Abstract
The airway epithelium of cigarette smokers undergoes dramatic remodeling with hyperplasia of basal cells (BC) and mucus-producing cells, squamous metaplasia, altered ciliated cell differentiation and decreased junctional barrier integrity, relevant to chronic obstructive pulmonary disease and lung cancer. In this study, we show that epidermal growth factor receptor (EGFR) ligand amphiregulin (AREG) is induced by smoking in human airway epithelium as a result of epidermal growth factor (EGF)-driven squamous differentiation of airway BC stem/progenitor cells. In turn, AREG induced a unique EGFR activation pattern in human airway BC, distinct from that evoked by EGF, leading to BC- and mucous hyperplasia, altered ciliated cell differentiation and impaired barrier integrity. Further, AREG promoted its own expression and suppressed expression of EGF, establishing an autonomous self-amplifying signaling loop in airway BC relevant for promotion of EGF-independent hyperplastic phenotypes. Thus, EGF-AREG interplay in airway BC stem/progenitor cells is one of the mechanisms that mediates the interconnected pathogenesis of all major smoking-induced lesions in the human airway epithelium.
Keywords: Airway basal cells, Epidermal growth factor receptor, Hyperplasia, Metaplasia, Chronic obstructive pulmonary disease
Introduction
The human airway epithelium is composed of four major cell types, for example, differentiated ciliated and secretory cells, intermediate cells (IC), and basal cells (BC) [1, 2]. The distribution of individual cell types in the airway epithelium is tightly controlled to ensure effective barrier function. Ciliated and mucus-producing secretory cells mediate the mucociliary escalator that continuously removes foreign particles and microbes from the airway surface [3], while BC function as stem/progenitor cells of the airway epithelium [2, 4, 5]. Under homeostatic conditions, airway BC are relatively quiescent, but in response to injury, self-renew and generate IC, which serve as precursors of ciliated and secretory cells [4–8]. The tight junctions (TJ) between adjacent differentiated cells form a semipermeable barrier that limits diffusion of solutes across the epithelium and physically protects the lung from inhaled pathogens [9].
Cigarette smoking, the major cause of chronic obstructive pulmonary disease (COPD) and lung cancer, alters virtually all components of the normal epithelial architecture, causing BC hyperplasia (expansion of BC and IC), squamous metaplasia (squamous cells replacing ciliated cells), cilia shortening, mucous hyperplasia (increased number of mucus-producing cells), and decreased TJ barrier integrity [10, 11]. These lesions often coexist in the same or adjacent airway areas, contributing to decreased mucociliary clearance, impaired barrier function, accumulation of mucus, airway obstruction, and pathogen colonization [2, 11].
Increasing evidence suggests that smoking-induced disordering of the airway epithelium begins with changes in airway BC stem/progenitor cells, mediated, at least in part, by exaggerated signaling through the epidermal growth factor receptor (EGFR) enriched in BC [11]. Smoking induces airway ciliated cells to produce epidermal growth factor (EGF) that shifts the fate of BC toward squamous metaplasia and epithelial-mesenchymal transition-like phenotype with decreased TJ barrier function [12]. However, EGF does not induce BC- or mucous hyperplasia [12], the phenotypes thought to be EGFR-dependent [13–16]. Thus, we hypothesized that another EGFR ligand might be involved in activation of BC in the airways of smokers, which, together with EGF, directs BC to form the entire spectrum of smoking-associated lesions. One plausible candidate could be amphiregulin (AREG), an EGF-like growth factor that interacts with EGFR but with a lower affinity compared to EGF [17, 18]. Earlier studies have identified distinct EGFR trafficking patterns induced by EGF and AREG in various epithelial cell lines: whereas EGF targets EGFR for lysosomal degradation, AREG causes EGFR recycling, potentially relevant to different biological functions exerted by these growth factors [19, 20]. AREG has been reported to increase cell proliferation and mediate upregulation of mucus-related genes in the mouse lung and airway epithelial cell lines [21–23].
In this study, we describe a novel mechanism that underlies interconnected pathogenesis of smoking-associated lesions in the human airway epithelium, which involves a crosstalk between EGF and AREG signaling in airway BC. AREG is upregulated by smoking in the airway epithelium as a result of EGF-induced squamous differentiation of BC. In turn, AREG induces a unique EGFR activation pattern in BC, distinct from that evoked by EGF, leading to BC- and mucous hyperplasia, altered ciliated cell differentiation, and impaired barrier integrity. Further, AREG promotes its own expression, establishing an autonomous self-amplifying signaling loop in airway BC relevant for promotion of EGF-independent hyperplastic phenotypes.
Materials and Methods
See Supporting Information Methods for detailed information.
Human Airway BC and Air-Liquid Interface
Airway BC were isolated from the large airway epithelium (LAE; 3rd-4th order bronchi) of healthy nonsmokers (n = 10 subjects; 3 males, 7 females; average age 37.8 ± 10.2) obtained by bronchoscopic brushings as previously described [24]. Subjects were recruited under a protocol approved by the Weill Cornell Medical College Institutional Review Board, with written informed consent. BC differentiation was assessed using the air-liquid interface (ALI) model [25]. Briefly, after BC reached 70%-80% confluence, cells were trypsinized and seeded at a density of 6 × 105 cells/cm2 onto a 0.4 µm pore-sized Transwells (Corning, Corning, NY, https://www.corning.com/) precoated with type IV collagen (Sigma, St. Louis, MO, http://www.sigmaaldrich.com/). When BC reached confluence, the apical surface was exposed to air (“ALI day 0”), and the ALI media consisting of 1:1 Dulbecco’s Modified Eagle Medium (DMEM)/Ham’s F12 and 2% Ultroser G serum substitute (BioSerpa S.A., Cergy-Saint-Christophe, France, https://www.pall.com/) was added from the basolateral side every other day till ALI day 28, when BC normally generate differentiated mucociliary airway epithelium [12].
Airway BC Stimulation
BC were cultured in ALI in the presence or absence (control) of the following stimuli added every other day separately or in combination from the basolateral ALI side: cigarette smoke extract (3% CSE) ± 1 hour pretreatment with EGFR inhibitor AG1478 (10 µM; Calbiochem, San Diego, CA) as detailed in Supporting Information Methods; EGF (10 ng/ml; Sigma, St. Louis, MO, http://www.sigmaaldrich.com/); AREG (10 ng/ml; R&D Systems, Minneapolis, MN, https://www.rndsystems.com/); neutralizing anti-AREG antibody or goat isotype control antibody (both 1 µg/ml; R&D Systems, Minneapolis, MN, https://www.rndsystems.com/). Amounts of AREG released into the basolateral ALI media were determined using the DuoSet ELISA Development kit (R&D Systems, Minneapolis, MN, https://www.rndsystems.com/). To study the effects of EGF-induced AREG, EGF (10 ng/ml) or media alone (control) were applied to the basolateral side at ALI day 0. After 48 hour of stimulation, the basolateral ALI supernatants were collected, and freshly collected supernatants were applied to the ALI cultures from the same donor starting day 2 ALI for 2 weeks ± neutralizing anti-EGF (0.5 µg/ml; R&D Systems, Minneapolis, MN, https://www.rndsystems.com/) or anti-AREG (see above) antibodies or their combinations (using goat IgG as a control). At various time-points, RNA was isolated for gene expression analysis; cytospins and sections of the ALI-derived epithelium were prepared and analyzed for general morphology and expression of various markers as detailed below. Epithelial barrier integrity was assessed by measuring transepithelial electric resistance (TER) using Millicell-ERS epithelial ohmmeter (Millipore, Bedford, MA, http://www.emdmillipore.com/) every other day when the media was changed. Barrier function was assessed using FITC-dextran flux assay [12].
Analysis of EGFR Activation
EGFR activation was assessed at various time-points after EGF or AREG treatment by Western analysis using rabbit monoclonal antibodies against phosphorylated (p-)EGFR (Tyr1173, clone 53A5, 1:1,000; Cell Signaling Technology, Beverly, MA), total EGFR (clone D38B1, 1:1,000; Cell Signaling Technology, https://www.cellsignal.com/), and mouse monoclonal GAPDH antibody (sc-32233; 1:5,000; Santa Cruz Biotechnology, Dallas, TX, https://www.scbt.com/) as described [12]. Signal intensity for p-EGFR and total EGFR was measured using ImageJ software (NIH), p-EGFR/total EGFR ratio was determined for each group and expressed as fold-change versus control for each time-point (see Supporting Information Methods for details). Cell surface EGFR expression was evaluated by FACS analysis using PE-conjugated anti-EGFR antibody (1:20; BD Pharmingen, San Diego, CA, http://www.bdbiosciences.com/) following standard protocols. In selected experiments, immunofluorescence (IF) analysis using rabbit monoclonal antibody against p-EGFR (Tyr1068, clone Y38, 1:50; Abcam, La Jolla, CA, http://www.abcam.com/) was performed for ALI day 14 samples.
Morphology, Immunohistochemistry, and Immunofluorescence Analysis
The large airway biopsy samples obtained by bronchoscopy from healthy nonsmokers and smokers and smokers with COPD (Supporting Information Table S1 for subject information) and ALI samples were assessed by immunohistochemistry (IHC) and IF using the methods and antibodies, and cilia length was measured as described in Supporting Information Methods. Images were captured by Zeiss Axiovert 200M microscope (Carl Zeiss, Jena, Germany, http://www.zeiss.com/) and analyzed by AxioVision Rel 4.8 software (Carl Zeiss, http://www.zeiss.com/). Alcian blue staining (Polysciences Inc., Warrington, PA, http://www.polysciences.com/) was used to visualize mucus-producing cells. For each sample, the value of cilia length was represented as the mean value of randomly selected 6 to 10 cilia on 10 randomly selected ciliated cells.
