Abstract
Background
Diagnosis of heart failure (HF) with preserved ejection fraction (HFpEF) is challenging and relies largely on demonstration of elevated cardiac filling pressures (pulmonary capillary wedge pressure, PCWP). Current guidelines recommend use of natriuretic peptides (NT-proBNP) and rest/exercise echocardiography (E/e’ ratio) to make this determination. Data to support this practice is conflicting.
Methods
Simultaneous echocardiographic-catheterization studies were prospectively conducted at rest and during exercise in subjects with invasively-proven HFpEF (n=50) and participants with dyspnea but no identifiable cardiac pathology (n=24).
Results
NT-proBNP levels were below the level considered to exclude disease (≤125 pg/ml) in 18% of subjects with HFpEF. E/e’ ratio was correlated with directly measured PCWP at rest (r=0.63, p<0.0001) and during exercise (r=0.57, p<0.0001). While specific, current guidelines were poorly sensitive, identifying only 34–60% of subjects with invasively-proven HFpEF based upon resting echocardiographic data alone. Addition of exercise echocardiographic data (E/e’ ratio>14) improved sensitivity (to 90%) and thus negative predictive value, but decreased specificity (71%).
Conclusions
Currently proposed HFpEF diagnostic guidelines based upon resting data are poorly sensitive. Adding exercise E/e’ data improves sensitivity and negative predictive value but compromises specificity, suggesting that exercise echocardiography may help rule out HFpEF. These results question the accuracy of current approaches to exclude HFpEF based upon resting data alone and reinforce the value of exercise testing using invasive and noninvasive hemodynamic assessments to definitively confirm or refute the diagnosis of HFpEF.
Clinical trial registration NCT01418248 https://clinicaltrials.gov/ct2/results?term=NCT01418248&Search=Search
Keywords: diagnosis, diastolic stress test, exercise, heart failure
INTRODUCTION
Approximately one-half of patients with heart failure (HF) have a preserved ejection fraction (HFpEF).1 Diagnosis of HFpEF is straightforward when patients are acutely decompensated. However, among stable people presenting with chronic dyspnea, diagnosis is challenging and relies upon identifying direct or indirect evidence of elevated left ventricular (LV) filling pressures.1–4 To make matters more complex, many patients with HFpEF display normal LV filling pressures at rest, with abnormalities that develop only during physiologic stresses like exercise.5–7 Invasive hemodynamic exercise testing has emerged as the gold standard to diagnose or exclude HFpEF in patients with exertional dyspnea of unclear etiology,7 but cost, risk, and the requirement for specialized training and equipment may limit its broad application in practice and in clinical trials.
The ACC/AHA guidelines define HFpEF as clinical signs and symptoms of HF, preserved EF, and no other obvious explanation for symptoms.8 This scheme works well for patients with a high likelihood of disease based upon clinical indicators of congestion such as jugular distention, gallop sounds, or edema.9 To address the patients without overt congestion, more recent guidelines statements from the ESC and ASE/ EACVI require objective evidence of high LV filling pressures, such as elevations in plasma natriuretic peptide (NP) levels and the ratio of transmitral E to mitral annular e’ velocities (E/e’).2–4 While this approach is supported by some studies, others have raised serious questions regarding their accuracy.5, 9–17 Importantly, no study has rigorously tested these more recently proposed algorithms for diagnosis of HFpEF using gold standard, invasive data.
Accordingly, we performed a trial testing the performance characteristics of these diagnostic algorithms for HFpEF and the incremental utility of adding exercise echocardiography to improve diagnostic performance in patients presenting with normal EF and unexplained dyspnea.
METHODS
Consecutive subjects referred to the Mayo Clinic catheterization laboratory for invasive exercise right heart catheterization because of the indication of exertional dyspnea of unclear cause were prospectively enrolled between August 2011 and July 2013. Some participant data from this study has been published,6, 18, 19 but not as it relates to the diagnostic evaluation of HFpEF. Written informed consent was provided by all patients prior to participation in study-related procedures. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written. The study was approved by the Mayo Clinic Institutional Review Board and the study was registered (NCT01418248).
Study Population
All patients referred for exercise stress testing in the evaluation of exertional dyspnea were approached for participation in this trial. The current analysis is restricted to subjects with normal LVEF and either HFpEF or non-cardiac dyspnea (NCD). HFpEF was defined by typical clinical symptoms (dyspnea, fatigue), normal LVEF (≥50%), elevated left heart filling pressures (pulmonary capillary wedge pressure, PCWP) at rest (>15mmHg) and/or with exercise (≥25mmHg), and exclusion of alternative causes of the clinical syndrome of HF: primary cardiomyopathies (hypertrophic, infiltrative or restrictive), constrictive pericarditis, high output heart failure, significant valvular heart disease (>moderate regurgitation or >mild stenosis), pulmonary embolism and right ventricular myopathies. Subjects with NCD (n=24) were required to display no cardiac pathology after thorough clinical evaluation, imaging and invasive assessment, including normal rest and exercise mean pulmonary artery (PA) pressures (rest<25mmHg, exercise<40mmHg) and normal rest-exercise PCWP (criteria above).
Study Protocol
After providing consent, subjects underwent history, physical examination and comprehensive resting echocardiogram to enable the sonographer to identify optimal imaging windows. Cardiac catheterization was then performed in the supine position with simultaneous echocardiography and expired gas analysis at rest and during cycle ergometry exercise. The first stage of exercise (20 Watts, W) was performed for 5 minutes and followed by graded 10W increments in workload (3 minute stages) to subject-reported exhaustion.
Catheterization Protocol
Patients were studied on their chronic medications in the fasted state after minimal sedation as previously described.5, 6, 18–21 Right heart catheterization was performed through a 9 Fr sheath via the right internal jugular vein. Pressures in the right atrium (RA), right ventricular (RV), PA, and PCWP were measured at end expiration (mean of ≥3 beats) using 2 Fr high fidelity micromanometer-tipped catheters (Millar Instruments, Houston, TX) advanced through the lumen of a 7 Fr fluid-filled catheter (Balloon wedge, Arrow). Mean micromanometer pressures were calibrated to mean fluid-filled pressures at the beginning and throughout each case to avoid baseline drift. Transducers were zeroed at mid-axilla, measured by laser calipers in each patient.
Pressure tracings from the entire study were digitized (250 Hz) and stored for offline analysis by one investigator experienced in exercise hemodynamic assessment (BAB). Mean RA and PCWP were taken at mid A wave. PCWP position was verified by typical waveforms, appearance on fluoroscopy, and direct oximetry (PCWP blood saturation≥94%). Arterial blood pressure (BP) was measured through a 4–6 Fr radial arterial cannula throughout the tests. Arterial-venous O2 content difference (AVO2diff) was measured directly as the difference between systemic arterial and PA O2 content (=saturation*hemoglobin*1.34). Oxygen consumption (VO2) was measured from expired gas analysis (MedGraphics, St. Paul, MN) to calculate cardiac output (CO), by the direct Fick method (CO= VO2÷AVO2diff) at baseline, 20W and peak exercise. Stroke volume (SV) was determined from the quotient of CO and heart rate (HR).
Echocardiography
Comprehensive two-dimensional, M-mode, Doppler and tissue Doppler echocardiography was performed according to contemporary guidelines by experienced sonographers.4 Echocardiographic data was obtained simultaneously with invasive assessment at rest and during all stages of exercise. All studies were interpreted offline and in a completely blinded fashion by a single investigator with extensive experience in resting and exercise echocardiographic assessment (GCK).
Early (E) transmitral filling velocities were measured at the mitral leaflet tips by pulse wave Doppler. Tissue Doppler echocardiography was performed to measure early (e’) diastolic tissue velocities at the septal and lateral mitral annulus. The mean of the septal and lateral E/e’ ratio was used as the primary estimate of PCWP. All measures represent the mean of measurements from 3 beats for subjects in sinus rhythm and the mean of 5 beats for subjects in atrial fibrillation.
