Abstract
An intracellular complement system (ICS) has recently been described in immune and nonimmune human cells. This system can be activated in a convertase-independent manner from intracellular stores of the complement component C3. The source of these stores has not been rigorously investigated. In the present study, Western blotting identified a band corresponding to C3 in freshly isolated human peripheral blood cells that was absent in corresponding cell lines. One difference between native cells and cell lines was the time absent from a fluid-phase complement source; therefore, we hypothesized that loading C3 from plasma was a route of establishing intracellular C3 stores. We found that many types of human cells specifically internalized C3(H2O), the hydrolytic product of C3, and not native C3, from the extracellular milieu. Uptake was rapid, saturable, and sensitive to competition with unlabeled C3(H2O), indicating a specific mechanism of loading. Under steady-state conditions, approximately 80% of incorporated C3(H2O) was returned to the extracellular space. These studies identify an ICS recycling pathway for C3(H2O). The loaded C3(H2O) represents a source of C3a, and its uptake altered the cytokine profile of activated CD4+ T cells. Importantly, these results indicate that the impact of soluble plasma factors should be considered when performing in vitro studies assessing cellular immune function.
Introduction
The complement system’s component C3 is the most abundant complement protein in blood and the central player in all three activation pathways (classical, alternative, and lectin). Complement engagement resulting in C3 cleavage to its biologically active fragments C3a (an inflammatory mediator) and C3b (an opsonin) is essential to innate immune protection from bacterial infections (1–3). C3 activation is traditionally thought to occur exclusively in the extracellular space.
Until the past few years, intracellular C3 activation was not considered. Several recent reports, however, have indicated a role for intracellular C3 in mediating key events for host defense and cell survival. Our group has described the presence of C3 stores and intracellular C3 activation in human CD4+ T cells (4). In that study, C3a and C3b were generated from an intracellular C3 source, leading to cellular consequences, including cell activation and lineage development. Importantly, a correlation between hyperactive intracellular C3a generation and IFN-γ production by CD4+ T cells from autoimmune arthritis patients was demonstrated, indicating that dysregulated intracellular C3 activation is likely to play an important role in disease. Additionally, a role for intracellular C3 has been highlighted recently in the literature through studies demonstrating that internalization of C3b-opsonized pathogens or cells can direct a proinflammatory immune response upon entering the intracellular milieu (5, 6). Apoptotic cells have been shown to internalize factor H (FH), which regulated endogenous intracellular C3 activation, resulting in enhanced apoptotic cell–surface opsonization. Also, the internalized FH complexed with nucleosomes and enhanced their clearance by phagocytes, thereby limiting inflammation (7).
The generation of C3a and C3b by human CD4+ T cells (8) is essential for the development of Th1 cell–mediated responses through autocrine stimulation of the C3aR and the complement membrane regulator CD46, respectively (9, 10). To further define the mechanism governing C3a and C3b generation by CD4+ T cells, Liszewski et al. (4) demonstrated that activation occurs inside the CD4+ T cell and that the processing into C3a and C3b is convertase independent, but cathepsin L (CTSL) dependent. Further, CTSL-driven C3a generation is required for CD4+ T cell survival, and shuttling of C3a to the cell surface is necessary for Th1 effector cell differentiation and proinflammatory cytokine production (4). In a follow-up to this study, the Kemper group demonstrated that intracellular C3b generation (and autocrine CD46 stimulation) is required for metabolic reprogramming that allows Th1 cell activation and contraction (11). Further, this group suggested that such reprogramming also provides for a novel “complement-metabolism-inflammasome” axis (12). Together, these studies establish an important role for intracellular C3 activation in normal CD4+ T cell immune function. Moreover, intracellular C3 stores are present in many other cell types, suggesting this phenomenon may be of broad physiologic significance (4).
Despite growing evidence indicating the importance of intracellular C3 stores in human T cell homeostasis (4, 8, 11, 12), the source and composition of these stores have not been rigorously investigated. Initially, we set out to characterize the composition of the intracellular C3 stores. Unexpectedly, we recognized a difference in the C3 species present in peripheral blood cells (PBCs) compared with cell lines by Western blotting (WB). Since a major distinction between cell lines and native cells is the time away from a source of human C3, we hypothesized that cells may “load” C3 from the surrounding milieu.
In the current study, we demonstrate that one route of establishing intracellular C3 stores is uptake from the extracellular space via plasma. We characterize the C3 uptake process and show that this is a generalized phenomenon, not being limited to a specific or few cell types. Interestingly, we establish that cells specifically load C3(H2O), not native C3. In a process known as tickover, a small amount of C3 continuously activates in the fluid phase by hydrolysis of the internal thioester bond. If it does not covalently bind to a target within microseconds, C3(H2O) is formed (13). Here, we show that, following uptake, a majority of C3(H2O) loaded is returned to the cell exterior. Additionally, the C3(H2O) remaining provides an intracellular source of C3a. These results point to a C3(H2O) recycling pathway being a major player in the intracellular complement system (ICS) that operates continuously in vivo.
Results
Human cell lines load C3 from an exogenous source.
In experiments designed to further characterize the intracellular C3 stores identified in a previous publication (4), we observed by WB that human peripheral blood B lymphocytes (B cell) (Figure 1A) contained native-like C3. In contrast, Farage, a cell line derived from a human B cell lymphoma (Figure 1A), lacked detectable native-like C3 by WB (Figure 1A). This was not pro-C3, a single chain predominantly pre-Golgi form that can be detected in cells secreting C3, because under reducing conditions, the C3 α and β chains (Figure 1A) were generated (14). This distinction between PBCs and their respective cell lines was not limited to B cells, but was true for several other PBCs tested, including CD4+ T cells versus Jurkat, a human T lymphocyte cell line (not shown). Therefore, PBCs contain stores of native-like C3 that were not detected in cultured cell lines of related cell types by WB.
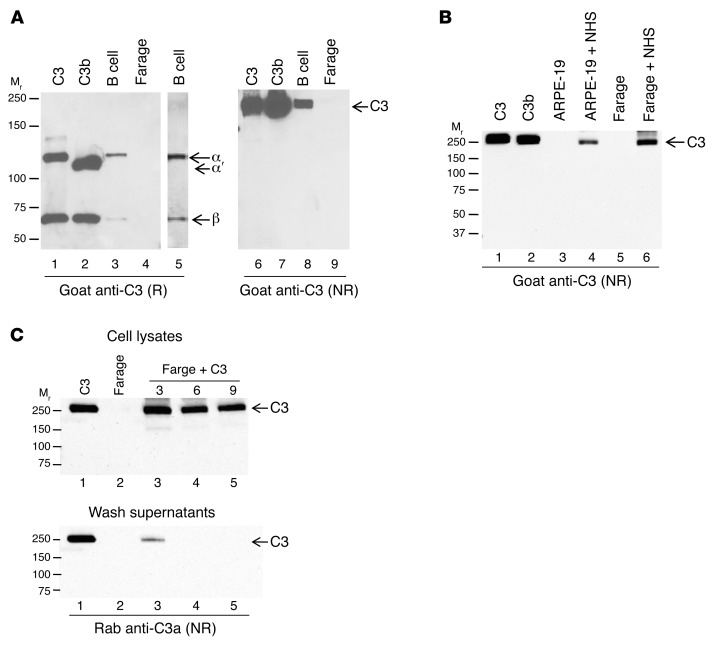
Figure 1. Human cell lines demonstrate uptake of C3 from an exogenous source.
(A) Whole cell lysates from peripheral blood B lymphocytes (B cell) and a human B cell line (Farage) were prepared and analyzed for C3 under reducing (R) and nonreducing (NR) conditions by WB. Lane 5 is a 4-fold longer exposure of lane 3. C3, 30 ng; C3b, 30 ng; B cells, 7.4 × 104 cell equivalents; Farage, 6.3 × 105 cell equivalents. (B) Two human cells lines, ARPE-19 and Farage, were incubated in 10% NHS for 30 minutes to assess whether they take up C3. C3, 30 ng; cell lysates, 2.4 × 105 cell equivalents. (C) Farage cells were incubated for 15 minutes with commercially available purified C3 and washed 3 (lane 3), 6 (lane 4), or 9 (lane 5) times prior to preparation of cell lysates. Cell lysates (top panel), final wash supernatants (bottom panel). C3, 30 ng; cell lysates, 2.4 × 105 cell equivalents; wash supernatants, 20 μl (of 100 μl). Representative blot of 4 (A and B) or 2 (C) independent experiments.
A major difference between PBCs and cultured cells is that the latter have not been exposed to a source of human C3 for many generations. We therefore hypothesized that PBCs may load C3 from blood and/or synthesize and store C3. Consequently, Farage and ARPE-19, a human retinal epithelial cell line, were incubated with 10% normal human serum (NHS) and assessed for C3 by WB. As expected, Farage and ARPE-19 cell lines grown under standard culture conditions did not contain a detectable native-like C3 band at 190 kDa (Figure 1B). However, consistent with our “loading” hypothesis, after a short exposure to 10% NHS, Farage and ARPE-19 cells demonstrated a band corresponding to C3 (Figure 1B), identical in Mr to C3 observed in primary B lymphocytes (see Figure 1A). The same results were observed whether these cell lines were exposed to plasma or whole blood (Supplemental Figure 1A; supplemental material available online with this article; https://doi.org/10.1172/JCI89412DS1).