Gene Expression Analysis
RNA was processed and expression of selected genes was analyzed by TaqMan real-time RT-PCR using specific TaqMan gene expression assays (Applied Biosystems, Foster City, CA, https://www.thermofisher.com/us/en/home/brands/applied-biosystems.html) using standard protocols. Normalized expression was determined using the ΔΔCt method using 18S rRNA as endogenous control. In some analyses, normalized expression levels were presented as fold-change, relative to one of the study group. Genome-wide Pearson correlation analysis was performed using our previously described set of LAE samples obtained from healthy nonsmokers (n = 21) and healthy smokers (n = 31) [12] to identify genes whose expression in the human LAE positively (r ≥ 0.5, p < .05) correlated with the AREG gene expression using GeneSpring version 7.3.1 (Agilent technologies, Palo Alto, CA, http://genespring-support.com/). Biologic categories enriched among the AREG coexpressed genes were identified using DAVID and GATHER analytic tools as previously described [24].
Results
AREG is Upregulated in the Areas of Smoking-Associated Airway Remodeling In Vivo
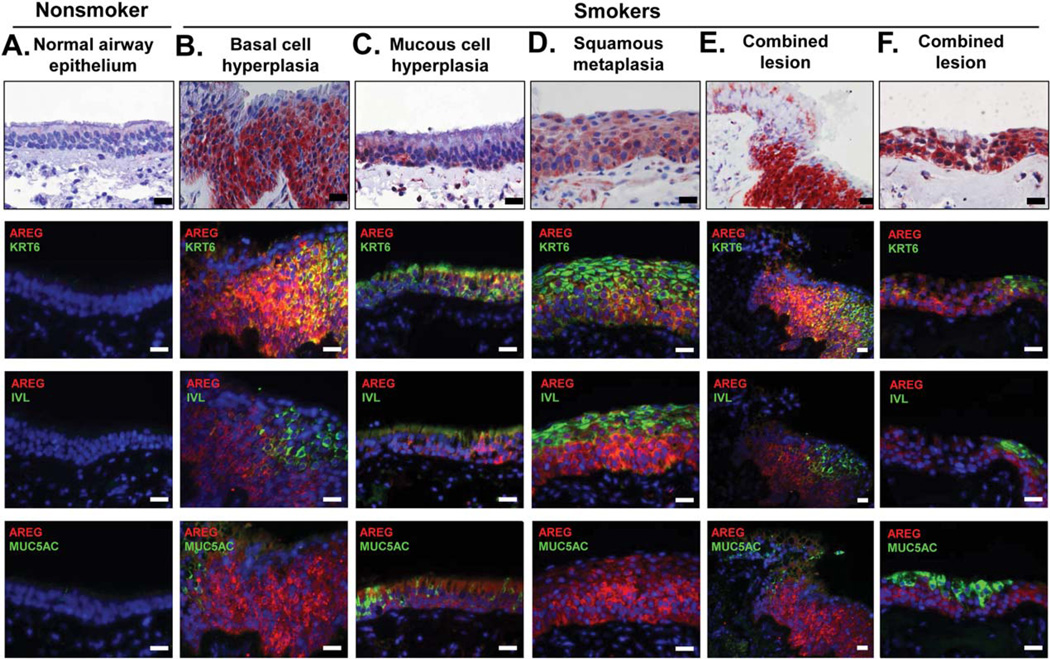
AREG expression was barely detectable in the normal human airway epithelium (Fig. 1A and Supporting Information Fig. S1A–S1G) but was markedly upregulated in the airway epithelium of smokers in association with squamous metaplasia, BC hyperplasia, and mucous cell hyperplasia (Fig. 1B-1E and Supporting Information Fig. S1A–S1D). In these remodeled regions, AREG was predominantly expressed in cells positive for keratin 6 (KRT6), a BC/IC population associated with squamous differentiation [26, 27]. KRT6+ cells were absent in the normal airway epithelium (Fig. 1A and Supporting Information Fig. S1B) but contributed to virtually all smoking-related lesions characterized by loss of the normal pseudostratified mucociliary morphology (Fig. 1B, 1C, 1E, and Supporting Information Fig. S1B, S1C). In the remodeled airway epithelium of both healthy and COPD smokers, ~50% of AREG+ cells also expressed KRT6 (Supporting Information Fig. S1H), and there was a significant correlation (r2 = 0.67, p < .01) between the proportions of cells expressing these two markers (Supporting Information Fig. S1I). Although AREG+ cells were frequently detected in the airways of smokers with squamous metaplasia, only a minor subset (≤30%) of these cells coexpressed involucrin (IVL), a marker of squamous cells, whereas the majority of AREG+ cells were found in the expanded KRT6+ BC/IC compartment beneath the layer of apical squamous cells (Fig. 1B, 1D, 1E; and Supporting Information Fig. S1D, S1E, S1H).
Figure 1.
Upregulation of AREG in the airway epithelium of smokers. Airway samples with indicated histologic phenotypes analyzed using immunohistochemistry (upper row) or immunofluorescence (IF) for AREG and markers of squamous (IVL), BC/IC (KRT6), and mucous-producing (MUC5AC) cells. Each vertical panel (A–F) shows representative images of the adjacent or nearby sections from the same specimen. In IF, nuclei are stained with DAPI (blue). Scale bars-20 µm. See Supporting Information Figure S1 for more examples and quantification data. Abbreviations: AREG, amphiregulin; IVL, involucrin; KRT6, keratin 6.
AREG was also upregulated in the airways of smokers with mucous hyperplasia (Fig. 1C, Supporting Information Fig. S1F, S1G). However, also in these lesions, KRT6+ BC, IC and occasionally ciliated cells, but not mucus-producing cells, were the major source of AREG. In the remodeled airway epithelium of smokers, <20% of AREG+ cells were positive for MUC5AC, the marker of mucus-producing cells (Supporting Information Fig. S1H). BC hyperplasia with expansion of AREG+ and KRT6+ BC and IC often accompanied “combined lesions” containing more than one remodeling phenotype, for example, squamous and mucous cell metaplasia, both characterized with loss of normal ciliated cell morphology, and the degree to which epithelial architecture was deranged in these lesions was proportional to AREG+ cell number (Fig. 1E, 1F).
Although hyper-/metaplastic airway lesions are commonly associated with smoking, they can also be observed in non-smokers, and, conversely, some regions of the airway epithelium of smokers are histologically intact [28, 29]. In the airway epithelium of nonsmokers with BC hyperplasia and squamous metaplasia, AREG+ was also upregulated, but the frequency of AREG+ cells was lower than in similar lesions of smokers (14.0 ± 6.2% vs. 54.6 ± 15.9%, p < .001; Supporting Information Fig. S1J). Although, similar to smokers, expanded BC/IC were the major source of AREG in the remodeled airway epithelium of nonsmokers, KRT6 expression in these cells was not detected (Supporting Information Fig. S1J). In histologically intact airway epithelium of smokers, AREG+ cells were detected with markedly lower frequency (2.7 ± 1.8%; Supporting Information Fig. S1K), suggesting that emergence and expansion of AREG+ cells are associated with airway epithelial remodeling promoted by smoking rather than direct effect of smoking.
Cigarette Smoke Induces AREG Expression via EGFR Signaling in Airway BC
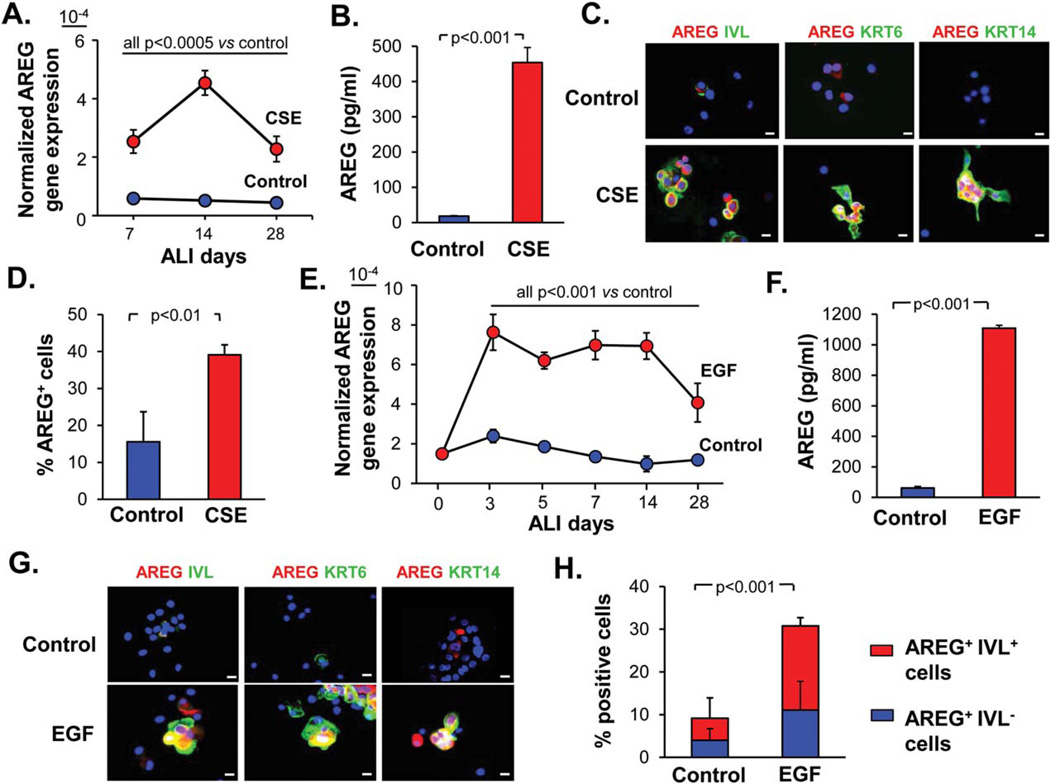
To determine whether cigarette smoke can upregulate AREG in the airway epithelium by acting via BC and altering the differentiation potential of these stem/progenitor cells, BC isolated from the human large airways were exposed to CSE during differentiation in ALI. AREG expression was increased in the airway epithelium derived from CSE-treated BC within the first week of ALI culture, and CSE-induced upregulation maintained during the entire process of BC differentiation (Fig. 2A). Particularly high level of AREG upregulation was reached during the first 2 weeks of CSE exposure (Fig. 2A), accompanied by secretion of ~10-times higher amount of AREG protein at day 14 of ALI (Fig. 2B) compared to the control group. In CSE-treated cultures, AREG was detected in KRT5+ BC (Supporting Information Fig. S2A), IVL+ squamous-like cells (Fig. 2C and Supporting Information Fig. S2B), KRT6+ and KRT14+ cells (Fig. 2C), a subset of BC/IC activated in response to injury and during squamous differentiation [4, 30]. CSE-induced AREG was largely detected in the cytoplasm and occasionally in the nuclei of BC and squamous-like cells (Supporting Information Fig. S2A, S2B). The proportion of AREG+ cells significantly increased after CSE exposure (Fig. 2D). AREG expression was prevented when a selective EGFR tyrosine kinase inhibitor AG1478 was applied to airway BC prior to CSE exposure between days 5 and 11 of ALI (Supporting Information Fig. S2C), which was accompanied by decreased AREG protein secretion (Supporting Information Fig. S2D), suggesting that EGFR signaling in airway BC mediates, at least in part, smoking-induced AREG upregulation during the early phase of BC differentiation.
