Abstract
AIM
To evaluate the numbers of different subsets of monocytes and their associations with the values of clinical measures in mild acute pancreatitis (MAP) patients.
METHODS
The study included one group of 13 healthy controls and another group of 24 patients with new-onset MAP. The numbers of different subsets of monocytes were examined in these two groups of subjects by flow cytometry. The concentrations of plasma interleukin (IL)-10 and IL-12 were determined by cytometric bead array. The acute physiology and chronic health evaluation (APACHE) II scores of individual patients were evaluated, and the levels of plasma C-reactive protein (CRP) as well as the activities of amylase and lipase were measured.
RESULTS
In comparison with that in the controls, significantly increased numbers of CD14+CD163-, CD14+CD163-MAC387+ M1 monocytes, but significantly reduced numbers of CD14+CD163+IL-10+ M2 monocytes were detected in the MAP patients (P < 0.01 or P < 0.05). Furthermore, significantly higher levels of plasma IL-10 and IL-12 were observed in the MAP patients (P < 0.01 for all). More importantly, the levels of plasma CRP were positively correlated with the numbers of CD14+CD163- (R = 0.5009, P = 0.0127) and CD14+CD163-MAC387+ (R = 0.5079, P = 0.0113) M1 monocytes and CD14+CD163+CD115+ M2 monocytes (R = 0.4565, P = 0.0249) in the patients. The APACHE II scores correlated with the numbers of CD14+CD163+CD115+ (R = 0.4581, P = 0.0244) monocytes and the levels of plasma IL-10 (R = 0.4178, P = 0.0422) in the MAP patients. However, there was no significant association among other measures tested in this population.
CONCLUSION
Increased numbers of CD14+CD163- and CD14+ CD163-MAC387+ monocytes may contribute to the pathogenesis of MAP, and increased numbers of CD14+CD163+CD115+ monocytes may be a biomarker for evaluating the severity of MAP.
Keywords: Mild acute pancreatitis, Monocyte, Cytokine, Acute physiology and chronic health evaluation II score, C-reactive protein
Core tip: This is the first study on the numbers of different phenotypes of peripheral blood monocytes in patients with new-onset mild acute pancreatitis (MAP). Increased numbers of CD14+CD163- and CD14+CD163-MAC387+ M1 monocytes were positively correlated with the levels of plasma C-reactive protein (CRP), which suggest that pro-inflammatory monocytes may participate in the pathogenesis of MAP. The increased numbers of CD14+CD163+CD115+ M2 monocytes were positively correlated with the plasma CRP levels and the acute physiology and chronic health evaluation II scores, suggesting that the numbers of CD14+CD163+CD115+ monocytes may be a valuable biomarker for evaluating the severity of MAP. Our findings may provide new insights into the pathogenic process and immunoregulation of MAP.
INTRODUCTION
Mild acute pancreatitis (MAP), the mild form of acute pancreatitis, accounts for 80% of AP, and is mainly caused by gallstones and alcohol abuse[1]. Once inflammation damages the pancreas, the inactive digestive enzymes, such as trypsinogen, are activated, leading to autodigestion of the pancreas and triggering AP[2]. Immunocompetent cells, including monocytes and macrophages, participate in the development and progression of AP[3-5]. However, how monocytes regulate the pathogenesis of AP has not been clarified.
Monocytes are circulating white blood cells (WBCs), which differentiate into macrophages when they enter the tissue[6]. Macrophages can be classically activated as M1 or alternatively activated as M2 cells[7]. The M1 macrophages can produce many types of pro-inflammatory cytokines, including interleukin (IL)-12, IL-1β and IL-6, and defense against infectious pathogens and tumors. The M2 macrophages are characterized by expressing anti-inflammatory cytokines (e.g., IL-10) and many other factors that regulate immune responses and tissue repair, but may promote tumor growth and metastasis[7,8]. Different types of macrophage responses and their relative IL-10 and IL-12 have been associated with different types of inflammatory diseases and are valuable for evaluating disease severity[9,10]. The M1 and M2 classification is initially proposed for macrophages, and can be extended to human peripheral blood monocytes[11]. The expression of both M1 and M2 markers is detected in circulating peripheral blood mononuclear cells (PBMCs), and the different types of polarized monocytes in peripheral blood are associated with different diseases[11-13]. However, little is known on whether the numbers of peripheral blood different types of polarized monocytes and the levels of plasma IL-10 and IL-12 can be valuable for evaluating the severity of MAP.
Peripheral blood monocytes and macrophages express CD14, which is one of the pattern-recognition receptors and a co-receptor of toll-like receptor[14,15]. MAC387 antibody can recognize calprotectin, a complex of intracellular myeloid-related proteins (MRPs) 8 and MRP14. The blood-derived MAC387+ monocytes/macrophages are recently infiltrating monocytes/macrophages and are important inflammatory components[16-18]. CD163 is a hemoglobin scavenger receptor expressed by peripheral blood monocytes and macrophages, polarizes monocytes/macrophages toward M2-phenotype and is crucial for the resolution of inflammation[19,20]. CD115 is macrophage colony-stimulating factor receptor (CSF-1R) and engagement of CSF-1R by CSF-1 polarizes toward M2-type macrophages and enhances their function[21]. CD115 is expressed by human monocytes. How different subsets of M1 and M2 monocytes regulate the pathogenesis of MAP has not been explored. Notably, anti-CD115 can inhibit inflammatory osteolysis and has potential anti-tumor effect, suggesting that CD115+ M2 monocytes/macrophages are associated with inflammation and tumor growth[21-23]. However, there is no report on the numbers of CD115+ monocytes in MAP patients.
In this study, we characterized the numbers of different subsets of monocytes and the levels of plasma IL-10 and IL-12 in patients with newly diagnosed MAP and health controls. Furthermore, we analyzed the potential association of the numbers of different subsets of monocytes and the levels of plasma IL-10 and IL-12 with the levels of plasma C-reactive protein (CRP) and the acute physiology and chronic health evaluation (APACHE) II scores in MAP patients.