To further assess whether C3 loads into cells, we incubated Farage cells with commercially available purified human C3. Similar to the results obtained with NHS, incubation with purified C3 demonstrated a C3-like band (Figure 1C). To check that the C3 detected is not secondary to carryover, we washed Farage cells 3, 6, or 9 times prior to preparing the lysates. The C3 did not dilute out of the lysates, even after it was undetectable in the wash supernatants (Figure 1C). Note that, as shown in Figure 1C, we employed a polyclonal Ab to C3a, which gives the same results as the goat anti-C3 pAb, i.e., the C3 in the cells contains C3a and this further establishes that it is native C3 and/or C3(H2O).
Cells specifically take up C3(H2O), not native C3.
In a review of the literature, we located one report describing intracellular uptake of C3 by the α2-macroglobulin receptor (α2MR) (15). In that article, the α2MR was reported to specifically mediate uptake of C3(H2O). Under physiologic conditions, C3 turns over in blood at about 1% to 2% per hour to generate C3 with a cleaved thioester bond (13). Almost all of this transiently active C3 does not become bound to a target and therefore is inactivated by combining with H2O (C3[H2O]).
To determine whether C3(H2O) was the form being internalized in our experiments, we compared uptake of commercially available C3 that has undergone several freeze/thaw cycles (generates more C3[H2O]) to that of C3 freshly prepared in our laboratory. We detected minimal or no uptake of “fresh” C3 by Farage cells (Figure 2A). To further support the hypothesis that C3(H2O), but not native C3, is taken up, we compared the uptake efficiency of commercially available C3 versus C3 inactivated by treatment with methylamine C3(H2O] analog, C3[MA] ref. 16). After incubation with 5 μg/ml of either C3 or C3(MA), Farage cells loaded approximately 10× more C3(MA) compared with C3, indicating that cells were loading the thioester-inactivated fraction of the C3 (Figure 2B). Since a fraction of the commercially available C3 is of the form C3(H2O), we used a lower concentration of C3 and C3(MA), as shown in Figure 2B, to ensure we would not reach saturation of loading with the C3 due to its percentage as C3(H2O). This would result in an inability to detect a difference in uptake compared with C3(MA). These data support the conclusion that C3(H2O), not native C3, is preferentially loaded into cells. There is a dramatic conformational change upon tickover of C3 to C3(H2O) (17–19). Thus, and importantly, this points to uptake being mediated by a mechanism that specifically recognizes the conformation of C3(H2O).
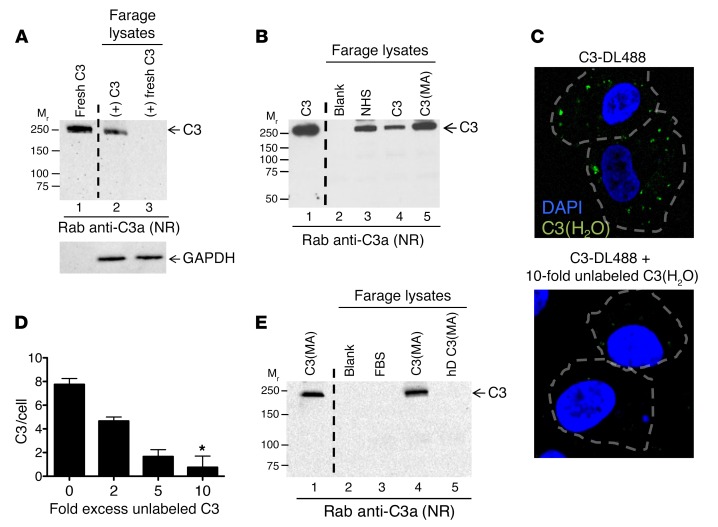
Figure 2. Loading of C3(H2O) is specific.
Uptake of C3(H2O) versus native C3 was investigated by incubating Farage cells with (A) 20 μg/ml of a commercially available C3 preparation (C3) or a freshly purified C3 preparation (fresh C3) or (B) 5 μg/ml commercially available C3 or C3(MA). Cell lysates were prepared and analyzed for uptake by WB. GAPDH, loading control. C3, 20 ng (lane 1); cell lysates, 2.4 × 105 cell equivalents. (C) Labeled C3 (C3-DL488) was utilized to visualize C3 loading into cells and determine whether uptake is sensitive to competition with unlabeled protein. ARPE-19 cells were loaded for 5 minutes without (top panel) or with (bottom panel, 10-fold) 2-, 5-, or 10-fold excess concentrations of unlabeled C3(H2O) followed by C3-DL488 (50 μg/ml) for an additional 15 minutes and visualized by confocal microscopy. Original magnification, ×60. C3-DL488 uptake per cell is quantified in part D. n = 3 representative cells per condition. Blue, DAPI; green, C3-DL488. *P < 0.05 compared with no excess of unlabeled C3(H2O) by 1-way ANOVA with Dunn’s multiple comparison test. (E) To determine whether denatured C3(MA) is internalized, Farage cells were incubated with 10 μg/ml C3(MA) or heat-denatured C3(MA) (hD-C3[MA]). C3, 30 ng (lane 1); cell lysates, 4 × 105 cell equivalents (lanes 3–5). (A, B, and E) Blots are representative of 2 independent experiments.
To further characterize the specificity of C3(H2O) uptake, intracellular C3(H2O) loading by Farage and ARPE-19 cells was visualized by incubating cells with Dylight 488–conjugated C3 (C3-DL488) and analyzed by confocal microscopy (Figure 3A). Following labeling of purified C3, C3-DL488, like C3(H2O), does not contain an intact thioester bond, as assessed by autolytic cleavage (Supplemental Figure 1B). ARPE-19 cells rapidly loaded the C3-DL488, and the uptake could be specifically inhibited in a dose-dependent manner by the addition of increasing concentrations of unlabeled C3(H2O) to the culture media (Figure 2, C and D). We then heat denatured C3(H2O), which abrogated loading, pointing out that C3(H2O) uptake requires its 3D structure (Figure 2E). We next showed that loading has species specificity in that human cell lines incubated with normal mouse serum did not take up mouse C3 (Supplemental Figure 1C). Taken with the preceding data demonstrating specificity of uptake for C3(H2O) compared with native C3, these results strongly suggest that C3(H2O) internalization occurs via a specific recognition system.
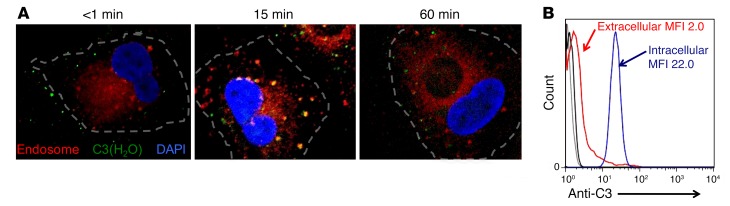
Figure 3. C3 is localized to intracellular compartments following rapid uptake.
(A) Representative confocal microscopy images showing that exogenously added C3 colocalizes to early endosomes. ARPE-19 cells were incubated with C3-DL488 (50 μg/ml, green) for 5 minutes, washed, and then incubated for an additional 1, 15, or 60 minutes in normal growth media. C3-DL488–loaded cells were washed, fixed, and permeabilized at the indicated time points and then stained for early endosomes (Rab5, red). Blue, DAPI. Representative images are from 3 independent experiments. Original magnification, ×60. (B) C3 is predominately intracellular in freshly isolated CD4+ T cells by flow cytometry. Ab, goat anti-C3. Gray and black histograms, isotype controls; red histogram, extracellular; blue histogram, intracellular. Representative of 5 independent experiments.
Intracellular location of C3(H2O).
One common mechanism for internalization of a ligand is clathrin-coated pit–mediated endocytosis. This results in localization of the cargo into early endosomes. To follow the path of internalized C3(H2O), we performed a kinetic evaluation of loading in ARPE-19 cells (Figure 3A) and CD4+ T cells (Supplemental Figure 2), utilizing C3-DL488, a marker for early endosomes (Rab5), and confocal microscopy. Immediately following exposure to C3-DL488 (<1 minute), the majority of the C3 was detected in a pattern suggesting association with the plasma membrane. Within 15 minutes after loading, the C3-DL488 colocalized to early endosomes, characteristic of clathrin-coated pit–mediated endocytosis. An hour after loading of C3-DL488, it no longer colocalized with Rab5 and a portion of the C3-DL488 was lost, consistent with our subsequent results demonstrating recycling of the loaded C3(H2O) (see Figure 5G).
