Abstract
Background
Chronic inflammation and oxidative stress are hallmarks of chagasic cardiomyopathy (CCM). In this study, we determined if microparticles (MPs) generated during Trypanosoma cruzi (Tc) infection carry the host's signature of the inflammatory/oxidative state and provide information regarding the progression of clinical disease.
Methods
MPs were harvested from supernatants of human peripheral blood mononuclear cells in vitro incubated with Tc (control: LPS treated), plasma of seropositive humans with a clinically asymptomatic (CA) or symptomatic (CS) disease state (vs. normal/healthy [NH] controls), and plasma of mice immunized with a protective vaccine before challenge infection (control: unvaccinated/infected). Macrophages (mφs) were incubated with MPs, and we probed the gene expression profile using the inflammatory signaling cascade and cytokine/chemokine arrays, phenotypic markers of mφ activation by flow cytometry, cytokine profile by means of an ELISA and Bioplex assay, and oxidative/nitrosative stress and mitotoxicity by means of colorimetric and fluorometric assays.
Results
Tc- and LPS-induced MPs stimulated proliferation, inflammatory gene expression profile, and nitric oxide (∙NO) release in human THP-1 mφs. LPS-MPs were more immunostimulatory than Tc-MPs. Endothelial cells, T lymphocytes, and mφs were the major source of MPs shed in the plasma of chagasic humans and experimentally infected mice. The CS and CA (vs. NH) MPs elicited >2-fold increase in NO and mitochondrial oxidative stress in THP-1 mφs; however, CS (vs. CA) MPs elicited a more pronounced and disease-state-specific inflammatory gene expression profile (IKBKB, NR3C1, and TIRAP vs. CCR4, EGR2, and CCL3), cytokine release (IL-2 + IFN-γ > GCSF), and surface markers of mφ activation (CD14 and CD16). The circulatory MPs of nonvaccinated/infected mice induced 7.5-fold and 40% increases in ∙NO and IFN-γ production, respectively, while these responses were abolished when RAW264.7 mφs were incubated with circulatory MPs of vaccinated/infected mice.
Conclusion
Circulating MPs reflect in vivo levels of an oxidative, nitrosative, and inflammatory state, and have potential utility in evaluating disease severity and the efficacy of vaccines and drug therapies against CCM.
Keywords: Chagasic cardiomyopathy, Microparticles, Metabolic inflammatory gene expression profile, Macrophage activation
Introduction
Trypanosoma cruzi (TC) is endemic in Latin America and is an emerging infection in the USA [1]. The prevalence of human Tc infection is approximately 20 million, and 120 million are at risk of infection in Latin America [1]. Vectors carrying Tc are wide-spread in the USA, and the Centers for Disease Control and Prevention estimate that >300,000 infected individuals are living in the USA [2]. Unfortunately, exposure to Tc remains undetected for several years, when patients then display cardiac insufficiency due to tissue fibrosis, ventricular dilation, and arrhythmia [3]. Chagasic cardiomyopathy (CCM) results in a loss of 2.74 million disability-adjusted life-years, and >15,000 deaths due to heart failure per year [1].
Macrophages (mφs) are the immune cells that are essential for controlling Tc infection [4, 5]. It is suggested that mφ-derived peroxynitrite, a strong cytotoxic agent that is formed by the reaction of nitric oxide (∙NO) with superoxide (O2−∙), plays a major role in the direct killing of Tc[6]. Infected experimental animals and humans also elicit strong adaptive immunity, constituted of antiparasite lytic antibodies, type 1 cytokines, and antigen-specific cytolytic T lymphocytes [review [7]]. These immune responses are capable of keeping the parasite burden under control but lack the ability to achieve pathogen clearance [8], leading to low-level parasite persistence.
In recent years, we and other study groups have shown that Tc invasion elicits functional changes in mitochondrial respiratory chain complexes, and this initial insult continues and serves as a major source of the increased production of O2−∙ radicals in the heart [9]. Control of Tc-induced reactive oxygen species (ROS) by using chemical antioxidants or by genetically enhancing the expression of superoxide dismutase (SOD2) results in an improvement in cardiac mitochondrial respiratory chain function in chagasic mice [10, 11]. Importantly, SOD2-overexpressing mice also exhibit a lower degree of inflammatory infiltrates and mitochondrial damage that is otherwise pronounced in chagasic myocardium [12]. These studies suggest that Tc-induced ROS are not only associated with chronic oxidative stress but may also signal the activation and recruitment of inflammatory infiltrate in the chagasic heart.
The role of ROS in signaling inflammatory immune responses in Chagas disease is not completely understood. Extensive infiltration of gp91phox+ (NOX2 component) mφ clusters associated with oxidative adducts has been noted in chagasic hearts [13, 14]. Macrophages in vitro incubated with heart homogenates or the plasma of infected mice were found to elicit a proinflammatory response, demonstrated by an increased production of ROS, ∙NO, and TNF-α [15]. Heart homogenates of chagasic mice or of normal mice in vitro, oxidized with H2O2 or peroxynitrite, are recognized by antibodies present in the sera of the Tc-infected host [15]. These studies suggest that oxidative stress-induced adducts are potentially responsible for the chronic activation of inflammatory mφs and non-Tc-specific antibody response in Chagas disease. The practical and ethical limitations in obtaining cardiac biopsies, however, prohibit using ROS production and ROS-induced antigen generation and mφ activation as early indicators for identifying patients at risk of developing clinically symptomatic CCM.
Microparticles (MPs) are small vesicles harboring ligands, receptors, active lipids, or RNA/DNA from their cell of origin [16]. In pathological conditions, a stimulus that triggers MP formation regulates the selective sorting of constituents and the composition of MPs, and, consequently, the biological information that they transfer [17]. Thus, MPs can play roles in intercellular communication and be able to modulate important cellular regulatory functions. There is no literature or information in the public domain on the potential effects of MPs on the molecular mechanisms implicated in the pathophysiology of CCM.
In this study, we aimed to determine whether circulating MPs generated during Tc infection carry the host's signature of inflammatory/oxidative pathology and provide information regarding clinical disease severity. We isolated MPs released in the (1) supernatants of human peripheral blood mononuclear cells in vitro infected with Tc and (2) plasma of human subjects who were characterized as seropositive with clinically asymptomatic (CA) or symptomatic (CS) Chagas disease. We then employed high-throughput transcriptomic and physiological approaches to study the mφ response to MPs. We also used MPs from a mouse model of vaccination and chronic disease to determine if mφ response to circulating MPs provides an indication of cure from infection.
Materials and Methods
Ethics Statement
All animal experiments were performed according to the National Institutes of Health Guide for the Care and Use of Experimental Animals, and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Texas Medical Branch (UTMB), Galveston (protocol No. 0805029).
The collection of human peripheral blood samples was approved by the institutional review board at the UTMB, Galveston (protocol No. IRB13-0367) and the ethics committee at the Universidad Nacional de Salta, Salta, Argentina. A written informed consent was obtained from all individuals visiting the Cardiologic Unit of San Bernardo Hospital in Salta Argentina for clinical service. The leftover blood samples collected for clinical purpose were decoded and deidentified before they were provided for research purposes.
Human Samples
The Tc-specific antibodies were analyzed in all sera samples by using the Chagatest ELISA recombinant (v4.0) and Chagatest HAI kits (Wiener, Rosario, Argentina). Sera samples were considered seropositive if both tests identified the presence of anti-Tc antibodies. Electrocardiography (ECG, 12-lead at rest and 3-lead with exercise) and transthoracic echocardiography were performed for evaluating the heart function in all individuals. Normal healthy (NH, n = 10) controls were seronegative and exhibited no history or clinical symptoms of heart disease. Seropositive individuals were grouped as CA (n = 10) when they exhibited none-to-minor ECG abnormalities, no left ventricular dilatations, and normal ejection fraction of 55–70%. Seropositive individuals were categorized as CS (n = 10) when they displayed a varying degree of ECG abnormalities, systolic dysfunction (ejection fraction <55%), left ventricular dilatation (diastolic diameter ≥57 mm), and/or potential signs of heart failure [18].
Mice, Immunization, and Challenge Infection
All chemicals used in the study were of molecular grade, and purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise specified. C57BL/6 female mice (wild type) were purchased from Harlan Laboratories (Indianapolis, IN, USA). Tc (SylvioX10/4) and C2C12 cells (an immortalized, mouse-derived myoblast cell line) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and Tc trypomastigotes (infective stage) were propagated in C2C12 cells. The Tc antigens TcG2 and TcG4 were used as vaccine candidates and have been described in detail previously [19, 20]. Mice (n = 5/group/experiment; 2 experiments) were injected in the quadriceps muscle with TcG2 and TcG4 antigens that were delivered as a DNA-prime/protein-boost vaccine [19, 20]. Two weeks after immunization, they were challenged with Tc trypomastigotes (10,000/mouse), and sacrificed at approximately 120 days after infection, corresponding to the chronic disease phase [19, 20]. Plasma samples were subjected to isolation of circulating MPs (described below). Protein levels were determined by using the Bradford protein assay (Bio-Rad, Hercules, CA, USA).