Noninvasive Diagnosis of HFpEF
To evaluate the diagnostic performance of current algorithms and the potential value for exercise echocardiography, the diagnosis of HFpEF was first coded from resting echocardiographic data alone using contemporary diagnostic schemes as proposed by the European Society of Cardiology (ESC) and separately according to recommendations from the American Society of Echocardiography/ European Association of Cardiovascular Imaging (ASE/EACVI) for the assessment of diastolic dysfunction (Supplemental Figure 1A, B).2, 4
Next, the diagnosis of HFpEF was coded after adding in the data obtained from exercise echocardiography according to the ASE/EACVI.4 Specifically, the latter guidelines stipulate that diastolic dysfunction (in this case, HFpEF) can be coded if average E/e’>14 or septal E/e’>15 during exercise, peak TR velocity is >2.8 m/sec, and septal e’ velocity is <7 cm/sec (if only lateral e’ was acquired, values <10 cm/sec considered abnormal).4 Cases were coded as HFpEF if either 20W or peak criteria (or both stages) were met. We also investigated the diagnostic value of adding exercise E/e’ alone (without requiring corroboratory evidence from TR velocity).
Statistical Analysis
Results are reported as mean (SD), median (IQR) or number (%). Within-group differences are assessed by paired t test or repeated measures ANOVA. Between-group differences were compared by unpaired t test, Wilcoxon rank sum test, χ2 or Fisher’s exact test as appropriate. Regression was used to assess correlation between invasive and noninvasive hemodynamic measures. Correlations are reported using the Spearman rank coefficient. C-statistics were derived from logistic regression analysis using HFpEF as the outcome.
RESULTS
Subject Characteristics
Of the 108 subjects enrolled in this prospective trial, 1 withdrew consent prior to study, 1 developed transient heart block requiring transvenous pacing, and 32 did not meet entry criteria because of HFrEF, significant valvular heart disease, or Group 1 pulmonary hypertension. Of the remaining 74 participants, 24 (32%) were found to have NCD and 50 (68%) met prospectively-defined criteria for HFpEF.
Compared to NCD, subjects with HFpEF were older, more obese, hypertensive, anemic, and had more impaired renal function (Table 1). LV structure and EF were similar in HFpEF and NCD. Peak exercise capacity reflected by maximal workload achieved was lower in HFpEF (36±15 vs 70±29 W, p<0.0001). While NT-proBNP levels were higher in HFpEF than NCD (Table 1), 18%, 30% and 40% of subjects with invasively-proven HFpEF displayed NT-proBNP levels that fell below the thresholds considered to exclude HFpEF according to ESC consensus guidelines and/or eligibility criteria for enrollment in clinical trials (≤125, <225, and <300 pg/ml, respectively).2, 3
Table 1.
Baseline Characteristics
| NCD (n=24) |
HFpEF (n=50) |
P value | |
|---|---|---|---|
| Age (years) | 61±12 | 70±11 | 0.004 |
| Female, n (%) | 11 (46) | 27 (54) | 0.6 |
| Body mass index (kg/m2) | 27.2±4.4 | 34.4±6.9 | <0.0001 |
| Prior HF hospitalization n (%) | 0 (0) | 0 (0) | 1.0 |
| Comorbidities | |||
| Coronary disease, n (%) | 6 (25) | 18 (36) | 0.4 |
| Diabetes mellitus, n (%) | 5 (21) | 18 (36) | 0.3 |
| Hypertension, n (%) | 15 (63) | 47 (94) | 0.001 |
| Current atrial fibrillation, n (%) | 2 (8) | 8 (16) | 0.5 |
| Medications | |||
| ACEI or ARB, n (%) | 10 (42) | 33 (66) | 0.048 |
| Beta-blocker, n (%) | 11 (46) | 33 (66) | 0.10 |
| Loop Diuretic, n (%) | 5 (21) | 20 (40) | 0.12 |
| Laboratories | |||
| Hemoglobin (gm/dl) | 13.9±1.2 | 12.6±1.3 | <0.0001 |
| Creatinine (gm/dl) | 1.0 (0.8,1.1) | 1.2 (0.9,1.6) | 0.01 |
| NT-proBNP (pg/ml) | 90 (44,429) | 406 (139,1257) | 0.0008 |
| Physical Exam | |||
| JVP (<8/8-12/12-16/>16 cm), n | 24/0/0/0 | 26/15/2/7 | 0.0007 |
| Rales, n | 0 | 2 | 1.0 |
| S3 gallop, n | 0 | 1 | 1.0 |
| Edema (none/1+/≥2+), n | 23/1/0 | 30/13/7 | 0.006 |