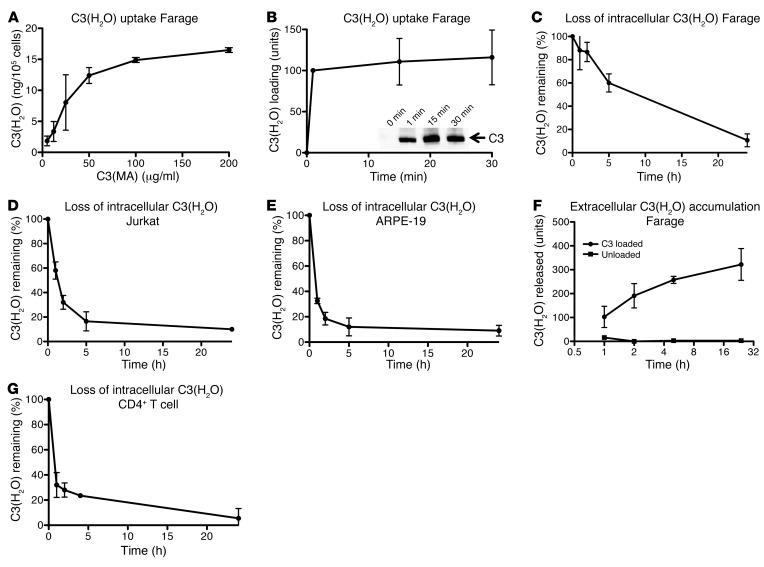
Figure 5. Characterization of C3(H2O) recycling.
(A) The amount of C3(H2O) uptake was assessed by WB and quantified by densitometry. Farage cells were incubated for 1 hour with increasing concentrations of C3(MA) in 10% C3-depleted serum. Densitometry was used to determine ng of C3(MA) taken up per 105 cell equivalents. (B) The kinetics of loading were determined by incubating Farage cells with 5 μg/ml C3(MA) for increasing lengths of time and analyzed by WB. Densitometry was used to assess the amount of C3(H2O) present in the cells at each time point. Inset is a representative WB. (C–E) The kinetics of C3(H2O) loss from established stores was assessed in Farage (C), Jurkat (D), and ARPE-19 (E) cells. Cells were incubated for 1 hour with 20 μg/ml C3, washed, and plated in the absence of C3 (normal growth media) for the indicated time points. Densitometric scanning of WB was used to determine the amount of C3(H2O) remaining in the cells compared with cells lysed immediately after loading. (F) The amount of C3(H2O) released from loaded (C3 loaded) or unloaded (unloaded) Farage cells into the supernatant was assessed by WB at the indicated time points. (G) The kinetics of C3(H2O) loss from freshly isolated CD4+ T cells that contained C3(H2O) stores loaded in vivo was assessed. The isolated CD4+ T cells were plated in complete media containing 10% FBS and 25 U/ml rhIL-2, and cell lysates were prepared at the indicated time points. Densitometric scanning was used to determine the amount of C3(H2O) remaining in the cells compared with cells lysed immediately after isolation. (A–G) Data (mean ± SD) are from 2 independent experiments.
To augment the observation that C3(H2O) was internalized, we performed colocalization studies with surface marker CD46 at 15 minutes after C3(H2O) loading. We did not detect colocalization with the plasma membrane marker CD46, confirming the intracellular location of the loaded C3(H2O) (Supplemental Figure 3). Additionally, we performed flow cytometry with a goat pAb to C3 on freshly isolated CD4+ T cells and detected intracellular C3(H2O), but minimal C3, on the plasma membrane (Figure 3B). These data further establish that cell lines can rapidly “load” C3(H2O) from an exogenous source into the cell interior and, as the C3(H2O) is transferred into the cell, it migrates in early endosomes. In addition, the detection of C3(H2O) in primary human cells, but not cultured human cell lines, indicates that in vivo cells continually take up, store, and process C3(H2O) derived from plasma and/or the interstitial milieu.
α2MR-independent uptake of C3(H2O).
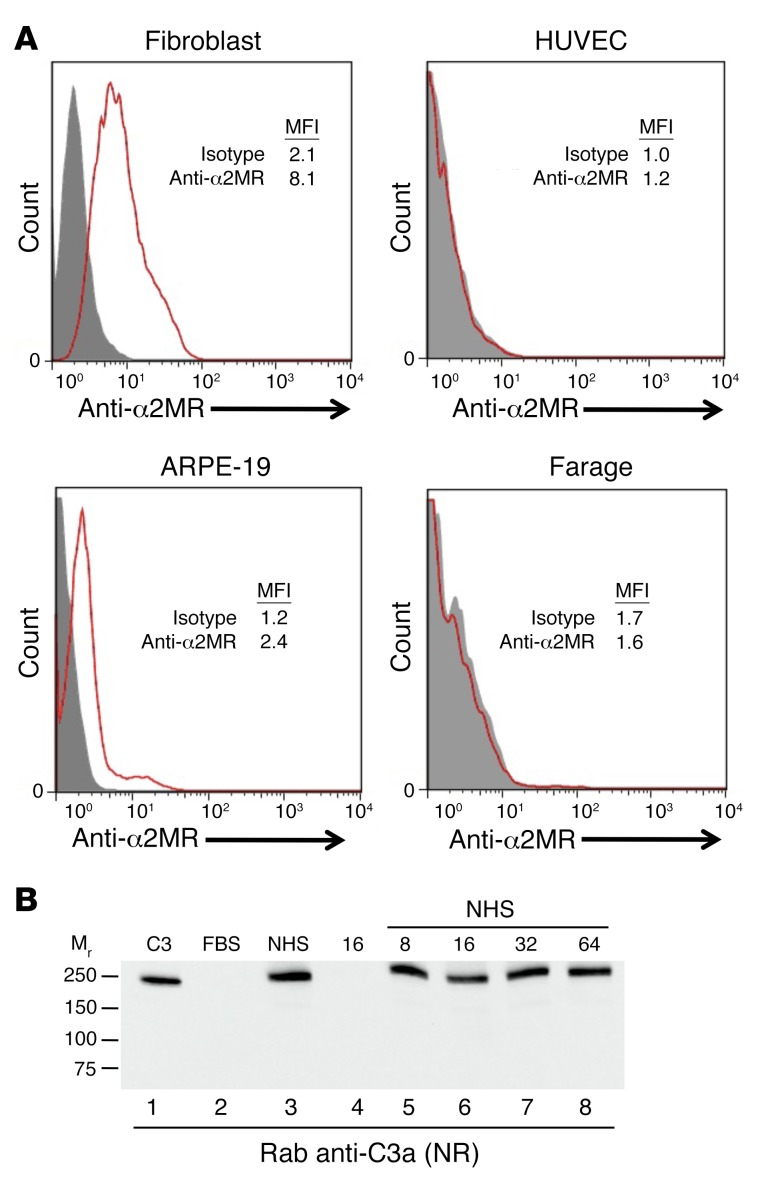
Since α2MR-mediated uptake of C3(H2O) by human fibroblasts has been previously reported (15), we evaluated its expression on the cell lines used in this study. Consistent with the literature, fibroblasts expressed the α2MR, while HUVECs did not (Figure 4A) (20). The ARPE-19 cell line expressed the α2MR at low levels (Figure 4A). Although Farage cells did not express the α2MR, they rapidly took up C3(MA), indicating that uptake is not mediated exclusively through this receptor. To characterize α2MR-mediated uptake on fibroblasts, the receptor was blocked with increasing concentrations of a mAb prior to adding C3(H2O) to the culture media. Even on α2MR-expressing fibroblasts and at all mAb concentrations used, we detected only a modest decrease (~15%) in C3(H2O) uptake (Figure 4B). Taken together, these data strongly support an α2MR-independent mechanism of uptake.
Figure 4. α2MR-independent uptake of C3.
(A) Analysis of α2MR expression on the indicated cell lines by flow cytometry. Gray-filled histogram, isotype control; red histogram, anti-α2MR. Results are representative of 2 independent experiments. (B) The α2MR was blocked on fibroblasts with the indicated concentrations of anti-α2MR mAb (μg/ml) prior to incubation with 10% NHS (lanes 5–8). C3, 10 ng (lane 1); 2.4 × 105 cell equivalents (lanes 2–8). Results are representative of 3 independent experiments.
We next sought to identify the receptor that mediates uptake. We considered several other candidate receptors and regulatory proteins, including complement receptors that bind C3b and C3(H2O). To competitively inhibit binding, we employed a strategy in which we separately preincubated cells with a mAb to each protein prior to loading with C3(H2O). We evaluated uptake in cell lysates by WB. Specifically, we utilized mAbs to inhibit binding by CD46 (also known as membrane cofactor protein [MCP]), complement receptor 1 (CR1), complement receptor 2 (CR2), and complement receptor 3 (CR3) (Supplemental Figure 4, B and C). In addition to known C3b-binding proteins, we blocked sortilin, which shares common ligands with the LDLR gene family (i.e., the α2MR) (Supplemental Figure 4D). In each case, uptake occurred comparably to that in controls. Thus, the above candidate proteins are not responsible for C3(H2O) loading.
C3(H2O) recycles to the cell exterior.
Because we noticed decreasing quantities of C3(H2O) in PBCs, apparently depending on the length of time removed from a C3 source, we were interested in assessing how long such loaded C3(H2O) stores were maintained intracellularly. To establish C3(H2O)-loading parameters, we first investigated the magnitude of uptake. After a 1-hour incubation, Farage cells were saturated with approximately 15 ng of C3(H2O) per 105 cells (Figure 5A). Next, we investigated the time course of C3(H2O) uptake. Farage cells loaded C3(H2O) rapidly, with C3(H2O) detected in cell lysates after being exposed to C3(MA) for even 1 minute (Figure 5B). To investigate the kinetics of loss, Farage cells (Figure 5C) and Jurkat cells (Figure 5E) were loaded with C3(H2O), washed, and plated onto FBS-containing culture media for the indicated time points. At each time point, the supernatants were collected, and cell lysates were prepared and then analyzed by WB with a rabbit anti-C3a pAb. Although the kinetics varied by cell type, Farage (Figure 5C), Jurkat (Figure 5D), and ARPE-19 (Figure 5E) cells all demonstrated rapid depletion of the internalized C3(H2O) stores.
