Abstract
Glaucoma is a complex group of diseases wherein a selective degeneration of retinal ganglion cells (RGCs) leads to irreversible loss of vision. A comprehensive approach to glaucomatous RGC degeneration may include stem cells to functionally replace dead neurons through transplantation and understand RGCs vulnerability using a disease in a dish stem cell model. Both approaches require the directed generation of stable, functional, and target-specific RGCs from renewable sources of cells, i.e., the embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). Here, we demonstrate a rapid and safe, stage-specific, chemically defined protocol that selectively generates RGCs across species, including human, by recapitulating the developmental mechanism. The de novo generated RGCs from pluripotent cells are similar to native RGCs at the molecular, biochemical, functional levels. They also express axon guidance molecules, and discriminate between specific and non-specific targets, and are non-tumorigenic.
Graphical Abstract
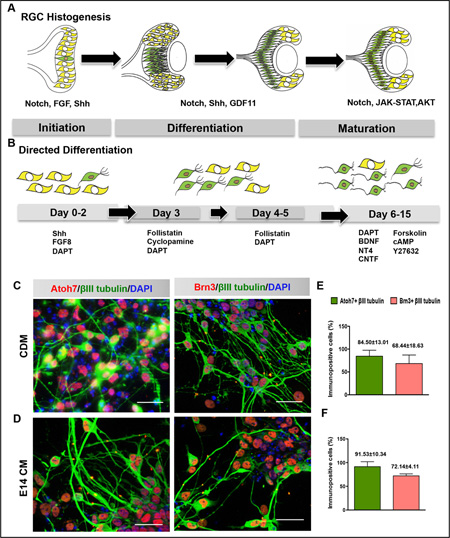
Recapitulation of developmental mechanism for de novo generation of RGCs. Schematic of retinal development and cell-extrinsic factors involved in RGC differentiation (A). Schematic representation of sequential steps involved in initiation, differentiation and maturation of in vitro generated retinal progenitor cells along RGC lineage by stage-specific manipulation of signaling pathways using recombinant growth factors and small molecules (B). mESC-RPCs, under both conditions differentiated into RGCs as ascertained by cells expressing Atoh7+βIII tubulin and Brn3+βIII tubulin immunoreactivities (C–F). Each bar represents mean ± s.d. Scale bar: 20µm. Abbreviations: RPC, retinal progenitor cells; RGC, retinal ganglion cells; Shh, sonic Hedgehog; FGF8, fibroblast growth factor 8; DAPT, (N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester); BDNF, brain-derived neurotrophic factor; NT4, neurotrophin-4; CNTF, cilliary neurotrophic factor; cAMP, Adenosine-3′,5′-cyclic monophosphate; CM, conditioned medium; CDM, chemically defined medium.
INTRODUCTION
Glaucoma represents a group of diseases, which are associated with multiple risk factors and genetic variants [1]. The unifying theme is the progressive degeneration of retinal ganglion cells (RGCs) that carry information from the retina to the brain for visual perception, and their degeneration leads to irreversible loss of vision. It is projected that by 2020, approximately 80 million people worldwide will suffer from open angle glaucoma and angle closure glaucoma [2]. Currently, there is no effective treatment for RGC degeneration. Neuroprotective approaches, proven successful in animal models of glaucoma [3, 4], are of limited practical use as their efficacy has not been demonstrated in clinics and they do not address RGCs that have already succumbed to the disease [5]. A comprehensive approach requires answers to the following questions: (A) Why are RGCs vulnerable in glaucoma? (B) How can degenerated cells be functionally replaced? The answers may lie in stem cell technology, wherein underlying pathological mechanisms can be studied in a dish, using patient-specific, induced pluripotent (iPS) stem cells [6–9] and degenerated RGCs may be replaced using ex-vivo stem cell approaches [10].
The success of these approaches depends upon de novo generation of RGCs from pluripotent cells with stable molecular, cellular, and physiological phenotypes. The reproducibility of directed RGC generation, ascertained at multiple levels, is essential for unambiguous screening of the pathological phenotype in patient-specific iPS cells. Most importantly from the perspective of ex-vivo stem cell approaches, these RGCs should be able to discriminate between specific and non-specific targets to establish functional connections with bona fide ones. The efficient and reproducible generation of stable RGCs requires the development of a protocol that recapitulates a normal RGC developmental mechanism, generates cells with guidable axons, and is clinically safe. Current published protocols for RGC generation have exploited the default potential of ES/iPS cells to generate optic vesicle-like structure containing complements of all retinal cell types, rather than predominantly RGCs [8, 11, 12].
Here, we present a chemically defined approach, based on developmental mechanism, that leads to directed differentiation of retinal progenitors cells (RPCs) along the RGC lineage. This approach is comprehensively tested on RPCs derived from both mouse and human pluripotent cells in parallel with native RPCs. We demonstrate that the step-wise temporal recruitment of Shh, Notch, FGF, and TGFβ signaling allows native or pluripotent cell-derived RPCs to recapitulate the hierarchical gene expression that underlies initiation, differentiation, and maturation of RGCs, enabling directed acquisition of RGC phenotypes. The resulting RGCs, like those differentiated from the native RPCs, were defined by small soma and extensive processes and expressed multiple RGC-specific markers. The similarity in transcriptional signatures between RGCs differentiated from the pluripotent RPCs and RGCs enriched from the retina further reflected the stability of the acquired phenotype at the molecular and genomic levels. Examination of these cells at the physiological levels revealed electrophysiological properties characteristic of RGCs [13–15]. A species-specific difference in the physiological maturity of RGCs was observed, dependent on the expression of REST (Repressor Element 1-Silencing Transcription Factor), a negative regulator of RGC differentiation [16]. Silencing of its expression in mouse ES cell derived RGCs (mESC-RGCs) restored the physiology observed in human iPSC derived RGCs (hiPSC-RGCs). More importantly, from the viewpoint of ex-vivo stem cell approach for RGC degeneration, the de novo-generated RGCs expressed a battery of molecules necessary for axonal guidance to seek appropriate targets. Our method led to complete silencing of pluripotency genes: these cells failed to form teratomas, demonstrating their safety for clinical use. Taken together, our approach, based on developmental principles, can efficiently and reproducibly generate stable and safe RGCs for a disease in dish model of glaucoma, providing a practical and safe ex-vivo stem cell approach.
MATERIALS & METHODS
Detailed materials and methods are described in the supplemental materials and methods.
Generation of RPCs
Mouse embryonic stem cells (mESCs) (D3) were maintained on mitotically inactivated mouse embryonic fibroblasts as previously described [17]. A human iPSC line, derived from the foreskin fibroblasts, was maintained in feeder-free conditions as previously described [18]. Differentiation of mESCs along retinal lineage was induced as described previously [19]. Retinal induction of hiPSCs was performed using the protocol published previously [20]. E18 rat RPCs were enriched using neurosphere assay as previously described [21, 22].
Differentiation of RPCs into RGCs
RPCs derived from E18 retina/mESCs and human iPSCs were plated onto PDL/Laminin, and matrigel coated dishes, respectively and cultured in basal medium with the following stage-specific inducers/growth factors: RGC differentiation was initiated by treating cells for 2 days with Shh (250ng/ml), FGF8 (100ng/ml), and DAPT (3µM); RGCs differentiation was facilitated by treatment with Follistatin (100ng/ml), Cyclopamine (0.25µg/ml) and DAPT (3µM) for 1 day, followed by Follistatin (100ng/ml) and DAPT (3µM) for 2 days. Finally, RGCs maturation and survival was promoted by supplementing medium with BDNF (100ng/ml), Forskolin (5µM), NT4 (5ng/ml), CNTF (10ng/ml) cAMP (400µM), Y27632 (10µM) and DAPT (3µM) for the next 10 days. Medium was changed every 2–3 days.