MP Isolation
Human blood samples were drawn in EDTA-containing Vacutainer cell preparation tubes (BD Biosciences, San José, CA, USA). The tubes were centrifuged for 20 min each at 270 and 1,000 g to separate plasma. Plasma samples were subjected to 3 series of centrifugation at 15,000 g for 15 min each, and pelleted MPs were washed with RPMI media and stored at −80°C. A similar protocol was followed for isolating the MPs from murine plasma samples.
In some experiments, buffy coat obtained after separation of plasma was subjected to Ficoll Hypaque™ density gradient (GE Healthcare, Pittsburgh, PA, USA), and centrifuged at 400 g for 30 min. The enriched human peripheral blood mononuclear cell (PBMC) pellets were washed with RPMI media, seeded in 24-well plates (0.5–1 × 106 cells/well/mL), and incubated in triplicate with Tc (cell-to-parasite ratio, 1:3) or LPS (100 ng/mL) in RPMI media/10% FBS media at 37°C/5% CO2 for 48 h. MPs from the supernatants were collected as above.
Treatment of Macrophages with MPs
THP-1 human monocytes (ATCC TIB-202) were suspended in complete RPMI media and incubated at 37°C/5% CO2 for 24 h in the presence of 50 ng/mL phorbol-12-myristate-13-acetate (PMA, Sigma-Aldrich), and then for 48 h in complete RPMI media without any stimulus to generate the resting mφs [21]. RAW264.7 murine mφs (ATCC TIB-71) were cultured in high-glucose Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA), 2 mmol/L glutamine, 100 IU/mL penicillin, and 100 µg/mL streptomycin (Corning, Corning, NY, USA). THP-1 (human) or RAW264.7 (murine) mφs were seeded in 6-well (1 × 106 cells/well), 24-well (5 × 105 cells/well), or 96-well (1 × 104 cells/well) plates, or in Nunc Lab-Tek II chamber slides (1 × 104 cells/well, Thermo Scientific, Waltham, MA, USA) and incubated for 2 h to allow the cells to adhere. Serum-free media was added, and mφs were incubated in triplicate with MPs isolated from human or mouse plasma (10% plasma equivalent) or from media of Tc-infected cells (10% media equivalent). Macrophages (± MPs) were incubated for 1, 12, 24, or 48 h and cells and supernatants were stored at −80°C.
Gene Expression Profiling by Real-Time RT-qPCR
The quantitative expression profiling of a panel of 91 human genes involved in the inflammatory signaling cascade was performed by using custom-designed arrays printed by Sigma-Aldrich. Full details of the arrays were previously described [22]. Gene expression profiling for human cytokines/chemokines was performed by using an in-house PCR array that consisted of 89 genes [23]. All primer sequences are available upon request.
THP-1 mφs were seeded in 12-well plates (1 × 106 cells/well) and incubated in triplicate with MPs for 12 h. Cells were suspended in TRIzol reagent, and total RNA was extracted and precipitated by chloroform/isopropanol/ethanol method. DNA that could contaminate the RNA preparation was removed by deoxyribonuclease I (DNase I) treatment (Ambion, Austin, TX, USA). Total RNA absorbance at 260 and 280 nm was read by using a NanoDrop ND-1000 spectrophotometer (Wilmington, DE, USA) to assess the quality (OD260/280 ratio >2.0) and quantity (OD260 of 1 = 40 µg/mL RNA). First-strand cDNA was synthesized from DNaseI-treated 1-μg RNA sample using the iScript™ cDNA synthesis kit (Bio-Rad) and diluted 5-fold with nuclease-free ddH2O. Quantitative real-time PCR was performed in a 20-µL reaction containing 1 µL cDNA, 10 µL SYBR Green master mix (Bio-Rad), and 500 nM of each gene-specific oligonucleotide. The thermal cycle conditions were 94°C for 30 s followed by 60°C for 1 min, for 40 cycles. The PCR Base Line Subtracted Curve Fit mode was applied for determining the threshold cycle, Ct, using iCycler iQ real-time detection system software (Bio-Rad). For each target gene, Ct values were normalized to the Ct values for the β-actin (ACTB) and β-glucuronidase (GUSB) reference genes. The relative expression level of each target gene was calculated according to the 2−ΔΔCt method, where ΔCt represents the Ct (target) - Ct (reference), and ΔΔCt represents ΔCt (sample) - ΔCt (no treatment control or control MP treatment) [21].
Cell Viability
THP-1 mφs were seeded to 96-well plates (1 × 104 cells/200 µL/well), and incubated in triplicate with serum-free media (± MPs) for 24 h. Cells were loaded with 10% (v/v) AlamarBlue (Life Technologies, Carlsbad, CA, USA) during the last 3 h of incubation. AlamarBlue metabolism by functional mitochondria, resulting in cleavage of resazurin into fluorescent resorufin (Ex560 nm/Em590 nm) was recorded by using a SpectraMax M5 spectrophotometer (Molecular Devices, Sunnyvale, CA, USA).
ROS and ∙NO Levels
Reactive oxygen species (ROS) were monitored by using 2′,7′ dichlorodihydrofluorescein diacetate (H2DCFDA, Invitrogen) fluorescent probe. Briefly, THP-1 mφs were seeded in 96-well plates (1 × 104 cells per well), were allowed to adhere for 2 h, and were then incubated for 1 h in triplicate with MPs. Supernatants were harvested, and cells were washed and loaded with 10 μM of H2DCFDA in 100 μL of serum-free media, and incubated in the dark for 30 min at 37°C. Cells were washed 3 times with phenol-red-free media and H2DCFDA oxidation by intracellular ROS, resulting in the formation of fluorescent dichlorodihydrofluorescein (DCF, Ex498 nm/Em598 nm), was recorded by fluorimetry. Cells treated with 0.1–1 μM H2O2 were used as a positive control.
The ∙NO level (an indicator of inducible ∙NO synthase activity) was monitored by Griess reagent assay. Briefly, samples were reduced with 0.01 unit/100 μL of nitrate reductase, and incubated for 10 min with 100 μL of 1% sulfanilamide made in 5% phosphoric acid/0.1% N-(1-napthyl) ethylenediamine dihydrochloride (1:1, v/v). Formation of diazonium salt was monitored at 545 nm by spectrophotometry (standard curve: 2–50 μM sodium nitrite) [24].
Mitochondrial Membrane Potential and ROS
To examine the changes in mitochondrial membrane potential, mφs were seeded and MPs were added in triplicate. Macrophages were incubated for 24 h with the MPs, washed, and then with 10 µM 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide (JC-1, Molecular Probes, Eugene, OR, USA) for 30 min. Cells were washed twice with cold PBS to remove the excess dye, suspended in serum-free/phenol-red-free RPMI, and fluorescence was measured as above. JC-1 dye in respiring mitochondria is converted from green (Ex485 nm/Em530 nm) to red (Ex530 nm/Em580 nm) fluorescent J-aggregates and provides a sensitive in dication of the changes in mitochondrial membrane potential (ΔΨm).
To measure mitochondrial ROS production, mφs (± MPs) were incubated in the dark for 30 min with 5 µM MitoSOX Red (Invitrogen). MitoSOX Red oxidation by mitochondrial ROS, resulting in red fluorescence (Ex518 nm/Em605 nm), was detected by fluorimetry.
Cytokine Levels
A Bio-Plex Pro Human Cytokine 17-plex assay (Bio-Rad M5000031YV) was employed to profile the concentration of human cytokines and chemokines. Briefly, THP-1 mφs were seeded in 48-well plates in 500 µL media and MPs were added in triplicate. After incubation for 24 h, culture supernatants (50 µL) were transferred in duplicate to the plates precoated with cytokine-specific antibodies conjugated with different color-coded beads, and the plates were incubated for 1 h, washed, and then sequentially incubated with 50 µL of biotinylated cytokine-specific detection antibodies and streptavidin-phycoerythrin conjugate. Fluorescence was recorded using a SpectraMax M5 microplate reader, and cytokine/chemokine concentrations were calculated with Bio-Plex Manager software (v5) by using a standard curve derived from recombinant cytokines (2–32,000 pg/mL).
In some experiments, THP-1 mφs seeded in 24-well plates (5 × 105 cells/well/mL) were incubated in triplicate with human MPs for 48 h. Culture supernatants were utilized for the measurement of cytokine release (IL-1β, IL-4, IL-10, IFN-γ, and TNF-α) using human cytokine optEIA™ ELISA kits (Pharmingen, San Diego, CA, USA). Likewise, supernatants from RAW264.7 mφs incubated for 48 h with MPs isolated from normal, chagasic and vaccinated/chagasic mice were analyzed for IL-1β, IL-4, IL-10, IFN-γ, and TNF-α levels by using murine cytokine optEIA™ ELISA kits (Pharmingen).
