Western blot, we measured protein level of PTEN and the phosphorylated downstream effectors in the IBZ. The data show that compared with liposome and Ex-Con treatment, Ex-miR-17-92+ treatment significantly reduced the PTEN level (Fig 5A-1) and increased the phosphorylation of Akt (Fig 5A-2) and mTOR (Fig 5A-3), and the phosphorylation of GSK-3β (Fig 5A-4). Double immunofluorescence staining shows that p-GSK-3β was localized in the IBZ neurons (Fig 5B). Since GSK-3β inhibits axonal outgrowth, and phosphorylation of GSK-3β inactivates its function, our data suggest that, Ex-miR-17-92+ treatment targets PTEN and subsequent activation of the downstream PI3K/Akt/mTOR signaling pathway to inactivate GSK-3β in neurons.
Fig 5.
MiR-17-92 cluster elevated MSC exosomes negatively regulate the PTEN expression and subsequently activate the PI3K/Akt/mTOR signaling pathway. Panel A shows compared with liposome and Ex-Con treatment, Ex-miR-17-92+ treatment significantly downregulated the PTEN protein level in the IBZ (1) and subsequently increased the phosphorylation of Akt (2) and mTOR (3), and then increased the phosphorylation of GSK-3β (4) in the IBZ. Double immunofluorescence staining shows p-GSK-3β locates in the IBZ neurons (B). LP: MCAO rats treated with liposome; EV: MCAO rats treated with Ex-Con; 17-92: MCAO rats treated with Ex-miR-17-92+. *P<0.05, compared to EV. Mean±SE, n=3/group. Scale bar=50 µm.
Discussion
MSC exosomes are hypothesized as the major paracrine effectors of MSCs, since they are involved in cell-to-cell communication by encapsulating and transferring a large number of functional factors including regulatory RNAs, proteins or lipids6, 10, 11 to the recipient cells. In our previous studies, we found that MSC generated exosomes mediate therapeutic benefits of MSC therapy for stroke7, and the therapeutic impact of MSC exosomes may be attributed in-part to their miRNA contents6, 8. Functional benefits derived from MSC treatment of stroke may also, in-part, be attributed to the induction of the sonic hedgehog (Shh) pathway in brain parenchymal cells27, and we and others have demonstrated that Shh stimulates the miR-17-92 cluster15, 28. Based on our in vitro data that over-expression of the miR-17-92 cluster promotes axonal outgrowth of primary cortical neurons cultured in a microfluidic chamber16, and the important role of the miR-17-92 cluster in mediating neural progenitor cell function, including cell proliferation and cell death15, we selected the miR-17-92 cluster as a target for our studies of using tailored exosomes with modified miRNA content for the promotion of brain plasticity and enhancing functional recovery from stroke. Our in vitro studies then demonstrated, the tailored MSC exosomes containing elevated miR-17-92 cluster further enhances axonal growth compared to native MSC exosomes17. Here, our in vivo study demonstrates for the first time, that MSC harvested exosomes engineered to elevate the miR-17-92 cluster augment the control MSC exosome mediated improvement of neurological outcome for stroke recovery, and increase neural remodeling including neurogenesis, oligodendrogenesis and neurite plasticity. Since frozen sections of the cerebral tissue were obtained and primarily used for specific Western blot and histological analyses, H&E staining for analysis of volume of cerebral infarction was not performed in the present study. Although we and others have demonstrated that volumes of cerebral infarction are not altered by the MSC and MSC exosome treatments when treatment was initiated 24 hours post stroke7, 29, 30, we cannot exclude the possibility that treatment with miR-17-92 enriched MSC exosomes at 24 hours post stroke also evokes some neuroprotective benefits in the ischemic boundary region.
In the present study, we found that the miR-17-92 members increased in the MSC exosomes after transfection, and our previous studies have demonstrated that miRNA content in the MSC exosome transferred to recipient cells in vitro and in vivo8, 9, 17, 31. Also, we have found that a validated miR-17-92 target, PTEN, was downregulated in the brain tissue, which suggests that the miR-17-92 cluster members transferred to cerebral tissue after treatment with the miR-17-92 enriched MSC exosomes, and thereby contributed to recovery. The increase of miR-17-92 cluster level in MSCs may alter profiles of the proteins, lipids and the RNAs in the MSCs, as well as their exosome cargos. We also do not exclude the possibility that enriching the exosome miR-17-92 cluster likely alters the myriad of protein/RNA/lipid content of exosomes, and secondary changes in exosome content induced by introducing of miR-17-92 may contribute to the enhancement of plasticity and neurological recovery post stroke, possibly, via direct modification of the recipient cells or indirectly by affecting recipient cell targeting and/or uptake. However, it is evident that the enrichment of the miR17-92 cluster as a perturbation to the complex web of exosome cargo facilitates recovery, given that PTEN, a major target of the miR-17-92, is concomitantly altered with miR-17-92. The importance of our current study, is that it demonstrates, for the first time, that tailoring the exosome content with targeted miRNAs may be a viable option to promote restorative neurological processes after stroke. Investigation of the contributions of secondary changes of (possibly hundreds of) proteins, lipids and RNAs as a result of selectively enriching the miR 17-92 cluster is warranted.