Immunocytochemical Analysis
Immunocytochemistry analysis was performed as published elsewhere [19]. Sholl analysis was performed with the software ImageJ using the plugin Sholl Analysis (v1.50) with a 20 µm ring interval from neural rosettes [23].
RESULTS
Developmental signaling pathways for RGC differentiation
Among seven different cell types in the vertebrate retina, RGCs are specified first, regardless of species, suggesting an underlying evolutionarily conserved mechanism for their generation. The mechanism may involve an instructive niche that facilitates differentiation of early RPCs along the RGC lineage. In support of this premise, it has been demonstrated that retinal cells isolated from the initial stage of early histogenesis, either from a chick, rat, or mouse retina, elaborate potent RGC-promoting activities capable of inducing differentiation of retinal and non-retinal progenitors, including those derived from pluripotent cells into RGCs [22, 24–26]. Together, these observations suggested that RPCs are malleable in response to their environment through cell-to-cell interactions. Based on this premise, we designed an approach that recapitulated the cell-signaling dependent initiation, differentiation, and maturation of RGCs in vitro (Fig. 1A, B). This approach was exhaustively tested on RPCs derived from the rat retina and mESCs first before applying on those derived from hiPSCs.
Figure 1. Recapitulation of developmental mechanism for de novo generation of RGCs.
Schematic of retinal development and cell-extrinsic factors involved in RGC differentiation (A). Schematic representation of sequential steps involved in initiation, differentiation and maturation of in vitro generated retinal progenitor cells along RGC lineage by stage-specific manipulation of signaling pathways using recombinant growth factors and small molecules (B). mESC-RPCs, under both conditions differentiated into RGCs as ascertained by cells expressing Atoh7+βIII tubulin and Brn3+βIII tubulin immunoreactivities (C–F). Each bar represents mean ± s.d. Scale bar: 20µm. Abbreviations: RGC, retinal ganglion cells; Shh, sonic Hedgehog; FGF8, fibroblast growth factor 8; DAPT, (N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester); BDNF, brain-derived neurotrophic factor; NT4, neurotrophin-4; CNTF, cilliary neurotrophic factor; cAMP, Adenosine-3′,5′-cyclic monophosphate; CM, conditioned medium; CDM, chemically defined medium.
Evidence has emerged that the initiation of RGC differentiation begins in the center of the developing retina, presumably coordinated by FGF, Shh, and Notch signaling pathways (Fig. 1A). For example, it has been observed that transient FGF signaling by FGF8 and FGF3 facilitates initial RGC differentiation in the central retina [27, 28]. Shh is likely to be similarly involved. However, beyond the initiation of RGC differentiation, Shh may promote proliferation, thus maintaining RPCs for subsequent differentiation [27–30]. A coordinated decrease in Notch signaling is essential for RPCs to commit along the RGC lineage [31, 32]. Based on these observations, our strategy to initiate RGC differentiation in vitro included transiently activating FGF and Shh signaling and inhibiting Notch activities for the first 48 hours in culture, as outlined in Fig. 1B. Subsequently, FGF8 was removed from the culture and cyclopamine added to inhibit the Shh elaborated by the nascent RGCs that could adversely affect differentiation. Notch signaling, like Shh signaling, was kept inhibited to prevent any drift of committed precursors back into the proliferative mode. To keep the committed precursors on the RGC differentiation track, we left the TGFβ pathway inhibited, given that activation of the TGFβ pathway by GDF11, secreted by differentiating RGCs, has been observed to be a potent inhibitor of RGC differentiation [33]. The survival of nascent RGCs depends upon neurotrophins to prevent the activation of programmed cell death (PCD) [34]. To facilitate RGC maturation without excessive cell death, BDNF, NT4, and CNTF, all known to prevent PCD in RGCs [35–38], were included with general promoters of cell survival, Forskolin and Rock inhibitor [35, 39, 40].
We first tested the three-stage chemically defined medium (CDM) protocol on late RPCs, which are developmentally constrained from giving rise to early born neurons such as RGCs [31] (Supporting Information Fig. S1A). The CDM protocol was examined in parallel to that of a previously described method involving the induction of RGCs under conditioned media obtained from E14 rat retinal cells (E14 CM) [19]. We observed that the majority of late RPCs under the CDM protocol expressed immunoreactivities corresponding to RGC-specific markers, Atoh7, Islet1, and Brn3++βIII tubulin+ at the end of day 15 in culture (Supporting Information Fig. S1B, D). The numbers of cells expressing RGC-specific immunoreactivities were comparable when late RPCs were differentiated under E14CM (Supporting Information Fig. S1C, E). Temporal analysis of the pattern of gene expression during RGC differentiation revealed decreasing levels of transcripts corresponding to RPCs and concordant increase of those encoding regulators (Atoh7, Brn3b, Isl1) and mature markers (Thy1) of RGCs (Supporting Information Fig. S1F). Whole cell patch recordings and recordings in lose-patch conditions of RGCs differentiated under the influence of CDM revealed robust, inward, voltage-gated sodium, and outward potassium currents (Supporting Information Fig. S2A–B) and spontaneous currents (Supporting Information Fig. S2C), respectively, similar to those observed in cells differentiated along the RGC lineage under the influence of E14 CM (Supporting Information Fig. S2D–F). Having confirmed CDM influence in conferring the RGC phenotype on late RPCs, we carried out a comprehensive examination of its ability to differentiate RPCs derived from mESCs along the RGC lineage. As a reference for the efficiency, stability, and fidelity of RGC differentiation, the method was again examined in parallel with RPC induction under the influence of E14 CM [19]. Under both protocols Atoh7++βIII tubulin+ (Supporting Information Fig. S3A) and Brn3++βIII tubulin+ (Supporting Information Fig. S3B) cells were first detected at day 3, albeit at a low number. Their number increased steadily: at day 15 of differentiation, 84.50±13.013 % and 68.44±18.639 % of the total cells were Atoh7++βIII tubulin+ and Brn3++βIII tubulin+ (Fig. 1C–F), respectively. The Atoh7++βIII tubulin+ cells reflected an immature state, and therefore their numbers were relatively higher than the latter. Similarly, the number of cells expressing the matured RGC marker, Thy1, confirmed for RGC lineage by the co-expression of Brn3, was lower than Atoh7++βIII tubulin+ and Brn3++βIII tubulin+ cells (Fig. 2C, E). These cells (mESC-RGCs), regardless of the protocol, displayed small soma and long processes. Together, the immunocytochemical analyses suggested that CDM was equivalent to E14CM in promoting the expression of RGC-specific markers in mESC-RPCs.
Figure 2. Temporal expression pattern of RGC regulatory genes during RGC differentiation under the influence of CDM.
A schematic representation of gene regulatory hierarchy for RGC specification and differentiation is given in (A). Q-PCR analysis revealed a temporal increase in transcripts corresponding to RGC regulators (Atoh7, Brn3B and Islet1) and mature markers (Thy1 and GAP43) under the influence of CDM (B) and E14 CM (D). A subset of cells expressed Brn3+Thy1 immunoreactivities corroborating the acquisition of mature phenotype under both conditions (C, E). Scale bar: 20µm.