Flow Cytometry
To evaluate changes in the expression of surface markers in response to MPs, THP-1 mφs were seeded in 6-well plates (1 × 106 cells/well/mL), and incubated in triplicate with MPs (10% serum equivalent) for 24 h. Cells were harvested, pelleted and suspended in 50 μL of stain buffer (PBS with 2% FBS). Suspended cells were stained for 30 min with antibody cocktails containing human peridinin chlorophyll protein (PerCP)-anti-CD14, allophycocyanin (APC-Cy7)-anti-CD16, APC-anti-CD206, phycoerythrin (PE-Cy7)-anti-CD64, PE-Cy5-anti-CD80, and V-450-anti-CD200 fluorescence-conjugated antibodies (BD Biosciences, Franklin Lakes, NJ, USA). The stained cells were washed, fixed with 2% paraformaldehyde, and then analyzed by 6-color flow cytometry on an LSRII Fortessa cell analyzer. Cells stained with isotype-matched IgGs were used as controls. Macrophages were gated based on parameters of forward and side light scatter and data acquisition was performed on a minimum of 10,000-gated events. Data were analyzed using FlowJo software (v7.6.5, TreeStar, San Carlo, CA, USA). The mean fluorescence intensity was derived from fluorescence histograms, and was adjusted for background with isotype-matched controls.
To evaluate the cellular origin of the MPs, these were isolated from the media of human PBMCs in vitro infected with Tc and from the plasma of clinically characterized human subjects as described above. The MPs were also isolated from the plasma of normal and experimentally infected mice. They were resuspended in annexin V binding buffer and labeled for 30 min on ice with APC- or FITC-conjugated anti-annexin V antibody. Simultaneously, MPs were labeled with mouse or human PerCP-anti-CD14, APC-Cy7-anti-CD61, PE-Cy7-anti-CD62E, V-450-anti-troponin, FITC-anti-CD4, and PE-anti-CD8 fluorescence-conjugated antibodies (5–10 μL/sample, e-Biosciences). Stained MPs were washed with cold PBS, and flow cytometry was performed on an LSRII Fortessa cell analyzer. In order to separate true events from background noise and unspecific binding of antibodies to debris, we defined MPs as particles that were <1 μm in diameter and had positive staining for annexin V.
Statistical Analysis
All in vitro and in vivo experiments were repeated at least twice, and conducted with triplicate observations per sample, and data are expressed as mean ± SEM. All data were analyzed using InStat v3 (GraphPad, La Jolla, CA, USA). Data were analyzed by the Student t test (for comparison of 2 groups) and one-way analysis of variance (ANOVA) with the Tukey post hoc test (for comparison of multiple groups). Significance is presented as * p < 0.05, ** p < 0.01, and *** p < 0.001.
Results
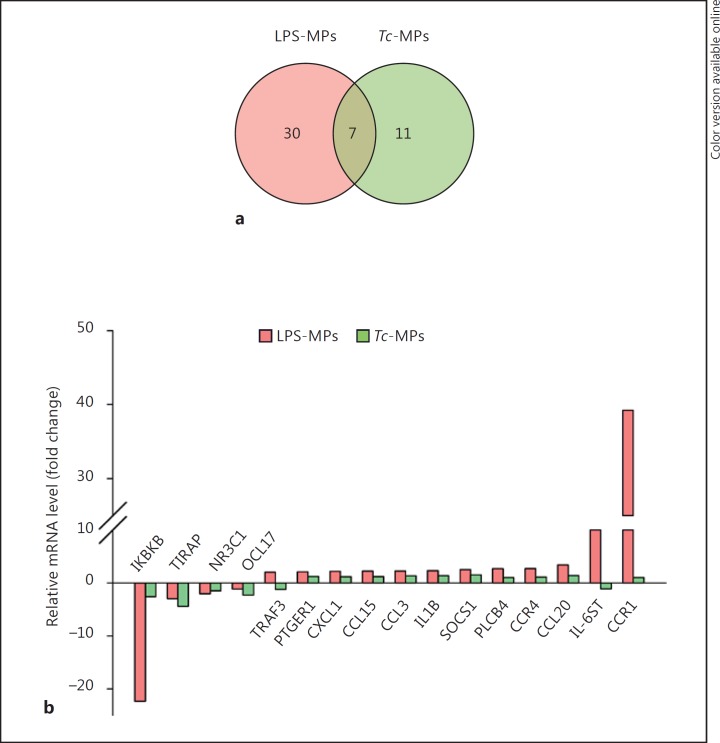
We first utilized an in vitro system to determine if Tc infection produces MPs capable of activating mφs. For this, human PBMCs were incubated for 48 h with Tc and supernatants were centrifuged to harvest the Tc-induced MPs (Tc-MPs). PBMCs were also incubated with LPS or media alone for 48 h, and supernatants were centrifuged to harvest the LPS-induced MPs (LPS-MPs) and nontreated/control MPs (Con-MPs), respectively. We incubated THP-1 mφs for 12 h with MPs, and first performed qRT-PCR analysis using the inflammatory signaling cascade and cytokine/chemokine arrays to probe the expression of 180 genes (including the housekeeping genes). The data were normalized to housekeeping genes, and the relative change in gene expression in THP-1 mφs incubated with sample MPs (vs. Con-MPs) was calculated. These data showed that 37 genes (30 upregulated, 7 downregulated) and 18 genes (5 upregulated, 13 down-regulated) were differentially expressed (≥|1.5| fold change, p < 0.05) in THP-1 mφs incubated with LPS-MPs and Tc-MPs, respectively, when compared to that noted in mφs incubated with Con-MPs (online suppl. Table S1; for all online suppl. material, see www.karger.com/doi/10.1159/000451055). Of these, 7 genes were differentially expressed (↓TIRAP, ↓IKBKB, ↓C3, ↓NR3C1, ↑SOCS1, ↑CXCL5, ↑IL-10) by both LPS-MPs and Tc-MPs, and 11 genes (↓CCL17, ↓TP53, ↓IL-6, ↓EGR2, ↓NR2C2, ↓EGFR, ↓PTGER2, ↓TNFSF18, ↓INSR, ↑IL-4, ↑IL-2RA) were differentially expressed in mφs in a Tc-MPs-specific manner (Fig. 1a; online suppl. Table S1). The top molecules that were differentially expressed >2-fold in THP-1 mφs by LPS-MPs and Tc-MPs in comparison to Con-MPs are shown in Figure 1b.
Fig. 1.
Macrophage gene expression profile response to MPs released by T. cruzi (Tc)-infected cells. Human PBMCs were incubated for 48 h with media alone, LPS or Tc, and supernatants were centrifuged to harvest the control (Con-MPs), LPS-induced (LPS-MPs), and Tc-induced MPs (Tc-MPs) MPs. THP-1 mφs were incubated in triplicate with MPs for 12 h. Total RNA from each sample was reverse transcribed, and cDNA was used for real-time PCR with the inflammation signaling cascade and cytokine/chemokine arrays. The differential mRNA level was normalized to housekeeping genes, and the fold change in gene expression was calculated (online suppl. Table S1). a Venn diagram of comparative analysis of gene expression profile (≥|1.5| fold change) induced by LPS-MPs and Tc-MPs in comparison to that noted in Con-MP-treated mφs. b Mean differential expression of top molecules (≥|2.0| fold change) induced by LPS-MPs and Tc-MPs (vs. Con-MPs) in THP-1 mφs.
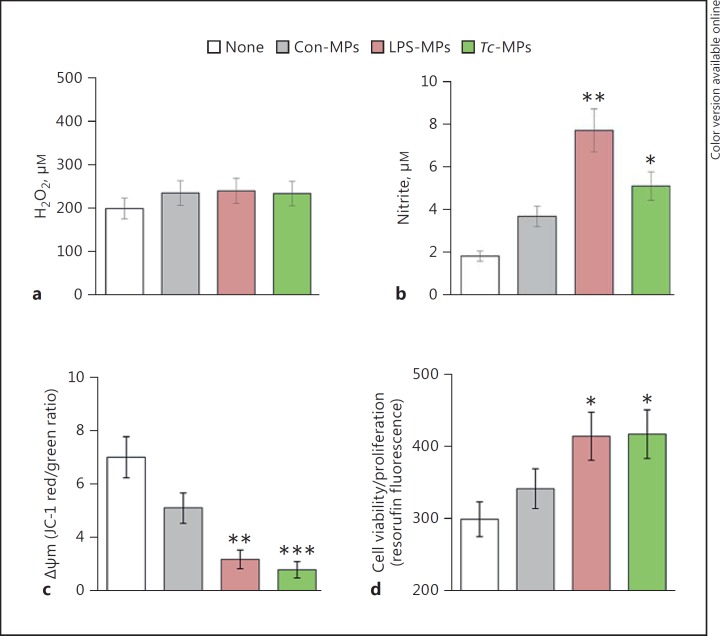
To assess if Tc-MPs elicited a functional response in immune cells, we incubated the THP-1 mφs with MPs for 1 h, and examined the oxidative/nitrosative response and mitochondrial stress levels. No increase in H2O2 release (amplex red assay) was induced by LPS-MPs and Tc-MPs when compared to that noted in mφs incubated in media alone or with Con-MPs (Fig. 2a). Macrophages incubated with LPS-MPs and Tc-MPs exhibited a 3.2-fold and 1.7-fold increase in nitrite release, respectively, compared to THP-1 mφs incubated in media alone (Fig. 2b, p < 0.05). JC-1 forms J-aggregates (red) in mitochondria and JC-1 red/green ratio provides a sensitive indicator of cellular stress. The LPS-MPs and Tc-MPs elicited a 56 and 60% decline in JC-1 red/green ratio, respectively (Fig. 2c, p < 0.01). Macrophage activation is followed by cell proliferation. Resazurin (alamarBlue) metabolism to fluorescent resorufin by mitochondrial aerobic respiration provides a sensitive measure of cell viability and proliferation. THP-1 mφs incubated with LPS-MPs and Tc-MPs for 24 h exhibited 38% increase in resorufin fluorescence compared to that noted in mφs incubated in media alone (Fig. 2d, p < 0.05). Con-MPs elicited a 26% decline in JC-1 red/green ratio (p > 0.05) and no proliferation in THP-1 mφs. Together, the results presented in Figures 1 and 2 suggested that Tc-MPs elicited the expression of some of the genes indicative of inflammatory activation in THP-1 mφs, and the immune activation was associated with an increase in nitrite release and cell proliferation and a decline in mitochondrial membrane potential in THP-1 mφs. The LPS-MPs were more immunostimulatory than the Tc-MPs, demonstrated by the greater induction of proinflammatory gene expression and nitrite release by THP-1 mφs.