| LV Structure & Function | |||
| LV diastolic dimension (mm) | 48±6 | 48±6 | 0.7 |
| LV mass index (gm/m2) | 88±20 | 86±23 | 0.7 |
| LV ejection fraction (%) | 60±9 | 62±8 | 0.4 |
| LA volume index (ml/m2) | 29 (20,46) | 37 (31,54) | 0.036 |
Data are mean ± SD, median (interquartile range), or n (%). ACEI indicates angiotensin-converting enzyme inhibitors; ARB, angiotensin-receptor blockers; HFpEF, heart failure with preserved ejection fraction; LA, left atrial; LV, left ventricular; NCD, non-cardiac dyspnea; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Baseline Invasive and Noninvasive Hemodynamics
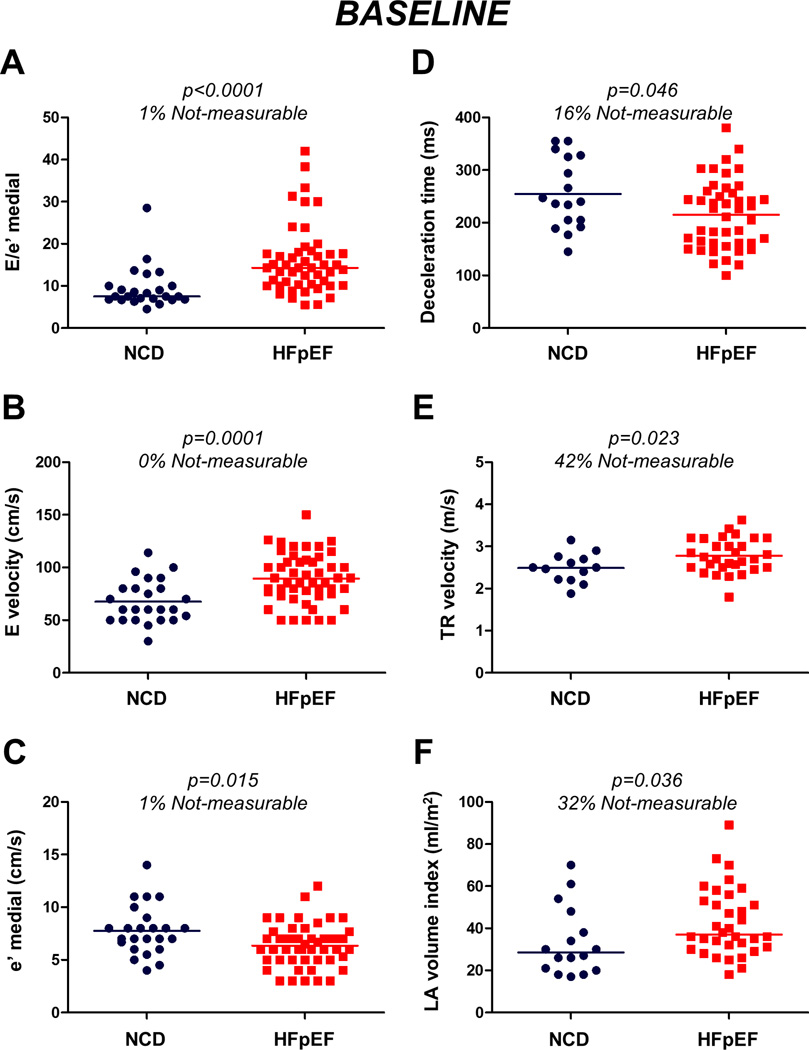
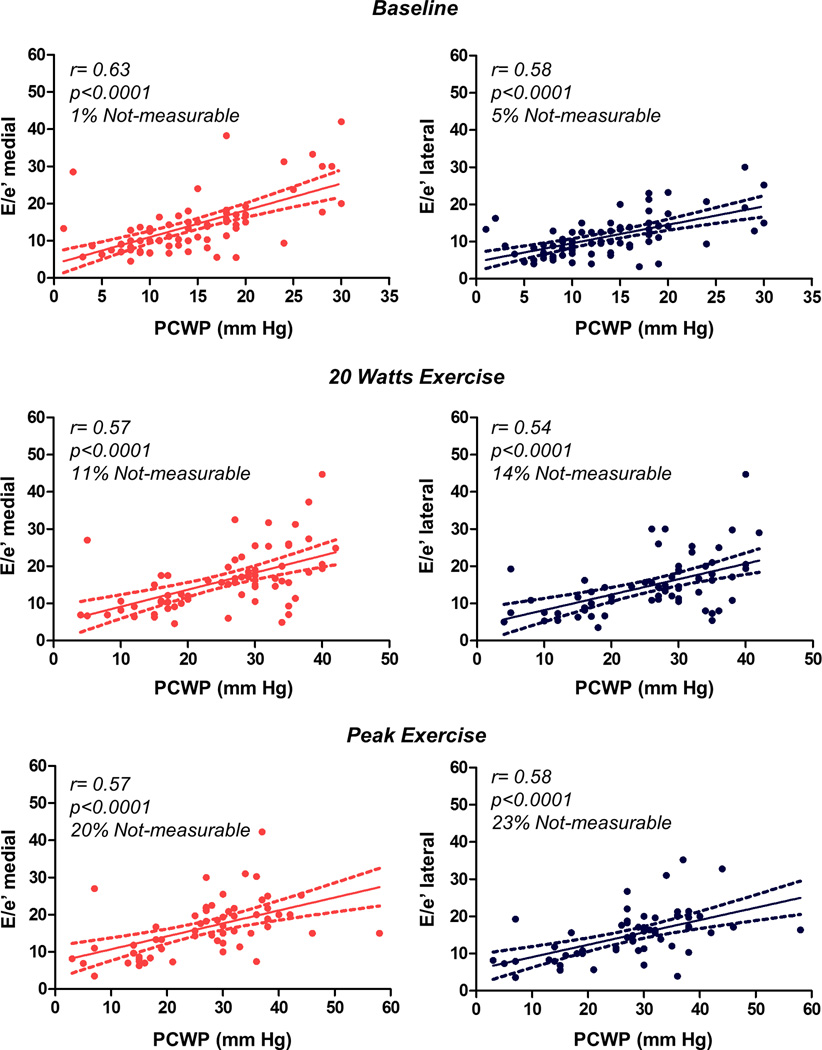
Subjects with HFpEF displayed higher right and left heart filling pressures with higher pulmonary artery pressures by catheterization compared to NCD (Table 2). Medial and lateral E/e’ data were obtainable in almost all subjects at rest (99% and 95% respectively). As expected, LV diastolic function was impaired in subjects with HFpEF compared to NCD, with higher transmitral E velocity, shorter deceleration time, lower medial and lateral e’ velocities, higher E/e’ ratio, larger left atrial (LA) volume index, and higher TR velocity (Tables 1 and 2). However, despite significant group differences, there was substantial overlap in these echocardiographic indices at rest (Figure 1). Medial and lateral E/e’ ratios were modestly correlated with directly measured PCWP at rest (r=0.63 and 0.58, p<0.0001; Figure 2).
Table 2.
Baseline and Exercise Invasive and Noninvasive Hemodynamics
| Baseline | 20 W Exercise | Peak Exercise | ||||
|---|---|---|---|---|---|---|
| NCD | HFpEF | NCD | HFpEF | NCD | HFpEF | |
| Invasive Hemodynamics | ||||||
| Heart rate (bpm) | 68±13 | 67±10 | 91±14 | 88±14 | 121±18 | 97±15* |
| Systolic BP (mmHg) | 139±24 | 149±21 | 167±29 | 175±26 | 184±25 | 185±30 |
| RA pressure (mmHg) | 4±2 | 10±4* | 8±3 | 21±5* | 8±4 | 22±6* |
| PA mean pressure (mmHg) | 16±4 | 27±8* | 25±7 | 47±10* | 27±6 | 48±8* |
| PCWP (mmHg) | 7±3 | 17±6* | 14±5 | 31±5* | 14±5 | 34±6* |
| Cardiac output (l/min) | 5.2±1.8 | 5.1±1.2 | 8.2±1.8 | 6.8±2.0† | 11.8±3.9 | 8.1±2.8* |
| Cardiac index (l/min*m2) | 2.7±0.8 | 2.4±0.6 | 4.3±0.9 | 3.2±1.0* | 6.0±1.7 | 3.8±1.3* |
| Echocardiographic Indices | ||||||
| E velocity (cm/s) | 68±20 | 89±24* | 85±27 | 114±24* | 109±28 | 127±25‡ |
| E/A ratio | 1.0±0.6 | 1.2±0.6 | 1.1±0.4 | 1.4±0.4‡ | 0.9±0.2 | 1.4±0.5† |
| Deceleration time (ms) | 255±67 | 215±65‡ | 183±83 | 167±46 | 155±46 | 163±53 |
| Medial e’ velocity (cm/s) | 8±2 | 6±2‡ | 9±3 | 7±2† | 12±5 | 7±3* |
| Lateral e’ velocity (cm/s) | 9±2 | 7±2† | 11±4 | 7±2† | 14±5 | 8±3* |
| Medial E/e’ ratio | 8 (7,10) | 14 (10,18)* | 10 (7,12) | 17 (15,24)* | 8 (7,12) | 20 (15,22)* |
| Lateral E/e’ ratio | 7 (5,9) | 13 (10,15)* | 8 (6,12) | 16 (12,20)* | 8 (6,10) | 17 (13,20)* |
| Average E/e’ ratio | 8 (7,10) | 13 (10,17)* | 9 (7,13) | 17 (14,21)* | 8 (7,12) | 18 (14,20)* |
| TR velocity, m/s | 2.5±0.3 | 2.8±0.4‡ | 2.9±0.5 | 3.2±0.4‡ | 2.8±0.6 | 3.4±0.4‡ |
Data are mean ± SD or median (interquartile range).
P<0.001 vs. NCD.
P<0.01 vs. NCD.
P<0.05 vs. NCD.
A indicates late mitral diastolic inflow velocity; BP, blood pressure; E, early mitral diastolic inflow velocity; e′, early diastolic mitral annular velocity; PA; pulmonary artery; PCWP, pulmonary capillary wedge pressure; RA, right atrial; TR, tricuspid regurgitation; and other abbreviations as in Table 1.
Figure 1. Baseline echocardiographic parameters shown in heart failure with preserved ejection fraction (HFpEF) and non-cardiac dyspnea (NCD).
(A) medial E/e’ ratio, (B) mitral E velocity, (C) medial e’ velocity, (D) deceleration time, (E) tricuspid regurgitant (TR) velocity, and (F) left atrial (LA) volume index. Notably, there was substantial overlap in these rest echocardiographic parameters.
Figure 2. Correlations between Invasive Filling pressures and Echocardiographic Estimates.