We next assessed whether the C3(H2O) loss was due to degradation, release, or a combination thereof. The supernatants from the Farage cells described above were evaluated via WB. Interestingly, an increase in C3 in the supernatant of Farage cells previously loaded with C3 roughly corresponded to the loss of C3 in the cell lysates (compare Figure 5F and Figure 5C). Supernatants from Farage cells not loaded with C3 contained much smaller amounts of C3 that were minimally, if at all, altered over time (Figure 5F), therefore establishing that C3 detected in supernatants from loaded cells was not derived from biosynthesis. Based on our findings that Farage cells internalized approximately 15 ng/105 cells at saturation (Figure 5A) and secreted approximately 12 ng/ml of media/105 cells 24 hours after being removed from a C3 source, we concluded that unstimulated Farage cells secrete approximately 80% of the C3(H2O) and thus may have degraded or metabolized up to approximately 20%.
To determine whether primary cells dissipated C3 stores in a time frame similar to that of cultured cells, CD4+ T cells were analyzed by WB for C3 after their removal from peripheral blood. Due to the time required to isolate CD4+ T cells (~3 hours), C3 loss at each time point cannot be directly compared with that of Jurkat cells (Figure 5D). However, in line with what was found with the latter, CD4+ T cells contained approximately 30% of the detectable C3 by 1 hour and approximately 5% by 24 hours (Figure 5G). These data indicate that both primary cells and cultured cell lines lost their C3(H2O) stores fairly rapidly upon removal from a C3 source. This supports the concept that in vivo cells are homeostatically internalizing, processing, and secreting C3(H2O).
C3(H2O) processing by cofactor proteins.
Based upon our finding, shown in Figure 5, demonstrating that cells take up C3(H2O), releasing approximately 80% intact and retaining approximately 20%, we further explored the fate of the internalized/retained protein. Two possibilities were considered. First, the C3(H2O) could be inactivated and degraded similarly to the regulation of C3(H2O) that deposits on a cell membrane. In the latter case, inactivation (i.e., iC3[H2O]) results from cofactor activity (CA) in which cleavage is mediated by plasma factor I (FI) along with a cofactor protein (such as CD46 on the cell surface or FH from the plasma). Consistent with this possibility, we observed cleavage fragments by WB in Farage cells incubated with purified C3 in the presence of C3-depleted serum for longer time periods (Supplemental Figure 5). A second possible fate of internalized C3(H2O) would be as a source for the generation of C3a (similar to our earlier findings, ref. 4) by an intracellular protease such as CTSL.
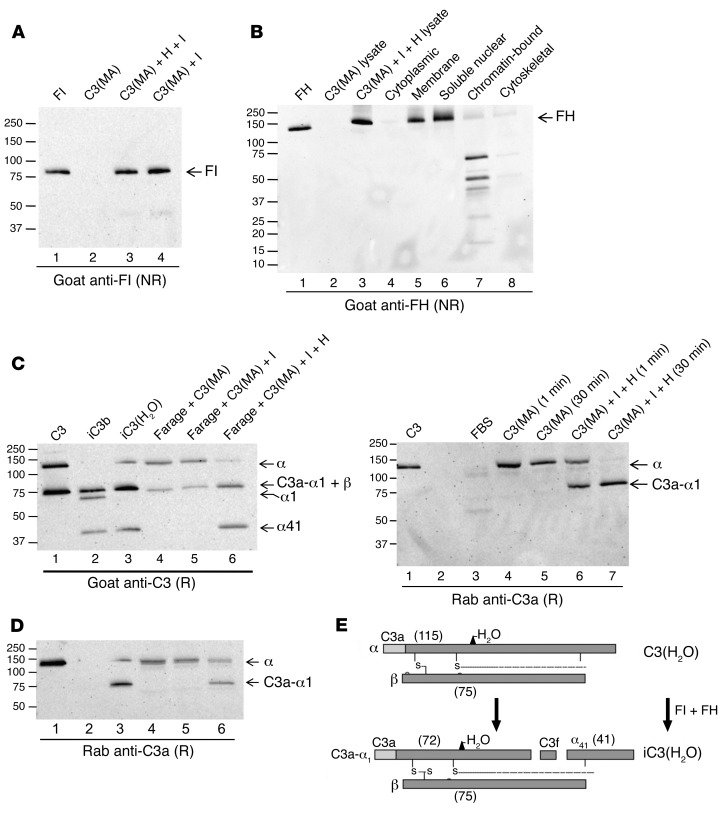
To further examine the first possibility, we determined by WB that Farage cells do not possess endogenous stores of FH or FI (not shown). Next, we considered the possibility that FH and FI also might be taken up by cells, especially in light of a recent finding that apoptotic cells take up FH (7). Thus, we incubated purified components with cells and washed and performed WBs on cell lysates. FI was taken up by Farage cells (Figure 6A) by itself and when incubated with FH. FH also was taken up (Figure 6B). Analysis of subcellular fractions, prepared from Farage cells incubated with C3(MA), FH, and FI by WB, showed FH in the membrane and soluble nuclear fractions (Figure 6B). These are FH-like proteins, FH fragments, or crossreacting bands. Notably, we identified a putative nuclear localization sequence (NLS) spanning complement control proteins CCP1 and CCP2 in FH (LRKCQKRPCGH). By FACS analysis, FH was not observed on the cell membrane (not shown). Thus, we demonstrated FH and FI in Farage cell lysates after exogenous addition to cell cultures. Our results indicate that even nonapoptotic cells can take up not only FH, but also FI.
Figure 6. C3(H2O) processing by cofactor proteins.
Whole cell lysates (A and B) and subcellular fractions (B) were prepared from Farage cells incubated with C3(MA), with or without FH and FI, and analyzed by WB with a goat anti-FI pAb (A) or a goat anti-FH pAb (B). Nonreducing conditions. FH, 20 ng; cell equivalents, 3 × 105. Representative of 2 independent experiments. (C) Farage cells were incubated for 15 minutes with 15 μg/ml C3(MA) alone or plus FI (I) (2 μg/ml), or FI and FH (H) (25 μg/ml). The resulting cell lysates were analyzed by WB with a goat anti-C3 pAb (top panel) or a rabbit anti-C3a pAb (bottom panel). Under reducing conditions. C3, iC3b and iC3(MA), 20 ng; cell lysates, 4 × 105 cell equivalents loaded. Representative of 3 independent experiments. (D) Lysates prepared from Farage cells incubated with C3(MA) alone or with FH and FI for the times indicated were analyzed for uptake and CA by WB with a rabbit anti-C3a pAb. Reducing conditions. C3, 70 ng; C3a, 30 ng; cell lysates, 4 × 105 cell equivalents loaded. Representative of 2 independent experiments. (E) Schematic depicting the cleavage and inactivation of C3(H2O) resulting from CA. These experiments were also performed with fibroblasts, and the same results were obtained.
Next, we loaded Farage cells with C3(H2O) in the presence of FH and FI and found cleavage products in lysates consistent with CA. When Farage cells were incubated with C3(MA) alone or C3(MA) with FI, we detected no fragmentation, but rather the expected intact C3 α and β chains (Figure 6C), suggesting that CD46 expressed by Farage did not serve as a cofactor for FI-mediated cleavage of loaded C3(MA). However, in the presence of loaded FI and FH, we observed C3(MA) cofactor–mediated cleavage. That is, incubation of all 3 with Farage cells resulted in the anticipated breakdown fragments, i.e., the α41 fragment and the C3a-retaining fragment that migrates at approximately 75 kDa (Figure 6C). These fragments are identical to purified iC3(H2O) (Figure 6, C and E). Since the WB employed goat Ab that does not distinguish the comigrating β chain (75 kDa) from C3a-α1 (72 kDa) (Figure 6C), we also probed the samples with rabbit anti-C3a polyclonal Ab and verified the presence of C3a-α1 (Figure 6C).
The requirement for addition of exogenous FH and FI for CA suggested that cultured Farage cells do not synthesize or contain endogenous stores of these proteins that are accessible to the loaded C3(H2O). A time course of C3(H2O) α chain degradation indicated that no cleavage of C3(MA) occurs unless FH and FI are also loaded (Figure 6D). Further, CA begins quickly (~1 minute) and is completed by 30 minutes. These studies were also performed with fibroblasts, and the same results were obtained. These data indicate that cells take up FH and FI and that these proteins then can mediate intracellular cleavage of the loaded C3(H2O).