MATERIALS AND METHODS
Patients and controls
A total of 24 patients with new-onset MAP were recruited at the inpatient service of the Department of Gastroenterology, the Second Part of the First Hospital of Jilin University (Changchun, China) from July 2015 to March 2016. All patients met the Atlanta criteria of MAP[24]. Another 13 sex-, age- and ethnicity-matched healthy subjects were recruited from the Physical Examination Center of our hospital during the same period, and served as the controls. These controls had no history of autoimmune diseases, chronic inflammatory diseases, or allergies. The disease severity of individual patients was evaluated by APACHE II score. Patients were excluded if she/he had a history of malignant tumor, autoimmune diseases, any of other chronic inflammatory diseases, or had received immunosuppressive drugs within the past 3 mo. Written informed consent was obtained from individual subjects. The experimental protocol was established according to the guidelines of the Declaration of Helsinki and was approved by the Human Ethics Committee of Jilin University. The demographic and clinical characteristics of individual participants are shown in Table 1.
Table 1.
Demographic and clinical characteristics of participants
| Parameter | MAP patients | Healthy controls |
| No. | 24 | 13 |
| Age (yr) | 41 (25-60) | 40 (26-59) |
| Sex, female/male | 10/14 | 6/7 |
| BMI | 24.5 | 23.6 |
| WBC (109/L) | 9.34 (5.46-16.34)1 | 6.79 (4.87-9.3) |
| Neutrophils (109/L) | 6.92 (3.50-14.16)1 | 4.16 (3.01-6.15) |
| Monocytes (109/L) | 0.61 (0.23-1.94) | 0.51 (0.37-0.61) |
| AMY (U/L) | 302.8 (30.8-1845)1 | 73.05 (24.5-158.3) |
| LPS (U/L) | 1135 (40.08-3200)1 | 72.38 (6-150.4) |
| CRP (mg/L) | 16.62 (1.04-45.87)1 | 3.70 (0.43-7.25) |
| APACHE II score | 6 (4-9) | NA |
Data are median (range) or real case number.
P < 0.05 vs the controls. Normal values: WBC: 3.50-9.50 (109/L), Monocytes: 0.1-0.6 (109/L), AMY: 8-220 (U/L), LPS: 0-190 (U/L), CRP: 0-10 (mg/L). MAP: Mild acute pancreatitis; BMI: Body mass index; WBC: White blood cell counts; AMY: Amylase; LPS: Lipase; CRP: C-reactive protein; APACHE: Acute physiology and chronic health evaluation; NA: Not available.
Clinical data
The clinical data of each subject were collected from the hospital records. These data included age, sex, height, body weight, body mass index (BMI) and laboratory tests. Individual subjects were subjected to routine laboratory tests for full blood cell counts, the concentrations of plasma CRP, amylase (AMY) and lipase activities. The levels of plasma CRP were determined by scatter turbidimetry using a Siemens special protein analyzer (Siemens Healthcare Diagnostics Products, GmbH, Munich, Germany). The concentrations of plasma AMY and lipase were determined by ADVIA 1650 biochemical analyzer (Bayer, Pittsburg, PA, United States).
Flow cytometry analysis
Heparinized fasting venous blood samples (6 millilitres, mL) were collected from the median cubital vein of individual MAP patients (within 72 h after upper abdominal pain occurred) and control subjects, and PBMCs were isolated by density-gradient centrifugation using Ficoll-Paque Plus (Amersham Biosciences, Little Chalfont, United Kingdom). PBMCs at 1 × 106/tube were stained in duplicate with BV510-anti-CD14, PE-anti-CD115 (BD Biosciences, Franklin Lakes, NJ, United States), PE/Cy7-anti-CD163 and APC/Cy7-anti-206 (Biolegend, San Diego, CA, United States) in the dark at 4 °C for 30 min. After being washed, the cells were fixed and permeabilized using a fixation/permeabilization kit (BD Biosciences), followed by intracellular staining with FITC-anti-MAC387 (Abcam, Cambridge, United Kingdom). The fluorescence- and isotype-matched antibodies served as negative controls.
To detect the function, PBMCs (106 cells/well) were stimulated in duplicate with 50 ng/mL of lipopolysaccharide, phorbol myristate acetate and 1.0 μg/mL of ionomycin (Sigma-Aldrich, St. Louis, United States) in 10% fetal bovine serum RPMI 1640 (complete medium) for 2 h at 37 °C in 5% CO2 and exposed to Brefeldin A (GolgiPlug; BD Biosciences) for 4 h, as described previously in a study from our laboratory[15,25]. After being washed, the cells were stained with BV510-anti-CD14 and PE/Cy7-anti-CD163, fixed and permeabilized using the permeabilization solution, followed by intracellular staining with BV421-anti-IL-12 and PE-CF594-anti-IL-10 (BD Biosciences). The real positive and negative cells were distinguished by fluorescence minus one (FMO) and the cells were stained with all of the fluorochromes, except for the one that was being measured. The percentages of different subsets of monocytes were characterized on a FACSAria II (Becton, Dickinson and Company, Franklin Lakes, NJ, United States) and the data were analyzed by FlowJo software (v5.7.2; TreeStar, Ashland, OR, United States). Finally, the numbers of different subsets of monocytes were calculated, based on the numbers of monocytes in individual subjects and expressed as the numbers of cells per mL.
Cytometric bead array analysis of plasma cytokines
The concentrations of plasma IL-10 and IL-12 were determined by Cytometric bead array (CBA), according to the manufacturer’s protocol (BD Biosciences) with minor modification. Briefly, plasma samples (50 μL) from individual subjects were subjected in duplicate to analysis of the levels of plasma IL-10 and IL-12 using the CBA kit on a FACSAria II (Beckton, Dickinson and Company). The concentrations of plasma cytokines were quantified using the CellQuestPro and CBA software (Becton, Dickinson and Company). The limitation of detection for IL-10 and IL-12 is 3.3 pg/mL and 1.9 pg/mL, respectively.
Statistical analysis
Data are expressed as median and range. The difference between two groups was analyzed by the Mann-Whitney U nonparametric test. The relationship between variables was evaluated using the Spearman rank correlation test. All the statistical analyses were performed by the SPSS version 19.0 software. A two-sided P value of < 0.05 was considered statistically significant.