Amongst subjects with obtainable data, medial and lateral E/e’ ratios were modestly correlated with directly measured pulmonary capillary wedge pressure (PCWP) at rest, submaximal (20W) and peak exercise. The feasibility of obtaining diagnostic quality echocardiographic measurements decreased during exercise.
Exercise Echo-Catheterization Correlation
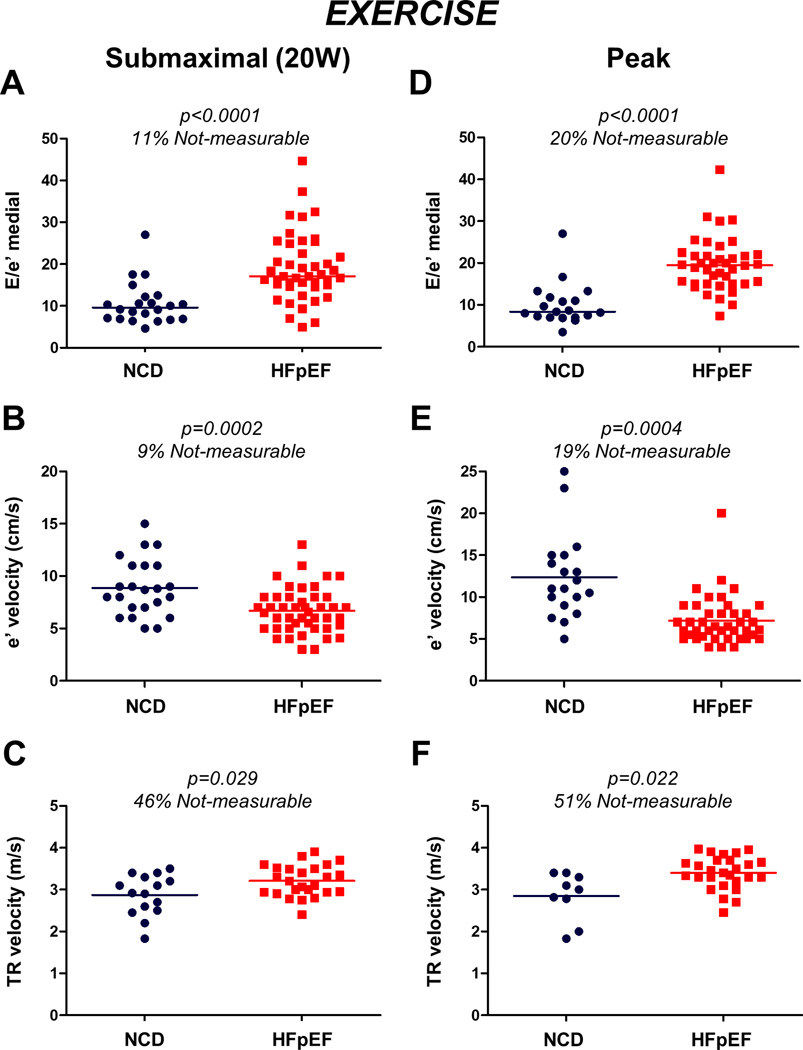
During submaximal (20W) and peak exercise, subjects with HFpEF displayed higher left and right heart filling pressures, higher PA pressures and lower CO and cardiac index compared to NCD (Table 2). The feasibility of obtaining diagnostic quality echocardiographic measurements decreased during exercise, with the medial and lateral E/e’ obtainable in 89% and 86% of subjects at 20W exercise, and 80% and 77% at peak exercise. TR velocities were obtainable in 54% of participants at 20W exercise and 49% at peak exercise.
Medial E/e’ was higher, medial e’ velocity was lower, and TR velocity was higher in HFpEF patients compared to NCD during exercise. However, there was again substantial overlap between HFpEF and NCD in the echocardiographic indicators of diastolic function during exercise, with the E/e’ ratio showing the best separation between groups (Figure 3).
Figure 3. Echocardiographic hemodynamic and Ventricular function indices during Exercise.
(A–C) Compared to NCD, medial E/e’ was higher, medial e’ velocity was lower, and TR velocity was higher in HFpEF patients during submaximal (20W) and (D–F) peak exercise. However, there was still substantial overlap between HFpEF and NCD in these echocardiographic markers during exercise, with the E/e’ ratio showing the best separation between the groups. Abbreviations as in Figure 1.
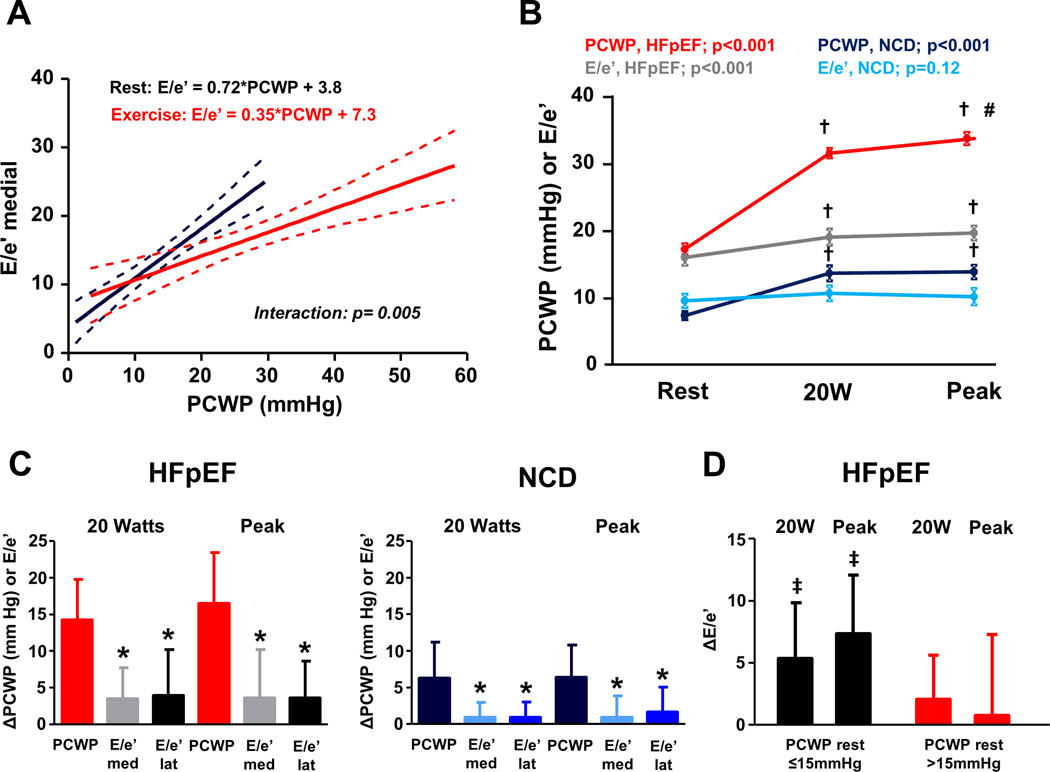
Amongst subjects with obtainable data, modest correlations between E/e’ and PCWP were again observed at 20W and peak exercise (Figure 2). However, as compared to rest, exercise E/e’ underestimated PCWP during exercise (higher PCWP for any E/e’ value, interaction p=0.005, Figure 4A). Absolute unit increases in E/e’ during exercise were much lower than unit changes in PCWP in both HFpEF and NCD subjects, indicating lower dynamic range for E/e’ (Figure 4B, C). Medial E/e’ ratio was found to increase during exercise in HFpEF patients with normal rest filling pressures (p<0.001), but there was no change in E/e’ amongst HFpEF subjects with elevated resting PCWP (p=0.15, Figure 4D).
Figure 4. Relationships between invasive and noninvasive estimated filling pressures during exercise.