Figure 2.
Cigarette smoke and EGF induce AREG in the airway epithelium. (A): Normalized AREG gene expression in the epithelium derived from airway basal cells (BC) treated every other day from day 0 of ALI with 3% CSE or untreated (control) BC at indicated time-points of ALI (mean ± SD); representative of three experiments. (B): AREG protein levels determined by ELISA in the basolateral supernatants of the epithelium derived from BC after 14 days of ALI as described in A (mean ± SD). (C): IF analysis of cytospins of epithelial cells generated from airway BC as described in B for expression of AREG (red) and indicated squamous-related markers (green); scale bars-20 µm. (D): AREG+ cells (% of total) in the samples derived in ALI and analyzed by IF as shown in C (mean ±SD). (E): Normalized AREG gene expression in the epithelium derived from EGF-treated (10 ng/ml) and control BC at different time points of ALI (mean ± SD). See Supporting Information Figure S2E for summary of three independent experiments. (F): AREG ELISA of the basolateral supernatants of the epithelium derived from BC after 14 days of ALI as described in E (mean ±SD). (G): IF analysis of cytospins of epithelial cells generated from airway BC as described in F for expression of AREG (red) and indicated squamous-related markers (green); scale bars-20 µm. (H): Quantification of AREG+ and IVL+ cells (% of total cells) in samples described in G; p value shows significance of difference in the total AREG+ and AREG+/IVL+ cells between the groups (mean±SD). All panels represent data derived from ≥3 independent experiments. Abbreviations: ALI, air-liquid interface; AREG, amphiregulin; CSE, cigarette smoke extract; EGF, epidermal growth factor; IVL, involucrin; KRT6, keratin 6.
EGF-Induced Squamous Differentiation of Airway BC Generates AREG-Expressing Cells
We have previously shown that EGF is induced by smoking and shifts airway BC fate toward a squamous phenotype with IVL+, KRT6+, and KRT14+ BC/IC [12], that is, cell populations, where AREG was highly expressed in the smokers’ airways in vivo (Fig. 1B-1E) and in response to CSE in vitro (Fig. 2C), suggesting that smoking-induced EGF might stimulate generation of AREG+ cells. Indeed, treatment of airway BC with EGF markedly increased and maintained AREG expression throughout the entire course of BC differentiation (Fig. 2E and Supporting Information Fig. S2E), and resulted in a >10-fold increased AREG secretion compared to the control group (Fig. 2F). EGF-induced AREG was detected in IVL+ squamous cells, and KRT6+ and KRT14+ squamous-like cells (Fig. 2G). About 30% of cells in the airway epithelium derived from EGF-treated BC expressed AREG compared to ~10% in the control group, and the majority of these AREG+ cells coexpressed squamous cell marker IVL (Fig. 2H). Thus, EGF-induced differentiation of airway BC toward the squamous phenotype generates AREG-producing cells.
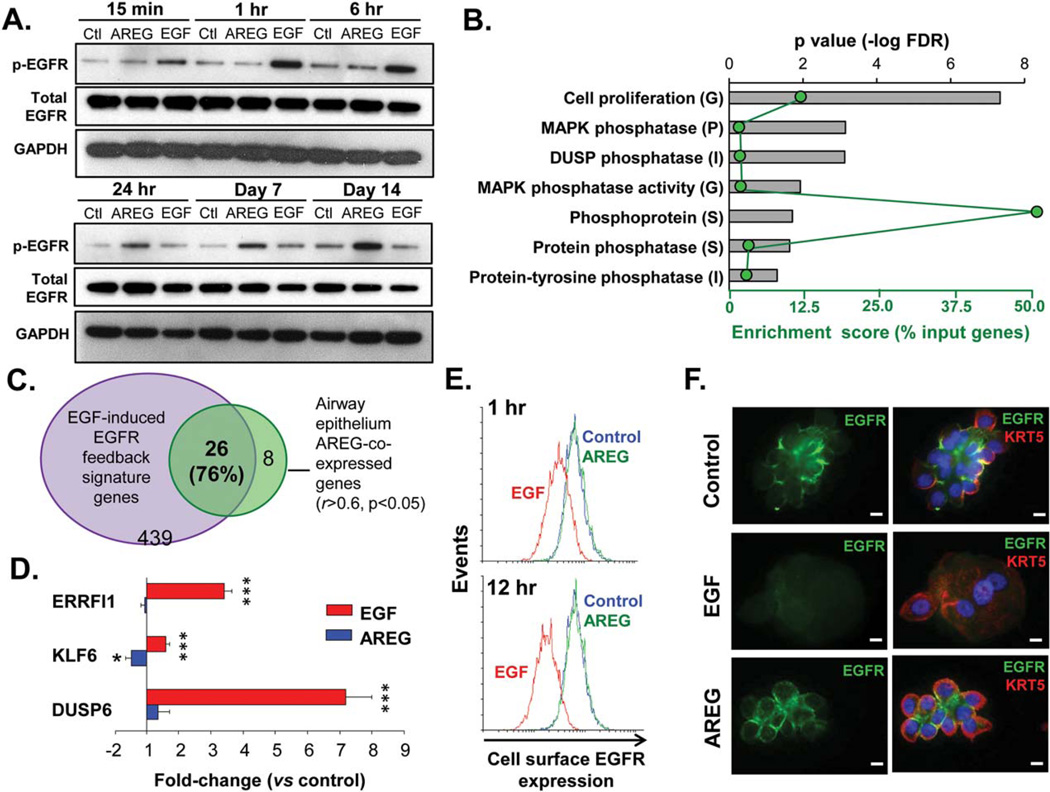
AREG and EGF Differently Activate EGFR in Airway BC
To determine the patterns of EGFR activation in airway BC in response to AREG and EGF, we applied these growth factors to BC cultured in ALI. EGF strongly induced EGFR phosphorylation (at Tyr1073) early after stimulation (from 15 minutes to 1 hour), whereas AREG activated EGFR at this phosphorylation site only at the later time-points (from day 7 to day 14), when the effect of EGF on EGFR activation was no longer significant (Fig. 3A; Supporting Information Fig. S3A for quantification data). AREG-induced EGFR activation at later time-points was further confirmed by IF analysis of the epithelium derived after 14 days of ALI culture from AREG-treated BC using the antibodies against EGFR phosphorylated at Tyr1068 (Supporting Information Fig. S3B), an EGFR phosphorylation site that has been implicated in AREG signaling in the airway epithelium [31].
Figure 3.
Distinct patterns of EGFR activation by EGF and AREG. (A): Western analysis of phospho-EGFR (Tyr1173), total EGFR and GAPDH protein levels in airway epithelial cells derived from basal cells (BC) treated with EGF or AREG (both 10 ng/ml) versus unstimulated BC at indicated time-points; representative of >3 independent experiments (see Supporting Information Fig. S3A for quantification data). (B): Top seven categories enriched in the AREG-coexpressed airway epithelial gene set (r ≥ 0.5, p < .05) annotated using Gene Ontology (G) and protein family classifications (P, Protein Information Resource; I, InterPro; S, The Human Spliceosome Protein-Protein Interaction Resource) using GATHER (for G, additional criteria: Beyes factor >6) and DAVID (for G, P, I, S); ranked by false discovery rate-corrected p value (top axis; bars); green dots represent enrichment score (% input genes; bottom axis). (C): Venn diagram: overlap of the top AREG-coexpressed airway epithelial genes (r ≥ 0.6, p < .05) with the EGFR feedback signature. (D): Fold-change in expression of the EGFR feedback genes (see text for full gene names) in the epithelium derived from BC during 28 days of air-liquid interface (ALI) ± basolaterally added EGF or AREG versus control (mean ± SD; n= 5 independent experiments). (E): FACS analysis: histogram plots of the cell surface EGFR expression in control basal cells (BC) (blue) and BC treated with AREG or EGF; at 1 hours and 12 hours after stimulation; representative of three independent experiments. (F): IF images of cytospins of epithelial cells derived from BC after 14 days of ALI culture ± AREG or EGF for expression of EGFR and BC marker KRT5. Nuclei are stained with DAPI (blue). Scale bars-10 µm. See Supporting Information Figure S3C for more examples. Abbreviations: AREG, amphiregulin; DUSP, dual specificity protein phosphatase; EGFR, epidermal growth factor receptor.
Consistent with differentiation-associated decrease in proportion of EGFR-expressing BC, the total EGFR protein levels declined at the later ALI time-points (Fig. 3A, lower panel). However, EGF, but not AREG, further decreased the total EGFR amount (Fig. 3A, lower panel), consistent with the known ability of EGF to cause rapid EGFR down-modulation [19, 32, 33] and suggesting that AREG-activated EGFR might escape this negative regulation.