RESULTS
Increased numbers of M1 monocytes in MAP patients
To understand the importance of monocytes, 24 MAP patients and 13 controls were recruited. Among all the patients, 7 cases were induced by alcohol, 11 cases by hypertriglyceridemia, and 6 by cholelithiasis, and the length of hospital stay was 7-10 d. There was no significant difference in the distribution of age, sex and BMI as well as the number of PBMCs between the MAP patients and controls (Table 1). The numbers of peripheral blood WBCs and neutrophils were significantly higher in the patients than those in the controls (P < 0.05; Table 1). The values of AMY, lipase and CRP were significantly higher in the MAP patients than that in the controls. In addition, MAP patients displayed variable values of APACHE II score.
The numbers of peripheral blood CD14+CD163- M1 and CD14+CD163+ M2 monocytes in the MAP patients and controls were characterized by flow cytometry. As shown in Figure 1, the numbers of CD14+CD163- M1 monocytes were significantly higher in the patients than that in the controls (P = 0.0049). In contrast, there was no significant difference in the numbers of CD14+CD163+ M2 monocytes between the MAP patients and controls (P = 0.8362). Collectively, increased numbers of CD14+CD163- M1 monocytes existed in MAP patients.
Figure 1.
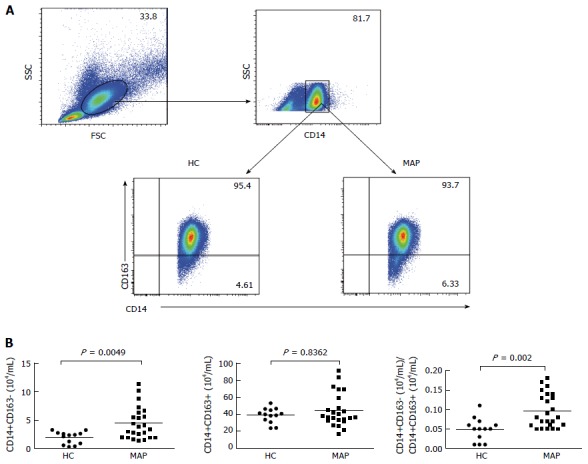
Flow cytometry analysis of peripheral blood monocytes. Peripheral blood mononuclear cells (PBMCs) were obtained from individual subjects and stained in duplicate with BV510-anti-CD14 and PE/Cy7-anti-CD163 or isotype control antibodies. The cells were gated initially on living mononuclear cells and then on CD14+ monocytes. Subsequently, the frequency of CD14+CD163- M1 and CD14+CD163+ M2 monocytes were determined by flow cytometry and the numbers of each subset of monocytes were calculated, based on total numbers of monocytes. Data are representative charts of flow cytometry and expressed as the mean values of individual subjects (n = 13 for the healthy controls, n = 24 for the patients). A: Flow cytometry analysis; B: Quantitative analysis. The horizontal lines indicate the median values for individual groups. FSC: Forward scatter; SSC: Side scatter; HC: Healthy control; MAP: Mild acute pancreatitis.
Increased numbers of MAC387+ M1 monocytes in the MAP patients
The blood-derived MAC387+ monocytes/macrophages are recently infiltrating monocytes/macrophages and CD14+CD163-MAC387+ monocytes/macrophages are important inflammatory components[16-18]. To further understand the importance of monocytes, the numbers of peripheral blood CD14+CD163-MAC387+ and CD14+CD163+MAC387+ cells in the MAP patients and controls were characterized by flow cytometry (Figure 2). The numbers of CD14+CD163-MAC387+ cells in the MAP patients were significantly higher than that in the controls (P = 0.0091). However, there was no statistically significant difference in the numbers of CD14+CD163+MAC387+ cells between the MAP patients and controls (P = 0.9619). Thus, increased numbers of MAC387+ M1 monocytes were present in the MAP patients.
Figure 2.
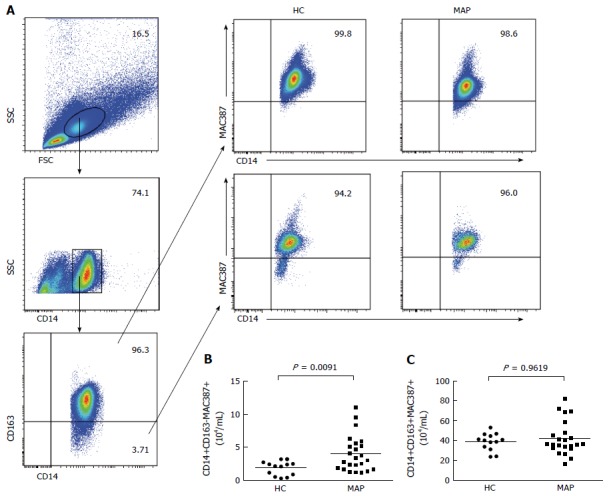
Flow cytometry analysis of peripheral blood MAC387+ monocytes. Peripheral blood mononuclear cells (PBMCs) were isolated from individual subjects and stained in duplicate with BV510-anti-CD14 and PE/Cy7-anti-CD163 or isotype controls. After being washed, the cells were fixed and permeabilized using a fixation/permeabilization kit, followed by intracellular staining with FITC-anti-MAC387. The numbers of peripheral blood CD14+CD163+MAC387+ and CD14+CD163-MAC387+ monocytes were analyzed by flow cytometry. Data are representative FACS charts and expressed as the mean numbers of each type of cells in individual subjects. The horizontal lines indicate the median values for each group. FSC: Forward scatter; SSC: Side scatter; HC: Healthy control; MAP: Mild acute pancreatitis.
Increased numbers of CD115+ M2 monocytes in the MAP patients
Although there was no statistically significant difference in the numbers of CD14+CD163+ M2 monocytes between the MAP patients and controls, there are different subsets of M2 monocytes with different surface markers and varying functions[15,26]. M2 monocytes can express CD206 (the mannose receptor) and/or CD115[15,27]. To understand the importance of different subsets of M2 monocytes, the numbers of peripheral blood CD206+ and/or CD115+ M2 monocytes in the MAP patients and controls were further analyzed by flow cytometry. As shown in Figure 3, the numbers of CD14+CD163+CD115+ and CD14+CD163+CD115+CD206+ monocytes in the MAP patients were significantly higher than those in the controls (P = 0.0072 and P < 0.0001, respectively). However, there was no statistically significant difference in the numbers of CD14+CD163+CD206+ monocytes between the MAP patients and controls (P = 0.9873). Together, increased numbers of CD115+ M2 monocytes were present in the MAP patients.