Hierarchical regulatory gene expression for RGC differentiation
The specification and differentiation of RGCs in the developing vertebrate retina requires temporal activation of hierarchical gene expression. Recent studies suggest that in this complex genetic hierarchy, instructive information is likely to flow through the nodes that are occupied by Atoh7, Isl1, and Brn3b (Fig. 2A) [41–45]. We were interested to know whether the CDM was capable of influencing this RGC gene regulatory network, essential if the RGC phenotype displayed at the end of day 15 were to remain stable. A temporal expression analysis during RGC induction under CDM and E14CM revealed a decrease in levels of transcripts corresponding to Rx and Pax6 (data not shown) and reciprocal increase in Atoh7 over time, suggesting a progressive commitment of RPCs and their specification along the RGC lineage (Fig. 2B, D). The temporal increase in transcripts corresponding to Brn3b/Islet1 and GAP43/Thy1 suggested their differentiation and maturation into RGCs, respectively. The presence of cells expressing immunoreactivities of both Brn3 and Thy1 at day 15 in vitro corroborated gene expression analysis of the acquisition of the matured RGC phenotype (Fig. 2C, E). We were next interested in whether mESC-RGCs differentiated under the two different conditions had acquired the similar genomic signature of the native RGCs. We immune-panned mESC-RGCs differentiated under the influence of CDM and E14CM using Thy1.2 antibodies [19]. The two populations of mESC-RGCs, CDM-RGCs and CM-RGCs, were first subjected to RNA-seq analysis (Fig. 3A). A comparison of transcriptional profiles between CDM- and E14CM-derived RGCs by Scatterplot using the log2 of expression levels as fragment per kilo base of transcripts per million mapped reads (FPKM) revealed similar patterns of expression between the two populations, where Spearman correlation co-efficiency (ρ) was 0.96 (Fig. 3B). Next, the transcription profiles of CDM-RGCs were compared with those obtained from RGCs, similarly enriched from the PN2 mouse retina (Retinal-RGCs). The analysis found high correlations (ρ=0.83) between the de novo generated and native RGC population transcriptomes (Fig. 3C). Analysis of number of genes expressed (FPKM>0.5) revealed that the CDM-RGCs shared 80.7% of those with the retinal-RGCs (Fig. 3D) and their GO analysis represented shared biological processes, for example, those associated with neuronal differentiation, axonogenesis, cell cycle and cytoskeleton regulation (Fig. 3E). The shared expression included 382 RGC-associated genes (e.g., Atoh7, Brn3b, Isl1, Shh, SMARCA2, Irx2, Robo2, NRP1, SNCG, RPF1, EphA4, WT1) previously identified using different approaches [42, 46, 47] (data not shown). To identify the key pathways encoded by the expressed gene sets, analysis of the curated KEGG pathway repositories was carried out. The enriched KEGG signaling pathways included those involved in RGC differentiation (e.g., Notch and Shh pathways), survival (e.g., Neurotrophin signaling and JAK-STAT pathways), and communication (axon guidance and mTOR signaling pathways) (Fig. 3F).
Figure 3. Global gene expression pattern in de novo generated and native RGCs.
A Schematic representation of Thy1.2 antibody-based enrichment of RGCs, derived under the influence of E14CM (CM-RGCs), CDM (CDM-RGCs) and from PN2 mouse retina (Retinal-RGCs) (A). Correlation of expressed genes by Scatterplot between CM-RGCs and CDM-RGCs (B) and CDM-RGCs and retinal-RGCs (C). ρ = Spearman correlation coefficiency. Venn diagram of genes expressed (FPKM>0.5) by CDM-RGCs and retinal RGCs alone and by both (D). GO analysis of biological processes that are significantly enriched within the shared expressed genes (p values; Bonferroni) (E). The enriched KEGG signaling pathways reveal those involved in RGC development and biology (F).
Functional properties of RGCs and the role of REST
Next, we wanted to know if the de novo differentiated mESC-RGCs possessed pan-neuronal and RGC-specific electrophysiological properties. Examination of current profiles by whole cell, patch clamped recordings yielded different results for mESC-RGCs generated under the two different conditions. When currents were evoked by a series of voltage steps (−100mV to +20mV) in an identical condition of holding potential of −90mV, 30% of mESC-RGCs under CDM displayed only delayed and sustained outward potassium currents, and no fast inward currents corresponding to voltage-gated sodium channel were observed (Fig. 4A). In contrast, 62.5% of mESC-RGCs under E14CM revealed the presence of fast inward and delayed but sustained outward currents (Fig. 4C). Examination of the fast inward and outward currents in mESC-RGCs generated under E14CM showed an I-V relationship typical of voltage–gated sodium and delayed potassium currents, respectively (Fig. 4D). Such I-V relationships, observed in isolated native RGCs [13–15], could not be detected in mESC-RGCs derived under the influence of CDM conditions due to the absence of voltage-gated sodium currents (Fig. 4B). We argued that the non-neuronal electrophysiological signature of mESC-RGCs derived under CDM despite acquiring RGC-specific molecular and chemical phenotypes could be due to a less than favorable stoichiometric ratio of the expressed RGC-specific genes required to confer functional maturity. Such a notion could be explained by the inhibitory effects on hierarchical gene expression imposed by residual expression of REST, a global inhibitor of neuronal genes that has emerged as a known negative regulator of RGC differentiation[16]. To see if REST is involved in the functional maturity of RGCs, we first determined its relative expression in E18-RGCs and mESCs-RGCs under CDM conditions. We observed significantly higher levels of transcripts corresponding to REST in the latter than in the former (Supporting Information Fig. S4). When we reduced REST transcript levels in RPCs by shRNA-mediated REST loss-of-function (LOF) and then subjected them to RGC differentiation (Fig. 4E), we observed a significant increase in levels of transcripts corresponding to Atoh7, Brn3b, and Islet1, compared to controls (Fig. 4F). Subsequent whole cell patch clamp recording of differentiated cells revealed prominent voltage-dependent sodium currents, which had been previously absent (Fig. 4G) and had displayed a symmetrical RGCs-like I-V relationship (Fig. 4H). Furthermore, we found 66.6% of REST LOF cells capable of generating depolarization evoked action potential when clamped in loose patch conditions (Fig. 4I). Together, these observations suggested that inhibition of REST is essential for functional maturity of mESCs-RGCs.
Figure 4. Restoration of functional maturity in mESC-RGCs generated under the influence of CDM following REST-LOF.
Whole cell patch clamp analysis of cells, performed at the end of differentiation, revealed that mESCs-RGCs induced by CDM displayed only delayed outward currents but no inward currents (A) and lacked typical I-V relationship due to absence of voltage gated sodium currents (B). mESC-RGCs induced by E14 CM displayed fast inward and delayed outward currents in 62.5% of cells (C) and demonstrated I-V curve indicative of typical voltage-gated sodium channels and delayed potassium currents, respectively (D). mESCs-RPCs were transduced with REST shRNA + GFP/ Control GFP lentivirus and subjected to RGC differentiation using CDM (E). REST shRNA transduced cells showed significant increase in the expression of RGCs specific markers (Atoh7, Brn3b and Islet1) and decrease in the expression of REST, as compared to controls (F). Whole cell patch clamp of recorded mESC-RGCs after REST knockdown showed voltage dependent sodium currents (G), typical IV curve of sodium and potassium currents (H), and evoked action potential in 66.6% of cells (I).