Fig. 2.
Functional response of mφs incubated with Tc-MPs. THP-1 mφs were incubated in triplicate with medium only (none) or with Con-MPs, LPS-MPs, and Tc-MPs for 1 h. Supernatants were utilized to measure ROS release by amplex red assay (a) and ∙NO production by Griess reagent assay (b). c THP-1 mφs were loaded with a JC-1 probe, and change in mitochondrial membrane potential was measured as J aggregates (red)/J monomers (green) ratio by fluorimetry. d THP-1 mφs were incubated with MPs or media alone for 21 h, and then loaded with AlamarBlue for 3 h. The cell proliferation and viability was determined by resorufin fluorescence. Data are shown as mean value ± SEM, and significance is presented as * p < 0.05, ** p < 0.01, *** p < 0.001 (no treatment vs. MP treatment).
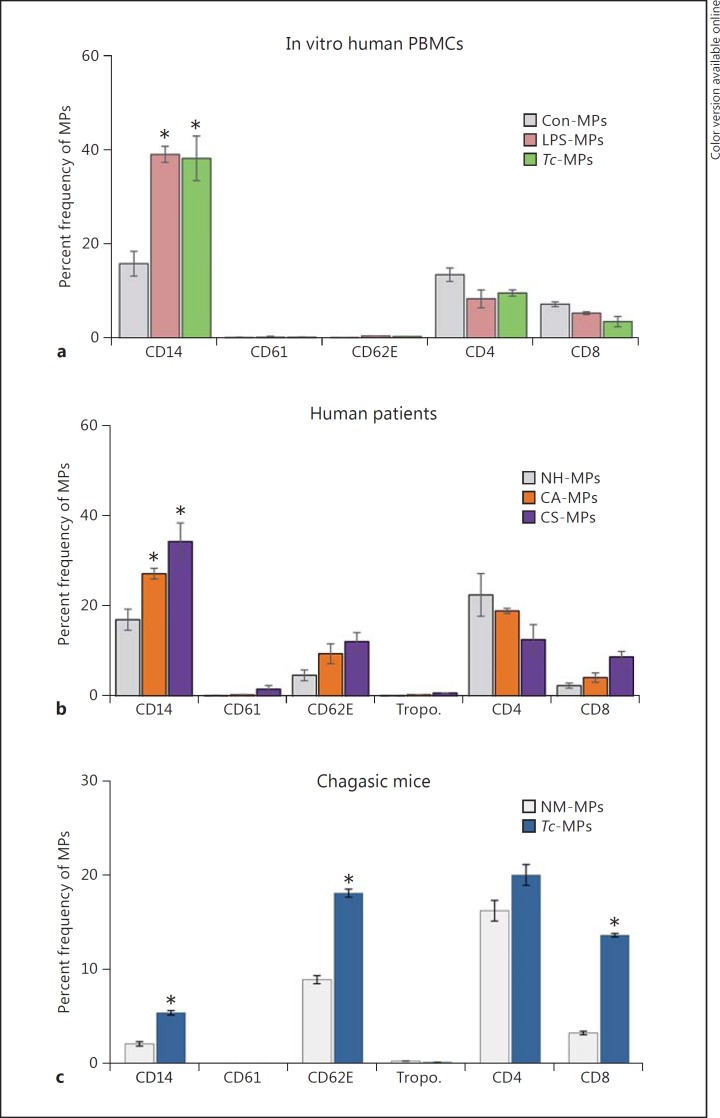
To assess the cellular source of the membranes for MPs released during Tc infection, we took a 3-prong approach. Firstly, Tc-MPs were isolated from the supernatants of human PBMCs incubated for 48 h with Tc, stained with fluorescence-conjugated antibodies against cell-specific markers, and analyzed by flow cytometry. These data showed that MPs released by human PBMCs upon Tc infection were primarily of monocyte/mφ origin (Fig. 3a). Secondly, we examined the phenotype of circulating MPs from chagasic patients. For this, MPs were isolated from the plasma of NH individuals and seropositive subjects characterized as CA and CS for heart disease (n = 10 per group), and analyzed, as above (Fig. 3b). These data showed that MPs of platelet (CD61+) or cardiomyocyte (troponin+) origin constituted <2% of the total circulating MPs in the plasma of normal and chagasic individuals (Fig. 3b). A majority of circulating MPs in the blood of all the human subjects, irrespective of infection and disease status, were of monocyte/mφ (CD14+), endothelial (CD62E+), and CD4+/CD8+ T lymphocyte origin (CD14 ≥ CD4 > CD62 = CD8; Fig. 3b). The chagasic subjects exhibited a substantial increase in the frequency of circulatory MPs that were CD14+/CD14hi (up to 2-fold, mφ marker), CD62E+/CD62Ehi (2- to 3-fold, endothelial marker), or CD8+/CD8hi (4.2-fold, T lymphocytes) (Fig. 3b, p < 0.05). Thirdly, MPs were isolated from the plasma of mice experimentally infected with Tc, and analyzed by flow cytometry. As noted in human chagasic patients, mice chronically infected with Tc exhibited 2.3-fold, 2-fold and 4.5-fold increases in circulatory MPs of monocytes/mφ (CD14+), endothelial (CD62E+), and CD8+ T lymphocyte origin, respectively, compared to that noted in the plasma of normal mice (Fig. 3c, p < 0.05). Together, these results suggested that circulatory MPs originating from activated endothelial cells, mφs, and CD8+ T cells were enhanced in chagasic patients and chronically infected mice.
Fig. 3.
Phenotype of MPs induced by Tc infection and chronic Chagas disease. a, b MPs were harvested from in vitro infected human PBMCs (as in Fig. 1, 2). Plasma samples from seropositive chagasic subjects categorized as CA and CS, and NH controls (n = 10 per group) were centrifuged (Materials and Methods) and MPs were harvested. Human MPs were labeled with fluorescence-conjugated antibodies against human molecules and analyzed by flow cytometry. The frequency of surface markers of various cellular origin on MPs isolated from control and Tc-infected PBMCs (a) and chagasic subjects (b) are shown. c C57BL/6 mice were challenged with Tc (10,000 parasites/mouse, n = 5 mice/group/experiment, 2 experiments), and plasma MPs were collected at day 120 after infection, corresponding to the chronic disease phase. The MPs were labeled with fluorescence-conjugated antibodies against mouse molecules and analyzed by flow cytometry. Bar graphs (mean ± SEM) show the percent frequency of surface markers of various cellular origin on MPs isolated from media of Tc-infected PBMCs or the plasma of chronically infected human patients and experimental mice. Significance (* p < 0.05) is plotted with respect to normal controls.