Exosome therapy, by minimizing potential adverse effects of administering potentially replicating and thrombosis mediating cells32, provides a novel treatment for stroke, with the possibility to replace cell-based therapy6, 33, 34. We have previously demonstrated that exosomes harvested from cells likely provide the therapeutic effects of cell-based therapies7, 35. However, the complexities and questions associated with therapeutic action present in cell-based therapy are also evident in exosome therapy, e.g. the distribution, localization and half-life of administered exogenous exosomes, as well as questions of potential systemic and immune/inflammatory system modulating effects. Exosomes penetrate the brain blood barrier (BBB)36, 37; however, the detailed mechanisms of doing so are unclear. Given the difficulty in measurements, the cellular and organ distribution of the IV administered exosomes were not determined in the current study. It is likely, as with the use of cell-based therapies, that most of the exosomes are distributed in other organs, like the liver38, 39. However, given that the molecular target of the miR-17-92 in brain, i.e. PTEN, responds to the exosome administration, it is likely that exosomes do enter brain. We do not exclude the possibility that like cell-based therapies, exosome therapy modulates the immune/inflammatory system, which in concert with the induction of neuroplasticity or independently, contributes to the therapeutic efficacy. Investigation of the exosome contributions on systemic effects and the modulation on immune/inflammatory system by enriching the miR 17-92 cluster in exosomes is warranted. Although angiogenesis after ischemic stroke correlates with improvement of functional outcome in both animal models and in human patients with stroke40, and our previous data show that treatment with naïve MSC exosomes increases angiogenesis7; in the current study, we did not observe angiogenesis induced by miR-17-92 cluster enriched exosome. A possible explanation is that individual members of the miR-17-92 cluster are either pro- or anti-angiogenic, and the net effect of the cluster may be context dependent41, 42. Thus, with the miR-17-92 enrichment there is a balanced pro- or anti-angiogenic effect which may mitigate the net induction of angiogenesis.
Our present study only focused on the miR-17-92 cluster targeting the PTEN signaling pathway, since PTEN is a validated target of miR-17-92 cluster and the miR-17-92/PTEN axis controls neurite remodeling16, 25, cell proliferation and differentiation15, 26. Our previous in vitro data demonstrated that over-expression of the miR-17-92 cluster promoted axonal outgrowth of primary cortical neurons cultured in a microfluidic chamber, via down-regulating of PTEN and subsequent activation of the PI3K/Akt/mTOR signaling pathway16. In vivo, stroke induces limited axonal outgrowth in the peri-infarct region, which was closely associated with an increase in myelin proteins24. The miR-17-92 cluster also regulates oligodendrogenesis during development24 and promotes stroke-induced neurogenesis. The PTEN/PI3K/mTOR signaling pathway mediates axonal regeneration after spinal cord injury43. GSK-3β plays an essential role in axon regeneration44, and inactivation of GSK-3β promotes axonal growth and recovery in the CNS45. Expression of PTEN and activation of its downstream proteins, Akt, mTOR, and GSK-3β in the peri-infarct region were measured by means of Western blots and immunohistochemistry. We found that exosomes encapsulating elevated miR-17-92 cluster down-regulated PTEN, as well as activated the PTEN downstream proteins, Akt, and mTOR, and inhibited GSK-3β activity by increasing the phosphorylation of GSK-3β, the inactivated form of GSK-3β. Further studies on the quantification of mRNA levels of PTEN in neurons, and connective tissue growth factor (CTGF) and Tsp1 in astrocytes, are warranted.
Summary
We demonstrate, for the first time, that IV treatment of stroke with exosomes tailored to encapsulate an increase of a specific miRNA cluster gene, the miR-17-92 cluster, significantly increases neurogenesis, oligodendrogenesis, and neural plasticity, and significantly augments the therapeutic benefits for stroke treated with control exosomes. The molecular bases for these restorative changes, may in-part be attributed to the miR-17-92 cluster down-regulation of PTEN expression and subsequent activation of PTEN downstream proteins, Akt, and mTOR, as well as inhibition of GSK-3β activity. This study demonstrates that stroke may be treated with exosomes whose content is engineered to amplify neural plasticity and enhance functional recovery. This novel therapeutic approach may find application for other forms of neural injury or disease.