Differentiation of human iPSCs into functional RGCs
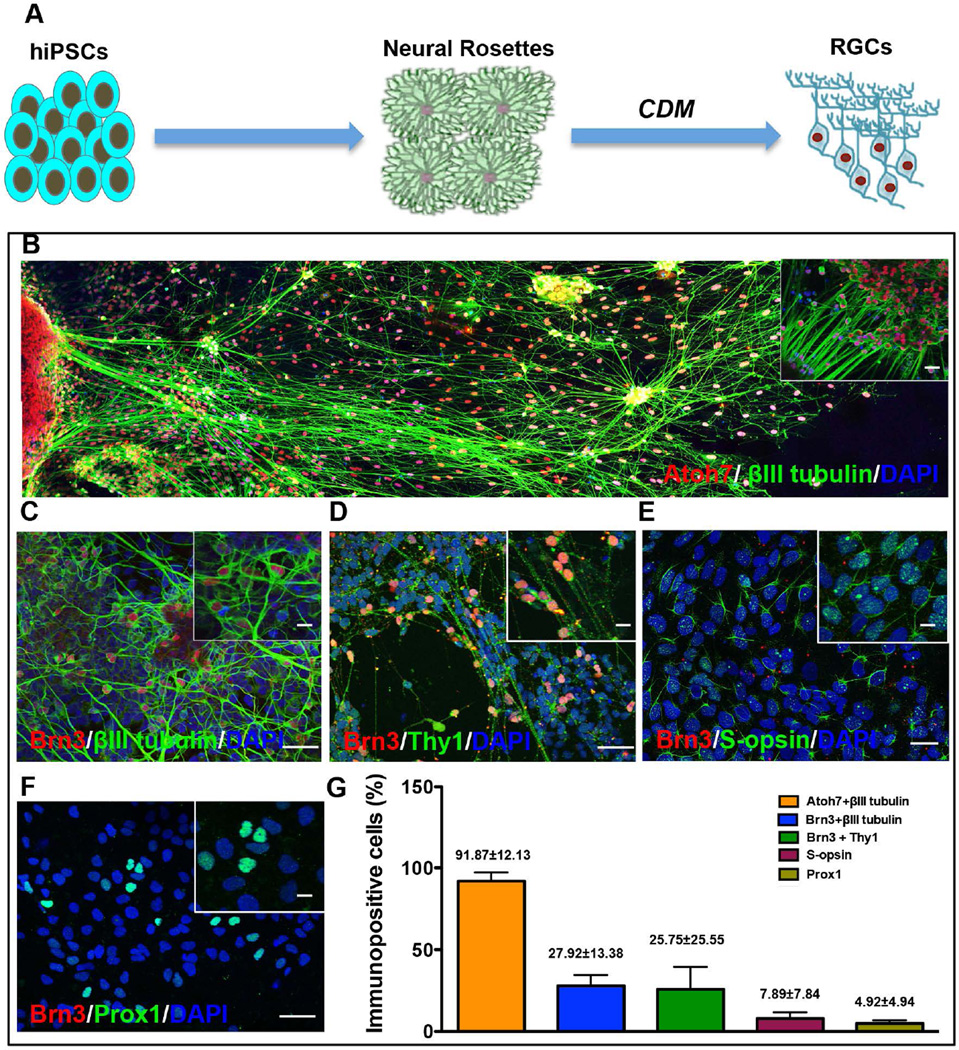
Next, we examined whether or not the CDM was equally effective in differentiating RPCs derived from human pluripotent cells into RGCs (hiPSC-RGCs). hiPSCs were cultured in enhanced IGF signaling and reduced BMP and Wnt signaling to obtain neural rosettes, as previously described [20]. Immunocytochemical examination of neural rosettes revealed cells co-expressing RPC markers, Rx and Pax6 (Supporting Information Fig. S5A–C, D). The proportion of cells expressing immunoreactivities corresponding to Pax6 and Rx was 91.27±2.92% (Supporting Information Fig. S5D). Results obtained by immunocytochemical analysis were corroborated by a significant increase in levels of transcripts corresponding to Rx and Pax6, compared to those in uninduced hiPSCs (Supporting Information Fig. S5E, F). Exposure of these cells to three-stage CDM protocol led to a remarkable change in their morphology, with small soma and elongated network of processes at day 15 in culture (Fig. 5A–B). Immunocytochemical analysis revealed the majority of these cells (91.87±12.13%) co-expressed Atoh7 and βIII tubulin immunoreactivities. The proportions of cells co-expressing Brn3 and βIII tubulin and Brn3 and Thy1 were 27.92±13.38% and 25.75±25.55%, respectively (Fig. 5C, D, G). In contrast, a minor proportion of cells expressed immunoreactivities corresponding to other early born neurons, s-opsin (7.89±7.84) (cone photoreceptors) and Prox1 (4.92±4.94) (horizontal cells), suggesting RGC differentiation of hiPSCs (Fig. 5E–G). This premise was further supported by the absence of cells expressing rhodopsin, a marker corresponding to rod photoreceptors and a Muller glia specific marker, Glast (data not shown). Analysis of cell-type-specific gene expression corroborated these results. The expression of Rx and Pax6 decreased significantly at day 15 in culture, compared to controls, demonstrating the loss of RPC properties in the presence of CDM (Supporting Information Fig. S6). In contrast, levels of transcripts corresponding to Atoh7, Brn3b, and Islet1 increased ~1.5 fold, and those of Thy1 doubled in cells at day 15 in culture versus controls. These observations and others, such as these cells also expressed the vesicular glutamate transporter 2 (VGluT2), the vesicular glutamate transporter expressed in RGCs [48] (Supporting information Fig. S7A), suggested that CDM induced directed differentiation of hiPSC-RPCs along the RGC lineage. We used electrophysiolgical analysis of hiPSC-RGCs to test for the hallmarks of neuronal function in cells with neuronal morphology. These cells backfilled with Lucifer yellow-expressed Brn3b, identifying them as RGCs (Fig. 6A). Whole-cell, voltage-clamp recordings of these cells revealed the presence of fast inward Na+ currents and outwardly-rectifying K+ currents (Fig. 6B). Na+ currents were reversibly blocked by tetrodotoxin (1 µM) and had mean amplitude of 655 ± 341 pA measured at −26 mV (n = 7) (Fig. 6C). Examination of fast inward currents and outwardly rectifying currents displayed an I-V relationship typical of voltage–dependent Na+ currents and delayed potassium currents (Fig. 6D). In current clamp recordings from cells with particularly large INa (n = 2 cells tested; peak INa = 2064 and 2191 pA), depolarizing current injections evoked action potentials (Fig. 6E). These results suggest that the three-stage CDM approach was effective in generating functional RGCs across species by recruiting normal developmental mechanisms.
Figure 5. Differentiation of hiPSC into RGCs using CDM.
Schematic showing experimental protocol used for stepwise differentiation of hiPSCs into RGCs (A). A panoramic view of five images of hiPSC-RGCs coexpressing Atoh7 and βIII tubulin immunoreactivities, revealed long and complex network of processes (B). Immunostaining showing a subset of cells coexpressing immunoreactivities corresponding to Brn3+βIII tubulin (C, G) and Brn3 +Thy1 (D, G). A minor subset of Brn3 negative cells expressed S-opsin (E, G) and Prox1 immunoreactivities (F, G), corresponding to early born retinal neurons, cone photoreceptors and horizontal cells, respectively. Scale bar: Panels B-F 50µm; Inset: 20µm.
Figure 6. Electrophysiological properties of hiPSC-RGCs.
Whole cell patch clamp recording of Brn3+ hiPSC-RGCs, identified by backfilling of cells with lucifer yellow (LY) (A), revealed fast inward and delayed outward currents, evoked by depolarizing steps (−66 to +4 mV) from a holding potential of −76 mV; the former being TTX sensitive (B, C). The fast inward and outward currents showed I-V relationship typical of voltage-gated sodium (INa) and delayed potassium (IK) currents, respectively (n=7 cells) (D). Voltage traces from the same cell in a current-clamp recording showed responses to depolarizing and hyperpolarizing current injections (E). Scale bar: 20µm.