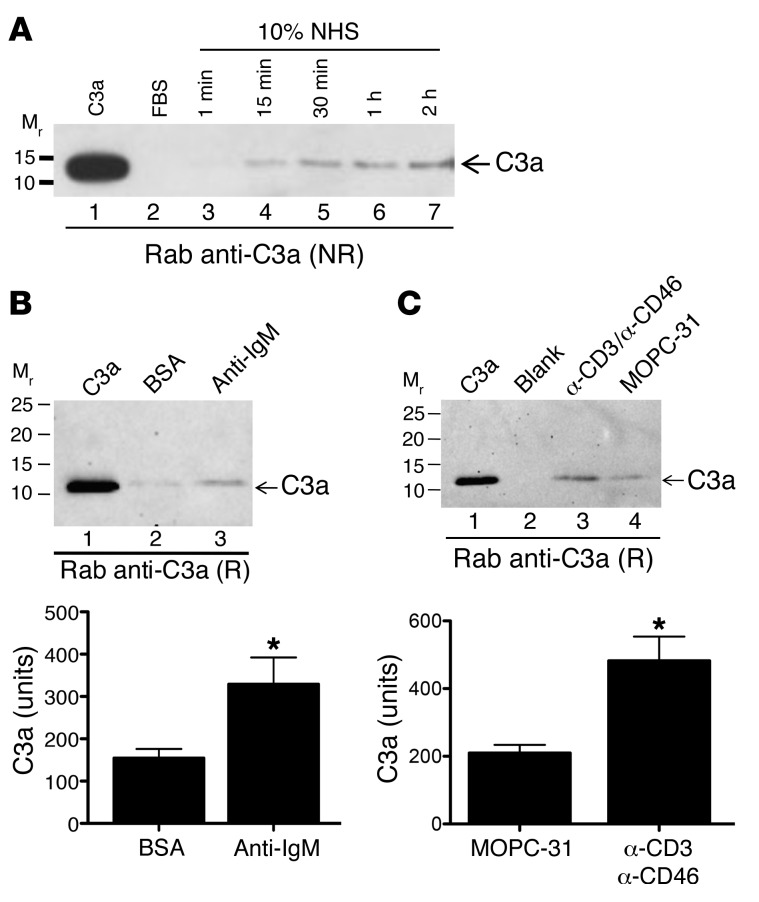
C3a is generated from loaded C3(H2O) and is augmented by cell activation.
In addition to degradation by CA, a second fate of loaded C3(H2O) is to contribute to the generation of C3a. Since previous reports described the intracellular generation of C3a (4, 11), we examined whether C3a was generated following uptake from C3(H2O). Intracellular C3a was detected in resting Farage cells by 15 minutes after exposure to NHS, and its generation progressively increased over 2 hours (Figure 7A). C3a generation from loaded C3(H2O) could be detected in multiple cell types (not shown). Although we could not detect C3a generation by WB in the absence of uptake, previous studies have detected a scant amount of C3a via flow cytometry from intracellular stores in cells not exposed to C3(H2O) (4). This indicates that there are 2 sources of intracellular C3 stores, i.e., C3a can be generated after uptake of C3(H2O) from blood or from synthetic C3 stores. In agreement with the latter, we detected C3 mRNA in a survey of cell lines (Supplemental Figure 6). In addition, C3a generation was observed following uptake of purified C3(MA), indicating that it is due to intracellular cleavage and not a result of other serum factors or convertase activity (not shown). As further evidence for the conclusion that uptake is not convertase dependent, we show uptake in the presence of EDTA (Supplemental Figure 7).
Figure 7. Intracellular C3a generation from loaded C3(H2O) is augmented following cell activation.
(A) Cell lysates from Farage cells incubated in 10% NHS for the indicated time points were analyzed for C3a generation by WB. To determine whether cell activation modulates C3a production, Farage cells (B) were incubated on anti-IgM– or BSA-coated plates or CD4+ T cells (C) were incubated on anti-CD3/CD46– or MOPC-31–coated plates for 3 hours in the presence of 10% NHS. The resulting cell lysates were assessed for C3a production by WB. Densitometric scanning of C3a production is expressed as mean ± SD in bottom panels. n = 3–4 from at least 2 independent experiments. *P < 0.05, 2-tailed Student’s t test. (A–C) Reducing conditions. C3a, 30 ng; cell lysates, 2 × 105 cell equivalents loaded.
To extend this observation and examine biological relevance, we asked whether C3a generation from loaded C3(H2O) was modulated in activated cells. Farage cells were incubated on anti-IgM–coated or BSA-coated (nonactivated control) plates for 3 hours in the presence of 10% NHS. The resulting cell lysates were analyzed for C3a generation by WB with a rabbit pAb to C3a. Compared with nonactivated controls, C3a generation significantly increased in activated Farage cells (Figure 7B). This points to a potentially important role for C3(H2O) uptake and subsequent C3a generation in B cell activation and function.
To determine whether this phenomenon is of broader significance, we investigated C3a generation by activated CD4+ T cells. CD4+ T cells were incubated for 3 hours on anti-CD3/CD46–coated or MOPC-31–coated (nonactivated control) plates in the presence of 10% NHS. C3a generation was evaluated by WB with a rabbit pAb to C3a. As with the Farage cell line, C3a generation significantly increased in activated CD4+ T cells compared with nonactivated controls (Figure 7C), and in the absence of NHS, C3a generation was not detectable by WB (not shown). Therefore, the observed increase in C3a generated under activating conditions was from loaded C3(H2O). A CTSL-dependent mechanism for generation of C3a from intracellular C3 stores in CD4+ T cells has been previously described (4), representing one mechanism for generation of C3a from loaded C3(H2O). In support of this, we have demonstrated generation of C3a from C3(MA) by CTSL in vitro (Supplemental Figure 8A). Additionally, we showed more efficient cleavage of C3(MA) compared with native C3 by CTSL in vitro (Supplemental Figure 8B). Since the protease responsible for C3a generation from C3(H2O) is likely cell type specific, we also demonstrated cleavage of C3(H2O) by elastase (Supplemental Figure 8C) and granzyme B (Supplemental Figure 8D).
C3(H2O) uptake modulates CD4+ T cell cytokine production.
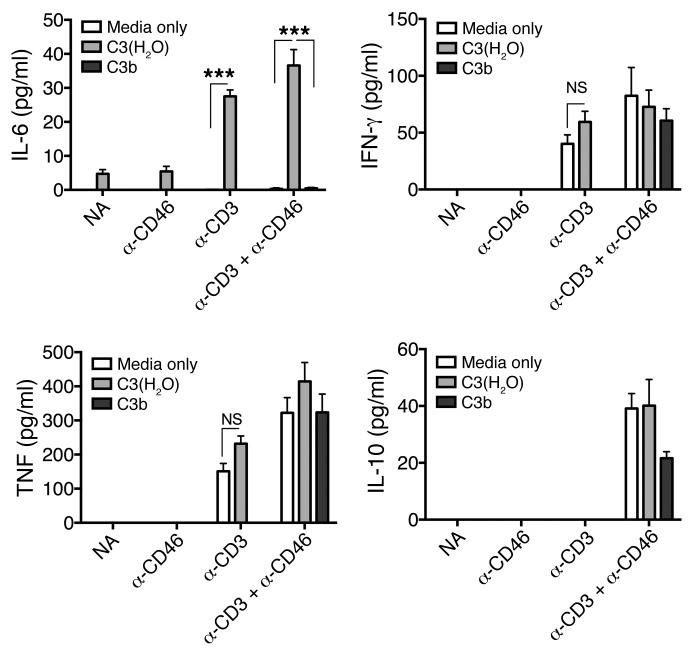
Previous work has demonstrated that shuttling of intracellular-generated C3a (with the C3aR) to the cell surface results in a proinflammatory phenotype (IFN-γ production) by CD4+ T cells (4). Since we detected C3a generation from loaded C3(H2O) that was increased following CD4+ T cell stimulation (Figure 7C), we investigated the effect of C3(H2O) uptake on Th1/Th2 cytokine production by stimulated CD4+ T cells. We costimulated CD4+ T cells via the T cell receptor (mAb OKT-3) and CD46 (mAb TRA-2-10) in the presence/absence of C3(H2O) and assessed production of 4 cytokines involved in the Th1 response or contraction. We detected induction of IL-6 in the presence of C3(H2O) uptake that was significantly augmented by crosslinking the T cell receptor (Figure 8). This result was specific for C3(H2O), as purified C3b did not augment IL-6 production. These data highlight one example of how the C3(H2O) recycling pathway contributes to immune defense. The generation of IL-6, a cytokine with pleiotropic effects, could influence immune responses as well as regulate metabolic and regenerative processes (21). Thus, our data suggest the importance of evaluating the impact of soluble plasma factors when performing in vitro studies assessing cellular immune function.
Figure 8. C3(H2O) uptake modulates the cytokine profile of activated CD4+ T cells.
Supernatants from CD4+ T cells incubated for 18 hours on anti-CD3– (mAb OKT-3), anti-CD46– (mAb TRA-2-10), anti-CD3 plus anti-CD46–, or nonactivated-coated (NA) (MOPC-31) plates in the presence of C3(H2O), C3b, or media only were assessed for IL-6, IFN-γ, TNF, and IL-10 by CBA. Experiments with C3b were only performed with NA and α-CD3+α-CD46 activated cells. Data are expressed as mean ± SEM. n = 6 from 2 healthy donors in 2 independent experiments run in triplicate. ***P < 0.001 by 1-way ANOVA with Bonferroni’s multiple comparison test.
