Abstract
Background and Purpose
Slow recruitment in acute stroke trials hampers the evaluation of new therapies and delays the adoption of effective therapies into clinical practice. This systematic review evaluates whether recruitment efficiency and rates have increased in acute stroke trials from 1990–2014.
Methods
Acute stroke trials from 2010–2014 were identified by a search of PubMed, Medline, the Cochrane Database of Research in Stroke, and the Stroke Trials Registry. These trials were compared to a previously published dataset of trials conducted from 1990–2004.
Results
The median recruitment efficiency of trials from 1990–2004 was 0.41 participants/site/month compared to 0.26 participants/site/month from 2010–2014 (p=0.14). The median recruitment rate of trials from 1990–2004 was 26.8 participants/month compared to 19.0 participants/month from 2010–2014 (p=0.13).
Conclusions
For acute stroke trials, neither recruitment efficiency nor recruitment rates have increased over the past 25 years and, if anything, have declined.
Indexing: acute stroke trials, recruitment rate, recruitment efficiency
Subject: cerebrovascular disease/stroke
Introduction
Recruitment for randomized controlled trials (RCTs) is often far slower than investigators predict, which threatens the financial feasibility of studies and delays the evaluation and adoption of potentially beneficial new therapies.1,2 Trials of acute interventions for ischemic stroke are particularly vulnerable to slow recruitment given the narrow time window for intervention, associated risks of complications, and reliance upon surrogate decision-makers when patients are incapacitated.3–6 A large systematic review of stroke trials from 1990–2004 found a median recruitment efficiency of only 0.52 participants/site/month.7 Predictors of lower recruitment were more study sites, shorter maximum time to intervention, exclusion of mild strokes, and enrollment across multiple countries. Clinical investigators have worked to improve recruitment in recent years, relying upon waivers of consent, stroke networks, and even telemedicine assessments. Yet, despite adoption of these methods, it is not clear that recruitment has improved. This systematic review evaluates whether recruitment efficiency and rates have increased in newer acute stroke trials (2010–2014) compared to older trials (1990–2004).
Methods
This review analyzes RCTs of interventions for ischemic stroke within 12 hours of symptom-onset. The older trials (1990–2004) that serve as a comparator for analysis were included in a systematic review by Elkins et al.7 The 32 trials in that review were all hospital-based (as opposed to field-based) trials with more than 300 participants, 18 of which had interventions applied within 12 hours of symptom-onset. Our search strategy for identifying newer trials (2010–2014) has been reported elsewhere8 and is consistent with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement.9 Our search yielded 36 trials, 10 of which were hospital-based trials with more than 300 study participants occurring within 12 hours of symptom-onset (Supplemental Figure I; Supplemental Table I). Thus, a total of 28 trials are included in this review: 18 older trials (1990–2004) and 10 newer trials (2010–2014). The following variables were abstracted from each trial: number of participants and sites, trial duration, region, intervention, maximum time to intervention, publication year, recruitment efficiency, and recruitment rate. Recruitment efficiency was defined as the number of participants enrolled per site per month. Recruitment rate was defined as the number of participants enrolled per month across all sites. Categorical variables were compared by Fisher’s exact test. Continuous variables were compared by Student t-test and ANOVA. A Wilcoxon-rank sum test was used to compare medians. All hypothesis tests were two-sided. Stata version 14.1 (StataCorp, College Station, TX) was used for analysis.