Target specificity and safety of de novo-generated human RGCs
The utility of hiPSC-RGCs in ex-vivo stem cell approaches required functional maturity of differentiated cells to elaborate guidable axons that can reach bonafide targets for recovering lost vision. Therefore, we examined whether or not hiPSC-RGCs express receptors for axon guidance molecules. RT-PCR analysis of hiPSC-RGCs revealed that they expressed transcripts corresponding to ROBO2, which facilitates guidance within the retina and at the optic chiasm, DCC, which is required for the exit of the axons at the optic disc, NEUROPILIN 1 (NRP1), for keeping the axons coalesced and EPHs for establishing the spatial gradient of connections in the superior colliculus [49]. We observed that the expression of transcripts corresponding to these molecules, which was not detectable in un-induced hiPSCs and hiPSC-RPCs, was activated in hiPSC-RGCs under the influence of CDM (Fig. 7A). We also examined the expression of GAP43, a cytoplasmic protein essential for guiding axons from the optic chiasm into the optic tracts (Supporting Information Fig. S6) [49]. Immunocytochemical analysis of select guidance molecules revealed the expression of immunoreactivities corresponding to ROBO2 and GAP43 in hiPSC-RGCs, corroborating the PCR results at the cellular levels (Supporting Information Fig. S7B–C). Together, these observations suggested that hiPSC-RGCs had the molecular make-up necessary to respond to guidance cues for target selection. To test the premise that these cells possess the target specificity of native RGCs, we cultured CFDA-labeled hiPSC-RGCs across cell aggregates from either the superior colliculus (SC) or inferior colliculus (IC), obtained from the rat midbrain [19]. Given the fact that retino-colliculi connections are phylogenetically old [50], molecules mediating these connections are expected to be evolutionarily conserved and thus functional across the species. We observed that the CFDA-labeled hiPSC-RGCs elaborated long processes in the presence of SC explants and oriented them toward the SC cells, the specific target of RGC axons in the mid brain (Fig. 7B). The esterified fluorchrome complexes of CFDA do not stain the newly elaborated process and remain trapped within the cell body, thus highlighting it. The CFDA-stained hiPSC-RGCs, when cultured in the presence of the IC cells (a target of the auditory neurons), elaborated short processes that failed to orient themselves toward the explants (Fig. 7C). Sholl analysis of the processes emanating from hiPSC-RGCs (neural rosettes identified as center of analysis) revealed a significant increase in their length when cultured in the proximity of SC cells, compared to those close to IC cells (Fig. 7D). We next wanted to know if the presence of SC cells affected the function of hiPSC-RGCs. In cells co-cultured with SC explants for 9 days, depolarizing steps revealed the presence of outwardly rectifying potassium currents and fast inward sodium currents that were reversibly blocked by TTX (1 µM) (Fig. 7E). Measured at −26 mV, INa had a mean amplitude of 1261 ± 222 pA (n = 8 cells), twice that recorded from hiPSC-RGCs in non-co-culture conditions (Fig. 7F). Examination of fast inward currents and outwardly rectifying currents displayed an I-V relationship typical of voltage–dependent Na+ currents and delayed potassium currents (Fig. 7G). In current-clamp recordings, depolarizing current injections evoked action potentials in these cells (8/8 cells) (Fig. 7H). Action potentials were somewhat variable in amplitude, peaking at ~0 mV (−3.3 ± 4.4 mV; range: +17 to −24 mV). The action potential amplitude positively correlated with the amplitude of the sodium current (r = 0.74, p<0.05, Pearson correlation). In a handful of recorded cells (3/6 cells tested) a 2-second puff of L-glutamate onto the soma evoked a small inward current (12.6 ± 2.0 pA, n = 3) that was reversibly inhibited by the AMPA/Kainate antagonist CNQX (30 µM), suggesting that those cells expressed functional ionotropic glutamate receptors (Fig. 7I). Together, these results suggested that hiPSC-RGCs possessed the molecular and cellular wherewithal for axonal guidance and were capable of discriminating specific and non-specific targets while maintaining their physiological properties, features essential for an ex-vivo stem cell approach to RGC degeneration. Also essential in this regard was ensuring that hiPSC-RGCs had fully differentiated and lost the propensity of the iPS cells to form tumors. To test this premise we first screened hiPSC-RGCs for the expression of pluripotency genes. Q-PCR analysis of hiPSCs and hiPSC-RGCs revealed that transcripts corresponding to Oct4 and Nanog, easily detected in the former, were absent in the latter (Supporting Information Fig. S8A). These observations suggested that the three-stage CDM protocol effectively silenced the pluripotency genes and therefore generated hiPSC-RGCs that might not be tumorigenic. To test this, hiPS cells/ hiPSC-RGCs were injected in NOG mice. Those injected with hiPSCs formed teratomas in 6 weeks and those with hiPSC-RGCs remained free of teratomas at 12 weeks post-injection, when the recipient mice were sacrificed (Supporting Information Fig. S8B, C). Our results demonstrate that the three-stage CDM protocol could efficiently generate hiPSC-RGCs with the molecular, cellular, and functional attributes of native RGCs, capable of target specificity and safe for ex-vivo replacement of degenerated RGCs in glaucomatous neuropathy.
Figure 7. Target specificity of hiPSC-RGCs.
RT-PCR analysis revealed the expression of transcripts corresponding to axon guidance molecules (ROBO2, DCC, NRP1, EPHA3, EPHA4, EPHB2 and EPHB3) in hiPSC-RGCs compared to un-induced iPSCs and hiPSC-RPCs (A). CFDA-tagged hiPSC-RGCs elaborated long processes toward SC cells aggregate (B), compared to the one co-cultured with IC cells aggregate (C). Sholl analysis of processes of hiPSC-RGCs co cultured with SC and IC cell aggregates, displayed a significant difference in the length of processes elaborated towards SC/IC cells aggregate (D). Whole cell patch clamp recording of hiPSC-RGCs co-cultured with SC cells aggregate, revealed fast inward and delayed outward currents, evoked by depolarizing steps (−66 to +24 mV) from a holding potential of −76 mV; the former being TTX sensitive (E, F). The fast inward and outward currents showed I-V relationship typical of voltage-gated sodium (INa) and delayed potassium (IK) currents, respectively (n=8 cells) (G). Voltage traces from the same cell in a current-clamp recordings showed responses to depolarizing and hyperpolarizing current injections (H). Inward currents evoked in response to a 2-second puff of L-glutamate (1 mM) in control conditions, in the presence of 30 µM CNQX, and following washout of CNQX (I). Scale bar: 50µm. M= marker lane.
DISCUSSION
The selective degeneration of RGCs in glaucoma leads to irreversible blindness. The universal vulnerability of RGCs in this complex group of disorders remains unexplained and practical strategies to replace degenerated cells remain elusive. Pluripotent stem cell technology may shed light on the intractable degenerative disease. The patient-specific iPSCs, may reveal why these neurons are susceptible to glaucoma risk factors and lead to better diagnosis and formulation of therapeutic approaches. The ex-vivo stem cell approaches using RGCs derived from pluripotent cells may replace degenerated neurons. The success of both cases depends on the generation of RGCs in a dish that faithfully resembles their native counterparts in structure, function, and in existing as final output neurons of the retina that are capable of discriminating between authentic and non-specific targets.
Several approaches have been reported for the de novo generation of RGCs, the majority of them based on the default retinal potential of pluripotent cells, exposed when they are cultured in conditions that facilitate the formation of optic vesicle-like structures [8, 11, 12, 51, 52]. This is a reproducible approach wherein retinal cells are generated in an evolutionarily conserved, temporal sequence observed in vivo. Early born neurons including RGCs are differentiated earlier than late born neurons, e.g., rod photoreceptors. However, this method and its variations do not promote directed differentiation of RPCs into RGCs; therefore, the end stage culture represents complements of all retinal cell types, including Muller glia, which may not be desirable for generating an RGC disease model or for ex-vivo stem cell approaches [51]. Several studies have also shown that in the optic vesicle-like structures, expression of RPC (e.g., Pax6, Rx, and Chx10) and or photoreceptor (e.g., Crx) regulators [8, 11, 12, 53–55] remain up-regulated, raising the issue of the existence of cells in sustained transitional states. This is supported by a recent observation that Brn3-positive cells generated by a similar method co-expressed retinal progenitor markers [8]. Such transitional states may affect phenotype stability and function.