(A) Compared to rest, exercise E/e’ underestimated PCWP during exercise. 95% CI on regression lines are for the best-fit mean line. Interaction p value reflects the difference in slopes in the regression between E/e’ and PCWP at rest and during exercise. (B) Absolute values of PCWP and medial E/e’ during exercise for HFpEF and NCD. Error bars indicate SEM. P values at the top reflect a 1-way repeated measures ANOVA testing whether PCWP or E/e’ changes during exercise stages in HFpEF and NCD. †p<0.05 vs at rest; #p<0.05 vs 20 W exercise. (C) Absolute unit increases (i.e. changes from baseline, Δ) in both medial and lateral E/e’ were much lower than unit changes in PCWP in both HFpEF and NCD at 20W (left) and peak exercise (right). Error bars indicate SD. *p<0.05 vs PCWP. (D) Compared to HFpEF with elevated resting PCWP, changes with exercise (Δ) in medial E/e’ were greater in HFpEF with normal rest filling pressures both submaximal (20W) and peak exercise. Error bars indicate SD. ‡p<0.05 vs. HFpEF with rest PCWP >15mmHg. Abbreviations as in Figures 1 and 2.
Invasive and Noninvasive Approaches to Diagnose HFpEF
By definition, resting cardiac catheterization enabled diagnosis of HFpEF with perfect specificity, but misclassified 22 (44%) of the HFpEF patients as NCD based upon the absence of high PCWP at rest (Table 3, Figure 5). Addition of exercise invasive data thus significantly reclassified subjects beyond resting invasive data alone, confirming the incremental utility of invasive exercise testing beyond resting catheterization alone.5 Intriguingly, use of PCWP with submaximal exercise (20W) was nearly as effective as peak exercise data to discriminate HFpEF and NCD (Table 3).
Table 3.
Diagnosis of HFpEF According To Contemporary Guidelines
| Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
Indeter (%) | Positive LR |
Negative LR |
C-statistic | |
|---|---|---|---|---|---|---|---|---|
| ASE/EACVI algorithm | 34 (22–48) |
83 (64–93) |
94 (74–99) |
53 (37–68) |
24 | 2.0 | 0.8 | 0.65 (0.57–0.72) |
| ESC criteria | 60 (46–72) |
75 (55–88) |
83 (68–92) |
47 (32–63) |
0 | 2.4 | 0.5 | 0.68 (0.55–0.78) |
| ESC + Leg raise E/e’ | 56 (42–69) |
79 (60–91) |
85 (69–93) |
46 (32–61) |
0 | 2.7 | 0.6 | 0.68 (0.56–0.77) |
| ESC + Ex Echo | 70 (56–81) |
75 (55–88) |
85 (72–93) |
55 (38–70) |
0 | 2.8 | 0.4 | 0.73 (0.60–0.82) |
| ESC + Ex E/e’ alone | 90 (79–96) |
71 (51–85) |
87 (75–93) |
77 (57–90) |
0 | 3.1 | 0.1 | 0.80 (0.68–0.89)* |
| ESC + 20W E/e’ alone | 80 (67–89) |
88 (69–96) |
93 (81–98) |
68 (50–81) |
0 | 6.7 | 0.2 | 0.84 (0.73–0.91)* |
| Rest Cath | 56 (42–69) |
100 (86–100) |
100 (88–100) |
52 (38–66) |
0 | +∞ | 0.4 | 0.78 (0.70–0.84) |
| Rest Cath + 20W Cath | 94 (84–98) |
100 (86–100) |
100 (92–100) |
89 (72–96) |
0 | +∞ | 0.1 | 0.97 (0.91–0.99)† |
| Rest Cath + Ex Cath | 100 (93–100) |
100 (86–100) |
100 (93–100) |
100 (86–100) |
0 | +∞ | 0 | 1.00 (1.00–1.00)† |
Mean values with (95% CI) shown.
p<0.05 vs ESC
p<0.001 vs rest cath for comparisons of c-statistics.
ASE/EACVI indicates the American Society of Echocardiography/European Association of Cardiovascular Imaging; ESC, the European Society of Cardiology; Ex Cath, exercise catheterization; Ex Echo, exercise echocardiography; LR, likelihood ratio; NPV, negative predictive value; PPV, positive predictive value; Indeter, indeterminate; other abbreviations as in Tables 1 and 2.
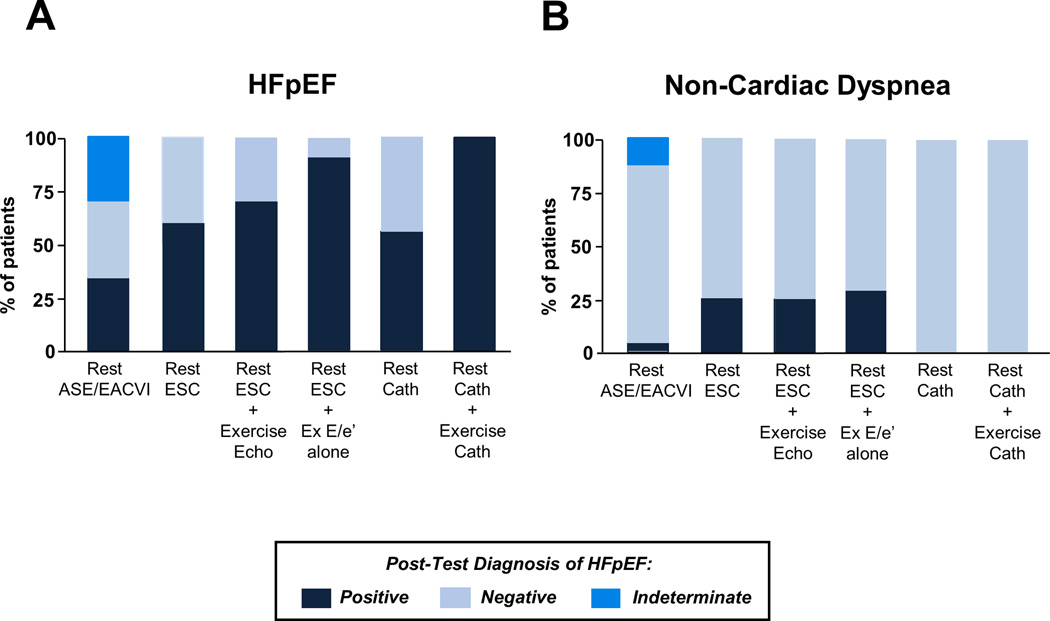
Figure 5. Proportion of subjects with HFpEF and NCD identified according to the different guideline recommended algorithms.
Dark blue indicates subjects diagnosed with true HFpEF (A) or true NCD (B) diagnosed as having HFpEF (dark blue) or NCD (light blue) using the different schemes. ASE/EACVI indicates the American Society of Echocardiography/ European Association of Cardiovascular Imaging algorithm; Cath, catheterization; Echo, echocardiography; ESC, the European Society of Cardiology criteria; Ex, exercise; and other abbreviations as in Figure 1.
Next, we evaluated the performance of noninvasive rest and stress echocardiography algorithms endorsed by current guidelines. The ASE/EACVI algorithm to diagnose diastolic dysfunction4 based upon resting echocardiography was poorly sensitive but fairly specific when used to identify or exclude HFpEF, with high positive predictive values (PPV) but low negative predictive values (NPV, Table 3, Figure 5). The low sensitivity for this algorithm was caused by a large number of indeterminate subjects (Supplemental Figure 1) where incomplete data was available to characterize them (e.g. unable to assess TR velocity) and by the substantial number of participants with high PCWP despite normal E/e’ values (Figure 2).
Next we applied the ESC algorithm for HFpEF diagnosis, which does not include indeterminate categories (Supplemental Figure 1).3 The ESC criteria displayed higher sensitivity than the ASE/EACVI algorithm, but also had poor sensitivity and thus low NPV (Table 3). Adding exercise echocardiography criteria based upon the ASE/EACVI guidelines4 to the ESC algorithm increased sensitivity modestly to diagnose HFpEF (Table 3, Figure 5) but NPV remained poor.