Results
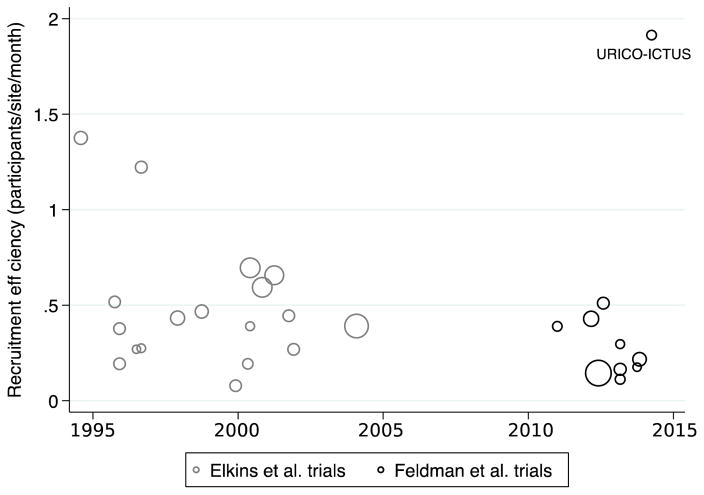
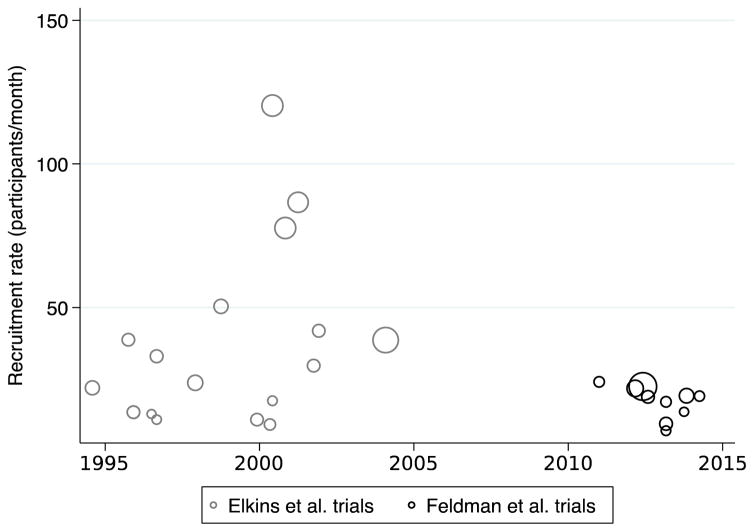
The median recruitment efficiency was 0.41 participants/site/month from 1990–2004 and 0.26 participants/site/month from 2010–2014 (p=0.14; Table 1; Figure 1). The median recruitment rate was 26.8 participants/month from 1990–2004 and 19.0 participants/month from 2010–2014 (p=0.13; Figure 2). The mean number of sites, individuals, and maximum time to intervention in each trial were similar during the two time periods. There were more thrombolytic trials (7 trials versus 1 trial) and fewer endovascular trials (0 trials versus 2 trials) in the earlier time period.
Table 1.
Comparison of historic stroke trials (1990–2004) with newer trials (2010–2014)
| 1990–2004 (n=18 trials) | 2010–2014 (n=10 trials) | p-value | |
|---|---|---|---|
|
| |||
| Number of individuals per trial, mean (SD) | 903 (626) | 824.2 (812) | 0.78 |
|
| |||
| Number of sites per trial, mean, (SD) | 81.4 (47.9) | 71.9 (49.7) | 0.62 |
|
| |||
| Intervention type, number of trials (%) | 0.09 | ||
| Thrombolysis | 7 (38.9) | 1 (10.0) | |
| Neuroprotectant | 9 (50.0) | 7 (70.0) | |
| Monoclonal antibody | 1 (6.3) | 0 (0) | |
| Fibrinogen-reducing agent | 1 (6.3) | 0 (0) | |
| Endovascular | 0 (0) | 2 (20.0) | |
|
| |||
| Maximum time to intervention (hours), mean (SD) | 6.22 (2.4) | 6.47 (2.7) | 0.81 |
|
| |||
| Overall recruitment rate (participants/month), median (IQR) | 26.8 (13.5–41.9) | 19.0 (13.7–21.8) | 0.13 |
|
| |||
| Overall recruitment efficiency (subjects/site/month), median (IQR) | 0.41 (0.27–0.59) | 0.26 (0.16–0.43) | 0.14 |
Figure 1. Recruitment efficiency of acute stroke trials by publication year (1990–2014).