Figure 3.
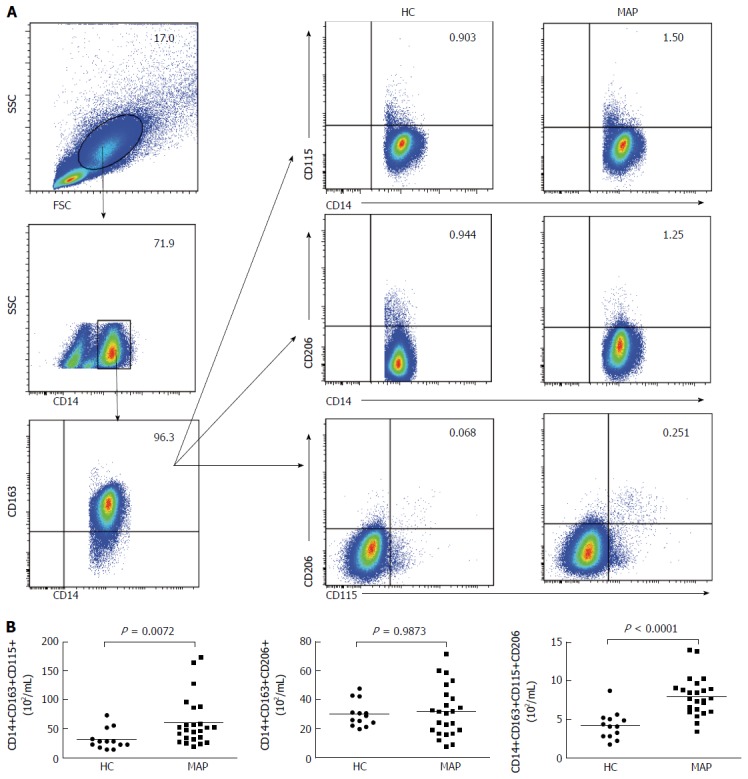
Flow cytometry analysis of peripheral blood CD206+ and CD115+ M2 monocytes. Peripheral blood mononuclear cells (PBMCs) were isolated from individual subjects and stained in duplicate with BV510-anti-CD14, PE/Cy7-anti-CD163, PE-anti-CD115, and APC/Cy7-anti-206 or isotype controls. The numbers of peripheral blood CD14+CD163+CD206+, CD14+CD163+CD115+ and CD14+CD163+CD206+CD115+ M2 monocytes were analyzed by flow cytometry. Data shown are representative FACS charts and expressed as the mean numbers of each type of cells per mL of blood in individual subjects. The horizontal lines indicate the median values for each group. FSC: Forward scatter; SSC: Side scatter; HC: Healthy control; MAP: Mild acute pancreatitis.
Increased cytokine production in the MAP patients
M1 monocytes can produce IL-12 and are critical for inflammation. In contrast, M2 monocytes produce IL-10, which controls inflammation and promotes tumor growth. To understand the functions of M1 and M2 monocytes in the pathogenic process of MAP, the numbers of peripheral blood IL-12+ M1 monocytes and IL-10+ M2 monocytes were characterized by flow cytometry. There was no significant difference in the fluorescent intensity of anti-IL-12 and anti-IL-10 signals in monocytes between the MAP patients and controls (data not shown). As shown in Figure 4, the numbers of IL-12+ M1 monocytes were significantly higher in the MAP patients than the controls (P = 0.0202). Conversely, the numbers of IL-10+ M2 monocytes were significantly lower in the MAP patients than the controls (P = 0.022). As a result, the ratios of the numbers of CD14+CD163-IL-12+ to CD14+CD163+IL-10+ monocytes in the MAP patients were significantly higher than that in the controls. Further CBA analysis indicated that the concentrations of plasma IL-12 and IL-10 were significantly higher in the MAP patients than that in the controls (P = 0.0065 and P = 0.0001, respectively). Hence, increased levels of plasma pro-inflammatory IL-12 and anti-inflammatory IL-10 existed in the MAP patients.
Figure 4.
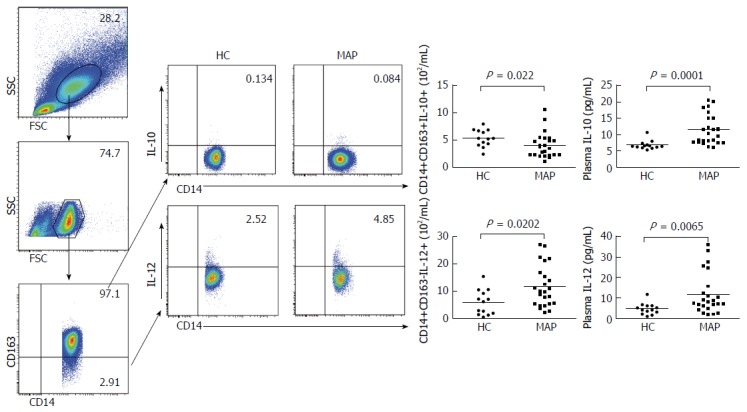
Analysis of peripheral blood IL-12+ M1 and IL-10+ M2 monocytes and the concentrations of plasma IL-10 and IL-12. Peripheral blood mononuclear cells (PBMCs) were isolated from individual subjects and stimulated with lipopolysaccharide /phorbol myristate acetate/ionomycin in vitro. The cells were stained in duplicate with BV510-anti-CD14 and PE/Cy7-anti-CD163 or isotype controls. The cells were fixed, and permeabilized, followed by intracellular staining with BV421-anti-IL-12 and PE-CF594-anti-IL-10. The numbers of peripheral blood CD14+CD163-IL-12+ M1 and CD14+CD163+IL-10+ M2 monocytes in individual subjects were determined by flow cytometry. The concentrations of plasma IL-10 and IL-12 in individual subjects were determined by cytometric bead analysis. Data are representative FACS charts or the mean numbers of each type of cells and the mean values of each type of cytokine in individual subjects. The horizontal lines indicate the median values for each group. FSC: Forward scatter; SSC: Side scatter; HC: Healthy control; MAP: Mild acute pancreatitis.