Discussion
Processing of C3 into the activation fragments C3a and C3b was traditionally thought to occur predominately if not exclusively in the extracellular space. A recent report by our group in collaboration with the Kemper laboratory demonstrated intracellular C3 activation from C3 stores in human cells by a convertase-independent process (4). However, in this report, the source of the intracellular C3 stores was not examined. Herein we provide evidence that one route of establishing these C3 stores is through intracellular loading from blood. We also describe the C3(H2O) uptake process and its recycling to the extracellular milieu in several cell types, suggesting that this process is of broad significance.
Our results are consistent with a specific, receptor-mediated mechanism. This was evident by exclusive uptake of C3(H2O) and not native C3. These 2 proteins differ primarily but strikingly in conformation (17–19). Additionally, uptake of labeled C3(H2O) was competitively reduced/inhibited with unlabeled protein. Further, we have demonstrated that denatured C3(H2O) was not taken up, suggesting that recognition of its 3D structure is essential. Also, mouse C3(H2O) was not loaded into human cell lines, indicating that this process is species specific. C3(H2O) was located in a plasma membrane–associated pattern within 1 minute of exposure and then colocalized with early endosomes by 15 minutes after loading. Finally, uptake was a rapid and saturable process. Our next goals include identifying the receptor and further elaborating the intracellular handling and processing of internalized C3(H2O).
To our knowledge, only one previous report has described cellular uptake of C3(H2O) (15). This article suggested that uptake of C3(H2O) is a means for its clearance — in other words, a straightforward degradation pathway. However, C3(H2O) is not inert. First, it can combine with factor B (FB) to initiate an alternative pathway (AP) feedback loop (cleavage of C3 and C5 via C3 and C5 convertases, respectively). Second, it can be proteolytically modified (both intracellularly and extracellularly) to liberate C3a and probably other C3 α and β chain fragments with biological activity (22). Here, we demonstrate that this uptake allows for recycling of the C3(H2O), provides a source of C3a, and affects cell activation phenomena. In agreement with the report by Meilinger et al. (15), we show that cells specifically internalize C3(H2O) and not native C3.
In contrast with Meilinger et al. (15), we did not find that uptake is mediated exclusively through the α2MR. We showed this by 2 methods. First, human cell lines that do not express the α2MR still loaded C3(H2O). Second, blocking this receptor utilizing α2MR-expressing human fibroblasts did not substantially abrogate uptake of C3(H2O). Notably, the dominant binding protein identified in the plasma membrane fraction by Meilinger et al. (15) was an unidentified approximately 80-kDa species, not the α2MR. This supports our results showing that an unidentified receptor mediates uptake of C3(H2O) in human cells, either alone or in conjunction with the α2MR.
Additionally, much of the work by Meilinger et al. (15) was performed in rodents, whereas we have focused exclusively on human cells (15). In a single experiment using human fibroblasts, they demonstrated that receptor-associated protein (RAP), a protein that impairs binding to the α2MR, blocked C3(H2O) uptake. However, RAP does not exclusively block binding to the α2MR (23), and other binding partners of RAP are potential candidate receptors mediating uptake in human fibroblasts. Further, blocking of candidate receptors CD46, CR1, CR2, and CR3 did not reduce binding. Taken together, these data suggest that C3(H2O) has additional receptors on the plasma membrane responsible for mediating its uptake. Identifying the receptor or receptors will be a focus of ongoing studies.
While FH uptake by apoptotic cells has recently been reported (7), we discovered that, in addition to C3(H2O), healthy cells also load FH. Despite synthesis and storage of FH in certain cell types (24–27), we did not detect stores in the Farage cell line prior to exposure to a source of FH. Interestingly, after subcellular fractionation of Farage cells loaded with FH, we detected it in the soluble nuclear fraction. We also identified a putative NLS spanning the first and second repeats of FH. This result is in line with a recent report demonstrating FH binding to nucleosomes of apoptotic cells (7). Whether this predicted NLS mediates FH nuclear targeting and additional roles of FH in the nucleus are interesting areas for investigation. Notably, another member of the RCA-containing protein family, CD46, also contains a NLS in its cytoplasmic tail and targets to the nucleus (11, 28).
In addition to demonstrating FH uptake by normal cells and cell lines, we discovered that the same cell populations load FI. This plasma serine protease is required for FH or CD46 to mediate CA. In line with this finding and following internalization, FH and FI function inside the cell in processing of loaded C3(H2O). Thus, FH and FI cleaved the C3(H2O) α chain to produce iC3(H2O) (see Figure 6, A and E). It has been reported that C3a can be liberated from C3(H2O) by both trypsin and the C3 convertase (22). Although this cleavage mediated by a C3 convertase was less efficient than from the native C3, we show that CTSL, a physiologically relevant intracellular protease, cleaves C3(H2O) more efficiently that native C3 (Supplemental Figure 8B). Intracellularly, we hypothesize that C3a production could occur from both C3(H2O) and iC3(H2O). We speculate that C3a may be liberated more efficiently from iC3(H2O) compared with C3(H2O), providing one possible role for FH and FI loading. This role of loaded FH is supported by the results of Martin et al., who demonstrated that apoptotic cell–loaded FH enhanced CTSL-mediated endogenous C3 cleavage (2016, ref. 7). These data point to a process whereby C3(H2O), FH, and FI are internalized and produce iC3(H2O). Subsequently, C3a and other fragments can be released from iC3(H2O) by intracellular proteases and become available for immune modulation and effector functions (see Supplemental Figure 9). Further clarifying the intracellular processing of C3(H2O) is a current area of investigation in our laboratory.
To our surprise, a large fraction of the C3(H2O) taken up by multiple cell types was returned to the media. It did not appear to be modified, as the Mr was identical to that of C3(H2O) and C3a and the β chain remained attached. These data are therefore consistent with a C3-recycling pathway involving C3(H2O). This saturable pathway likely operates continuously in vivo (see Supplemental Figure 9). We believe this is a novel pathway of C3 metabolism that operates in many (if not most) cell types, as we demonstrated in PBCs and a variety of cultured cell lines. Our hypothesis is that cells take advantage of this source to trigger not only intracellular but also interstitial recognition events that lead to cell activation phenomena and result in enhanced proinflammatory and survival phenotypes. There are several biological advantages for such a system. First, this represents a source of C3a that is required for Th1 differentiation in CD4+ T cells (4, 29). Second, although C3(H2O) lacks a reactive thioester and therefore cannot covalently attach to a pathogen, it can serve as the nidus to initiate the AP feedback loop to generate a C3 convertase. Such a process could enhance the recent observation that pathogens opsonized with C3 extracellularly and then gain entrance into the cell interior, where they can activate an inflammasome pathway (6). These previous studies were performed in the absence of a source of human complement and therefore did not take into account C3(H2O) loading. Indeed, we speculate that in the presence of a source of C3(H2O), cells that are challenged with an intracellular pathogen will respond more rapidly and vigorously.
Herein, we report that C3a production occurs from C3(H2O) following uptake. Additionally, we show that C3a production from loaded C3(H2O) is increased under activating conditions in both human CD4+ T cells and the human B cell line Farage, supporting the concept that this uptake process plays an important role in cellular responses. The significance of intracellular C3a generation is underscored by previous work from our group showing that CD4+ T cells from autoimmune arthritis patients had an increased generation of C3a (as compared with healthy controls) due to an overactive ICS and that this correlated with an increased Th1 response (4). Importantly, our results establish that C3(H2O) uptake by CD4+ T cells alters the induced cytokine profile. Specifically, we demonstrate IL-6 production that is significantly (Figure 8, P < 0.01) augmented by CD3 crosslinking in the presence of C3(H2O). Our results, combined with previous work establishing the importance of intracellular C3a in immune defense and homeostasis, point to an essential role for C3(H2O) uptake and processing in the immune response. Further, generation of IL-6 could affect processes ranging from inflammation to metabolic reprogramming (21). Highlighting the importance of IL-6 in regulation of the immune response, the dysregulated production of IL-6 plays a pathological role in several autoimmune diseases. Further, a humanized IL-6 receptor Ab, tocilizumab, is therapeutically beneficial in several inflammatory diseases, including rheumatoid arthritis and Castleman’s disease (reviewed in ref. 30). It has been previously suggested from studies in Il6–/– and C3–/– mice that IL-6 and C3 are linked in regulation of germinal center development and Ab production (31), and our current results provide additional mechanistic insight into this process. In view of the multiple possible effects of the induced IL-6, a more in-depth investigation is underway to explore this phenomenon.
Currently, most in vitro cell activation studies reported in the literature have been performed in traditional media containing 10% FCS, and therefore, no human C3(H2O) was available for uptake and intracellular processing. Additionally, other plasma components may be required to maintain cellular homeostasis or participate in cell activation phenomena. A role for soluble plasma factors should be considered when performing in vitro studies assessing cellular activation phenomena on cells isolated from blood prior to study.