The area of individual markers is proportional to the number of participants per trial. URICO-ICTUS: Safety and efficacy of uric acid in patients with acute stroke.
Figure 2. Recruitment rate of acute stroke trials by publication year (1990–2014).
The area of individual markers is proportional to the number of participants per trial.
Among older trials, the mean recruitment efficiency for thrombolytic trials was 0.31 participants/site/month (n=7) compared to 0.61 participants/site/month for non-thrombolytic trials (n=11) (p=0.07). The mean recruitment rate for older thrombolytic trials was 21.6 participants/month versus 45.5 participants/month in older non-thrombolytic trials (p=0.11).
Discussion
Neither recruitment rate nor efficiency has increased in acute stroke trials over the past 25 years. An outlier among newer trials is URICO-ICTUS (with a recruitment efficiency of 1.91 participants/site/month).10 One distinguishing feature of URICO-ICTUS was that only higher-volume stroke centers (which gave alteplase to at least 50 patients per year) were included. The remainder of newer trials either required thrombolytic treatment for 20 or fewer stroke patients per year or made no explicit mention of such a requirement. With only high-volume stroke centers in URICO-ICTUS, recruitment efficiency was high, though recruitment rate was comparable to other trials.
The declining trends in recruitment efficiency and rate over time are not attributable to differences in the maximum time to intervention or the mean number of sites, as these parameters were comparable across both time periods. Moreover, they are not attributable to variation in the number of trials testing thrombolytic interventions. Though older trials were more likely to test thrombolytic interventions, recruitment efficiency and rate were both lower in thrombolytic trials when compared to non-thrombolytic trials. This suggests that recruitment may have been faster in older trials despite (not because of) the higher number of thrombolytic trials among older studies.
One possible explanation for our findings is the growth of community hospitals equipped to treat acute stroke patients (e.g. Primary Stroke Centers in the US). These hospitals may be less likely to transfer acute stroke patients to academic centers, which, in turn, may limit the pool of candidates for clinical trials. Another explanation is that trial protocols have become more complex. Though the number of study sites per trial have remained relatively constant, the number of endpoints, exclusion criteria, procedures, and follow-ups in neurology trials have increased.11 With more steps required to enroll patients and deliver interventions, recruitment may have slowed. Thirdly, given the heterogeneity of both trial designs and interventions studied, recruitment into acute stroke trials may have been affected by factors that are simply not captured by our analysis (including shifting financial incentives and inclusion of international sites). Finally, the two sets of trials from which this review draws were generated by different search criteria and may have therefore selected for different types of trials.
A recent meta-analysis of methods to improve recruitment in RCTs across fields (beyond neurology trials) found that opt-out strategies and open-trial designs were effective in increasing recruitment.2 The first of these strategies has been pursued in several recent stroke trials testing field-based interventions, the most prominent of which tested thrombolytic agents on mobile stroke units.12 The recruitment efficiency of that trial was 10.5 participants/site/month, and the recruitment rate was 294.4 participants/month—both far higher than the trials in this review and indeed far higher than other recent field-based trials with opt-in enrollment.8 We specifically excluded field-based trials from this review to enable proper comparison of newer trials to those in the Elkins dataset, which included only hospital-based interventions. But field-based interventions designed to improve the speed with which stroke therapy is delivered may also enhance the recruitment of trials as more participants are reached before the time window for inclusion closes. Currently, only 5% of patients with ischemic strokes arrive to the hospital in time for thrombolytic therapy.13 Opt-out strategies raise challenging ethical questions, which merit further examination, but they have gained increasing acceptance by institutional review boards (IRBs) and the Food and Drug Administration.8 Whether such opt-out strategies become more prevalent in acute stroke trials remains to be seen.