A large number of subjects were classified as indeterminate using ASE/EACVI guidelines for exercise echocardiography (n=54, 73%). This was predominantly related to inability to assess diagnostic quality TR velocities during exercise (n=37, 50%). To reduce data missingness and simplify the evaluation, we then determined whether addition of exercise E/e’ alone using published cutoffs for abnormal (average E/e’>14 or septal E/e’>15) to the ESC algorithm3 would improve diagnostic performance. This decreased the number of unclassifiable exercise cases to 16 (22%). Addition of exercise E/e’ improved classification beyond the resting ESC criteria, with greater sensitivity and NPV (Table 3) but an increase in the false positive rate to 29% (Figure 5). Addition of exercise E/e’ during submaximal exercise only (20W) also improved upon the resting ESC criteria (Table 3), but had less sensitivity compared to using the entire exercise E/e’ data. Addition of E/e’ with passive leg raise did not improve group discrimination as compared to the ESC criteria (Table 3). All correlations and comparisons were similar and remained significant after excluding subjects in atrial fibrillation (data not shown).
DISCUSSION
The diagnosis of HFpEF can be challenging. Current recommendations for evaluation are based upon expert consensus opinion and have not been empirically tested. We performed a prospective, comprehensive simultaneous echocardiographic-cardiac catheterization study, conducted at rest and during exercise, to evaluate and compare the utility of current approaches proposed by the ESC and ASE/EACVI to diagnose or exclude HFpEF, and identify whether addition of exercise echocardiography to current algorithms enhances diagnostic performance. We found that normal NP levels, which are considered by some to exclude disease, were common in subjects with invasively-proven HFpEF. Diagnostic quality echocardiographic indices, in particular TR velocity, could not be obtained during exercise in a large proportion of participants, and even when feasibly obtained, these indices displayed substantial overlap between HFpEF and NCD. E/e’ ratio could be measured with greater completeness and was modestly correlated with directly measured PCWP at rest and during exercise. The ESC and ASE diagnostic algorithms for HFpEF were specific but displayed poor sensitivity. Addition of exercise E/e’ alone (>14) to the currently-proposed ESC diagnostic algorithm improved sensitivity and resulted in a significant net reclassification improvement. These data have several key implications: they question the utility of evaluations for HFpEF based upon resting data alone, they suggest that addition of exercise echocardiography may be useful to exclude HFpEF (when diagnostic quality images are unequivocally normal), and they reinforce the value of invasive diastolic stress testing to definitively confirm or refute the diagnosis of HFpEF in patients presenting with unexplained exertional dyspnea.
Noninvasive Diagnosis of HFpEF using Rest Data
Echocardiography plays a critical role in the evaluation of HFpEF. As expected, we observed many differences in echo-Doppler indices of diastolic relaxation, compliance and filling pressures comparing cases and controls. Despite these differences there was substantial overlap between groups (Figures 1, 3). Recent guidelines from the ESC3 have been proposed to diagnose or rule out HFpEF in clinical practice, but their accuracy is yet to be validated. Similarly, the ASE/EACVI writing committee has developed guidelines for the diagnosis of diastolic dysfunction,4 which were also tested in this study because diastolic dysfunction has been considered to be necessary to secure the diagnosis of HFpEF.2 While it is important to emphasize that diastolic dysfunction does not guarantee clinical HFpEF,1 it is also notable that >60% of subjects with invasively-confirmed HFpEF did not meet criteria for diastolic dysfunction as proposed by the ASE/EACVI guidelines.4 Thus, while the ASE/EACVI criteria are quite specific, there is limited sensitivity.
The E/e’ ratio is central to both algorithms, serving as a lynchpin in the non-invasive assessment of LV filling pressures.2, 4 Despite its widespread use, there is conflicting data on the accuracy of E/e’ to estimate PCWP. Early studies showed modest but significant correlations between E/e’ and filling pressures at rest.4, 11, 22 Ommen et al reported excellent specificity with E/e’, but there were many patients with high filling pressures despite normal E/e’,11 similar to what we observed at rest in the current study. Thus, it is reasonable to conclude that an elevated resting E/e’ strongly supports the presence of high PCWP and thus HFpEF, but that a normal resting E/e’ does not exclude HFpEF.
We observed that 18% of subjects with invasively-proven HFpEF displayed completely normal NT-proBNP (<125 pg/ml), a level proposed in the most recent ESC guideline statement to effectively exclude HF.3 A larger number of subjects with HFpEF (30% and 40%) displayed NT-proBNP levels below other partition values that have been suggested to rule out HFpEF (<225 and <300 pg/ml). While it has been suggested that normal NP levels can exclude HF in the outpatient setting,23 one cannot definitively rule out a disease without performing the gold standard test (invasive hemodynamic stress testing). In this light, two previous studies have clearly demonstrated that hemodynamic proof of HFpEF is present in a substantial number of patients with normal NT-proBNP levels, either when assessed at rest10 or during exercise.5 Indeed, it is well known that NP levels are lower in HFpEF than HFrEF. This observation is believed to be primarily related to 2 factors: lower wall stress owing to smaller chamber size and thicker ventricular walls, and greater prevalence of obesity which suppresses NP levels.1 Thus, the current data, in light of these prior studies, clearly demonstrate that like normal E/e’ ratios, normal NP levels do not exclude the diagnosis of HFpEF, particularly among patients with early stage HFpEF, which includes the majority of subjects participating in this study.
The Incremental Utility of Exercise Testing in HFpEF Evaluation
The diagnosis of HFpEF in people presenting with unexplained dyspnea is problematic because many afflicted patients develop pathologic elevations in the filling pressures only during exercise, in the absence of congestion at rest.5 Invasive hemodynamic exercise testing serves as the gold standard to make or refute the diagnosis of HFpEF in these patients, as confirmed in the current study, but is limited by increased cost, the requirement for specialized equipment and operator expertise, and relatively small but measurable risk.
These limitations form the basis for the noninvasive diastolic stress test to evaluate for HFpEF, which has been proposed as a means to enhance diagnosis of HFpEF and diastolic dysfunction.3, 4 While some groups have demonstrated significant correlations between E/e’ and filling pressures during exercise,12–14 others have observed little to no relationship between echocardiographic and invasive indices.15–17 Importantly, very little data is available evaluating simultaneous assessment, as in the current study.
We found that when diagnostic-quality E/e’ data could be obtained during exercise (80–85% of the time), there were modest correlations between E/e’ and directly-measured PCWP (r=0.5–0.6). Notably, the relationship shifted during exercise, such that PCWP values were higher for a given E/e’ with exercise as compared to rest (Figure 4). Sensitivity for the ESC algorithm3 was improved following addition of exercise E/e’, suggesting that adding exercise E/e’ alone may be useful to rule out HFpEF if it is completely normal. However, the false positive rate also increased with addition of exercise E/e’, suggesting that confirmatory invasive testing may be required if exercise E/e’ is abnormal in this population.
Rather than relying exclusively on E/e’ during exercise echocardiography, the ASE/EACVI has proposed an integrated multi-marker interpretation scheme based upon E/e’, septal e’, and TR velocity, which we tested in this study.4 We observe that application of this exercise echocardiography grading scheme only nominally improved HFpEF classification, and was not as robust as exercise E/e’ alone. This was related largely to the inability to obtain diagnostic-quality TR velocity during exercise in a number of patients. Thus while the ASE/EACVI algorithm is pathophysiologically sound, its complexity and reduced feasibility for obtaining complete data during exercise are significant shortcomings, and the current data suggest that it may be best to focus the exam on accurately measuring E/e’ during exercise rather than relying on too many different indices.