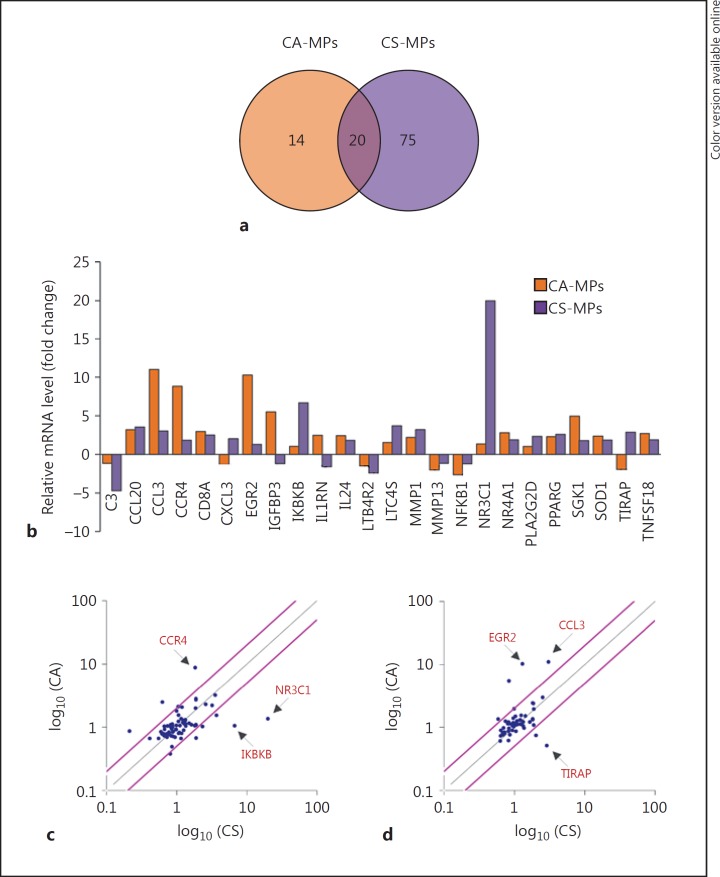
Next, we investigated if circulating MPs present in the plasma of chagasic patients elicited differential THP-1 mφ activation depending upon the clinical disease state. THP-1 mφs were incubated for 12 h with MPs isolated from the plasma of NH, CA, and CS subjects (n = 10 per group), and total RNA was isolated and reverse-transcribed. The cDNA samples from individuals within a group were pooled into 2 sets, and all samples were analyzed in duplicate by qPCR. The profiling of the gene expression in THP-1mφs using the inflammatory signaling cascade and cytokine/chemokine arrays showed that 34 genes (9 downregulated, 25 upregulated) and 95 genes (16 downregulated, 79 upregulated) were differentially expressed (≥|1.5| fold change, p ≤ 0.05) by CA-MPs and CS-MPs, respectively, with respect to the MPs of NH controls (online suppl. Table S1). Of these, 20 genes were differentially regulated in THP-1 mφs by both CA-MPs and CS-MPs. Furthermore, 14 genes (↑ADRB2, ↑CCR3, TGFBR1, ↑NR4A2, ↑IL-1B, ↑CSF1 ↑CCL17, ↑IL-6ST, ↑IGFBP3, ↑EGR2, ↓PIM3, ↓TRAF2, ↓MMP13, ↓NFKB1) were differentially expressed in a CA-MP-specific manner, and 7 genes (↑NR3C1, ↑IKBKB, ↑PLA2G2D, ↑CXCL3, ↓C3, ↓NR4A2, and ↓CD14, ≥|2.0| fold) were greatly changed in expression in a CS-MPs-specific manner (Fig. 4a, b). Scatter plots show that CA-MPs elicited maximal upregulation of CCR4, EGR2, and CCL3, while CS-MPs elicited maximal upregulation of IKBKB, NR3C1, and TIRAP in THP-1 mφs (Fig. 4c, d). These results suggest that the circulatory MPs from seropositive, Tc-infected humans elicit a disease-stage-specific inflammatory gene expression profile in THP-1 mφs.
Fig. 4.
Inflammatory gene expression profile of mφs in response to MPs of chagasic patients. Plasma MPs were isolated from seropositive chagasic subjects categorized as CA and CS, and NH controls (n = 10 per group). THP-1 mφs were incubated for 12 h with human MPs, and gene expression profiling was performed by qRT-PCR using the inflammation signaling cascade and cytokine/chemokine arrays. The differential mRNA level was normalized to housekeeping genes, and fold change in gene expression was calculated in comparison to NH-MP-treated mφs (online suppl. Table S1). a Venn diagram of differential inflammatory gene expression profile (≥|1.5| fold change) in mφs incubated with CA-MPs versus CS-MPs. b Mean differential expression of top molecules (≥|2.0| fold change) induced by CA-MPs and CS-MPs (vs. NH-MPs) in THP-1 mφs. c, d Scatter plots show the CA-MP-specific versus CS-MP-specific changes in mφ gene expression profile captured by qRT-PCR using the inflammatory signaling cascade (c) and cytokine/chemokine arrays (d). Pink lines mark the >2-fold difference in expression (see online version for colors).
To assess the phenotypic effect of human chagasic patients' MPs on mφs, we incubated THP-1 mφs for 24 h with NH-MPs, CA-MPs, and CS-MPs, and evaluated the surface expression of markers of mφ activation by flow cytometry and cell viability by alamarBlue assay. THP-1 mφs in vitro incubated with recombinant IFN-γ, IL-4, and IL-10 cytokines were used as controls. Flow cytometry analysis showed that IFN-γ-treated (proinflammatory) mφs were primarily CD80+/CD64+, and IL-4- and IL-10-treated (immunoregulatory) mφs were CD163+/CD206+. The IL-10-treated THP-1 mφs exhibited a CD16hi/CD200hi profile and the IL-4-treated mφs exhibited a CD16lo/CD200lo profile [25]. THP-1 mφs incubated with MPs isolated from the plasma of chagasic patients exhibited no significant change in cell viability and/or cell proliferation (online suppl. Fig. S1A). Furthermore, incubation of THP-1 mφs with CA-MPs resulted in no change in the expression of any of the surface markers indicative of IFN-γ-induced or IL-4/IL-10-induced phenotypes compared to that noted in mφs incubated with NH-MPs (online suppl. Fig. S1B). The CS-MP-induced CD14+ mφ population consisted of a higher number of CD16+ (35%↑)/CD16hi (24%↑), CD64+ (30%↑), and CD163hi (10%↑) mφs when compared to that noted with CA-MPs. We noted no statistically significant change in the population (as well as intensity) of the CD200+ and CD206+ mφ population when incubated with MPs from any of the subject group (online suppl. Fig. S1B, e, f). These data suggested that CS-MPs may induce a greater level of inflammatory activation of mφs than was induced by CA-MPs, evidenced by increased expression of CD14 and CD16.
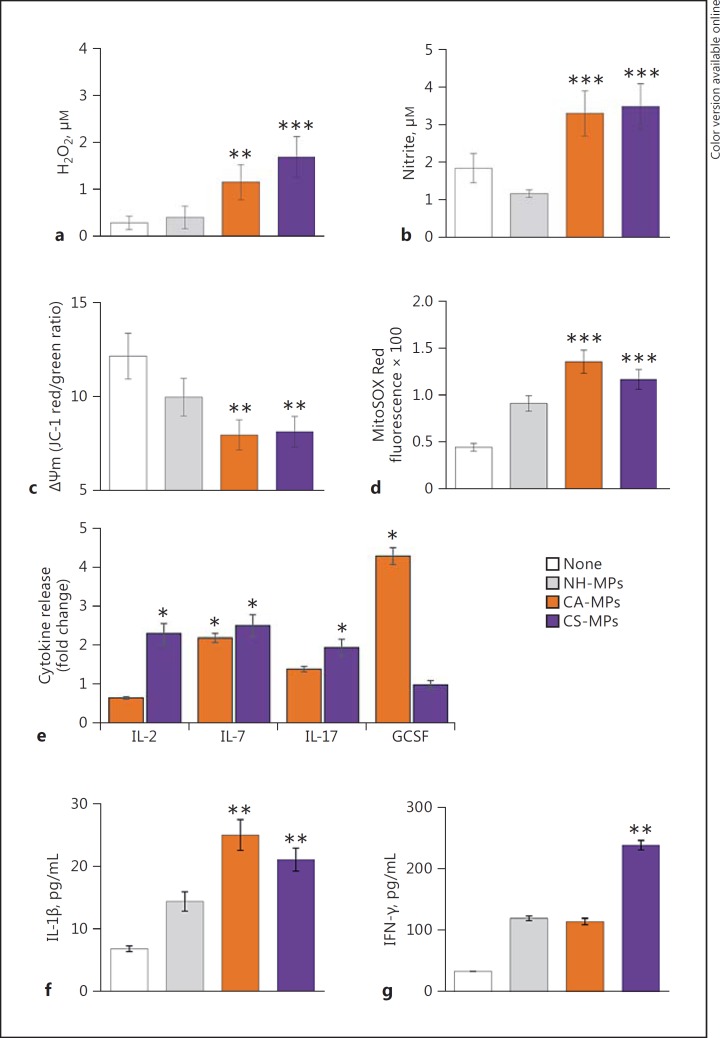
We investigated the functional response of mφs to chagasic patients' MPs at 1, 24, and 48 h after stimulation. The ROS and ∙NO generation and mitochondrial stress were examined in THP-1 mφs incubated for 1 h with plasma MPs from NH, CA, and CS human subjects (n = 10 per group). The THP-1 mφs incubated with CA-MPs or CS-MPs exhibited a 3.8- to 5.6-fold increase in ROS release (Fig. 5a, p < 0.01) and a 2.8- to 3.1-fold increase in ∙NO levels (Fig. 5b, p < 0.001) when compared to that noted in mφs incubated with NH-MPs or media only. Further, THP-1 mφs incubated with CA-MPs and CS-MPs, in comparison to mφs incubated with media or NH-MPs, exhibited a 33–35% decline in mitochondrial membrane potential (Fig. 5c, p < 0.01) and a 2.6- to 3-fold increase in mitochondrial ROS production (Fig. 5d, p < 0.001). A Bio-Plex Multiplex Human Cytokine Assay was employed to evaluate the cytokine release in supernatants of THP-1 mφs incubated for 24 h with MPs from NH, CA, and CS subjects (n = 10 per group). These data showed an increase of up to 2-fold in the release of IL-7 by mφs incubated with CA-MPs and CS-MPs (vs. NH-MPs). The CA-MPs also elicited a 4-fold increase in GCSF levels, while CS-MPs elicited >2-fold increase in IL-2 and IL-17 cytokines in THP-1 mφs (Fig. 5e). In another set of experiments, THP-1 mφs were incubated for 48 h with MPs from NH, CA, and CS subjects (n = 10 per group), and cytokine release in supernatants was evaluated by ELISA. We noted an increase of approximately 20% in IL-4 release and no change in IL-10 release in mφs incubated with CA-MPs or CS-MPs (vs. NH-MPs; data not shown). Furthermore, THP-1 mφs incubated for 48 h with CA-MPs exhibited a 75% increase in IL-1β production, and incubation with CS-MPs elicited a 50% increase in IL-1β and a 2-fold increase in IFN-γ release, respectively, compared to that noted in THP-1 mφs incubated with NH-MPs (Fig. 5f, g, p < 0.01). Together, the results presented in Figure 5 suggest that: (a) circulatory MPs from CA and CS subjects elicited oxidative and nitrosative stress and cytokine (i.e., IL-1β and IL-7) release in THP-1 mφs compared to when mφs were incubated with media alone or NH-MPs, and (b) GCSF and IL-2/IFN-γ are released by THP-1 mφs in a CA-MP- and CS-MP-specific manner, respectively.