Therefore, our goal was to develop a CDM-based protocol, underpinned by a normal RGC development mechanism, wherein RPCs generated from pluripotent cells were constrained to preferentially differentiate along the RGC lineage. In this method, CDM influenced two interacting processes required for RGC differentiation; it adversely affected the maintenance of RPCs as demonstrated by a temporal decrease in the expression of RX and Pax6, and channeled their commitment along the RGC lineage by activating the stage-specific expression of key RGC regulators, Atoh7, Brn3b, and Isl1. Their expression was detected at the specification (day2) of RPCs and later became stage-specific. For example, the expression of Atoh7 peaked during the early stages of differentiation of RGCs (day 5). In contrast, the expression of more distal regulators in the hierarchical gene regulatory network, Brn3b and Isl1 increased significantly at day 15, timed with RGC differentiation and maturation. This ability of CDM to reproducibly influence the RGC gene regulatory network in a stage-specific manner in both native and pluripotent cell-derived RPCs may underlie the acquisition of transcriptional signature and electrophysiological properties similar to that of native RGCs. However, a difference in species-specific response was observed when RPCs were exposed to CDM. While RGCs generated from rat RPCs and hiPSC-RPCs obtained functional maturity as ascertained by electrophysiological criteria, those from mESC-RPCs did not despite expression of all other RGC features. This was attributed to a persistent REST expression in the latter because a loss of function of REST restored functionality, similar to that of rat and hiPSC-RGCs. This observation suggests two possibilities. First, the inability of CDM to suppress REST expression in mouse pluripotent cells may be the function of species or pluripotent cell-specific (ESCs versus iPSCs) epigenetic signatures. Second, if REST remains expressed the stoichiometry of RGC-specific gene expression remains below the threshold of facilitating functional maturity.
The method presented here is rapid and efficient in generating RGCs. In 15 days RPCs were differentiated into matured RGCs, a faster rate than published methods of 40 to 90 days [8, 11, 12, 53, 56]. Quantification of cells at the end of differentiation revealed ~90% of total mouse and human cells expressed immunoreactivities corresponding to Atoh7 and βIII tubulin, demonstrating that the CDM committed the majority of cells along the RGC lineage. This was demonstrated by either the absence (Muller glia) or the presence of other retinal cell types (cone photoreceptors and horizontal cells) as a minor Brn3 negative population in the culture. Further, unlike the observation associated with the default pathway-based methods, immunoreactivities corresponding to Pax6 and Rx were not detected in Brn3 positive cells, demonstrating that the CDM effectively silenced the regulators of RPCs. Still, the transition of Atoh7++βIII tubulin+ positive cells into more matured RGCs, i.e., those expressing Brn3+/Brn3+Thy1+ immunoreactivities, was not synchronous and appeared delayed, regardless of species. It may be that recently committed RGCs remain in different stages of development and at that stage of culture only a subset of those have advanced to a stage of maturity, displayed by the co-expression of Brn3 and Thy1. Nevertheless, the pluripotent cell-derivatives at day 15 had irreversibly committed along lineage-specific differentiation so as to lose their atavistic tumorigenic potential.
The function of RGCs as projection neurons of the retina depends upon their ability to form contacts with central targets such as the LGN and SC for conscious and subconscious visual perception, respectively. This requires expression of guidance molecules that help RGC axons navigate to their targets. The generation of an accurate disease model of glaucomatous degeneration or ex-vivo stem cell therapy to replace degenerated RGCs necessitates the demonstration of RGCs’ ability to guide their axons to bona fide targets. The RGCs generated under the influence of CDM fulfill the criteria of projection neurons. They express a battery of guidance molecules confirming their molecular competence as native RGCs for guiding the axons within the retina, at the optic chiasm, and for their topographical connections in the central targets, SC and LGN. They also discriminate between cells from SC and IC by extending processes when exposed to the former not the latter. This suggests patient-specific or normal RGCs generated under the influence of CDM could be evaluated for the characteristics of native RGCs, with the potential to replace cells degenerated in glaucomatous neuropathy.
CONCLUSIONS
Our observations demonstrate that pluripotent cells, regardless of species and origin, can be directly and rapidly differentiated into functional, target-specific, and safe RGCs by recapitulating developmental mechanisms through stage-specific chemically defined conditions. These attributes of the de novo generated RGCs posit these cells as a valuable and practical reagent for understanding and addressing glaucomatous RGC degeneration.
Supplementary Material
A Schematic representation of E18 RPCs, enriched by neurosphere assay into RGCs under the influence of CDM and E14 CM (A). Immunocytochemical analysis of de novo generated RGCs, revealed a subset of cells expressing immunoreactivities corresponding to Atoh7, Islet1 and Brn3 +βIII tubulin positive cells (B, D) similar to E14 CM (C, E). Q-PCR analysis revealed temporal decrease in transcripts corresponding to Rx and Pax6 with increase in the expression of Atoh7, Brn3b, Islet1 and Thy1 (F). Scale bar: 50µm.
Whole cell patch recordings of RGCs differentiated under the influence of CDM revealed robust, inward, voltage-gated sodium, and outward potassium currents (A–B) and spontaneous currents (C) similar to those observed in cells differentiated along the RGC lineage under the influence of E14 CM (D–F).
Atoh7++ βIII tubulin+ and Brn3++ βIII tubulin+ cells are detected by day 3, the proportion of former being grater than the latter. The proportion of cells expressing RGC-specific immunoreactivities increased with time (A, B). Scale bar: 20µm.
QPCR analysis revealed that mESCs-RGCs expressed significantly high expression level of REST transcripts, compared to those derived from late RPCs (A).
Immunocytochemical analysis performed on neural rosettes on day 24 revealed the expression of immunoreactivities corresponding to Rx (A, D), Pax6 (B, D), and Rx+Pax6 in colonies (C, D). Q-PCR analysis on neural-rosette cells revealed significantly higher level of transcripts corresponding to Rx and Pax6, compared to that in un-induced iPSCs (E, F). Scale bar: 50µm.
Q-PCR analysis revealed a temporal decrease in the expression of Rx and Pax6 with concordant increase in transcripts corresponding to RGC regulators (Atoh7, Brn3b and Islet1) and mature markers (Thy1 and GAP43).
hiPSC-RGCs expressed immunoreactivities corresponding to vGluT2 (A) GAP43 (B) and ROBO2 (B). Cell nuclei were counterstained with DAPI. Scale bar: 20µm.
Q-PCR analysis revealed silencing of transcripts corresponding to pluripotency genes (Oct4 and Nanog) in hiPSC-RGCs compared to un-induced hiPSCs at the end of differentiation (A). Transplantation of hiPSCs and not hiPSC-RGCs led to tumor formation in NOG mice (B). Analysis of hiPSCs-derived teratoma demonstrated the presence of tissues of the three germ lineages (C). Scale bar: 100µm.
Acknowledgments
The research was supported by NIH/NEI: R01-EY022051 (IA), Research to Prevent Blindness, NIH/NEI:RO1 EY010145 (JCM) and P30 EY010572 (Casey Core grant). We thank Dr. Larisa Poluektova for providing NOG (NOD/Shi-scid/IL-2Rγnull) mice.
Footnotes
Author contributions:
P.T.: Conception and design, Collection and/or assembly of data, Data analysis and interpretation and Manuscript writing
D.C: Collection and/or assembly of data, Data analysis and interpretation
S.M.D.: Collection and/or assembly of data, Data analysis and interpretation
M.V.H.: Collection and/or assembly of data, Data analysis and interpretation
F.Q: Data analysis and interpretation
J.M.: Conception and design
A.R.: Provision of study material or patients
I.A.: Conception and design, Collection and/or assembly of data, Data analysis and interpretation and Manuscript writing
Disclosure of potential conflicts of interest: The authors indicate no conflicts of interest.