EGF-induced EGFR activation is controlled, at least in part, by a negative feedback transcriptional program [34, 35]. To gain insights into potential transcriptional mechanisms that may underlie the unique mode of EGFR activation by AREG, we performed genome-wide analysis that identified 227 genes, whose expression significantly correlated with that of the AREG gene (r ≥ 0.5; p < .05) in the human large airway epithelium (Supporting Information Table S1). Functional categories related to cell proliferation and phosphoprotein metabolism, for example, MAPK phosphatase, dual specificity protein phosphatase (DUSP), and protein-tyrosine phosphatase, were enriched among the AREG-coexpressed genes (Fig. 3B). Strikingly, 26 of the 34 (76%) unique annotated genes that had the highest degree of correlation (r ≥ 0.6; p<.05) with AREG, overlapped with the EGFR feedback signature (Fig. 3C). Six of these 26 genes (AREG, KLF6, HBEGF, ERRFI1, DUSP5, and VEGFA) were enriched in the human airway BC signature [24] and downregulated during BC differentiation [36] (GSE5264; Supporting Information Table S2). Individual EGFR feedback genes, for example, ERRFI1, KLF6 and DUSP6, were upregulated in the epithelium derived from EGF-treated, but not, or to a lesser extent, from AREG-treated, BC at day 14 of ALI (Fig. 3D), potentially responsible for the lack of effect of EGF on EGFR phosphorylation at the later time points after stimulation.
These findings suggest that AREG is a component rather than an activator of the EGF-inducible EGFR feedback program in the airway epithelium. However, by contrast to the classical EGFR feedback elements that usually terminate EGFR signaling, AREG activates EGFR in a unique manner, distinct from EGF, which does not lead to EGFR down-modulation. Indeed, whereas stimulation of BC with EGF decreased EGFR expression on the cell surface, indicative of EGFR internalization and/or degradation, AREG did not change it (Fig. 3E). Further, in the epithelia derived from EGF-treated BC during 14 days of ALI culture, expression of EGFR was barely detectable, whereas AREG treatment preserved EGFR expression on the BC surface (Fig. 3F and Supporting Information Fig. S3C). Together, AREG activates EGFR in the human airway epithelium in a unique, distinct from EGF manner, which is associated with stabilization of EGFR expression on the BC surface.
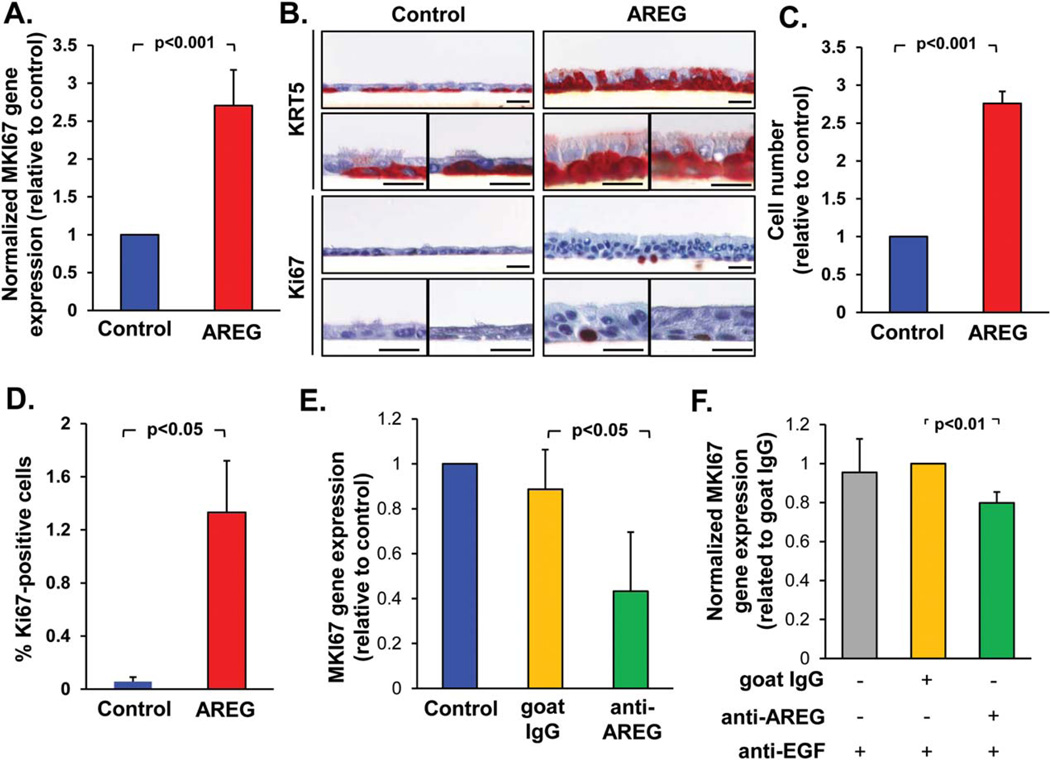
AREG Promotes Airway BC Proliferation and BC Hyperplasia
Based on the observations described above, we hypothesized that persistent activation of EGFR-expressing BC by AREG may promote smoking-related BC proliferation and hyperplasia. Consistent with this hypothesis, there was upregulation of the proliferation-related gene MKI67 in the epithelium derived from AREG-treated BC compared to control BC (Fig. 4A), accompanied by increased numbers of KRT5+ BC/IC and Ki-67+ BC (Fig. 4B). AREG-treated BC formed multilayered epithelium with histologic features of BC hyperplasia (Fig. 4B), with increased numbers of the total (Fig. 4C) and Ki-67+ (Fig. 4D) cells.
Figure 4.
AREG promotes airway basal cells (BC) proliferation and hyperplasia. (A): Expression of the MKI67 gene in the epithelium derived from airway BC after 28 days of air-liquid interface (ALI) in the presence of AREG relative to control group (mean±SD). (B): Morphology and immunohistochemistry of samples derived as described in A for BC marker KRT5 and proliferation marker Ki-67. Shown are three separate areas of the ALI cultures for each of marker; scale bars-20 µm. (C): Total cell number (normalized to the control group) and (D) % Ki-67+ cells in the samples derived from BC as described A in three randomly selected fields (mean ± SD). (E). MKI67 gene expression in the epithelium derived from BC during 14 days in ALI ± neutralizing anti-AREG antibody or goat IgG isotype control (both 1 µg/ml; normalized to control group; mean ± SD). (F): MKI67 gene expression in the airway epithelium derived from BC treated with EGF-conditioned media (EGF-CM; see text) ± neutralizing anti-AREG antibodies (1 µg/ml), and neutralizing anti-EGF antibody (0.5 µg/ml); normalized to the control IgG group (mean ± SD); for more data see Supporting Information Figure S4. All panels represent data derived from ≥3 independent experiments. Abbreviations: AREG, amphiregulin; EGF, epidermal growth factor.
Application of neutralizing anti-AREG antibody to airway BC during the ALI culture decreased MKI67 gene expression (Fig. 4E), suggesting that endogenously produced AREG may sustain BC proliferation. By contrast, stimulation of BC with EGF downregulated MKI67 expression and markedly reduced cell number, but, consistent with our previous study [12], promoted squamous phenotype with increased expression of IVL (Supporting Information Fig. S4A, S4B). To assess the effect of EGF-induced AREG, we exposed airway BC to the basolateral supernatant of BC treated with EGF during 2 weeks of ALI culture (EGF-conditioned medium, EGF-CM) that contained both EGF-induced AREG (Fig. 2F) and residual EGF. Consistent with antiproliferative effect of EGF, treatment of airway BC with EGF-CM suppressed expression of the MKI67 gene, which was increased when anti-EGF antibodies were added (Supporting Information Fig. S4C). However, addition of both anti-EGF and anti-AREG antibodies to EGF-CM significantly decreased MKI67 expression compared to anti-EGF antibody only (Fig. 4F), suggesting that EGF-induced AREG promotes airway BC proliferation.
AREG Promotes Mucous Cell Hyperplasia
Based on the observation that mucous hyperplastic lesions in the airways of smokers, particularly those combined with squamous metaplasia, are accompanied by increased number of AREG+ BC/IC, we hypothesized that AREG produced by BC undergoing squamous differentiation, as shown above, may promote mucous hyperplasia by skewing BC differentiation toward mucus-producing cells. Consistent with this hypothesis, AREG-treated BC generated airway epithelium with increased expression of genes typical for mucous differentiation, including mucins MUC5AC and MUC5B, trefoil factor TFF3, SAM pointed domain containing ETS transcription factor (SPDEF), and anterior gradient AGR2 (Fig. 5A). Although MUC5AC upregulation did not reach statistical significance, due to high variability and relatively low MUC5AC expression in this model, epithelium generated by AREG-treated BC contained ~3 times more MUC5AC-expressing and Alcian blue-positive mucus-producing cells (Fig. 5B-5D, Supporting Information Fig. S5A, S5B) versus control group, and in some areas had features of mucous cell hyperplasia (Fig. 5B).
Figure 5.
AREG promotes mucous cell differentiation and hyperplasia. (A): Fold-change in expression of mucous differentiation-related genes (see text for full gene names) in the epithelium derived from basal cells (BC) after 28 days of ALI culture in the presence of AREG versus untreated control (mean ± SD). (B): Cytopreps (top panels) and sections (remaining panels) of ALI samples derived from BC as described in A analyzed using immunohistochemistry for MUC5AC (see Supporting Information Fig. S5A for more examples) or Alcian blue staining (blue–mucus); scale bars-20 µm (representative of three experiments). (C): Quantification of MUC5AC-expressing cells in samples described in B (relative to the AREG group; mean±SD). (D): % Alcian blue-positive cells in AREG versus control groups described in B (mean ± SD; n = 3 experiments); arrows show Alcian blue-positive cells. (E): Fold-change in expression of indicated mucous differentiation-related genes in the epithelium derived from BC during 14 days of ALI in the presence of neutralizing anti-AREG antibody versus goat IgG control (mean±SD). (F): Expression of indicated mucous-related genes in the epithelium derived from BC treated as described in Figure 4F; normalized to control IgG group (mean ± SD); see more data in Supporting Information Figure S5C. All panels represent data derived from ≥3 independent experiments. Abbreviations: AGR2, anterior gradient 2; AREG, amphiregulin; SPDEF, SAM pointed domain containing ETS transcription factor.