Fig. 5.
Functional activation of mφs by MPs isolated from chagasic patients. MPs were harvested from the plasma of seropositive CA and CS subjects. MPs from seronegative NH individuals were used as controls. a–d THP-1 mφs were incubated in triplicate with medium only (none) or with NH-MPs, CA-MPs, and CS-MPs for 1 h. Cell-free supernatants were utilized to measure ROS levels by amplex red assay (a) and measure nitrate/nitrite levels by Griess reagent assay (b). c, d THP-1 mφs were incubated for 1 h with MPs as above. Cells were loaded with JC-1 or MitoSOX Red probes, and analyzed by fluorimetry. Changes in mitochondrial membrane potential measured as J aggregates (red)/J monomers (green) ratio (c) and MitoSOX Red fluorescence as a measure of mitochondrial ROS production (d) are shown. e Plasma-derived MPs from NH, CA, and CS subjects were added in triplicate to THP-1 mφs. Cells were incubated for 24 h, and supernatants were utilized in a Bioplex assay for a panel of 17 human cytokines and chemokines. f, g ELISA was performed to measure the IL-1β and IFN-γ levels in cell-free supernatants collected 48 h after incubation of THP-1 mφs with MPs. In all bar graphs, data are plotted as mean value ± SEM, and significance is presented as * p < 0.05, ** p < 0.01, *** p < 0.001 (media only or NH-MPs [controls] vs. CA-MPs or CS-MPs).
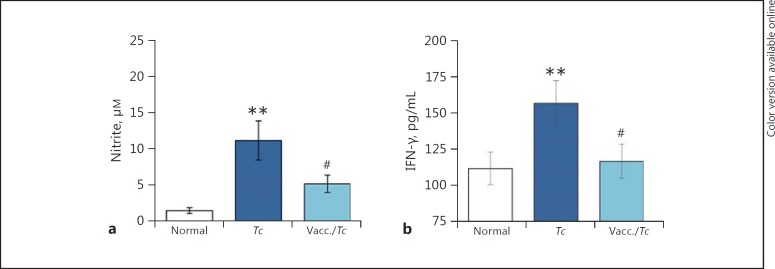
Finally, we determined if the observed differences in mφ activation by CA-MPs and CS-MPs were reflective of control of the parasite and disease. For this, we utilized an experimental model of vaccination against Tc infection. We have previously shown that a subunit vaccine composed of TcG2 and TcG4 antigens of Tc, delivered by DNA-prime/protein-boost approach, was efficacious in controlling parasite burden, myocarditis, and cardiac remodeling in mice [19]. We isolated MPs from the plasma of nonvaccinated/infected and vaccinated/infected mice at 120 days after infection (corresponding to a chronic disease state) and incubated the MPs with RAW264.7 mφs for 48 h. Our data showed that MPs isolated from the nonvaccinated/infected mice (vs. MPs from normal mice) elicited a 7.5-fold and 40% increase in ∙NO and IFN-γ production, respectively, in RAW264.7 mφs (Fig. 6a, b). In comparison, MPs isolated from the plasma of TcG2/TcG4-vaccinated/infected mice elicited a significantly lower level of ∙NO release and no IFN-γ production (Fig. 6a, b). Together, the results presented in Figure 6 suggest that vaccine-induced control of infection and disease is associated with a significant decline in the ability of MPs to induce the mφ activation of the ∙NO and IFN-γ response.
Fig. 6.
Nitrite and IFN-γ stimulation by MPs from chagasic mice (±anti-Tc vaccine). Mice were vaccinated with TcG2/TcG4 candidate antigens, delivered as DNA-prime/protein-boost vaccine (Materials and Methods). Two weeks after the last immunization, they were infected with Tc. MPs were harvested from the plasma of nonvaccinated/infected and vaccinated/infected mice 120 days after infection. MPs harvested from plasma of nonvaccinated/noninfected (normal) mice were used as controls. RAW264.7 mφs were incubated in triplicate for 48 h with MPs. Supernatants were utilized for measuring the nitrate/nitrite levels by Griess reagent assay (a) and measuring IFN-γ levels by ELISA (b). Data are plotted as mean value ± SEM (n = 5 mice/group/experiment, 2 experiments), and significance is presented as ** p < 0.01 (nonvaccinated/infected vs. normal controls) and #p < 0.05 (nonvaccinated/infected vs. vaccinated/infected).
Discussion
The currently available invasive tools are not practical for routine screening and monitoring the disease status or for predicting the risk of developing full-blown cardiac failure in Tc-infected individuals. The cure for chronic patients is routinely determined based upon the conversion to negative serology, which can take up to 8–10 years after treatment [26, 27] and occurs in <15% of treated adult subjects [28, 29]. Furthermore, the recently completed BENEFIT clinical trial concluded that trypanocidal therapy with benznidazole in patients with established Chagas cardiomyopathy significantly reduced the parasite detected in the serum but did not reduce cardiac clinical deterioration during a 5-year follow-up. These findings affirmed that conversion to negative serology is not synonymous with a cure [30, 31]. No easy-to-use diagnostic tests for determining a patient's risk of developing CS disease and the efficacy of a treatment in controlling infection or arresting disease progression are available. Therefore, in this study, our goal was to determine whether circulating MPs generated during Tc infection carry the host's signature of inflammatory/oxidative state and provide information regarding clinical disease severity. We utilized an in vitro system, samples from chagasic patients exhibiting different stages of disease development, and a murine model of Tc infection and cure by vaccination, and employed high-throughput transcriptomic and physiological approaches to study the mφ response to MPs. Our results suggest that MPs released by human PBMCs infected with Tc in vitro or circulatory MPs present in the plasma of chronically infected chagasic patients and experimentally infected mice were primarily of monocyte/mφ and lymphocyte (CD8 > CD4) origin, and exhibited an inflammatory phenotype. This was demonstrated by a decline in mitochondrial membrane potential and an increase in the proinflammatory gene expression profile, and IFN-γ and ∙NO production in mφs incubated with MPs derived from the 3 models of Tc infection. The key features of the mφ response to CS-MPs included a pronounced proinflammatory gene expression profile (Fig. 4), an increase in the expression of the NR3C1, IKBKB, and TIRAP genes, and substantially higher levels of IFN-γ release, while the mφ response to CA-MPs was captured by increased expression of CCR4, EGR2, and CCL3, and GCSF production. Furthermore, vaccinated mice, which were previously shown to control parasite persistence and chronic myocarditis [19, 20], produced MPs that elicited no ∙NO and less IFN-γ in mφs than was noted with MPs of nonvaccinated/infected mice. To the best of our knowledge, this is the first study demonstrating that circulating MPs predict the in vivo levels of the oxidative and inflammatory state and have potential utility in evaluating disease severity and the efficacy of vaccines and drug therapies against CCM.
Several Tc-derived molecules (e.g., glycosylphosphatidylinositols, mucin-like glycoproteins) act as TLR2 and TLR4 agonists and induce the production of ∙NO and inflammatory cytokines and chemokines via cells of the monocytic lineage [review [32]]. Other study groups have shown that TLR4−/− mφs are deficient in the production of trypanocidal ∙NO and ROS and fail to control parasite replication [33]. TLR3−/−, TLR7−/−, and TLR9−/− mice are also susceptible to Tc infection [34], and it has been suggested that Tc-DNA-dependent TLR/Myd88 plays an important role in bridging innate to acquired immunity in the context of the control of Tc infection. These studies support the role of innate immune cells (e.g., mφs, dendritic cells) in regulating Tc infection.