Correlation analysis of clinical parameters with different subsets of monocytes and cytokines in the MAP patients
Finally, the potential relationship between the values of clinical parameters and the numbers of different subsets of monocytes and the levels of cytokines was analyzed in the MAP patients. As shown in Figure 5, the levels of plasma CRP were positively correlated with the numbers of CD14+CD163- (R = 0.5009, P = 0.0127) and CD14+CD163-MAC387+ (R = 0.5079, P = 0.0113) M1 monocytes, and CD14+CD163+CD115+ (R = 0.4565, P = 0.0249) M2 monocytes, but not with CD14+CD163+ M2 monocytes in this population. Furthermore, the APACHE II scores were positively correlated with the numbers of CD14+CD163+CD115+ (R = 0.4581, P = 0.0244) M2 monocytes, and the levels of plasma IL-10 (R = 0.4178, P = 0.0422) in this population of patients. There was no other significant correlation among these measures in this population. Collectively, increased numbers of CD14+CD163- and CD14+CD163-MAC387+ M1, and CD14+CD163+CD115+ M2 monocytes and elevated levels of plasma IL-10 may contribute to imbalance of pro-inflammatory and anti-inflammatory responses during the process of MAP, and the numbers of CD14+CD163+CD115+ monocytes may serve as a biomarker for evaluating MAP severity.
Figure 5.
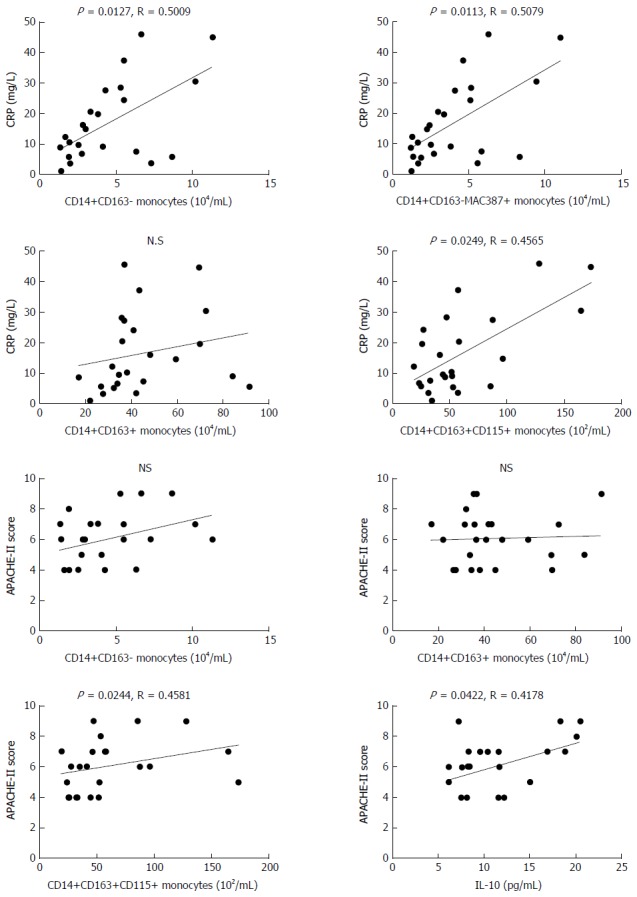
Correlation analysis. The potential correlations between the numbers of each subset of monocytes and APACHE II scores or CRP levels in those patients were analyzed by the Spearman rank correlation test. Data shown are the values of individual patients (n = 24). CRP: C-reactive protein; APACHE: Acute physiology and chronic health evaluation.
DISCUSSION
Monocytes and macrophages are important inflammatory cells and their activation is associated with the severity of AP[28]. In this study, the numbers of different subsets of peripheral blood monocytes and their potential association with disease severity were analyzed in 24 MAP patients. In comparison with healthy controls, significantly increased numbers of CD14+CD163-, CD14+CD163-MAC387+ and CD14+CD163-IL-12+ M1 monocytes and reduced numbers of CD14+CD163+IL-10+ monocytes were detected in the MAP patients. Furthermore, significantly higher levels of plasma IL-12 were observed in the MAP patients. More importantly, the levels of plasma CRP were positively correlated with the numbers of CD14+CD163- and CD14+CD163-MAC387+ M1 monocytes in the MAP patients. These data were consistent with a previous report of M1-polarized macrophages at the early stage in rats with AP[29]. Similarly, our previous study had found that peripheral blood polarized M1 monocytes are present in patients with tuberculous pleural effusion (TPE)[15]. Given that the levels of plasma CRP reflect the degrees of systemic inflammation, these findings support the notion that pro-inflammatory monocytes participate in the pathogenesis of MAP[30,31]. Hence, targeting pro-inflammatory M1 monocytes may be valuable for control of MAP.
MAC387+ macrophages are recently recruiting macrophages, and increased numbers of MAC387+ macrophages are associated with poor prognosis in cancer patients[16-18,32-34]. In this study, we found significantly increased numbers of circulating MAC387+ M1 monocytes in the MAP patients, similar to the findings in patients with encephalitis[16]. Furthermore, the numbers of CD14+CD163-MAC387+ M1 monocytes were positively associated with the levels of plasma CRP in the MAP patients. These data suggest that during the pathogenic process of MAP, continual differentiated M1 monocytes display in MAP patients, creating a positive feedback loop to strengthen pro-inflammatory responses. These novel findings may provide new insights into the pathogenesis of MAP.