Application of neutralizing anti-AREG antibody to control BC cultures in ALI decreased the expression of mucus-related genes (Fig. 5E), suggesting that endogenously produced AREG might promote homeostatic differentiation of airway BC toward the mucous cell lineage. However, EGF neither induced the expression of mucus-related genes nor increased the number of mucus-producing cells (Supporting Information Fig. S4A, S4B). To determine whether EGF-induced AREG is capable of promoting mucous-associated phenotype, airway BC were treated with EGF-conditioned medium (EGF-CM, see above), which, probably due to the presence of residual EGF, did not have consistent effect on mucus-related genes, apart from MUC5AC and SPDEF, which were upregulated (Supporting Information Fig. S5C). Addition of both anti-EGF and anti-AREG antibodies, but not anti-EGF only, to EGF-CM entirely blocked MUC5AC expression, and significantly decreased expression of mucous differentiation-related TFF3 and SPDEF (Fig. 5F and Supporting Information Fig. S5C), suggesting that EGF-induced AREG promotes the mucous phenotype.
AREG Suppresses Ciliated Cell Differentiation and Junctional Barrier Integrity
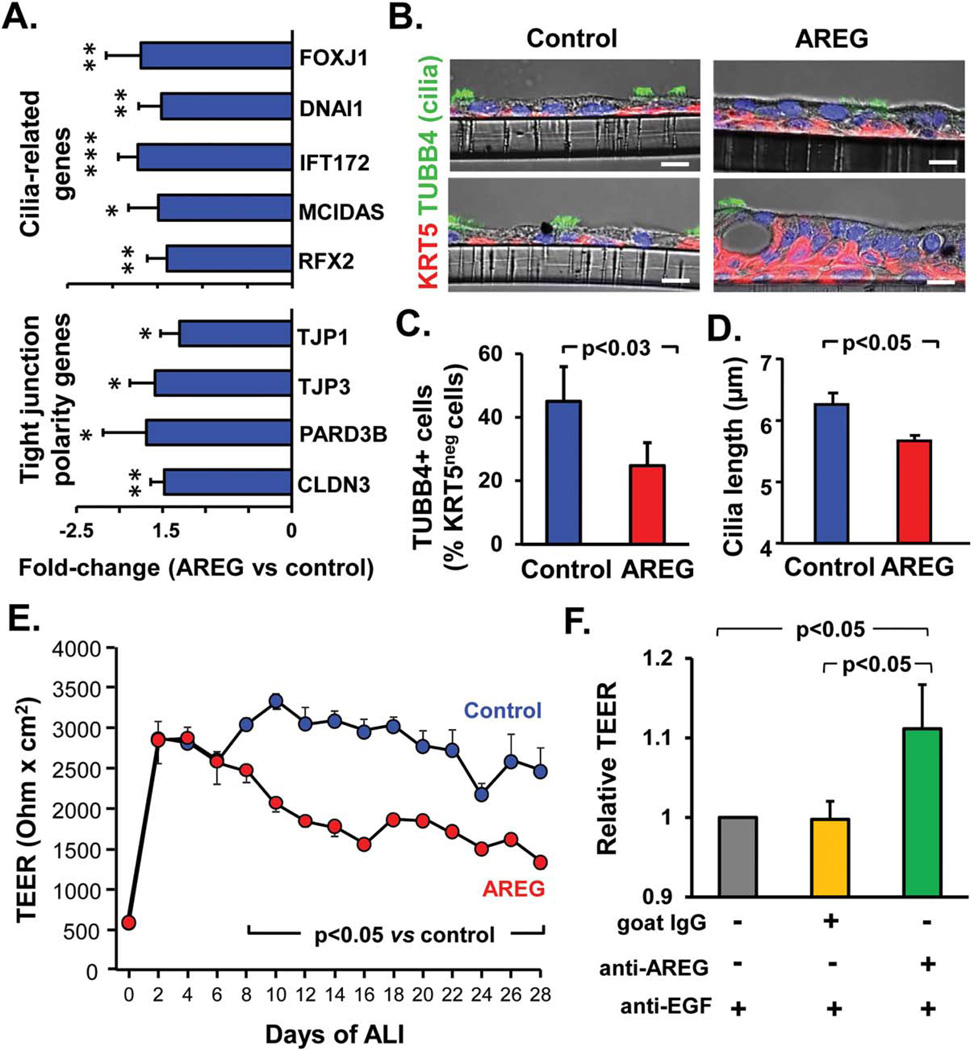
Next, we evaluated the effect of AREG on ciliated cell differentiation and junctional barrier integrity, features of normally differentiated airway epithelium suppressed in the airways of smokers in vivo and by EGF in vitro [12, 37–39]. Consistent with previous observations [12], EGF-treated BC formed airway epithelium with decreased expression of genes relevant to ciliated cell differentiation, for example, transcription factor forkhead box J1 (FOXJ1) and dynein DNAI1 (Supporting Information Fig. S4A). Similarly, the epithelium derived from AREG-treated BC had decreased expression of FOXJ1, DNAI1, intraflagellar transport-related gene IFT172, and transcription factors that control the early ciliogenesis program multicilin (MCIDAS) and regulatory factor RFX2 (Fig. 6A), and had lower overall frequency of ciliated cells compared to control cultures (Fig. 6B and Supporting Information Fig. S6A). Since decreased proportion of ciliated cells in AREG-derived epithelium can be due to increased number of BC, we determined proportion of ciliated cells among KRT5-negative, that is, non-BC, cells, which was also significantly lower in the AREG group (24.7 ± 7.3% vs. 45.0 ± 11.0% in the control group, p < .03; Fig. 6C).
Figure 6.
AREG alters ciliated cell differentiation and barrier integrity. (A): Fold-change in expression of the ciliated cell differentiation-related genes (FOXJ1, forkhead box protein J1; DNAI1, dynein intermediate chain 1, axonemal; IFT172, intraflagellar transport 172; MCI-DAS, multicilin, and RFX2, regulatory factor X, 2) and tight junction (TJ)- and epithelial polarity-related genes (TJ proteins (TJP) 1 and − 3, partitioning defective PARD3, and claudin CLDN3) in the epithelium derived from basal cells (BC) after 28 days of ALI ± AREG (mean 6SD). (B): Representative phase contrast IF images of sections of the airway epithelium derived in ALI as described in A stained for BC marker keratin 5 (KRT5) and cilia marker tubulin, beta 4 (TUBB4); blue–DAPI (nuclei); scale bars-10 µm. (C): % of ciliated (TUBB4-positive) cells (relative to all KRT5-negative, that is, non-BC, cells) in AREG versus control groups described in B (mean ± SD; n= 3 experiments). (D): Cilia length in epithelia derived from BC as described in A (mean ± SD). (E): TER of BC-derived epithelium at different time-points of ALI in AREG-treated versus control groups (mean ± SD; n= 4 replicates/group); see Supporting Information Figure S6B for summary of three independent experiments). (F): TER of airway BC-derived epithelium treated as described in Figure 4F measured at day 16 of ALI (mean ± SD). Panels A-D, and F represent data derived from ≥3 independent experiments. Abbreviations: ALI, air-liquid interface; AREG, amphiregulin; CSE, cigarette smoke extract; EGF, epidermal growth factor; TER, transepithelial resistance.
Further, whereas in the epithelium derived from control BC, cilia had an average length of 6.26 ± 0.32 µm, that is, similar to that in the normal human airways in vivo [39], AREG-treated BC generated airway epithelium with ciliated cells that had significantly shorter cilia with the length averaging at 5.66 ≥ 0.17 µm (Fig. 6D), mimicking the phenotype observed in the airways of smokers [39, 40].
Further, airway BC differentiating in the presence of AREG formed the epithelium with decreased expression of tight junction (TJ)- and epithelial polarity-related genes, such as TJ protein (TJP)1 and TJP3, claudin (CLDN)3, and the polarity complex PARD3 (Fig. 6A). This was accompanied by markedly reduced TER, a measure of TJ barrier integrity, in the AREG-treated group (Fig. 6E and Supporting Information Fig. S6B) and increased permeability as determined by increased para-cellular flux of apically added fluorescent-labeled dextran (Supporting Information Fig. S6C, S6D). Consistent with the ability AREG to suppress TJ barrier integrity, anti-AREG antibody significantly increased TER of the epithelium derived from BC exposed to EGF-CM (Fig. 6F and Supporting Information Fig. S6E). Thus, AREG suppresses TJ barrier formation and integrity in the human airway epithelium.
AREG Induces its Own Expression in Airway BC and Downregulates EGF
Based on the observation that AREG-activated EGFR can be maintained on the BC surface and that chronic stimulation of BC with AREG results in hyperplastic remodeling phenotypes not directly induced by EGF, we hypothesized that, similar to its known autocrine effects in various epithelial cell lines [20, 41, 42], AREG can establish a self-amplifying loop in airway BC that operates in EGF-independent manner. In agreement with this hypothesis, AREG promoted its own expression in airway BC (Supporting Information Fig. 7A, 7B, and Supporting Information Fig. S2A). AREG treatment resulted in ~10-times higher proportion of AREG+ cells, and >80% of these cells were KRT5+ BC (Fig. 7C). Consistent with this finding, expression of AREG was also detected in KRT5+ BC in the airway epithelium of smokers (Supporting Information Fig. S7A). Further, AREG neutralization markedly decreased CSE-induced AREG upregulation (Fig. 7D) and AREG+ cell frequency (Supporting Information Fig. S7B), indicating that AREG mediates its own expression in the airway epithelium following cigarette smoke exposure.
Figure 7.
Effect of AREG on the AREG and EGF gene expression. (A): AREG gene expression in the epithelium derived from airway basal cells (BC) during 28 days of ALI in the presence or absence of AREG relative to the control group (mean ± SD; n = 4 experiments). (B): Sections of the airway epithelium derived from BC during 28 days in ALI in the presence (lower) or absence (upper) of AREG analyzed by IF for expression of AREG and BC marker KRT5; arrows indicate AREG+ BC; representative of three experiments; scale bars-20 µm. (C): AREG+/KRT5+ and AREG+/KRT5 cells (% of AREG+ cells in the AREG-treated group) in samples derived from three independent experiments described in B; p value shows significance of difference in % of all AREG+ cells and AREG/CK5-double+ cells between the groups. (D): Normalized AREG gene expression in the airway epithelium derived from BC during 14 days of ALI culture in the presence or absence of 3% CSE, neutralizing anti-AREG antibodies or isotype control goat IgG (mean ± SD; n = 3 experiments); quantification of AREG+ cells for these experiments is shown in Supporting Information Figure S7B. (E): Normalized EGF gene expression in AREG-stimulated and control samples from 3 independent experiments described in A and B. Abbreviations: AREG, amphiregulin; CSE, cigarette smoke extract; KRT5, keratin 5.