Although we have shown that C3(H2O) uptake and processing occurs in many cell types, it is clear that an additional source of C3 is present within human cells. This is evident by the fact that intracellular C3 stores have been identified in C3-deficient individuals (4, 32–35) as well as in cell lines that have been long removed from a C3 source (4). One potential source, of course, is biosynthesis and storage of C3. In agreement with this, we demonstrated C3 mRNA in the cell lines examined (Supplemental Figure 6). Although most C3 is secreted, it has been shown that some cell types store C3, such as human neutrophils (36) and monocytes (37). The derivation and composition of these C3 stores have not been delineated. Whether these stores have overlapping or distinct roles from those established by C3(H2O) loading requires further investigation.
In retrospect, as we sought to understand the biologic function of C3(H2O) uptake, we considered the simple concept that C3(H2O) could be the original gateway to activation of the complement system in cells, on membranes and in the interstitium. In this scenario, a putative key innate immune role for C3(H2O) uptake into intracellular C3 stores is to assist in a rapid response of the host to danger. Thus, the C3 species within the cell and in the interstitium is readily available to generate C3a and other fragments to initiate substantial and rapid local complement activation at a site of injury or pathogen invasion. Indeed, such a mechanism could have been one of the earliest and quickest innate immune defenses developed, an adaptive remnant retained in modern times.
Finally, our data and other recent investigations now broaden the role of the newly discovered ICS (4, 11). The finding of a C3(H2O)-recycling pathway has important implications for our understanding of basic processes the cell utilizes for homeostasis and defense. These data may also suggest potential new therapeutic targets and strategies. We are just beginning to unravel this complex, intriguing, and important new system.
Methods
Cell lines, Abs, and proteins.
The Farage human B cell lymphoma and Jurkat human T cell leukemia cell lines (ATCC) were grown in RPMI 1640 with 10% heat-inactivated FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. The ARPE-19 retinal epithelial cell line (ATCC) was grown in DMEM:F12 with 10% heat-inactivated FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. Primary dermal fibroblasts (ATCC) were grown in basal medium supplemented with the Fibroblast Growth Kit–Low serum (ATCC). Human HUVECs (ATCC) were grown in vascular cell basal medium supplemented with the Endothelial Cell Growth Kit (ATCC). All cell lines were maintained at 37°C in 5% CO2. Rabbit anti-human C3a (A218) and goat anti-human C3 (A213) pAbs were obtained from Complement Technologies. Chicken anti-human C3 (GW20073F) pAb was from Sigma-Aldrich.
Purified human C3 was generated in house (38) or purchased from Complement Technologies (A113). Purified human C3b (A114), C3a (A118), FH (A137) and FI (A138), and C3-dpl serum (A314) were purchased from Complement Technologies. The anti-α2MR mAb (MA-8G1) used for flow cytometry and blocking experiments was purchased from Molecular Innovations. Ab for B lymphocyte activation (goat F[ab′]2 anti-human IgM, 2022-01) was purchased from SouthernBiotech. Abs for CD4+ T cell stimulation were generated in house (anti-CD3, OKT-3 or anti-CD46, TRA-2-10) (39).
Isolation and activation of human lymphocytes.
Primary human lymphocytes were isolated from whole blood samples. B lymphocytes were isolated from PBCs using MACS B Cell Isolation Kit II (Miltenyi Biotec). CD4+ T cells were isolated with the EasySep Human CD4+ T cell Isolation Kit (StemCell Technologies). CD4+ T cells (1 to 1.5 × 105 cells/well) were activated for 3 hours in the presence of 10% NHS or 10% FBS on 96-well culture plates coated with Abs to CD3 and CD46 (2 μg/ml). Cytokine production by activated CD4+ T cells was assessed by the BD Cytometric Bead Array (CBA) Human Th1/Th2 Cytokine Kit II. Farage B cells (1 to 1.5 × 105 cells/well) were activated for 3 hours in the presence of 10% NHS or 10% FBS on 96-well culture plates coated with anti-IgM (10 μg/ml).
Cell lysate preparation.
Cell lysates were prepared by resuspending cell pellets in cell lysis buffer (1% NP-40, 0.05% SDS, in PBS) with 2 mM PMSF and ProteaseArrest (G-Biosciences) and incubating for 20 minutes on ice. The resulting lysates were then centrifuged for 10 minutes at 11,200 g and supernatants collected, aliquoted, and stored at –80°C.
Methylamine and denaturing treatments.
C3 was treated with methylamine to hydrolyze the thioester bond and termed C3(MA) (16, 40). Briefly, purified C3 was incubated with 0.4 M methylamine hydrochloride (Sigma Aldrich) and 0.1 M Tris pH 8.0 for 1 hour at 37°C. To denature C3(MA), an aliquot was heated at 85°C for 2 minutes.
C3 loading and loss.
For C3 loading, 2 × 106 cells/ml were resuspended in serum-free medium containing 20 μg/ml C3(MA) (unless otherwise indicated). Cells were routinely incubated for 15 minutes at 37°C. Following incubation, cells were washed 3× with PBS. For C3 loss experiments, after loading, washed cells were plated in 48-well culture plates at 2 × 106 cells/ml in normal growth media containing 10% FBS and incubated for indicated times before the supernatants were collected, cell pellets washed, and cell lysates prepared. The C3 content of the cell lysates and supernatants was analyzed by WB. Briefly, samples were electrophoresed in reduced or nonreduced 4%–20% SDS-PAGE buffer (unless indicated otherwise), transferred to nitrocellulose, and probed with rabbit anti-human C3a (A218) or goat anti-C3 (A213) pAbs (Complement Technologies), followed by appropriate HRP-conjugated secondary Abs. Blots were visualized on a ChemiDoc XRS Imager (Bio-Rad).
FH and FI loading.
Similarly to what occurred in the C3 studies, 25 μg/ml FH and/or 4 μg/ml FI were added to the medium of cells plated at 2 × 106 cells/ml. After 15 minutes at 37°C incubation and washing (3×) with PBS, the FH and FI concentrations in cell lysates were analyzed by WB with a goat anti-FH (A237) pAb or a goat anti-FI (A238) pAb (Complement Technologies).
Enzymatic reactions.
Recombinant CTSL (R&D Systems) was activated according to the manufacturer’s instructions. In vitro cleavage of C3(MA) and C3 by CTSL was performed by incubating 500 ng C3(MA) or C3 with 2 ng or 300 ng CTSL for 1 hour at 37°C. In vitro cleavage by elastase (EPC Elastin Products Inc.) was performed by incubating 1 μg C3(MA) with increasing concentrations of elastase (0–1.3 μg/ml) for 1 hour at 37°C. In vitro cleavage by granzyme B (Enzo Life Sciences) was performed by incubating 50 ng C3(MA) with 50 ng/ml granzyme B for 1 hour at 37°C. Cleavage fragments were visualized by WB.
Confocal microscopy and colocalization analysis.
Confocal microscopy was performed as previously described (4). Briefly, fixed and permeabilized cells were stained with primary Abs for 30 minutes at room temperature (RT). Staining with secondary Abs was performed by incubation for 30 minutes at RT. Cells were mounted with ProLong Gold (Thermo Fisher) and incubated at 4°C overnight. For studies using fluorescent dye–conjugated C3, the C3 was labeled with Dylight 488 using the Dylight 488 Antibody Labeling Kit (Thermo Fisher). This labeling kit reacts with exposed N-terminal α amino groups or the ε amino groups of lysine residues to form stable amide bonds. Following labeling, the C3 is of the form C3(H2O) (see Supplemental Figure 1B). For colocalization studies, the Cellular Localization IF Ab Sampler Kit (catalog 4753, Cell Signaling) was employed. Images were obtained in the Imaging Facility, Department of Molecular Microbiology, WUSM, by confocal fluorescence microscopy. Samples were visualized with a Zeiss LSM880 with Airyscan laser scanning confocal microscope (Carl Zeiss Inc.) equipped with a 63×, 1.4 numerical aperture Zeiss Plan Apochromat oil objective.
Statistics.
All data were subjected to statistical analysis using Prism Software version 5 (GraphPad). Comparisons between 2 groups were performed by 2-tailed Student’s t test (parametric). Comparisons between multiple groups were performed using a 1-way ANOVA with Bonferroni’s multiple comparison test (parametric) or Dunn’s multiple comparison test (nonparametric). A P value of less than 0.05 was considered significant.
Study approval.
Blood was collected from healthy donors after written informed consent according to the guidelines of the WUSM Human Studies Committee.
Author contributions
JPA, ME, and MKL designed the research studies and wrote the manuscript. ME, MKL, and PB conducted experiments and acquired data. JPA, ME, and MKL analyzed and interpreted data. HSK reviewed the manuscript and provided helpful discussion.
Supplementary Material
Acknowledgments
Support was provided by the NIH (R01 GM0099111 and R01 AI041592 to JPA), an NIH Training grant in the Immunobiology of Rheumatic Disease (2T32 AR007279 to ME), and an NIH Training grant in the Principles of Pulmonary Research (5T32 HL007317 to HSK). Research reported in this publication is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the NIH (P30AR048335) and an NIH grant for the Washington University Institute of Clinical and Translational Sciences (3UL1 TR000448). The authors thank Richard Hauhart for preparing the purified C3 and Wandy Beatty and Brian Anthony in the Molecular Microbiology Imaging Facility at WUSM for expert technical assistance with confocal microscopy.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2017;127(3):970–981.https://doi.org/10.1172/JCI89412.