A further advance that may increase recruitment in acute stroke trials is the development of clinical trials networks like NIH StrokeNet, a network of more than 25 regional coordinating centers and over 200 hospitals that works to organize stroke research through a centralized IRB, streamlined contracting, and continuity of investigators and study personnel. NIH StrokeNet may prove to be a boon for recruitment in acute stroke trials by amortizing the costs of establishing and maintaining research infrastructure and supporting and developing professional research staff over multiple concurrent and successive clinical trials.
Despite these organizational advances, however, recruitment will still likely hinge on a multitude of competing factors. Further investigation is needed into methods that will improve recruitment in acute stroke trials. More timely completion of trials is vital for maintaining progress in the efforts to reduce the high morbidity and mortality of stroke.
Supplementary Material
Acknowledgments
Sources of Funding: Supported by the NIH (NIA, K23AG043553).
Footnotes
Conflicts of Interest: None.
Contributor Information
William B. Feldman, Brigham and Women’s Hospital, Department of Medicine.
Anthony S. Kim, University of California San Francisco, Weill Institute for Neurosciences, Department of Neurology.
Winston Chiong, University of California San Francisco, Weill Institute for Neurosciences, Department of Neurology, Memory and Aging Center.
References
- 1.McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7:9. doi: 10.1186/1745-6215-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Treweek S, Lockhart P, Pitkethly M, Cook JA, Kjeldstøm M, Johansen M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013;3:e002360. doi: 10.1136/bmjopen-2012-002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexandrov AV. Slow recruitment in clinical trials: failure is not an option! Int J Stroke. 2006;1:160. doi: 10.1111/j.1747-4949.2006.00046.x. [DOI] [PubMed] [Google Scholar]
- 4.Kasner SE, Del Giudice A, Rosenberg S, Sheen M, Luciano JM, Cucchiara BL, et al. Who will participate in acute stroke trials? Neurology. 2009;72:1682–1688. doi: 10.1212/WNL.0b013e3181a55fbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiong W, Kim AS, Huang IA, Farahany NA, Josephson SA. Testing the presumption of consent to emergency treatment for acute ischemic stroke. JAMA. 2014;311:1689–1691. doi: 10.1001/jama.2014.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiong W, Kim AS, Huang IA, Farahany NA, Josephson SA. Inability to consent does not diminish the desirability of stroke thrombolysis. Ann Neurol. 2014;76:296–304. doi: 10.1002/ana.24209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elkins JS, Khatabi T, Fung L, Rootenberg J, Johnston SC. Recruiting subjects for acute stroke trials: a meta-analysis. Stroke. 2006;37:123–128. doi: 10.1161/01.STR.0000195149.44390.aa. [DOI] [PubMed] [Google Scholar]
- 8.Feldman WB, Kim AS, Josephson SA, Lowenstein DH, Chiong W. Effect of waivers of consent on recruitment in acute stroke trials: A systematic review. Neurology. 2016;86:1543–1551. doi: 10.1212/WNL.0000000000002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 10.Chamorro A, Amaro S, Castellanos M, Segura T, Arenillas J, Martí-Fábregas, et al. Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): a randomised, double-blind phase 2b/3 trial. Lancet Neurol. 2014;13:453–460. doi: 10.1016/S1474-4422(14)70054-7. [DOI] [PubMed] [Google Scholar]
- 11.Silverman A. Increasing Therapeutic Complexity in CNS Clinical Trials: The need for Therapeutically Aligned Staff. J Clin Stud. 2014;6:40–42. [Google Scholar]
- 12.Ebinger M, Winter B, Wendt M, Weber J, Waldschmidt C, Rozanski M, et al. Effect of the use of ambulance-based thrombolysis on time to thrombolysis in acute ischemic stroke: a randomized clinical trial. JAMA. 2014;311:1622–1631. doi: 10.1001/jama.2014.2850. [DOI] [PubMed] [Google Scholar]
- 13.Kleindorfer D, de los Rios La Rosa F, Khatri P, Kissela B, Mackey J, Adeoye O. Temporal trends in acute stroke management. Stroke. 2013;44(suppl 1):S130–S131. doi: 10.1161/STROKEAHA.113.001457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.