We then asked whether AREG modulates the expression of EGF, the presence of which in the airway BC microenvironment can prevent AREG-mediated effects (Supporting Information Figs S4C, S5C) due to its higher affinity to EGFR [17, 18, 43, 44], and its ability to rapidly down-modulate EGFR (Fig. 3E, 3F) and inhibit BC proliferation (Supporting Information Fig. S4A). Strikingly, stimulation of airway BC with AREG resulted in ~2-fold-decreased EGF gene expression in the BC-derived airway epithelium (Fig. 7E). This may represent a negative feedback mechanism that contributes to a switch from CSE-induced squamous metaplasia [45], an EGF-mediated process responsible, at least in part, for the initial AREG upregulation, to EGF-independent hyperplasia driven by self-amplifying AREG signaling in BC. Indeed, by contrast to EGF, which promotes squamous differentiation of BC [12], AREG itself did not increase the expression of squamous-related genes, for example, IVL, KRT6A, KRT6B, and stratifin (Supporting Information Fig. S7C), or the number of IVL+ squamous cells (Supporting Information Fig. S2B). Consistent with previous studies [45], chronic exposure of differentiating BC to CSE induced, to various degree, molecular features of remodeling phenotypes induced by EGF and/or AREG (Supporting Information Fig. S7D). Thus, EGF-AREG interplay in airway BC drives the stepwise interconnected pathogenesis of histologically distinct remodeling phenotypes commonly observed in the airway epithelium of smokers with a progression from EGF-mediated squamous metaplasia that initially generates AREG-producing cells to EGF-independent AREG-driven BC- and mucous hyperplasia.
Discussion
The airway epithelium, which serves the primary lung tissue barrier provided by its pseudostratified architecture with balanced proportion of ciliated and mucus-producing cells that mediate mucociliary clearance, and BC stem/progenitor cells that maintain this architecture, undergoes remodeling in response to environmental stressors, one of which is cigarette smoking. Although airway epithelial remodeling, characterized by loss of the pseudostratified morphology and altered differentiation pattern, may represent compensatory response to injury not restricted to smoking [29, 30], persistence and progression of these lesions by chronic smoking-associated injury may decrease the host defense potential of the airway epithelium and underlie the early pathogenesis of smoking-induced lung disease, such as COPD and lung cancer [10, 11, 46]. In the airways of smokers, histologically distinct epithelial lesions often coexist in the same or adjacent areas, suggesting the possibility of interconnected pathogenesis of these lesions potentially driven by the common mechanism induced by smoking.
The present study identifies a novel mechanism that links the pathogenesis of all major smoking-associated remodeling phenotypes in the human airway epithelium. This mechanism involves an interplay between the two EGFR ligands, EGF and AREG, which induce distinct patterns of EGFR activation in airway BC stem/progenitor cells, and includes three interconnected events. First, smoking induces AREG expression as a result of EGF-driven squamous differentiation of airway BC. Second, AREG induces a unique activation of EGFR in BC, distinct from that evoked by EGF, and promotes EGF-independent lesions, for example, BC- and mucous hyperplasia, and, similar to EGF, suppresses ciliated cell differentiation and TJ barrier integrity. Third, AREG promotes its own expression in BC and negatively regulates EGF, establishing a self-reinforcing mechanism for sustained activation of airway BC.
The Origin and Nature of AREG-Expressing Cells in the Human Airway Epithelium
AREG is an EGF-like growth factor that was originally isolated from breast and colorectal carcinomas and extensively characterized as potent mitogen for various carcinoma cells [17, 43, 47, 48]. More recent studies have demonstrated that AREG can be also upregulated in nonmalignant epithelia of barrier organs, such as the lung and skin, and immune cells, including T cells and type 2 innate lymphoid cells, following tissue injury [27, 48]. Although it has been reported that injury and cigarette smoke can stimulate AREG expression in lung epithelial cells [21, 22, 42, 49], the mechanisms and biological consequences of AREG upregulation in the context of smoking-associated airway remodeling remained largely unknown.
In the present study, we show that AREG is barely detectable in the normal human airway epithelium, but is markedly upregulated in the regions of airway remodeling, where it is enriched in BC and IC. Although AREG was also detected in the remodeled airway epithelium of nonsmokers, pointing toward the link between AREG expression and airway remodeling [29], the frequency of AREG+ cells was markedly higher in the remodeled areas of airway epithelium of smokers, where these cells were enriched within the expanded BC/IC population expressing KRT6. This unique cell population, absent in the normal airways, has previously been shown to emerge from airway BC undergoing squamous differentiation [12, 27], suggesting that AREG+ cells in the airways of smokers might represent BC-derived precursors of squamous cells.
Indeed, stimulation of airway BC with CSE known to promote squamous differentiation of BC [45], resulted in generation of AREG+ cells that coexpressed squamous-related markers KRT6, IVL and KRT14. This effect was mediated by EGFR signaling and was reproduced by stimulating BC with EGF, a smoking-induced growth factor that induces squamous differentiation of airway BC [12]. However, AREG was only rarely detected in mature squamous cells, that is, those replacing ciliated cells in the apical epithelial layer during squamous metaplasia, suggesting that AREG upregulation occurs during the early/intermediate stages of BC differentiation toward the squamous phenotype.
Similar to other EGFR ligands, AREG is synthesized as a type I transmembrane protein precursor expressed on the cell surface prior to its shedding to the extracellular space. Following its upregulation following stimulation of BC with CSE or EGF, in addition to being released to the supernatant, increased amounts of AREG were detected in the cytoplasm and, occasionally, perinuclear/nuclear regions of cells, potentially indicative of its internalization. In favor of the latter possibility, it has been shown that 12-O-tetradecanoylphorbol-13-acetate, a known inducer of AREG expression and shedding, which, similar to CSE and EGF, induces squamous metaplasia in the airway epithelium [50], promotes internalization of unshed AREG to enable its interaction with nuclear proteins to regulate global gene expression [51]. Thus, it is possible that an alternative, EGFR-independent mechanism of AREG signaling may operate in the airway BC following its upregulation by the inducers of squamous differentiation.
Occasionally AREG was detected in ciliated cells positive for KRT6 and IVL. Although the nature of these cells remains unknown, it has been shown that ciliated cells can acquire squamous-like phenotype following injury [52, 53]. This suggests the possibility that ciliated cells that acquire squamous-like molecular phenotype in response to smoking-associated injury may represent an alternative source of AREG, in addition to AREG-expressing cells derived from BC undergoing smoking-induced squamous differentiation.
The Unique Pattern of AREG Signaling in Airway BC
Similar to EGF, AREG interacts with EGFR [17]. However, because the mature AREG protein is truncated at the C-terminus and lacks a conserved leucine residue essential for high affinity binding to EGFR, AREG is known to activate EGFR less strongly than does EGF [18]. Consistent with this concept, our data show that the pattern of AREG-induced EGFR activation in airway BC is distinct from that evoked by EGF. While EGF-induced EGFR activation in BC was rapid, the effect of AREG was detected only after 7 days, and BC continued to respond to AREG with robust EGFR activation till day 14 of ALI culture, when the stimulatory effect of EGF on EGFR activation was no longer significant.
Strikingly, genome-wide analysis identified that >75% of genes, whose expression in the human airway epithelium strongly correlated with the AREG gene are components of the EGFR feedback signature, a transcriptional program induced by EGF and negatively regulates EGFR signaling [34, 35]. By contrast to EGF, AREG did not induce individual components of this signature, suggesting that AREG-activated EGFR might escape this negative feedback mechanism. Indeed, earlier biochemical studies using epithelial cell lines have demonstrated that, compared to EGF, AREG-induced signaling does not target EGFR for degradation but, instead, promotes EGFR recycling to the plasma membrane [19, 20, 54, 55]. Here, we show that similar scenario holds true for human airway BC. Whereas stimulation of BC with EGF decreased cell surface EGFR expression, indicative of receptor internalization, AREG preserved EGFR expression on the BC surface potentially relevant to the ability of AREG to induce persistent EGFR signaling in airway BC. As discussed above, an alternative mechanism of AREG signaling may also exist, which may involve internalization of nonshed AREG and subsequent interaction with intracellular signaling partners independent from EGFR [51].
AREG as a Driver of Airway Epithelial Hyperplasia
Extensive evidence suggests that EGFR mediates a number of epithelial responses to cigarette smoke, such as overproduction of mucus, decreased TJ barrier integrity, and altered differentiation [11, 13]. We have previously shown that smoking induces EGF expression in ciliated cells, and activation of EGFR-expressing airway BC by EGF leads to squamous metaplasia and decreased barrier function [12]. However, EGF did not induce BC- or mucous cell hyperplasia, lesions that frequently accompany smoking-associated squamous metaplasia and, as shown here, characterized by accumulation of AREG+ cells. Consistent with earlier studies showing that AREG stimulates proliferation of various cell types [23, 24, 47], mediates expression of mucin-related genes in the mouse lung [21], and that AREG and EGF exert nonoverlapping effects in epithelial cell lines [20, 56], we found that AREG promoted the generation of BC- and mucous cell hyperplasia.
Whereas in our study AREG, but not EGF, induced expression of the genes related to mucus-related differentiation, Emomoto et al. [32] have shown that EGF, at a higher concentration (100 ng/ml), can upregulate the expression of the mucin MUC5AC gene in airway epithelial cells cultured in ALI in the presence of high amounts of retinoic acid (RA) added to the differentiation media. This observation suggests that changes in the local microenvironment may determine the outcome of EGFR signaling in airway BC. RA is a well-known suppressor of squamous metaplasia and inducer of mucous cell differentiation in the airway epithelium [57], whereas EGF is a potent inducer of squamous phenotype in BC [12]. In the present study, secondary, EGF-induced, AREG was able to promote mucous-producing phenotype only when residual EGF was neutralized in the media, mimicking the antisquamous effect of RA.