During the pathogenic process of MAP, inflammatory stimuli damage the pancreatic acinar cells to release many inflammatory cytokines and mediators, which recruit leukocyte infiltrates, including M2 monocytes/macrophages[28,35]. M2 monocytes/macrophages can produce anti-inflammatory molecules that control inflammation and promote tissue repair[36]. In this study, we found significantly increased numbers of CD14+CD163+CD115+ M2 monocytes and significantly elevated levels of IL-10 in MAP patients. More importantly, the numbers of CD14+CD163+CD115+ M2 monocytes were positively correlated with the levels of plasma CRP and the APACHE II scores in MAP patients. Furthermore, the levels of plasma IL-10 were positively correlated with the APACHE II scores in the MAP patients. However, our previous study had shown a decreased number of peripheral blood CD14+CD163+CD115+ M2 monocytes, which is not significantly associated with the clinical measures in TPE patients[15]. We speculate the different results may stem from the different diseases. Engagement of CD115 by CSF-1 is crucial for the survival, differentiation and possible activation of monocytes/macrophages, and can polarize macrophages toward M2-type[21,23]. Previous studies have shown that anti-CD115 can enhance anti-tumor immunity and ameliorate inflammation in humans and rodents[22,23,37-39]. The increased numbers of CD14+CD163+CD115+ M2 monocytes and elevated levels of plasma IL-10 may reflect a compensative response during the inflammatory process of MAP[40,41]. Alternatively, CD14+CD163+CD115+ monocytes may through unknown factors promote the pathogenesis of MAP. Given that APACHE II scores and the levels of plasma CRP are two standard measures for the severity of inflammatory diseases, the positive correlations suggest that the numbers of CD14+CD163+CD115+ monocytes and the levels of plasma IL-10 may be valuable for evaluating the disease severity in patients with MAP.
IL-10 is a key anti-inflammatory cytokine and can control inflammation[42]. IL-10 is associated with immunosuppression in the late phase of severe AP[43]. Previous studies have revealed elevated levels of plasma IL-10 and IL-12 in AP patients and that the levels of plasma IL-10 are biomarkers for evaluating the severity of AP[9,10,41,44]. In this study, we also observed significantly higher concentrations of plasma IL-10 and IL-12 in MAP patients. The levels of plasma IL-10 were positively correlated with the APACHE II scores in the MAP patients. However, we found significantly reduced numbers of CD14+CD163+IL-10+ M2 monocytes in the MAP patients. The contradictory data suggest that the elevated levels of plasma IL-10 may stem from other anti-inflammatory cells, such as bone marrow derived mesenchymal stem cells (MSCs), regulatory T and B cells as well as some types of dendritic cells[45]. Indeed, infusion with human MSCs inhibits pancreatitis and ameliorates tissue damage by enhancing regulatory T cell responses in a rat model of MAP[46] and restoration of regulatory B cells inhibits pancreatitis in CD19-/- mice[47]. Accordingly, it is possible that the elevated levels of plasma IL-10 from other anti-inflammatory cells may compensatively limit inflammation during the pathogenic process of MAP. We are interested in further investigating the immunoregulation during the pathogenesis of MAP.
In conclusion, our data indicated significantly increased numbers of pro-inflammatory CD14+CD163-, CD14+CD163-MAC387+ and CD14+CD163-IL-12+ M1 monocytes and reduced numbers of CD14+CD163+IL-10+ monocytes in the MAP patients. Furthermore, the levels of plasma CRP were positively correlated with the numbers of CD14+CD163- and CD14+CD163-MAC387+ M1 monocytes in the MAP patients. In addition, we detected significantly increased numbers of CD14+CD163+CD115+ monocytes and levels of plasma IL-10 in MAP patients. These data support the notion that pro-inflammatory monocytes participate in the pathogenesis of MAP. Moreover, the numbers of CD14+CD163+CD115+ monocytes were positively correlated with the levels of plasma CRP and the APACHE II scores in MAP patients, suggesting that the numbers of CD14+CD163+CD115+ monocytes may be a valuable biomarker for evaluating the severity of MAP. To the best of our knowledge, this was the first study on the numbers of peripheral blood different phenotypes of monocytes in patients with new-onset MAP. Our findings may provide new insights into the pathogenic process and immunoregulation of MAP. We recognized that our study had limitations, such as a relatively small sample size and the lack of longitudinally functional study of monocytes during the pathogenic process of MAP. We are interested in further investigating the values of these subsets of monocytes in a bigger population and in the moderate or severe acute pancreatitis to understand their roles in the pathogenesis of different types of AP.
ACKNOWLEDGMENTS
The authors would like to thank the members of the Department of Central Laboratory, The Second Part of First Hospital of Jilin University for their technical support.
COMMENTS
Background
Monocytes participate in the inflammatory process of mild acute pancreatitis (MAP) and their function is associated with the severity of MAP.
Research frontiers
To date, it is still unclear how different subsets of monocytes regulate the pathogenesis of MAP.
Innovations and breakthroughs
This study demonstrated that increased numbers of CD14+CD163- and CD14+CD163-MAC387+ M1 monocytes participate in the pathogenesis of MAP. Increased numbers of CD14+CD163+CD115+ M2 monocytes may be a valuable biomarker for evaluating the severity of MAP.
Applications
The present findings may provide new insights into the pathogenic process and immunoregulation of MAP.
Peer-review
The authors investigated the numbers of different subsets of monocytes and their associations with clinical markers of patients with MAP. They found that increased numbers of CD14+CD163- and CD14+CD163-MAC387+ M1 monocytes participated in the pathogenesis of MAP. Increased numbers of CD14+CD163+CD115+ M2 monocytes may be a valuable biomarker for evaluating the severity of MAP. These findings are interesting, and may provide new insights into the pathogenic process and immunoregulation of MAP.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Human Ethics Committee of the First Hospital of Jilin University.
Conflict-of-interest statement: The authors declare no financial or commercial conflicts of interest.
Data sharing statement: No additional data are available.