Indeed, the host is capable of controlling the acute parasitemia to barely detectable levels. Why the chronic inflammation then persists in the host is not completely understood. Our results in this study provide some clues as to the source of the stimulus contributing to the persistence of the inflammatory infiltrate in Chagas disease. We propose that after controlling the acute infection, the clearance of activated immune cells is necessary to maintain the homeostatic state. During this process, membranes shed by activated immune cells produce circulatory MPs and these MPs may potentially serve as damage-associated molecular patterns and activate immune cells by engaging TLRs and NLRs. This notion is supported by the observation that circulating MPs in chagasic patients and in the experimentally infected mice were composed of membranes shed by endothelial cells, mφs, and T lymphocytes, all of which are known to be activated during infection, and which contribute to the chronic inflammatory pathology in Chagas disease [reviews [35, 36]]. Furthermore, the MPs present in the plasma of chagasic humans and experimentally infected mice were capable of signaling a proinflammatory phenotype in the mφs, demonstrated by increased proliferation, ∙NO release, inflammatory gene expression, and cytokine (IL-1β, IFN-γ) production. The IL-12 and IL-18 cytokines are shown to induce IFN-γ in mφs [37], and the latter can contribute to the proinflammatory activation of mφs in an autocrine manner [38]. Additional studies evaluating the proteomic and functional profile of circulating MPs will provide insights into the pathological mechanisms that may contribute to generating MPs; however, our results provide a strong indication that the proinflammatory nature of the circulating MPs may, at least partially, contribute to the persistence of chronic inflammation during Chagas disease.
We have previously shown that the incubation of mφs with sera of chagasic mice elicited a proinflammatory phenotype (CD64hi CD80hi) and functional response (increased TNF-α/IFN-γ production) [25]. In this study, circulatory MPs from chagasic patients and experimentally infected mice produced a similar proinflammatory activation of mφs (Fig. 4, 5, 6), noted when mφs were incubated with complete sera or plasma of chagasic mice [25]. Incubation with MP-free sera or plasma from chagasic mice or patients elicited no response in mφs (data not shown). These results suggest that it is MPs, and not the soluble molecules present in the systemic circulation, that carry the proinflammatory signature of Chagas disease.
Tc infection mobilizes innate and adaptive immune responses that induce mφ activation and keep infection under control [39]. Experimental animals and humans elicit potent adaptive B and T cell immunity to Tc infection, and are capable of controlling the acute circulating and tissue parasite burden [35, 36, 40]. We showed that the MPs isolated from clinically symptomatic chagasic patients and experimentally infected mice elicited a substantial increase in cytokines (IL-1β, IL-7, and IFN-γ), ROS, and ∙NO production in mφs (Fig. 5, 6). These results suggest that circulatory MPs constitute a pathomechanism in chronic Chagas disease, and the therapies capable of preventing cellular injury (i.e., inhibiting the generation of MPs) or reprogramming the mφs (i.e., inhibiting proinflammatory cytokines, ROS, and ∙NO production) will be beneficial in halting the feedback cycle of mφ activation and the persistence of pathological inflammatory stress in Chagas disease. This notion is strongly supported by our findings that complete sera or MPs harvested from nonvaccinated/infected (vs. vaccinated/ infected) mice elicited a pronounced IFN-γ and ∙NO production response in mφs (Fig. 6) [25]. Likewise, the MPs of seropositive individuals who exhibited clinical disease induced a more robust proinflammatory gene expression profile, cytokine release (IL-1β, IL-2, and IFN-γ), and mitotoxic phenotype, while the MPs of seropositive individuals who had not yet developed clinical disease elicited a mild-to-moderate gene expression profile and no IFN-γ in THP-1 mφs (Fig. 4, 5).
∙NO release was suppressed in mφs stimulated with MPs from vaccinated/infected mice compared to the MPs from nonvaccinated/noninfected mice; however, we did not observe this trend with the MPs from CA versus CS patients, as the MPs from both groups elevated ∙NO release from THP-1 mφs. We speculate that the difference in the RAW264.7 versus THP-1 mφs' ability to respond to mouse versus human MPs, and the difference in the composition of MPs in mice and in humans, may contribute to the outcome that we observed, with respect to the control of ∙NO release when MPs from infected/vaccinated mice and CA humans were used. Resistance to Tc infection in humans and in mice may vary according to the genetic background of the host and the virulence of the parasite strain, and contribute to differential ∙NO production as well.
In summary, we used an in vitro system, an experimental model of vaccination, and human chagasic patients, and showed that MPs of monocyte/mφ and lymphocyte origin are produced during the course of Tc infection and chronic disease progression, and that these MPs elicit proinflammatory activation of mφs. Our results suggest that MP-induced activation of a differential inflammatory gene expression profile, cytokine release, and ∙NO production in mφs reflects the severity of the disease state in the chagasic host. These results give impetus for appraising the MP signature of a large number of individuals who exhibit varying degree of CCM severity. We hope that our MPs and in vitro cell-based assays can have the potential utility and power for identifying the risk of clinical disease development and evaluating the efficacy of therapies for controlling Chagas disease.
Funding Sources
This work was supported in part by grants from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (R01 AI054578 and R21 AI107227), and National Heart Lung Blood Institute (R01 HL094802) to N.J.G. L.S. and S.G. received a fellowship from the Summer Undergraduate Research Program and Sealy Center for Vaccine Development, respectively, at the UTMB Galveston. S.K. was awarded McLaughlin Endowment (UTMB) and American Heart Association pre-doc fellowships. M.P.Z. was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
Disclosure Statement
The authors have no competing interests.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Acknowledgements
We appreciate the consistent and long-standing support of Dr. Ines Vidal (biochemist of the Central Laboratory of San Bernardo Hospital, Salta, Argentina) and Federico Ramos and Alejandro Uncos (lab technicians) in sample collection and processing and serological testing. In addition, we thank Yolanda Huertas, Rosa Rodas, and Liliana Villagran (nurses and office secretary of the Cardiology Unit at San Bernardo Hospital, Salta, Argentina) for their help in patient recruitment and maintenance of clinical records. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper.
References
- 1.World Health Organization Chagas disease: control and elimination; in: Report of the Secretariat WHO, Geneva, 2010. UNDP/World Bank/WHO. http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_17-en.pdf.