AREG induced a number of EGF-mediated effects, for example, suppressed ciliated cell differentiation and decreased junctional barrier integrity. AREG suppressed the expression of transcriptional regulators of both the early (MCIDAS and RFX2) and late (FOXJ1) ciliogenesis programs [58] and promoted the generation of airway epithelium with lower frequency of ciliated cells and shorter cilia, a phenotype observed in the airways of smokers [39, 40]. By contrast to EGF, which suppresses the ciliated cell differentiation by inducing squamous phenotype [12], AREG did not activate the squamous differentiation program, and decreased proportion of ciliated cells in the AREG-derived epithelium was largely due to increased number of BC, undifferentiated IC and mucus-producing cells. The latter observation suggests that, similar to other mediators and pathways of mucous cell hyperplasia, for example, interleukin-13 and Notch [59, 60], AREG-EGFR signaling reciprocally suppresses ciliated cell differentiation.
EGF-AREG Interplay in Airway BC Links Smoking-Associated Remodeling Phenotypes
Our observation that AREG and EGF, by signaling via the same receptor in airway BC, exert both overlapping and unique effects contributing to the entire spectrum of smoking-associated lesions, suggests that the pathogenesis of these lesions might be connected via an interplay between these growth factors. How EGF and AREG interact with each other to ensure progression from EGF-induced squamous metaplasia to AREG-promoted hyperplastic phenotypes? Although EGF is important for initial induction of AREG, it competitively antagonizes AREG [61] and, due to its higher affinity to EGFR [18] and ability to robustly down-modulate EGFR expression on the BC surface, may limit the ability of AREG to stimulate BC. Further, by contrast to AREG, EGF suppresses BC proliferation and does not promote mucous hyperplasia and EGF-induced AREG upregulated the genes relevant to BC- and mucous hyperplasia only when EGF activity was neutralized. This implies that, in order to promote hyperplastic phenotypes, AREG, which is initially induced by smoking in an EGF-dependent mechanism, should operate in a milieu with tightly controlled EGF levels.
Thus, these should be an additional mechanism that maintains AREG expression in the airway epithelium independently from EGF and limits the effects of EGF once AREG is upregulated. Similar to various cells lines where AREG establishes autocrine self-reinforcing EGFR signaling [20, 23], AREG promoted its own expression in airway BC and, in addition, decreased EGF expression in BC-derived airway epithelium. Activation of this “late” AREG-dominant signaling in airway BC would relieve AREG from antagonistic effects of EGF and promote the shift from the “early” EGF-driven squamous metaplasia toward AREG-promoted hyperplastic lesions with increased production of mucus. Although squamous metaplasia is commonly observed in both healthy and COPD smokers [10], it is hyperplasia accompanied by overproduction of mucus that contributes to airway obstruction in COPD and correlates with the severity of disease [62]. Further, AREG is upregulated in a subset of particularly aggressive smoking-associated lung adenocarcinoma characterized by enrichment of the airway BC features [63] and mucinous histologic pattern [64]. Interestingly, no marked differences in AREG expression levels were found in the airways of smokers with COPD compared to healthy smokers. This implies that smoking-induced upregulation of AREG likely represents an early event in the pathogenesis of smoking-induced airway remodeling initiated prior to the establishment of clinically detectable disease.
Conclusion
Altogether, the present study identifies AREG as a novel smoking-associated mediator, which, in concert with EGF, induces all major smoking-related remodeling phenotypes in the human airway epithelium by altering the function of airway BC stem/progenitor cells. From the translational perspective, these data suggest that therapeutic modulation of EGFR signaling in airway BC by targeting its ligands EGF and AREG, represents a promising approach to prevent and treat smoking-induced lung diseases, such as COPD and lung cancer.
Supplementary Material
Significance Statement.
Cigarette smoking alters the architecture of the airway epithelium inducing stereotypic remodeling phenotypes, including squamous metaplasia, hyperplasia of basal and mucus-producing cells, altered differentiation of ciliated cells, and reduced barrier integrity. This study demonstrates that the pathogenesis of these smoking-associated lesions is linked by an interplay between the epidermal growth factor (EGF) and amphiregulin signaling in airway basal stem/ progenitor cells. Thus, therapeutic modulation of epidermal growth factor receptor signaling in airway basal stem/progenitor cells by targeting the smoking-induced EGF-amphiregulin axis may represent a novel approach to treat smoking-associated lung diseases, such as chronic obstructive pulmonary disease and lung cancer.
Acknowledgments
We thank A. Brekman, M.S. Walters, T. Fukui, M. Staudt for assistance with clinical sample collection, processing and/or analysis; and N. Mohamed for help in preparing the manuscript. These studies were supported, in part, by R01HL107882, UL1 TR000457, UL1 RR024143, R01 HL127393 and R01HL123544. RS was supported, in part, by The Parker B. Francis Foundation. K.G., I.C., and R.S. are currently affiliated to Department of Medicine Weill Cornell Medical College, New York, NY.
Footnotes
Author Contributions
W.L.Z., R.G.C., and R.S.: Conception and design; R.G.C. and R.S.: Financial support; R.G.C.: Administrative support; R.G.C.: Provision of study material or patients; W.L.Z., J.Y., I.W.C., and R.S.: Collection and/or assembly of data; W.L.Z., R.G.C., and R.S.: Data analysis and interpretation; W.L.Z., R.G.C., and R.S.: Manuscript writing; W.L.Z., J.Y., I.W.C., R.G.C., and R.S.: Final approval of manuscript.
Disclosure of Potential Conflict of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Crystal RG, Randell SH, Engelhardt JF, et al. Airway epithelial cells: Current concepts and challenges. Proc Am Thorac Soc. 2008;5:772–777. doi: 10.1513/pats.200805-041HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: A perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong KU, Reynolds SD, Watkins S, et al. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol. 2004;164:577–588. doi: 10.1016/S0002-9440(10)63147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rock JR, Onaitis MW, Rawlins EL, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rock JR, Gao X, Xue Y, et al. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell. 2011;8:639–648. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mori M, Mahoney JE, Stupnikov MR, et al. Notch3-Jagged signaling controls the pool of undifferentiated airway progenitors. Development. 2015;142:258–267. doi: 10.1242/dev.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardo-Saganta A, Law BM, Tata PR, et al. Injury induces direct lineage segregation of functionally distinct airway Basal stem/progenitor cell subpopulations. Cell Stem Cell. 2015;16:184–197. doi: 10.1016/j.stem.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneeberger EE, Lynch RD. The tight junction: A multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 10.Baraldo S, Turato G, Saetta M. Pathophysiology of the small airways in chronic obstructive pulmonary disease. Respiration. 2012;84:89–97. doi: 10.1159/000341382. [DOI] [PubMed] [Google Scholar]
- 11.Shaykhiev R, Crystal RG. Early events in the pathogenesis of chronic obstructive pulmonary disease. Smoking-induced reprogramming of airway epithelial basal progenitor cells. Ann Am Thorac Soc. 2014;11:S252–S258. doi: 10.1513/AnnalsATS.201402-049AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaykhiev R, Zuo WL, Chao I, et al. EGF shifts human airway basal cell fate toward a smoking-associated airway epithelial phenotype. Proc Natl Acad Sci USA. 2013;110:12102–12107. doi: 10.1073/pnas.1303058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgel PR, Nadel JA. Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax. 2004;59:992–996. doi: 10.1136/thx.2003.018879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemjabbar-Alaoui H, Sidhu SS, Mengistab A, et al. TACE/ADAM-17 phosphorylation by PKC-epsilon mediates premalignant changes in tobacco smoke-exposed lung cells. PLoS One. 2011;6:e17489. doi: 10.1371/journal.pone.0017489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemjabbar H, Li D, Gallup M, et al. Tobacco smoke-induced lung cell proliferation mediated by tumor necrosis factor alpha-converting enzyme and amphiregulin. J Biol Chem. 2003;278:26202–26207. doi: 10.1074/jbc.M207018200. [DOI] [PubMed] [Google Scholar]
- 16.Woodruff PG, Wolff M, Hohlfeld JM, et al. Safety and efficacy of an inhaled epidermal growth factor receptor inhibitor (BIBW 2948 BS) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:438–445. doi: 10.1164/rccm.200909-1415OC. [DOI] [PubMed] [Google Scholar]
- 17.Shoyab M, Plowman GD, McDonald VL, et al. Structure and function of human amphiregulin: A member of the epidermal growth factor family. Science. 1989;243:1074–1076. doi: 10.1126/science.2466334. [DOI] [PubMed] [Google Scholar]
- 18.Adam R, Drummond DR, Solic N, et al. Modulation of the receptor binding affinity of amphiregulin by modification of its carboxyl terminal tail. Biochim Biophys Acta. 1995;1266:83–90. doi: 10.1016/0167-4889(94)00224-3. [DOI] [PubMed] [Google Scholar]
- 19.Roepstorff K, Grandal MV, Henriksen L, et al. Differential effects of EGFR ligands on endocytic sorting of the receptor. Traffic. 2009;10:1115–1127. doi: 10.1111/j.1600-0854.2009.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willmarth NE, Baillo A, Dziubinski ML, et al. Altered EGFR localization and degradation in human breast cancer cells with an amphiregulin/EGFR autocrine loop. Cell Signal. 2009;21:212–219. doi: 10.1016/j.cellsig.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manzo ND, Foster WM, Stripp BR. Amphiregulin-dependent mucous cell metaplasia in a model of nonallergic lung injury. Am J Respir Cell Mol Biol. 2012;47:349–357. doi: 10.1165/rcmb.2011-0257OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Val S, Belade E, George I, et al. Fine PM induce airway MUC5AC expression through the autocrine effect of amphiregulin. Arch Toxicol. 2012;86:1851–1859. doi: 10.1007/s00204-012-0903-6. [DOI] [PubMed] [Google Scholar]