Peer-review started: October 8, 2016
First decision: December 19, 2016
Article in press: January 17, 2017
P- Reviewer: Camara-Lemarroy CR, Chvanov M, Rakonczay Z, Tonkin J S- Editor: Yu J L- Editor: Filipodia E- Editor: Zhang FF
References
- 1.Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143–152. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- 2.Shah AU, Sarwar A, Orabi AI, Gautam S, Grant WM, Park AJ, Shah AU, Liu J, Mistry PK, Jain D, et al. Protease activation during in vivo pancreatitis is dependent on calcineurin activation. Am J Physiol Gastrointest Liver Physiol. 2009;297:G967–G973. doi: 10.1152/ajpgi.00181.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saeki K, Kanai T, Nakano M, Nakamura Y, Miyata N, Sujino T, Yamagishi Y, Ebinuma H, Takaishi H, Ono Y, et al. CCL2-induced migration and SOCS3-mediated activation of macrophages are involved in cerulein-induced pancreatitis in mice. Gastroenterology. 2012;142:1010–1020.e9. doi: 10.1053/j.gastro.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 4.Sakai Y, Masamune A, Satoh A, Nishihira J, Yamagiwa T, Shimosegawa T. Macrophage migration inhibitory factor is a critical mediator of severe acute pancreatitis. Gastroenterology. 2003;124:725–736. doi: 10.1053/gast.2003.50099. [DOI] [PubMed] [Google Scholar]
- 5.Shrivastava P, Bhatia M. Essential role of monocytes and macrophages in the progression of acute pancreatitis. World J Gastroenterol. 2010;16:3995–4002. doi: 10.3748/wjg.v16.i32.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edin S, Wikberg ML, Dahlin AM, Rutegård J, Öberg Å, Oldenborg PA, Palmqvist R. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One. 2012;7:e47045. doi: 10.1371/journal.pone.0047045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregorić P, Doklestić K, Stanković S, Sijacki A, Karamarković A, Radenković D, Ivancević N, Bajec D. Interleukin-12 as a predictor of outcome in patients with severe acute pancreatitis. Hepatogastroenterology. 2014;61:208–211. [PubMed] [Google Scholar]
- 10.Gunjaca I, Zunic J, Gunjaca M, Kovac Z. Circulating cytokine levels in acute pancreatitis-model of SIRS/CARS can help in the clinical assessment of disease severity. Inflammation. 2012;35:758–763. doi: 10.1007/s10753-011-9371-z. [DOI] [PubMed] [Google Scholar]
- 11.Medeiros LT, Peraçoli JC, Bannwart-Castro CF, Romão M, Weel IC, Golim MA, de Oliveira LG, Kurokawa CS, Medeiros Borges VT, Peraçoli MT. Monocytes from pregnant women with pre-eclampsia are polarized to a M1 phenotype. Am J Reprod Immunol. 2014;72:5–13. doi: 10.1111/aji.12222. [DOI] [PubMed] [Google Scholar]
- 12.Babu S, Kumaraswami V, Nutman TB. Alternatively activated and immunoregulatory monocytes in human filarial infections. J Infect Dis. 2009;199:1827–1837. doi: 10.1086/599090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satoh N, Shimatsu A, Himeno A, Sasaki Y, Yamakage H, Yamada K, Suganami T, Ogawa Y. Unbalanced M1/M2 phenotype of peripheral blood monocytes in obese diabetic patients: effect of pioglitazone. Diabetes Care. 2010;33:e7. doi: 10.2337/dc09-1315. [DOI] [PubMed] [Google Scholar]
- 14.Zanoni I, Granucci F. Role of CD14 in host protection against infections and in metabolism regulation. Front Cell Infect Microbiol. 2013;3:32. doi: 10.3389/fcimb.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang Y, Hua SC, Qin GX, Xu LJ, Jiang YF. Different subsets of macrophages in patients with new onset tuberculous pleural effusion. PLoS One. 2014;9:e88343. doi: 10.1371/journal.pone.0088343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soulas C, Conerly C, Kim WK, Burdo TH, Alvarez X, Lackner AA, Williams KC. Recently infiltrating MAC387(+) monocytes/macrophages a third macrophage population involved in SIV and HIV encephalitic lesion formation. Am J Pathol. 2011;178:2121–2135. doi: 10.1016/j.ajpath.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukhtar RA, Moore AP, Tandon VJ, Nseyo O, Twomey P, Adisa CA, Eleweke N, Au A, Baehner FL, Moore DH, et al. Elevated levels of proliferating and recently migrated tumor-associated macrophages confer increased aggressiveness and worse outcomes in breast cancer. Ann Surg Oncol. 2012;19:3979–3986. doi: 10.1245/s10434-012-2415-2. [DOI] [PubMed] [Google Scholar]
- 18.Shabo I, Svanvik J. Expression of macrophage antigens by tumor cells. Adv Exp Med Biol. 2011;714:141–150. doi: 10.1007/978-94-007-0782-5_7. [DOI] [PubMed] [Google Scholar]
- 19.Etzerodt A, Moestrup SK. CD163 and inflammation: biological, diagnostic, and therapeutic aspects. Antioxid Redox Signal. 2013;18:2352–2363. doi: 10.1089/ars.2012.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tippett E, Cheng WJ, Westhorpe C, Cameron PU, Brew BJ, Lewin SR, Jaworowski A, Crowe SM. Differential expression of CD163 on monocyte subsets in healthy and HIV-1 infected individuals. PLoS One. 2011;6:e19968. doi: 10.1371/journal.pone.0019968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haegel H, Thioudellet C, Hallet R, Geist M, Menguy T, Le Pogam F, Marchand JB, Toh ML, Duong V, Calcei A, et al. A unique anti-CD115 monoclonal antibody which inhibits osteolysis and skews human monocyte differentiation from M2-polarized macrophages toward dendritic cells. MAbs. 2013;5:736–747. doi: 10.4161/mabs.25743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 24.Sarr MG. 2012 revision of the Atlanta classification of acute pancreatitis. Pol Arch Med Wewn. 2013;123:118–124. doi: 10.20452/pamw.1627. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Luo X, Lin Y, Tang X, Ling L, Wang L, Jiang Y. A Higher Frequency of CD14+ CD169+ Monocytes/Macrophages in Patients with Colorectal Cancer. PLoS One. 2015;10:e0141817. doi: 10.1371/journal.pone.0141817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaku Y, Imaoka H, Morimatsu Y, Komohara Y, Ohnishi K, Oda H, Takenaka S, Matsuoka M, Kawayama T, Takeya M, et al. Overexpression of CD163, CD204 and CD206 on alveolar macrophages in the lungs of patients with severe chronic obstructive pulmonary disease. PLoS One. 2014;9:e87400. doi: 10.1371/journal.pone.0087400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahmood DF, Abderrazak A, Couchie D, Lunov O, Diderot V, Syrovets T, Slimane MN, Gosselet F, Simmet T, Rouis M, et al. Truncated thioredoxin (Trx-80) promotes pro-inflammatory macrophages of the M1 phenotype and enhances atherosclerosis. J Cell Physiol. 2013;228:1577–1583. doi: 10.1002/jcp.24319. [DOI] [PubMed] [Google Scholar]