While assessment of E/e’ with passive leg raise was not incrementally useful, we observed fairly similar discriminatory ability of E/e’ with submaximal exercise (20W) as using E/e’ data from all stages of exercise (Table 3). However, sensitivity was reduced with this approach, leading to a lower negative predictive value, and suggesting that a peak effort noninvasive exercise test might be optimal. In contrast, measuring PCWP at 20W alone discriminated HFpEF from NCD with very high sensitivity and perfect specificity. While some might consider this finding as being sufficient to abandon maximal invasive exercise testing, it is important to consider that other valuable information can be obtained with peak testing, including insight on the roles of cardiac vs peripheral factors in limiting exercise capacity19, 24–28 and more detailed understanding of pulmonary vascular physiology.29, 30
Clinical Implications
The current data clearly show that many patients with HFpEF will be missed if the diagnosis relies exclusively on resting clinical data and echocardiography, because the sensitivities for contemporary diagnostic algorithms proposed by the ESC and ASE/EACVI were very low (34–60%). The sensitivity of resting cardiac catheterization was similarly poor in this series (56%), owing to the fact that filling pressures are frequently normal at rest in ambulatory patients with HFpEF.5, 6 Which individuals then should undergo noninvasive diastolic stress testing, and which should undergo the gold standard of invasive diastolic stress testing? As with all diagnostic tests, the answer depends upon Bayesian theory and the pretest probability of disease.
A patient with many features of HFpEF based upon initial evaluation (e.g. older age, typical comorbidities like obesity, specific signs and symptoms like jugular distention, peripheral edema, and orthopnea, clinical improvement with diuresis, cardiac limitation on non-invasive cardiopulmonary testing) has a very high pretest probability, meaning that even a negative exercise echocardiogram would not sufficiently reduce the post-test probability. Consistent with the ACC/AHA guidelines, the diagnosis of HFpEF can be made clinically in this circumstance and no further testing is required.8 Conversely, a patient with no features of HFpEF would have such a low pretest probability that even a positive exercise echocardiogram would not sufficiently increase the post-test probability to a level where one can feel secure in the diagnosis of HFpEF.
The noninvasive diastolic exercise stress test will be most useful in patients with intermediate pretest probabilities, such as the subjects enrolled in this study, where the high sensitivity afforded by exercise E/e’ provides reasonably strong negative predictive value to rule out HFpEF. For example, according to the current data, a finding of a normal exercise echocardiogram in a patient with a roughly 40% pretest probability of HFpEF would lower the post-test probability to 9%. It would be safe to conclude with a reasonably high degree of confidence that this patient does not have HFpEF. However, if this same patient had a positive exercise echocardiogram, the post-test probability would be only 67%, indicating a need for further confirmatory testing, because of the higher rate of false positive studies with exercise echocardiography.
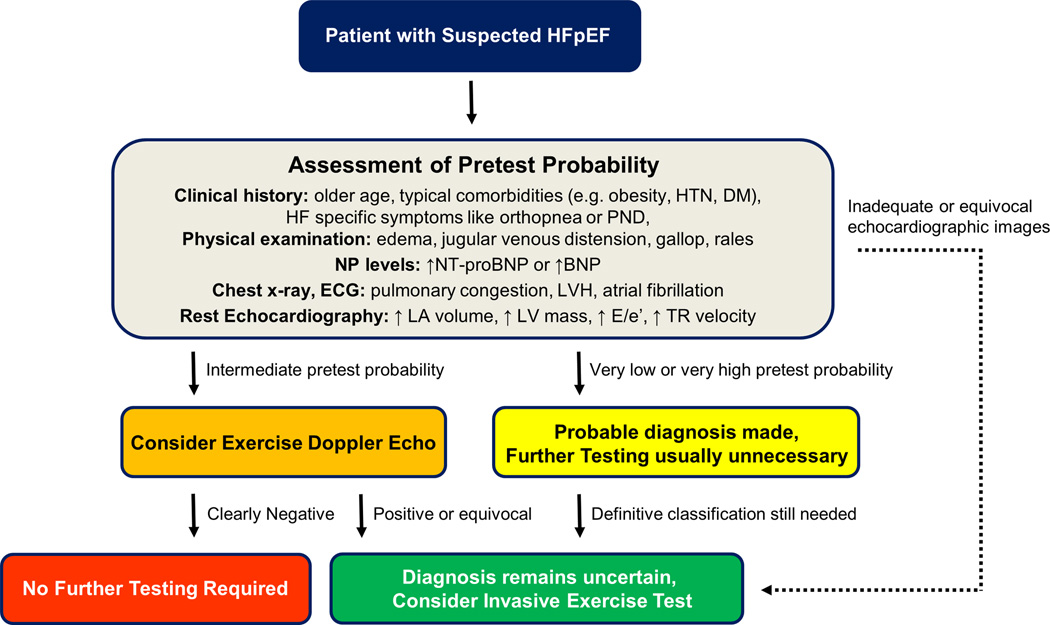
Accordingly, based upon these data, we propose a diagnostic approach as illustrated in Figure 6. Patients with very low or very high pretest probabilities should not undergo noninvasive diastolic exercise testing. If definitive diagnosis is necessary in these patients, the gold standard test should be used. In patients with intermediate pretest probability and adequate echocardiographic windows for imaging, it is reasonable to perform noninvasive diastolic stress testing. If this is negative, the likelihood of disease is low and further testing is probably not required. However, if the noninvasive diastolic stress test is abnormal, then the gold standard of invasive exercise stress testing should be performed to confirm the diagnosis given the higher false positive rate. If echocardiographic imaging is of poor quality or is equivocal, invasive exercise testing should also be performed.
Figure 6. Proposed diagnostic approach for HFpEF.
In a patient with normal EF suspected of HFpEF, assessment of pretest probability is performed based upon clinical characteristics, physical exam, natriuretic peptide levels, and rest imaging. Patients with very few or most of these characteristics are very unlikely or very likely (respectively) to have HFpEF, and the diagnosis can be made with reasonable confidence in these groups without further testing. If diagnosis is needed with certainty in this cohort, then the gold standard of invasive exercise testing should be performed because exercise echo results may not sufficiently change the post test probability if discordant with the pretest likelihood. Rest Doppler echocardiography (E/e’) has reasonable specificity but poor sensitivity for the diagnosis of HFpEF. For patients with intermediate pretest probability, exercise echocardiography focused on E/e’ should be considered. If this is normal, the likelihood of HFpEF is low and further testing is not likely required. However, a positive exercise echo should prompt consideration for exercise cath to confirm the diagnosis because of the higher false positive rate. An equivocal or nondiagnostic exercise echo should also prompt consideration of invasive diastolic stress testing to clarify the diagnosis. Invasive testing at rest has poor sensitivity and all patients with suspected HFpEF referred for cardiac catheterization should undergo invasive exercise testing if the PCWP at rest is normal. ECG indicates electrocardiogram; LV, left ventricular; NT-proBNP, N-terminal pro-B type natriuretic peptide; and other abbreviations as in Figures 1 and 5.
This approach may be useful to optimize the diagnostic evaluation for routine practice, and could even be applied as an alternate eligibility criterion for clinical trials, which currently require invasive confirmation or elevation in NP levels. If invasive exercise testing is not available at some centers due to the need for specialized equipment and dedicated staff, referral to a tertiary center where this testing is available should be considered.
Limitations
This is a single center study from a tertiary referral center and as such has inherent flaws relating to selection and referral bias. The sample size is moderate and this limits additional subgroup analyses. Further prospective validation of these diagnostic approaches is warranted, in particular the proposed algorithm shown in Figure 6, which is based upon but was not prospectively tested in this study. While echocardiography was performed by rigorously-trained, dedicated research sonographers, the performance in the supine, draped patient in the catheterization laboratory may have negatively impacted the availability and alignment of Doppler data, particularly tricuspid regurgitant velocities. Alternatively the proportion of patients in the community where diagnostic quality images can be obtained during exercise may be much lower than what we observed in this highly controlled environment. The diagnostic algorithm proposed relies upon being able to accurately estimate the pretest probability of HFpEF, which was not evaluated in this study but is currently done based upon clinical judgement alone. Further study is required to better quantify the pretest probability of HFpEF based upon clinical characteristics and resting echocardiographic data in order to optimally apply these data.