- 23.Hirota N, Risse PA, Novali M, et al. Histamine may induce airway remodeling through release of epidermal growth factor receptor ligands from bronchial epithelial cells. FASEB J. 2012;26:1704–1716. doi: 10.1096/fj.11-197061. [DOI] [PubMed] [Google Scholar]
- 24.Hackett NR, Shaykhiev R, Walters MS, et al. The human airway epithelial basal cell transcriptome. PLoS One. 2011;6:e18378. doi: 10.1371/journal.pone.0018378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fulcher ML, Randell SH. Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol Biol. 2013;945:109–121. doi: 10.1007/978-1-62703-125-7_8. [DOI] [PubMed] [Google Scholar]
- 26.Araya J, Cambier S, Markovics JA, et al. Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J Clin Invest. 2007;117:3551–3562. doi: 10.1172/JCI32526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi K, Coulombe PA. Defining a region of the human keratin 6a gene that confers inducible expression in stratified epithelia of transgenic mice. J Biol Chem. 1997;272:11979–11985. doi: 10.1074/jbc.272.18.11979. [DOI] [PubMed] [Google Scholar]
- 28.de Boer WI, Hau CM, van SA, et al. Expression of epidermal growth factors and their receptors in the bronchial epithelium of subjects with chronic obstructive pulmonary disease. Am J Clin Pathol. 2006;125:184–192. doi: 10.1309/W1AX-KGT7-UA37-X257. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh M, Ahmad S, Jian A, et al. Human tracheobronchial basal cells. Normal versus remodeling/repairing phenotypes in vivo and in vitro. Am J Respir Cell Mol Biol. 2013;49:1127–1134. doi: 10.1165/rcmb.2013-0049OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ooi AT, Mah V, Nickerson DW, et al. Presence of a putative tumor-initiating progenitor cell population predicts poor prognosis in smokers with non-small cell lung cancer. Cancer Res. 2010;70:6639–6648. doi: 10.1158/0008-5472.CAN-10-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enomoto T, Lu HQ, Yin M, et al. Evaluation of the efficacy and safety of olopatadine and fexofenadine compared with placebo in Japanese cedar pollinosis using an environmental exposure unit. J Investig Allergol Clin Immunol. 2009;19:299–305. [PubMed] [Google Scholar]
- 32.Citri A, Yarden Y. EGF-ERBB signalling: Towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 33.Lim RW, Hauschka SD. A rapid decrease in epidermal growth factor-binding capacity accompanies the terminal differentiation of mouse myoblasts in vitro. J Cell Biol. 1984;98:739–747. doi: 10.1083/jcb.98.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amit I, Citri A, Shay T, et al. A module of negative feedback regulators defines growth factor signaling. Nat Genet. 2007;39:503–512. doi: 10.1038/ng1987. [DOI] [PubMed] [Google Scholar]
- 35.Segatto O, Anastasi S, Alema S. Regulation of epidermal growth factor receptor signalling by inducible feedback inhibitors. J Cell Sci. 2011;124:1785–1793. doi: 10.1242/jcs.083303. [DOI] [PubMed] [Google Scholar]
- 36.Ross AJ, Dailey LA, Brighton LE, et al. Transcriptional profiling of mucociliary differentiation in human airway epithelial cells. Am J Respir Cell Mol Biol. 2007;37:169–185. doi: 10.1165/rcmb.2006-0466OC. [DOI] [PubMed] [Google Scholar]
- 37.Heijink IH, Brandenburg SM, Postma DS, et al. Cigarette smoke impairs airway epithelial barrier function and cell-cell contact recovery. Eur Respir J. 2012;39:419–428. doi: 10.1183/09031936.00193810. [DOI] [PubMed] [Google Scholar]
- 38.Shaykhiev R, Otaki F, Bonsu P, et al. Cigarette smoking reprograms apical junctional complex molecular architecture in the human airway epithelium in vivo. Cell Mol Life Sci. 2011;68:877–892. doi: 10.1007/s00018-010-0500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leopold PL, O’mahony MJ, Lian XJ, et al. Smoking is associated with shortened airway cilia. PLoS One. 2009;4:e8157. doi: 10.1371/journal.pone.0008157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hessel J, Heldrich J, Fuller J, et al. Intraflagellar transport gene expression associated with short cilia in smoking and COPD. PLoS One. 2014;9:e85453. doi: 10.1371/journal.pone.0085453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsao MS, Zhu H, Viallet J. Autocrine growth loop of the epidermal growth factor receptor in normal and immortalized human bronchial epithelial cells. Exp Cell Res. 1996;223:268–273. doi: 10.1006/excr.1996.0081. [DOI] [PubMed] [Google Scholar]
- 42.Richter A, O’donnell RA, Powell RM, et al. Autocrine ligands for the epidermal growth factor receptor mediate interleukin-8 release from bronchial epithelial cells in response to cigarette smoke. Am J Respir Cell Mol Biol. 2002;27:85–90. doi: 10.1165/ajrcmb.27.1.4789. [DOI] [PubMed] [Google Scholar]
- 43.Berasain C, Avila MA. Amphiregulin. Semin Cell Dev Biol. 2014;28:31–41. doi: 10.1016/j.semcdb.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Shoyab M, McDonald VL, Bradley JG, et al. Amphiregulin: A bifunctional growth-modulating glycoprotein produced by the phorbol 12-myristate 13-acetate-treated human breast adenocarcinoma cell line MCF-7. Proc Natl Acad Sci USA. 1988;85:6528–6532. doi: 10.1073/pnas.85.17.6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brekman A, Walters MS, Tilley AE, et al. FOXJ1 prevents cilia growth inhibition by cigarette smoke in human airway epithelium in vitro. Am J Respir Cell Mol Biol. 2014;51:688–700. doi: 10.1165/rcmb.2013-0363OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wistuba II, Gazdar AF. Lung cancer preneoplasia. Annu Rev Pathol. 2006;1:331–348. doi: 10.1146/annurev.pathol.1.110304.100103. [DOI] [PubMed] [Google Scholar]
- 47.Pommier G, Culouscou JM, Garrouste F, et al. CRGF: An autocrine growth factor associated with colorectal carcinomas. Ann N Y Acad Sci. 1988;551:382–384. doi: 10.1111/j.1749-6632.1988.tb22371.x. [DOI] [PubMed] [Google Scholar]
- 48.Zaiss DM, Gause WC, Osborne LC, et al. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity. 2015;42:216–226. doi: 10.1016/j.immuni.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Du B, Altorki NK, Kopelovich L, et al. Tobacco smoke stimulates the transcription of amphiregulin in human oral epithelial cells: Evidence of a cyclic AMP-responsive element binding protein-dependent mechanism. Cancer Res. 2005;65:5982–5988. doi: 10.1158/0008-5472.CAN-05-0628. [DOI] [PubMed] [Google Scholar]
- 50.Pfeifer AM, Lechner JF, Masui T, et al. Control of growth and squamous differentiation in normal human bronchial epithelial cells by chemical and biological modifiers and transferred genes. Environ Health Perspect. 1989;80:209–220. doi: 10.1289/ehp.8980209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isokane M, Hieda M, Hirakawa S, et al. Plasma-membrane-anchored growth factor pro-amphiregulin binds A-type lamin and regulates global transcription. J Cell Sci. 2008;121:3608–3618. doi: 10.1242/jcs.031443. [DOI] [PubMed] [Google Scholar]
- 52.Erjefalt JS, Erjefalt I, Sundler F, et al. In vivo restitution of airway epithelium. Cell Tissue Res. 1995;281:305–316. doi: 10.1007/BF00583399. [DOI] [PubMed] [Google Scholar]
- 53.Park KS, Wells JM, Zorn AM, et al. Trans-differentiation of ciliated cells during repair of the respiratory epithelium. Am J Respir Cell Mol Biol. 2006;34:151–157. doi: 10.1165/rcmb.2005-0332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baldys A, Gooz M, Morinelli TA, et al. Essential role of c-Cbl in amphiregulin-induced recycling and signaling of the endogenous epidermal growth factor receptor. Biochemistry. 2009;48:1462–1473. doi: 10.1021/bi801771g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stern KA, Place TL, Lill NL. EGF and amphiregulin differentially regulate Cbl recruitment to endosomes and EGF receptor fate. Biochem J. 2008;410:585–594. doi: 10.1042/BJ20071505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Solic N, Davies DE. Differential effects of EGF and amphiregulin on adhesion molecule expression and migration of colon carcinoma cells. Exp Cell Res. 1997;234:465–476. doi: 10.1006/excr.1997.3635. [DOI] [PubMed] [Google Scholar]
- 57.Gray TE, Guzman K, Davis CW, et al. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol. 1996;14:104–112. doi: 10.1165/ajrcmb.14.1.8534481. [DOI] [PubMed] [Google Scholar]
- 58.Choksi SP, Lauter G, Swoboda P, et al. Switching on cilia: Transcriptional networks regulating ciliogenesis. Development. 2014;141:1427–1441. doi: 10.1242/dev.074666. [DOI] [PubMed] [Google Scholar]
- 59.Danahay H, Pessotti AD, Coote J, et al. Notch2 is required for inflammatory cytokine-driven goblet cell metaplasia in the lung. Cell Rep. 2015;10:239–252. doi: 10.1016/j.celrep.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 60.Laoukili J, Perret E, Willems T, et al. IL-13 alters mucociliary differentiation and ciliary beating of human respiratory epithelial cells. J Clin Invest. 2001;108:1817–1824. doi: 10.1172/JCI13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson KJ, Mill C, Lambert S, et al. EGFR ligands exhibit functional differences in models of paracrine and autocrine signaling. Growth Factors. 2012;30:107–116. doi: 10.3109/08977194.2011.649918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 63.Fukui T, Shaykhiev R, Gosto-Perez F, et al. Lung adenocarcinoma subtypes based on expression of human airway basal cell genes. Eur Respir J. 2013;42:1332–1344. doi: 10.1183/09031936.00144012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hurbin A, Wislez M, Busser B, et al. Insulin-like growth factor-1 receptor inhibition overcomes gefitinib resistance in mucinous lung adenocarcinoma. J Pathol. 2011;225:83–95. doi: 10.1002/path.2897. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.