- 28.Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology. 2013;144:1230–1240. doi: 10.1053/j.gastro.2012.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu L, Yang F, Lin R, Han C, Liu J, Ding Z. Induction of m2 polarization in primary culture liver macrophages from rats with acute pancreatitis. PLoS One. 2014;9:e108014. doi: 10.1371/journal.pone.0108014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mansfield C. Pathophysiology of acute pancreatitis: potential application from experimental models and human medicine to dogs. J Vet Intern Med. 2012;26:875–887. doi: 10.1111/j.1939-1676.2012.00949.x. [DOI] [PubMed] [Google Scholar]
- 31.Folch-Puy E. Importance of the liver in systemic complications associated with acute pancreatitis: the role of Kupffer cells. J Pathol. 2007;211:383–388. doi: 10.1002/path.2123. [DOI] [PubMed] [Google Scholar]
- 32.Gebhardt C, Breitenbach U, Tuckermann JP, Dittrich BT, Richter KH, Angel P. Calgranulins S100A8 and S100A9 are negatively regulated by glucocorticoids in a c-Fos-dependent manner and overexpressed throughout skin carcinogenesis. Oncogene. 2002;21:4266–4276. doi: 10.1038/sj.onc.1205521. [DOI] [PubMed] [Google Scholar]
- 33.Kim HJ, Kang HJ, Lee H, Lee ST, Yu MH, Kim H, Lee C. Identification of S100A8 and S100A9 as serological markers for colorectal cancer. J Proteome Res. 2009;8:1368–1379. doi: 10.1021/pr8007573. [DOI] [PubMed] [Google Scholar]
- 34.Hermani A, Hess J, De Servi B, Medunjanin S, Grobholz R, Trojan L, Angel P, Mayer D. Calcium-binding proteins S100A8 and S100A9 as novel diagnostic markers in human prostate cancer. Clin Cancer Res. 2005;11:5146–5152. doi: 10.1158/1078-0432.CCR-05-0352. [DOI] [PubMed] [Google Scholar]
- 35.Xue J, Sharma V, Habtezion A. Immune cells and immune-based therapy in pancreatitis. Immunol Res. 2014;58:378–386. doi: 10.1007/s12026-014-8504-5. [DOI] [PubMed] [Google Scholar]
- 36.Porcheray F, Viaud S, Rimaniol AC, Léone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142:481–489. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chambers SK. Role of CSF-1 in progression of epithelial ovarian cancer. Future Oncol. 2009;5:1429–1440. doi: 10.2217/fon.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ide H, Seligson DB, Memarzadeh S, Xin L, Horvath S, Dubey P, Flick MB, Kacinski BM, Palotie A, Witte ON. Expression of colony-stimulating factor 1 receptor during prostate development and prostate cancer progression. Proc Natl Acad Sci USA. 2002;99:14404–14409. doi: 10.1073/pnas.222537099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayerle J, Dummer A, Sendler M, Malla SR, van den Brandt C, Teller S, Aghdassi A, Nitsche C, Lerch MM. Differential roles of inflammatory cells in pancreatitis. J Gastroenterol Hepatol. 2012;27 Suppl 2:47–51. doi: 10.1111/j.1440-1746.2011.07011.x. [DOI] [PubMed] [Google Scholar]
- 41.Vasseur P, Devaure I, Sellier J, Delwail A, Chagneau-Derrode C, Charier F, Tougeron D, Tasu JP, Rabeony H, Lecron JC, et al. High plasma levels of the pro-inflammatory cytokine IL-22 and the anti-inflammatory cytokines IL-10 and IL-1ra in acute pancreatitis. Pancreatology. 2014;14:465–469. doi: 10.1016/j.pan.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Gabryšová L, Howes A, Saraiva M, O’Garra A. The regulation of IL-10 expression. Curr Top Microbiol Immunol. 2014;380:157–190. doi: 10.1007/978-3-662-43492-5_8. [DOI] [PubMed] [Google Scholar]
- 43.Mentula P, Kylänpää ML, Kemppainen E, Jansson SE, Sarna S, Puolakkainen P, Haapiainen R, Repo H. Plasma anti-inflammatory cytokines and monocyte human leucocyte antigen-DR expression in patients with acute pancreatitis. Scand J Gastroenterol. 2004;39:178–187. doi: 10.1080/00365520310008278. [DOI] [PubMed] [Google Scholar]
- 44.Uehara S, Gothoh K, Handa H, Tomita H, Tomita Y. Immune function in patients with acute pancreatitis. J Gastroenterol Hepatol. 2003;18:363–370. doi: 10.1046/j.1440-1746.2003.02979.x. [DOI] [PubMed] [Google Scholar]
- 45.Ma X, Yan W, Zheng H, Du Q, Zhang L, Ban Y, Li N, Wei F. Regulation of IL-10 and IL-12 production and function in macrophages and dendritic cells. F1000Res. 2015;4:pii. doi: 10.12688/f1000research.7010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawakubo K, Ohnishi S, Fujita H, Kuwatani M, Onishi R, Masamune A, Takeda H, Sakamoto N. Effect of Fetal Membrane-Derived Mesenchymal Stem Cell Transplantation in Rats With Acute and Chronic Pancreatitis. Pancreas. 2016;45:707–713. doi: 10.1097/MPA.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 47.Qiu Z, Yu P, Bai B, Hao Y, Wang S, Zhao Z, Hang Z, Wang Q, Guo M, Feng Q, et al. Regulatory B10 cells play a protective role in severe acute pancreatitis. Inflamm Res. 2016;65:647–654. doi: 10.1007/s00011-016-0947-9. [DOI] [PubMed] [Google Scholar]