REFERENCES
- 1.Ahram DF, Alward WL, Kuehn MH. The genetic mechanisms of primary angle closure glaucoma. Eye (Lond) 2015;29:1251–1259. doi: 10.1038/eye.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danesh-Meyer HV, Levin LA. Neuroprotection: extrapolating from neurologic diseases to the eye. Am J Ophthalmol. 2009;148:186–191. e182. doi: 10.1016/j.ajo.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 4.Baltmr A, Duggan J, Nizari S, et al. Neuroprotection in glaucoma - Is there a future role? Exp Eye Res. 2010;91:554–566. doi: 10.1016/j.exer.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Dahlmann-Noor AH, Vijay S, Limb GA, et al. Strategies for optic nerve rescue and regeneration in glaucoma and other optic neuropathies. Drug Discov Today. 2010;15:287–299. doi: 10.1016/j.drudis.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Tucker BA, Solivan-Timpe F, Roos BR, et al. Duplication of TBK1 Stimulates Autophagy in iPSC-derived Retinal Cells from a Patient with Normal Tension Glaucoma. J Stem Cell Res Ther. 2014;3:161. doi: 10.4172/2157-7633.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minegishi Y, Iejima D, Kobayashi H, et al. Enhanced optineurin E50K-TBK1 interaction evokes protein insolubility and initiates familial primary open-angle glaucoma. Hum Mol Genet. 2013;22:3559–3567. doi: 10.1093/hmg/ddt210. [DOI] [PubMed] [Google Scholar]
- 8.Ohlemacher SK, Sridhar A, Xiao Y, et al. Stepwise Differentiation of Retinal Ganglion Cells from Human Pluripotent Stem Cells Enables Analysis of Glaucomatous Neurodegeneration. Stem Cells. 2016 doi: 10.1002/stem.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sterneckert JL, Reinhardt P, Scholer HR. Investigating human disease using stem cell models. Nat Rev Genet. 2014;15:625–639. doi: 10.1038/nrg3764. [DOI] [PubMed] [Google Scholar]
- 10.Levin LA, Ritch R, Richards JE, et al. Stem cell therapy for ocular disorders. Arch Ophthalmol. 2004;122:621–627. doi: 10.1001/archopht.122.4.621. [DOI] [PubMed] [Google Scholar]
- 11.Maekawa Y, Onishi A, Matsushita K, et al. Optimized Culture System to Induce Neurite Outgrowth From Retinal Ganglion Cells in Three-Dimensional Retinal Aggregates Differentiated From Mouse and Human Embryonic Stem Cells. Curr Eye Res. 2016;41:558–568. doi: 10.3109/02713683.2015.1038359. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka T, Yokoi T, Tamalu F, et al. Generation of retinal ganglion cells with functional axons from human induced pluripotent stem cells. Sci Rep. 2015;5:8344. doi: 10.1038/srep08344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipton SA, Tauck DL. Voltage-dependent conductances of solitary ganglion cells dissociated from the rat retina. J Physiol. 1987;385:361–391. doi: 10.1113/jphysiol.1987.sp016497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han Y, Jacoby RA, Wu SM. Morphological and electrophysiological properties of dissociated primate retinal cells. Brain Res. 2000;875:175–186. doi: 10.1016/s0006-8993(00)02614-7. [DOI] [PubMed] [Google Scholar]
- 15.Barres BA, Silverstein BE, Corey DP, et al. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. doi: 10.1016/0896-6273(88)90127-4. [DOI] [PubMed] [Google Scholar]
- 16.Mao CA, Tsai WW, Cho JH, et al. Neuronal transcriptional repressor REST suppresses an Atoh7-independent program for initiating retinal ganglion cell development. Dev Biol. 2011;349:90–99. doi: 10.1016/j.ydbio.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teotia P, Mohanty S, Kabra M, et al. Enhanced Reprogramming Efficiency and Kinetics of Induced Pluripotent Stem Cells Derived from Human Duchenne Muscular Dystrophy. PLoS Curr. 2015;7 doi: 10.1371/currents.md.a77c2f0516a8cb4809ffad5963342905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Claassen DA, Desler MM, Rizzino A. ROCK inhibition enhances the recovery and growth of cryopreserved human embryonic stem cells and human induced pluripotent stem cells. Mol Reprod Dev. 2009;76:722–732. doi: 10.1002/mrd.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parameswaran S, Dravid SM, Teotia P, et al. Continuous non-cell autonomous reprogramming to generate retinal ganglion cells for glaucomatous neuropathy. Stem Cells. 2015;33:1743–1758. doi: 10.1002/stem.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamba DA, McUsic A, Hirata RK, et al. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One. 2010;5:e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad I, Dooley CM, Thoreson WB, et al. In vitro analysis of a mammalian retinal progenitor that gives rise to neurons and glia. Brain Res. 1999;831:1–10. doi: 10.1016/s0006-8993(99)01376-1. [DOI] [PubMed] [Google Scholar]
- 22.Parameswaran S, Balasubramanian S, Babai N, et al. Induced pluripotent stem cells generate both retinal ganglion cells and photoreceptors: therapeutic implications in degenerative changes in glaucoma and age-related macular degeneration. Stem Cells. 2010;28:695–703. doi: 10.1002/stem.320. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci. 2009;29:476–482. doi: 10.1523/JNEUROSCI.4117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James J, Das AV, Bhattacharya S, et al. In vitro generation of early-born neurons from late retinal progenitors. J Neurosci. 2003;23:8193–8203. doi: 10.1523/JNEUROSCI.23-23-08193.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hegde GV, James J, Das AV, et al. Characterization of early retinal progenitor microenvironment: presence of activities selective for the differentiation of retinal ganglion cells and maintenance of progenitors. Exp Eye Res. 2007;84:577–590. doi: 10.1016/j.exer.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Del Debbio CB, Peng X, Xiong H, et al. Adult ciliary epithelial stem cells generate functional neurons and differentiate into both early and late born retinal neurons under non-cell autonomous influences. BMC Neurosci. 2013;14:130. doi: 10.1186/1471-2202-14-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCabe KL, Gunther EC, Reh TA. The development of the pattern of retinal ganglion cells in the chick retina: mechanisms that control differentiation. Development. 1999;126:5713–5724. doi: 10.1242/dev.126.24.5713. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Morales JR, Del Bene F, Nica G, et al. Differentiation of the vertebrate retina is coordinated by an FGF signaling center. Dev Cell. 2005;8:565–574. doi: 10.1016/j.devcel.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 29.Neumann CJ, Nuesslein-Volhard C. Patterning of the zebrafish retina by a wave of sonic hedgehog activity. Science. 2000;289:2137–2139. doi: 10.1126/science.289.5487.2137. [DOI] [PubMed] [Google Scholar]
- 30.Zhang XM, Yang XJ. Regulation of retinal ganglion cell production by Sonic hedgehog. Development. 2001;128:943–957. doi: 10.1242/dev.128.6.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.James J, Das AV, Rahnenfuhrer J, et al. Cellular and molecular characterization of early and late retinal stem cells/progenitors: differential regulation of proliferation and context dependent role of Notch signaling. J Neurobiol. 2004;61:359–376. doi: 10.1002/neu.20064. [DOI] [PubMed] [Google Scholar]
- 32.Nelson BR, Gumuscu B, Hartman BH, et al. Notch activity is downregulated just prior to retinal ganglion cell differentiation. Dev Neurosci. 2006;28:128–141. doi: 10.1159/000090759. [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Wu HH, Lander AD, et al. GDF11 controls the timing of progenitor cell competence in developing retina. Science. 2005;308:1927–1930. doi: 10.1126/science.1110175. [DOI] [PubMed] [Google Scholar]