Contributor Information
Michelle Elvington, Email: mlelvington@wustl.edu.
M. Kathryn Liszewski, Email: kliszews@wustl.edu.
Paula Bertram, Email: Pbertram@dom.wustl.edu.
Hrishikesh S. Kulkarni, Email: hkulkarn@wustl.edu.
References
- 1.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344(15):1140–1144. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 2.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344(14):1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 3.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liszewski MK, et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity. 2013;39(6):1143–1157. doi: 10.1016/j.immuni.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baudino L, et al. C3 opsonization regulates endocytic handling of apoptotic cells resulting in enhanced T-cell responses to cargo-derived antigens. Proc Natl Acad Sci USA. 2014;111(4):1503–1508. doi: 10.1073/pnas.1316877111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tam JC, Bidgood SR, McEwan WA, James LC. Intracellular sensing of complement C3 activates cell autonomous immunity. Science. 2014;345(6201):1256070. doi: 10.1126/science.1256070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin M, et al. Factor H uptake regulates intracellular C3 activation during apoptosis and decreases the inflammatory potential of nucleosomes. Cell Death Differ. 2016;23(5):903–911. doi: 10.1038/cdd.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardone J, et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat Immunol. 2010;11(9):862–871. doi: 10.1038/ni.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Friec G, et al. The CD46-Jagged1 interaction is critical for human TH1 immunity. Nat Immunol. 2012;13(12):1213–1221. doi: 10.1038/ni.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liszewski MK, Kemper C, Price JD, Atkinson JP. Emerging roles and new functions of CD46. Springer Semin Immunopathol. 2005;27(3):345–358. doi: 10.1007/s00281-005-0002-3. [DOI] [PubMed] [Google Scholar]
- 11.Kolev M, et al. Complement regulates nutrient influx and metabolic reprogramming during Th1 cell responses. Immunity. 2015;42(6):1033–1047. doi: 10.1016/j.immuni.2015.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arbore G, Kemper C. A novel “complement-metabolism-inflammasome axis” as a key regulator of immune cell effector function. Eur J Immunol. 2016;46(7):1563–1573. doi: 10.1002/eji.201546131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pangburn MK, Schreiber RD, Müller-Eberhard HJ. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. J Exp Med. 1981;154(3):856–867. doi: 10.1084/jem.154.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brade V, Hall RE, Colten HR. Biosynthesis of pro-C3, a precursor of the third component of complement. J Exp Med. 1977;146(3):759–765. doi: 10.1084/jem.146.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meilinger M, et al. Metabolism of activated complement component C3 is mediated by the low density lipoprotein receptor-related protein/alpha(2)-macroglobulin receptor. J Biol Chem. 1999;274(53):38091–38096. doi: 10.1074/jbc.274.53.38091. [DOI] [PubMed] [Google Scholar]
- 16.Tack BF, Harrison RA, Janatova J, Thomas ML, Prahl JW. Evidence for presence of an internal thiolester bond in third component of human complement. Proc Natl Acad Sci USA. 1980;77(10):5764–5768. doi: 10.1073/pnas.77.10.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishida N, Walz T, Springer TA. Structural transitions of complement component C3 and its activation products. Proc Natl Acad Sci USA. 2006;103(52):19737–19742. doi: 10.1073/pnas.0609791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen BJ, et al. Structures of complement component C3 provide insights into the function and evolution of immunity. Nature. 2005;437(7058):505–511. doi: 10.1038/nature04005. [DOI] [PubMed] [Google Scholar]
- 19.Chen ZA, et al. Structure of complement C3(H2O) revealed by quantitative cross-linking/mass spectrometry and modeling. Mol Cell Proteomics. 2016;15(8):2730–2743. doi: 10.1074/mcp.M115.056473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moestrup SK, Gliemann J, Pallesen G. Distribution of the alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein in human tissues. Cell Tissue Res. 1992;269(3):375–382. doi: 10.1007/BF00353892. [DOI] [PubMed] [Google Scholar]
- 21.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 22.Pangburn MK, Müller-Eberhard HJ. Relation of putative thioester bond in C3 to activation of the alternative pathway and the binding of C3b to biological targets of complement. J Exp Med. 1980;152(4):1102–1114. doi: 10.1084/jem.152.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bu G. The roles of receptor-associated protein (RAP) as a molecular chaperone for members of the LDL receptor family. Int Rev Cytol. 2001;209:79–116. doi: 10.1016/s0074-7696(01)09011-8. [DOI] [PubMed] [Google Scholar]
- 24.Turner NA, Sartain SE, Hui SK, Moake JL. Regulatory components of the alternative complement pathway in endothelial cell cytoplasm, factor H and factor I, are not packaged in Weibel-Palade bodies. PLoS One. 2015;10(3):e0121994. doi: 10.1371/journal.pone.0121994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ault BH, et al. Human factor H deficiency. Mutations in framework cysteine residues and block in H protein secretion and intracellular catabolism. J Biol Chem. 1997;272(40):25168–25175. doi: 10.1074/jbc.272.40.25168. [DOI] [PubMed] [Google Scholar]
- 26.Vĕtvicka V, Reed W, Hoover ML, Ross GD. Complement factors H and I synthesized by B cell lines function to generate a growth factor activity from C3. J Immunol. 1993;150(9):4052–4060. [PubMed] [Google Scholar]
- 27.Rayes J, et al. The interaction between factor H and VWF increases factor H cofactor activity and regulates VWF prothrombotic status. Blood. 2014;123(1):121–125. doi: 10.1182/blood-2013-04-495853. [DOI] [PubMed] [Google Scholar]
- 28.Liszewski MK, Tedja I, Atkinson JP. Membrane cofactor protein (CD46) of complement. Processing differences related to alternatively spliced cytoplasmic domains. J Biol Chem. 1994;269(14):10776–10779. [PubMed] [Google Scholar]
- 29.Ghannam A, Fauquert JL, Thomas C, Kemper C, Drouet C. Human complement C3 deficiency: Th1 induction requires T cell-derived complement C3a and CD46 activation. Mol Immunol. 2014;58(1):98–107. doi: 10.1016/j.molimm.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka T, Narazaki M, Ogata A, Kishimoto T. A new era for the treatment of inflammatory autoimmune diseases by interleukin-6 blockade strategy. Semin Immunol. 2014;26(1):88–96. doi: 10.1016/j.smim.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Kopf M, Herren S, Wiles MV, Pepys MB, Kosco-Vilbois MH. Interleukin 6 influences germinal center development and antibody production via a contribution of C3 complement component. J Exp Med. 1998;188(10):1895–1906. doi: 10.1084/jem.188.10.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghannam A, et al. Human C3 deficiency associated with impairments in dendritic cell differentiation, memory B cells, and regulatory T cells. J Immunol. 2008;181(7):5158–5166. doi: 10.4049/jimmunol.181.7.5158. [DOI] [PubMed] [Google Scholar]
- 33.Katz Y, Singer L, Wetsel RA, Schlesinger M, Fishelson Z. Inherited complement C3 deficiency: a defect in C3 secretion. Eur J Immunol. 1994;24(7):1517–1522. doi: 10.1002/eji.1830240709. [DOI] [PubMed] [Google Scholar]
- 34.Singer L, Whitehead WT, Akama H, Katz Y, Fishelson Z, Wetsel RA. Inherited human complement C3 deficiency. An amino acid substitution in the beta-chain (ASP549 to ASN) impairs C3 secretion. J Biol Chem. 1994;269(45):28494–28499. [PubMed] [Google Scholar]
- 35.Reis ES, Falcão DA, Isaac L. Clinical aspects and molecular basis of primary deficiencies of complement component C3 and its regulatory proteins factor I and factor H. Scand J Immunol. 2006;63(3):155–168. doi: 10.1111/j.1365-3083.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- 36.Botto M, Lissandrini D, Sorio C, Walport MJ. Biosynthesis and secretion of complement component (C3) by activated human polymorphonuclear leukocytes. J Immunol. 1992;149(4):1348–1355. [PubMed] [Google Scholar]
- 37.Einstein LP, et al. Biosynthesis of the third component of complement (C3) in vitro by monocytes from both normal and homozygous C3-deficient humans. J Clin Invest. 1977;60(5):963–969. doi: 10.1172/JCI108876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nickells MW, Atkinson JP. Characterization of CR1- and membrane cofactor protein-like proteins of two primates. J Immunol. 1990;144(11):4262–4268. [PubMed] [Google Scholar]
- 39.Wang G, Liszewski MK, Chan AC, Atkinson JP. Membrane cofactor protein (MCP; CD46): isoform-specific tyrosine phosphorylation. J Immunol. 2000;164(4):1839–1846. doi: 10.4049/jimmunol.164.4.1839. [DOI] [PubMed] [Google Scholar]
- 40.Seya T, Okada M, Nishino H, Atkinson JP. Regulation of proteolytic activity of complement factor I by pH: C3b/C4b receptor (CR1) and membrane cofactor protein (MCP) have different pH optima for factor I-mediated cleavage of C3b. J Biochem. 1990;107(2):310–315. doi: 10.1093/oxfordjournals.jbchem.a123044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.