Supplementary Material
Acknowledgments
We thank Cindi Roberts, Julie Landschoot-Ward, Qing-e Lu and Sue Santra for technical assistance on histology, Xia Shang for animal care, and Talan Zhang for assistance in statistical analysis.
Funding Sources:
Research reported in this publication was supported by National Institute of Neurological Disorders and Stroke of the NIH under award number RO1 NS088656 (MC), RO1 NS081189 (HX), and RO1 NS075156 (ZGZ). The content is solely the responsibility of the authors and does not the necessarily represent the official views of the National Institutes of Health.
Footnotes
Potential Conflicts of Interest:
None
References
- 1.Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. International journal of molecular sciences. 2014;15:4142–4157. doi: 10.3390/ijms15034142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia R, Hare JM. Mesenchymal stem cells: Future source for reparative medicine. Congestive heart failure (Greenwich, Conn. 2005;11:87–91. doi: 10.1111/j.1527-5299.2005.03618.x. quiz 92–83. [DOI] [PubMed] [Google Scholar]
- 3.Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, et al. Human marrow stromal cell therapy for stroke in rat: Neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- 6.Xin H, Li Y, Chopp M. Exosomes/mirnas as mediating cell-based therapy of stroke. Front Cell Neurosci. 2014;8:377. doi: 10.3389/fncel.2014.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, et al. Exosome-mediated transfer of mir-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556–1564. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, et al. Mir-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737–2746. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transplant. 2014;23:1045–1059. doi: 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- 11.Biancone L, Bruno S, Deregibus MC, Tetta C, Camussi G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol Dial Transplant. 2012;27:3037–3042. doi: 10.1093/ndt/gfs168. [DOI] [PubMed] [Google Scholar]
- 12.Mogilyansky E, Rigoutsos I. The mir-17/92 cluster: A comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell death and differentiation. 2013;20:1603–1614. doi: 10.1038/cdd.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, et al. Targeted deletion reveals essential and overlapping functions of the mir-17 through 92 family of mirna clusters. Cell. 2008;132:875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houbaviy HB, Murray MF, Sharp PA. Embryonic stem cell-specific micrornas. Dev Cell. 2003;5:351–358. doi: 10.1016/s1534-5807(03)00227-2. [DOI] [PubMed] [Google Scholar]
- 15.Liu XS, Chopp M, Wang XL, Zhang L, Hozeska-Solgot A, Tang T, et al. Microrna-17-92 cluster mediates the proliferation and survival of neural progenitor cells after stroke. J Biol Chem. 2013;288:12478–12488. doi: 10.1074/jbc.M112.449025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Ueno Y, Liu XS, Buller B, Wang X, Chopp M, et al. The microrna-17-92 cluster enhances axonal outgrowth in embryonic cortical neurons. J Neurosci. 2013;33:6885–6894. doi: 10.1523/JNEUROSCI.5180-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Chopp M, Liu XS, Katakowski M, Wang X, Tian X, et al. Exosomes derived from mesenchymal stromal cells promote axonal growth of cortical neurons. [published online ahead of print Mar 19, 2016] [Accessed November 8, 2016];Mol Neurobiol. 2016 doi: 10.1007/s12035-016-9851-0. http://link.springer.com/article/10.1007%2Fs12035-016-9851-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Powers C, Jiang N, Chopp M. Intact, injured, necrotic and apoptotic cells after focal cerebral ischemia in the rat. J Neurol Sci. 1998;156:119–132. doi: 10.1016/s0022-510x(98)00036-7. [DOI] [PubMed] [Google Scholar]
- 19.Ekanger LA, Ali MM, Allen MJ. Oxidation-responsive eu(2+/3+)-liposomal contrast agent for dual-mode magnetic resonance imaging. Chemical communications. 2014;50:14835–14838. doi: 10.1039/c4cc07027e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, et al. Atorvastatin induction of vegf and bdnf promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueno Y, Chopp M, Zhang L, Buller B, Liu Z, Lehman NL, et al. Axonal outgrowth and dendritic plasticity in the cortical peri-infarct area after experimental stroke. Stroke. 2012;43:2221–2228. doi: 10.1161/STROKEAHA.111.646224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu M, Chen J, Lu D, Yi L, Mahmood A, Chopp M. Global test statistics for treatment effect of stroke and traumatic brain injury in rats with administration of bone marrow stromal cells. J Neurosci Methods. 2003;128:183–190. doi: 10.1016/s0165-0270(03)00188-2. [DOI] [PubMed] [Google Scholar]