- 34.Guerin MB, McKernan DP, O'Brien CJ, et al. Retinal ganglion cells: dying to survive. Int J Dev Biol. 2006;50:665–674. doi: 10.1387/ijdb.062159mg. [DOI] [PubMed] [Google Scholar]
- 35.Meyer-Franke A, Kaplan MR, Pfrieger FW, et al. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 36.Cohen A, Bray GM, Aguayo AJ. Neurotrophin-4/5 (NT-4/5) increases adult rat retinal ganglion cell survival and neurite outgrowth in vitro. J Neurobiol. 1994;25:953–959. doi: 10.1002/neu.480250805. [DOI] [PubMed] [Google Scholar]
- 37.Spalding KL, Rush RA, Harvey AR. Target-derived and locally derived neurotrophins support retinal ganglion cell survival in the neonatal rat retina. J Neurobiol. 2004;60:319–327. doi: 10.1002/neu.20028. [DOI] [PubMed] [Google Scholar]
- 38.Ji JZ, Elyaman W, Yip HK, et al. CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: the possible involvement of STAT3 pathway. Eur J Neurosci. 2004;19:265–272. doi: 10.1111/j.0953-816x.2003.03107.x. [DOI] [PubMed] [Google Scholar]
- 39.Santos RC, Araujo EG. Cyclic AMP increases the survival of ganglion cells in mixed retinal cell cultures in the absence of exogenous neurotrophic molecules, an effect that involves cholinergic activity. Braz J Med Biol Res. 2001;34:1585–1593. doi: 10.1590/s0100-879x2001001200011. [DOI] [PubMed] [Google Scholar]
- 40.Lingor P, Tonges L, Pieper N, et al. ROCK inhibition and CNTF interact on intrinsic signalling pathways and differentially regulate survival and regeneration in retinal ganglion cells. Brain. 2008;131:250–263. doi: 10.1093/brain/awm284. [DOI] [PubMed] [Google Scholar]
- 41.Mu X, Klein WH. A gene regulatory hierarchy for retinal ganglion cell specification and differentiation. Semin Cell Dev Biol. 2004;15:115–123. doi: 10.1016/j.semcdb.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 42.Mu X, Fu X, Beremand PD, et al. Gene regulation logic in retinal ganglion cell development: Isl1 defines a critical branch distinct from but overlapping with Pou4f2. Proc Natl Acad Sci U S A. 2008;105:6942–6947. doi: 10.1073/pnas.0802627105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mu X, Fu X, Sun H, et al. A gene network downstream of transcription factor Math5 regulates retinal progenitor cell competence and ganglion cell fate. Dev Biol. 2005;280:467–481. doi: 10.1016/j.ydbio.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 44.Das AV, James J, Bhattacharya S, et al. SWI/SNF chromatin remodeling ATPase Brm regulates the differentiation of early retinal stem cells/progenitors by influencing Brn3b expression and Notch signaling. J Biol Chem. 2007;282:35187–35201. doi: 10.1074/jbc.M706742200. [DOI] [PubMed] [Google Scholar]
- 45.Wagner KD, Wagner N, Schley G, et al. The Wilms' tumor suppressor Wt1 encodes a transcriptional activator of the class IV POU-domain factor Pou4f2 (Brn-3b) Gene. 2003;305:217–223. doi: 10.1016/s0378-1119(02)01231-3. [DOI] [PubMed] [Google Scholar]
- 46.Qiu F, Jiang H, Xiang M. A comprehensive negative regulatory program controlled by Brn3b to ensure ganglion cell specification from multipotential retinal precursors. J Neurosci. 2008;28:3392–3403. doi: 10.1523/JNEUROSCI.0043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ivanov D, Dvoriantchikova G, Nathanson L, et al. Microarray analysis of gene expression in adult retinal ganglion cells. FEBS Lett. 2006;580:331–335. doi: 10.1016/j.febslet.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 48.Stella SL, Jr, Li S, Sabatini A, et al. Comparison of the ontogeny of the vesicular glutamate transporter 3 (VGLUT3) with VGLUT1 and VGLUT2 in the rat retina. Brain Res. 2008;1215:20–29. doi: 10.1016/j.brainres.2008.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erskine L, Herrera E. The retinal ganglion cell axon's journey: insights into molecular mechanisms of axon guidance. Dev Biol. 2007;308:1–14. doi: 10.1016/j.ydbio.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 50.Masterton RB, Glendenning KK. Phylogeny of the Vertebrate Sensory Systems. In: Masterton RB, editor. Sensory Integration. Boston, MA: Springer US; 1978. pp. 1–38. [Google Scholar]
- 51.Volkner M, Zschatzsch M, Rostovskaya M, et al. Retinal Organoids from Pluripotent Stem Cells Efficiently Recapitulate Retinogenesis. Stem Cell Reports. 2016;6:525–538. doi: 10.1016/j.stemcr.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakano T, Ando S, Takata N, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10:771–785. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 53.Sluch VM, Davis CH, Ranganathan V, et al. Differentiation of human ESCs to retinal ganglion cells using a CRISPR engineered reporter cell line. Sci Rep. 2015;5:16595. doi: 10.1038/srep16595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer JS, Howden SE, Wallace KA, et al. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. 2011;29:1206–1218. doi: 10.1002/stem.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meyer JS, Shearer RL, Capowski EE, et al. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2009;106:16698–16703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riazifar H, Jia Y, Chen J, et al. Chemically induced specification of retinal ganglion cells from human embryonic and induced pluripotent stem cells. Stem Cells Transl Med. 2014;3:424–432. doi: 10.5966/sctm.2013-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A Schematic representation of E18 RPCs, enriched by neurosphere assay into RGCs under the influence of CDM and E14 CM (A). Immunocytochemical analysis of de novo generated RGCs, revealed a subset of cells expressing immunoreactivities corresponding to Atoh7, Islet1 and Brn3 +βIII tubulin positive cells (B, D) similar to E14 CM (C, E). Q-PCR analysis revealed temporal decrease in transcripts corresponding to Rx and Pax6 with increase in the expression of Atoh7, Brn3b, Islet1 and Thy1 (F). Scale bar: 50µm.
Whole cell patch recordings of RGCs differentiated under the influence of CDM revealed robust, inward, voltage-gated sodium, and outward potassium currents (A–B) and spontaneous currents (C) similar to those observed in cells differentiated along the RGC lineage under the influence of E14 CM (D–F).
Atoh7++ βIII tubulin+ and Brn3++ βIII tubulin+ cells are detected by day 3, the proportion of former being grater than the latter. The proportion of cells expressing RGC-specific immunoreactivities increased with time (A, B). Scale bar: 20µm.
QPCR analysis revealed that mESCs-RGCs expressed significantly high expression level of REST transcripts, compared to those derived from late RPCs (A).
Immunocytochemical analysis performed on neural rosettes on day 24 revealed the expression of immunoreactivities corresponding to Rx (A, D), Pax6 (B, D), and Rx+Pax6 in colonies (C, D). Q-PCR analysis on neural-rosette cells revealed significantly higher level of transcripts corresponding to Rx and Pax6, compared to that in un-induced iPSCs (E, F). Scale bar: 50µm.
Q-PCR analysis revealed a temporal decrease in the expression of Rx and Pax6 with concordant increase in transcripts corresponding to RGC regulators (Atoh7, Brn3b and Islet1) and mature markers (Thy1 and GAP43).
hiPSC-RGCs expressed immunoreactivities corresponding to vGluT2 (A) GAP43 (B) and ROBO2 (B). Cell nuclei were counterstained with DAPI. Scale bar: 20µm.
Q-PCR analysis revealed silencing of transcripts corresponding to pluripotency genes (Oct4 and Nanog) in hiPSC-RGCs compared to un-induced hiPSCs at the end of differentiation (A). Transplantation of hiPSCs and not hiPSC-RGCs led to tumor formation in NOG mice (B). Analysis of hiPSCs-derived teratoma demonstrated the presence of tissues of the three germ lineages (C). Scale bar: 100µm.