Conclusions
Approaches to identify diastolic dysfunction and HFpEF from resting data are poorly sensitive, and the absence of elevation in surrogates of filling pressure like plasma NP levels or E/e’ does not rule out HFpEF. The E/e’ ratio is moderately correlated with directly-measured filling pressures at rest and during exercise, and addition of exercise E/e’ data to the ESC diagnostic guidelines improves sensitivity which may enable exclusion of HFpEF in patients with low to intermediate pretest probabilities of disease. Invasive hemodynamic testing may be highest yield in patients with inadequate imaging windows, equivocal noninvasive data, or with abnormal noninvasive exercise testing results to definitively confirm or refute the diagnosis of HFpEF.
Supplementary Material
Clinical Perspective.
What is New?
Diagnosis of HFpEF is straightforward when typical signs and symptoms are evident, but many patients present with unexplained dyspnea, and diagnosis is challenging in this group.
To address this challenge, expert consensus panels from the European Society of Cardiology and American and European Societies of Echocardiography have proposed diagnostic algorithms for HFpEF.
We show that while specific, these algorithms have poor sensitivity compared to the gold standard of invasive exercise testing.
Normal estimates of cardiac filling pressure, including NT-proBNP and Tissue Doppler E/e’, do not exclude HFpEF.
Addition of exercise E/e’ assessment increases the sensitivity of the ESC diagnostic algorithm.
What are the clinical implications?
These data question the accuracy of current approaches to diagnose HFpEF that are based only on resting assessments of cardiovascular structure and function.
We propose a new diagnostic approach where invasive and noninvasive diastolic stress testing can be used in the evaluation process to identify or rule out HFpEF amongst patients presenting with indeterminate dyspnea with much greater accuracy.
Acknowledgments
Funding
This research was supported by a competitive prospective grants award from the Mayo Clinic and Foundation. BAB is supported by RO1 HL128526 and U10 HL110262.
Footnotes
Disclosures
None
References
- 1.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: Pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: A consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the heart failure and echocardiography associations of the european society of cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 3.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 esc guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the european society of cardiology (esc) developed with the special contribution of the heart failure association (hfa) of the esc. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 4.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the american society of echocardiography and the european association of cardiovascular imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen MJ, Olson TP, Melenovsky V, Kane GC, Borlaug BA. Differential hemodynamic effects of exercise and volume expansion in people with and without heart failure. Circ Heart Fail. 2015;8:41–48. doi: 10.1161/CIRCHEARTFAILURE.114.001731. [DOI] [PubMed] [Google Scholar]
- 7.Maron BA, Cockrill BA, Waxman AB, Systrom DM. The invasive cardiopulmonary exercise test. Circulation. 2013;127:1157–1164. doi: 10.1161/CIRCULATIONAHA.112.104463. [DOI] [PubMed] [Google Scholar]
- 8.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 accf/aha guideline for the management of heart failure: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 9.From AM, Lam CS, Pitta SR, Kumar PV, Balbissi KA, Booker JD, Singh IM, Sorajja P, Reeder GS, Borlaug BA. Bedside assessment of cardiac hemodynamics: The impact of noninvasive testing and examiner experience. Am J Med. 2011;124:1051–1057. doi: 10.1016/j.amjmed.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 10.Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, Gheorghiade M, Shah SJ. Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:870–876. doi: 10.1016/j.amjcard.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of doppler echocardiography and tissue doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 12.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: A noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 13.Burgess MI, Jenkins C, Sharman JE, Marwick TH. Diastolic stress echocardiography: Hemodynamic validation and clinical significance of estimation of ventricular filling pressure with exercise. J Am Coll Cardiol. 2006;47:1891–1900. doi: 10.1016/j.jacc.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 14.Talreja DR, Nishimura RA, Oh JK. Estimation of left ventricular filling pressure with exercise by doppler echocardiography in patients with normal systolic function: A simultaneous echocardiographic-cardiac catheterization study. J Am Soc Echocardiogr. 2007;20:477–479. doi: 10.1016/j.echo.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56:855–863. doi: 10.1016/j.jacc.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 16.Bhella PS, Pacini EL, Prasad A, Hastings JL, Adams-Huett B, Thomas JD, Grayburn PA, Levine BD. Echocardiographic indices do not reliably track changes in left-sided filling pressure in healthy subjects or patients with heart failure with preserved ejection fraction. Circ Cardiovasc Imaging. 2011;4:482–489. doi: 10.1161/CIRCIMAGING.110.960575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos M, Rivero J, McCullough SD, West E, Opotowsky AR, Waxman AB, Systrom DM, Shah AM. E/e’ ratio in patients with unexplained dyspnea: Lack of accuracy in estimating left ventricular filling pressure. Circ Heart Fail. 2015;8:749–756. doi: 10.1161/CIRCHEARTFAILURE.115.002161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen MJ, Hwang SJ, Kane GC, Melenovsky V, Olson TP, Fetterly K, Borlaug BA. Enhanced pulmonary vasodilator reserve and abnormal right ventricular: Pulmonary artery coupling in heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8:542–550. doi: 10.1161/CIRCHEARTFAILURE.114.002114. [DOI] [PubMed] [Google Scholar]
- 19.Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37:3293–3302. doi: 10.1093/eurheartj/ehw241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borlaug BA, Koepp KE, Melenovsky V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2015;66:1672–1682. doi: 10.1016/j.jacc.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 21.Borlaug BA, Melenovsky V, Koepp KE. Inhaled sodium nitrite improves rest and exercise hemodynamics in heart failure with preserved ejection fraction. Circ Res. 2016;119:880–6. doi: 10.1161/CIRCRESAHA.116.309184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW. Assessment of mitral annulus velocity by doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–480. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 23.Zaphiriou A, Robb S, Murray-Thomas T, Mendez G, Fox K, McDonagh T, Hardman SM, Dargie HJ, Cowie MR. The diagnostic accuracy of plasma BNP and NT-proBNP in patients referred from primary care with suspected heart failure: Results of the uk natriuretic peptide study. Eur J Heart Fail. 2005;7:537–541. doi: 10.1016/j.ejheart.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, Borlaug BA. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:776–785. doi: 10.1093/eurjhf/hft026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santos M, Opotowsky AR, Shah AM, Tracy J, Waxman AB, Systrom DM. Central cardiac limit to aerobic capacity in patients with exertional pulmonary venous hypertension: Implications for heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8:278–285. doi: 10.1161/CIRCHEARTFAILURE.114.001551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, Houstis NE, Eisman AS, Hough SS, Lewis GD. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: The role of abnormal peripheral oxygen extraction. Circ Heart Fail. 2015;8:286–294. doi: 10.1161/CIRCHEARTFAILURE.114.001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis GD, Bossone E, Naeije R, Grunig E, Saggar R, Lancellotti P, Ghio S, Varga J, Rajagopalan S, Oudiz R, Rubenfire M. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation. 2013;128:1470–1479. doi: 10.1161/CIRCULATIONAHA.112.000667. [DOI] [PubMed] [Google Scholar]
- 30.Malhotra R, Dhakal BP, Eisman AS, Pappagianopoulos PP, Dress A, Weiner RB, Baggish AL, Semigran MJ, Lewis GD. Pulmonary vascular distensibility predicts pulmonary hypertension severity, exercise capacity, and survival in heart failure. Circ Heart Fail. 2016;9:e003011. doi: 10.1161/CIRCHEARTFAILURE.115.003011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.