- 2.Bern C, Montgomery SP. An estimate of the burden of Chagas disease in the United States. Clin Infect Dis. 2009;49:e52–e54. doi: 10.1086/605091. [DOI] [PubMed] [Google Scholar]
- 3.Tanowitz HB, Machado FS, Spray DC, Friedman JM, Weiss OS, Lora J, Nascimento D, Nunes MC, Garg NJ, Ribeiro AL. Developments in the manangement of chagasic cardiomyopathy. Expert Rev Cardiovasc Ther. 2016;13:1393–1409. doi: 10.1586/14779072.2015.1103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munoz-Fernandez MA, Fernandez MA, Fresno M. Activation of human macrophages for the killing of intracellular Trypanosoma cruzi by TNF-alpha and IFN-gamma through a nitric oxide-dependent mechanism. Immunol Lett. 1992;33:35–40. doi: 10.1016/0165-2478(92)90090-b. [DOI] [PubMed] [Google Scholar]
- 5.Plasman N, Metz G, Vray B. Interferon-gamma-activated immature macrophages exhibit a high Trypanosoma cruzi infection rate associated with a low production of both nitric oxide and tumor necrosis factor-alpha. Parasitol Res. 1994;80:554–558. doi: 10.1007/BF00933002. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez MN, Peluffo G, Piacenza L, Radi R. Intraphagosomal peroxynitrite as a macrophage-derived cytotoxin against internalized Trypanosoma cruzi: consequences for oxidative killing and role of microbial peroxiredoxins in infectivity. J Biol Chem. 2011;286:6627–6640. doi: 10.1074/jbc.M110.167247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardoso MS, Reis-Cunha JL, Bartholomeu DC. Evasion of the immune response by Trypanosoma cruzi during acute infection. Front Immunol. 2015;6:659. doi: 10.3389/fimmu.2015.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Tarleton RL. Parasite persistence correlates with disease severity and localization in chronic Chagas disease. J Infect Dis. 1999;180:480–486. doi: 10.1086/314889. [DOI] [PubMed] [Google Scholar]
- 9.Wen JJ, Dhiman M, Whorton EB, Garg NJ. Tissue-specific oxidative imbalance and mitochondrial dysfunction during Trypanosoma cruzi infection in mice. Microbes Infect. 2008;10:1201–1209. doi: 10.1016/j.micinf.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen J-J, Bhatia V, Popov VL, Garg NJ. Phenyl-alpha-tert-butyl nitrone reverses mitochondrial decay in acute Chagas disease. Am J Pathol. 2006;169:1953–1964. doi: 10.2353/ajpath.2006.060475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen J-J, Gupta S, Guan Z, Dhiman M, Condon D, Lui CY, Garg NJ. Phenyl-alpha-tert-butyl-nitrone and benzonidazole treatment controlled the mitochondrial oxidative stress and evolution of cardiomyopathy in chronic chagasic rats. J Am Coll Cardiol. 2010;55:2499–2508. doi: 10.1016/j.jacc.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhiman M, Wan X-X, Popov VL, Vargas G, Garg NJ. MnSODtg mice control myocardial inflammatory and oxidative stress and remodeling responses elicited in chronic Chagas disease. J Am Heart Assoc. 2013;2:e000302. doi: 10.1161/JAHA.113.000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhiman M, Garg NJ. NADPH oxidase inhibition ameliorates Trypanosoma cruzi-induced myocarditis during Chagas disease. J Pathol. 2011;225:583–596. doi: 10.1002/path.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhiman M, Garg NJ. P47phox−/− mice are compromised in expansion and activation of CD8+ T cells and susceptible to Trypanosoma cruzi infection. PLoS Pathog. 2014;10:e1004516. doi: 10.1371/journal.ppat.1004516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhiman M, Zago MP, Nunez S, Nunez-Burgio F, Garg NJ. Cardiac oxidized antigens are targets of immune recognition by antibodies and potential molecular determinants in Chagas disease pathogenesis. PLoS One. 2012;7:e28449. doi: 10.1371/journal.pone.0028449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burnouf T, Chou ML, Goubran H, Cognasse F, Garraud O, Seghatchian J. An overview of the role of microparticles/microvesicles in blood components: are they clinically beneficial or harmful? Transfus Apher Sci. 2015;53:137–145. doi: 10.1016/j.transci.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Shantsila E, Kamphuisen PW, Lip GY. Circulating microparticles in cardiovascular disease: implications for atherogenesis and atherothrombosis. J Thromb Haemost. 2010;8:2358–2368. doi: 10.1111/j.1538-7836.2010.04007.x. [DOI] [PubMed] [Google Scholar]
- 18.Wen JJ, Zago MP, Nunez S, Gupta S, Burgos FN, Garg NJ. Serum proteomic signature of human chagasic patients for the identification of novel protein biomarkers of disease. Mol Cell Proteomics. 2012;11:435–452. doi: 10.1074/mcp.M112.017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S, Garg NJ. Prophylactic efficacy of TcVac2R against Trypanosoma cruzi in mice. PLoS Negl Trop Dis. 2010;4:e797. doi: 10.1371/journal.pntd.0000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta S, Garg NJ. A two-component DNA-prime/protein-boost vaccination strategy for eliciting long-term, protective T cell immunity against Trypanosoma cruzi. PLoS Pathog. 2015;11:e1004828. doi: 10.1371/journal.ppat.1004828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dey N, Sinha M, Gupta S, Gonzalez MN, Fang R, Endsley JJ, Luxon BA, Garg NJ. Caspase-1/ASC inflammasome-mediated activation of IL-1β-ROS-NF-κB pathway for control of Trypanosoma cruzi replication and survival is dispensable in NLRP3−/− macrophages. PLoS One. 2014;9:e111539. doi: 10.1371/journal.pone.0111539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson RC, Guiheneuf F, Bahar B, Schmid M, Stengel DB, Fitzgerald GF, Ross RP, Stanton C. The anti-inflammatory effect of algae-derived lipid extracts on lipopolysaccharide (LPS)-stimulated human THP-1 macrophages. Mar Drugs. 2015;13:5402–5424. doi: 10.3390/md13085402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crean D, Cummins EP, Bahar B, Mohan H, McMorrow JP, Murphy EP. Adenosine modulates NR4A orphan nuclear receptors to attenuate hyperinflammatory responses in monocytic cells. J Immunol. 2015;195:1436–1448. doi: 10.4049/jimmunol.1402039. [DOI] [PubMed] [Google Scholar]
- 24.Kleinbongard P, Rassaf T, Dejam A, Kerber S, Kelm M. Griess method for nitrite measurement of aqueous and protein-containing samples. Methods Enzymol. 2002;359:158–168. doi: 10.1016/s0076-6879(02)59180-1. [DOI] [PubMed] [Google Scholar]
- 25.Gupta S, Silva TS, Osizugbo JE, Tucker L, Spratt HM, Garg NJ. Serum-mediated activation of macrophages reflects TcVac2 vaccine efficacy against Chagas disease. Infect Immun. 2014;82:1382–1389. doi: 10.1128/IAI.01186-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cancado JR. Long-term evaluation of etiological treatment of Chagas disease with benznidazole. Rev Inst Med Trop Sao Paulo. 2002;44:29–37. [PubMed] [Google Scholar]
- 27.Cancado JR. Criteria of Chagas disease cure. Mem Inst Oswaldo Cruz. 1999;94((suppl 1)):331–335. doi: 10.1590/s0074-02761999000700064. [DOI] [PubMed] [Google Scholar]
- 28.Viotti R, Vigliano C, Lococo B, Bertocci G, Petti M, Alvarez MG, Postan M, Armenti AH. Long-term cardiac outcome of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann Intern Med. 2006;144:724–734. doi: 10.7326/0003-4819-144-10-200605160-00006. [DOI] [PubMed] [Google Scholar]
- 29.Fabbro DL, Streiger ML, Arias ED, Bizai ML, del Barco M, Amicone NA. Trypanocide treatment among adults with chronic Chagas disease living in Santa Fe City (Argentina), over a mean follow-up of 21 years: parasitological, serological and clinical evolution. Rev Soc Bras Med Trop. 2007;40:1–10. doi: 10.1590/s0037-86822007000100001. [DOI] [PubMed] [Google Scholar]
- 30.Bautista-Lopez NL, Morillo CA, Lopez-Jaramillo P, Quiroz R, Luengas C, Silva SY, Galipeau J, Lalu MM, Schulz R. Matrix metalloproteinases 2 and 9 as diagnostic markers in the progression to Chagas cardiomyopathy. Am Heart J. 2013;165:558–566. doi: 10.1016/j.ahj.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A, Jr, Rosas F, Villena E, Quiroz R, Bonilla R, Britto C, et al. Randomized trial of benznidazole for chronic Chagas' cardiomyopathy. N Engl J Med. 2015;373:1295–1306. doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- 32.Rodrigues MM, Oliveira AC, Bellio M. The immune response to Trypanosoma cruzi: role of toll-like receptors and perspectives for vaccine development. J Parasitol Res. 2012;2012:507874. doi: 10.1155/2012/507874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliveira AC, de Alencar BC, Tzelepis F, Klezewsky W, da Silva RN, Neves FS, Cavalcanti GS, Boscardin S, Nunes MP, Santiago MF, et al. Impaired innate immunity in TLR4(−/−) mice but preserved CD8+ T cell responses against Trypanosoma cruzi in TLR4-, TLR2-, TLR9- or Myd88-deficient mice. PLoS Pathog. 2010;6:e1000870. doi: 10.1371/journal.ppat.1000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caetano BC, Carmo BB, Melo MB, Cerny A, dos Santos SL, Bartholomeu DC, Golenbock DT, Gazzinelli RT. Requirement of UNC93B1 reveals a critical role for TLR7 in host resistance to primary infection with Trypanosoma cruzi. J Immunol. 2011;187:1903–1911. doi: 10.4049/jimmunol.1003911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Machado FS, Dutra WO, Esper L, Gollob KJ, Teixeira MM, Weiss LM, Nagajyothi F, Tanowitz HB, Garg NJ. Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease. Semin Immunopathol. 2012;34:753–770. doi: 10.1007/s00281-012-0351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanowitz HB, Wen JJ, Machado FS, Desruisseaux MS, Robello C, Garg NJ. Trypanosoma cruzi and Chagas disease: innate immunity, ROS, and cardiovascular system. In: Gavins NE, Stokes KY, Vascular Responses to Pathogens, editors. Waltham: Academic Press/Elsevier; 2016. pp. pp 183–193. [Google Scholar]
- 37.Darwich L, Coma G, Pena R, Bellido R, Blanco EJ, Este JA, Borras FE, Clotet B, Ruiz L, Rosell A, et al. Secretion of interferon-gamma by human macrophages demonstrated at the single-cell level after costimulation with interleukin (IL)-12 plus IL-18. Immunology. 2009;126:386–393. doi: 10.1111/j.1365-2567.2008.02905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ribeiro-Gomes FL, Lopes MF, DosReis GA. Madame Curie Bioscience Database, edited by NCBI Bookshelf. Austin: Landes Bioscience; 2000. Negative Signaling and Modulation of Macrophage Function in Trypanosoma cruzi Infection. [Google Scholar]
- 40.Nagajyothi F, Machado FS, Burleigh BA, Jelicks LA, Scherer PE, Mukherjee S, Lisanti MP, Weiss LM, Garg NJ, Tanowitz HB. Mechanisms of Trypanosoma cruzi persistence in Chagas disease. Cell Microbiol. 2012;14:634–643. doi: 10.1111/j.1462-5822.2012.01764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data