- 23.Chklovskii DB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004;431:782–788. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- 24.Budde H, Schmitt S, Fitzner D, Opitz L, Salinas-Riester G, Simons M. Control of oligodendroglial cell number by the mir-17-92 cluster. Development. 2010;137:2127–2132. doi: 10.1242/dev.050633. [DOI] [PubMed] [Google Scholar]
- 25.Zou H, Ding Y, Wang K, Xiong E, Peng W, Du F, et al. Microrna-29a/pten pathway modulates neurite outgrowth in pc12 cells. Neuroscience. 2015;291:289–300. doi: 10.1016/j.neuroscience.2015.01.055. [DOI] [PubMed] [Google Scholar]
- 26.Andreas E, Hoelker M, Neuhoff C, Tholen E, Schellander K, Tesfaye D, et al. Microrna 17-92 cluster regulates proliferation and differentiation of bovine granulosa cells by targeting pten and bmpr2 genes. Cell Tissue Res. 2016;366:219–230. doi: 10.1007/s00441-016-2425-7. [DOI] [PubMed] [Google Scholar]
- 27.Ding X, Li Y, Liu Z, Zhang J, Cui Y, Chen X, et al. The sonic hedgehog pathway mediates brain plasticity and subsequent functional recovery after bone marrow stromal cell treatment of stroke in mice. J Cereb Blood Flow Metab. 2013;33:1015–1024. doi: 10.1038/jcbfm.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Northcott PA, Fernandez LA, Hagan JP, Ellison DW, Grajkowska W, Gillespie Y, et al. The mir-17/92 polycistron is up-regulated in sonic hedgehog-driven medulloblastomas and induced by n-myc in sonic hedgehog-treated cerebellar neural precursors. Cancer Res. 2009;69:3249–3255. doi: 10.1158/0008-5472.CAN-08-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steiner B, Roch M, Holtkamp N, Kurtz A. Systemically administered human bone marrow-derived mesenchymal stem home into peripheral organs but do not induce neuroprotective effects in the mcao-mouse model for cerebral ischemia. Neurosci Lett. 2012;513:25–30. doi: 10.1016/j.neulet.2012.01.078. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 31.Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, et al. Exosomes from marrow stromal cells expressing mir-146b inhibit glioma growth. Cancer Lett. 2013;335:201–204. doi: 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Han ZB, Song YP, Han ZC. Safety of mesenchymal stem cells for clinical application. Stem Cells Int. 2012;2012:652034. doi: 10.1155/2012/652034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marote A, Teixeira FG, Mendes-Pinheiro B, Salgado AJ. Mscs-derived exosomes: Cell-secreted nanovesicles with regenerative potential. Front Pharmacol. 2016;7:231. doi: 10.3389/fphar.2016.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lasser C. Exosomes in diagnostic and therapeutic applications: Biomarker, vaccine and rna interference delivery vehicle. Expert opinion on biological therapy. 2015;15:103–117. doi: 10.1517/14712598.2015.977250. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Chopp M, Zhang ZG, Katakowski M, Xin H, Qu C, et al. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2d and 3d conditions improves functional recovery in rats after traumatic brain injury. [published online ahead of print Aug 15, 2016] [Accessed November 8, 2016];Neurochem Int. 2016 doi: 10.1016/j.neuint.2016.08.003. http://www.sciencedirect.com/science/article/pii/S0197018616302510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of sirna to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 37.Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in danio rerio. Pharm Res. 2015;32:2003–2014. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 2016;7:7. doi: 10.1186/s13287-015-0271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Askar SF, Ramkisoensing AA, Atsma DE, Schalij MJ, de Vries AA, Pijnappels DA. Engraftment patterns of human adult mesenchymal stem cells expose electrotonic and paracrine proarrhythmic mechanisms in myocardial cell cultures. Circ Arrhythm Electrophysiol. 2013;6:380–391. doi: 10.1161/CIRCEP.111.000215. [DOI] [PubMed] [Google Scholar]
- 40.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- 41.Wang S, Olson EN. Angiomirs--key regulators of angiogenesis. Current opinion in genetics & development. 2009;19:205–211. doi: 10.1016/j.gde.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doebele C, Bonauer A, Fischer A, Scholz A, Reiss Y, Urbich C, et al. Members of the microrna-17-92 cluster exhibit a cell-intrinsic antiangiogenic function in endothelial cells. Blood. 2010;115:4944–4950. doi: 10.1182/blood-2010-01-264812. [DOI] [PubMed] [Google Scholar]
- 43.Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, et al. Pten deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eldar-Finkelman H, Martinez A. Gsk-3 inhibitors: Preclinical and clinical focus on cns. Front Mol Neurosci. 2011;4:32. doi: 10.3389/fnmol.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dill J, Wang H, Zhou F, Li S. Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the cns. J Neurosci. 2008;28:8914–8928. doi: 10.1523/JNEUROSCI.1178-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.