Abstract
Key points
In hypertensive adults (HTN), cardiovascular risk increases disproportionately during environmental cold exposure.
Despite ample evidence of dysregulated sympathetic control of the peripheral vasculature in hypertension, no studies have examined integrated neurovascular function during cold stress in HTN.
The findings of the present study show that whole‐body cold stress elicits greater increases in sympathetic outflow directed to the cutaneous vasculature and, correspondingly, greater reductions in skin blood flow in HTN.
We further demonstrate an important role for non‐adrenergic sympathetic co‐transmitters in mediating the vasoconstrictor response to cold stress in hypertension.
In the context of thermoregulation and the maintenance of core temperature, sympathetically‐mediated control of the cutaneous vasculature is not only preserved, but also exaggerated in hypertension. Given the increasing prevalence of hypertension, clarifying the mechanistic underpinnings of hypertension‐induced alterations in neurovascular function during cold exposure is clinically relevant.
Abstract
Despite ample evidence of dysregulated sympathetic control of the peripheral vasculature in hypertension, no studies have examined integrated neurovascular function during cold stress in hypertensive adults (HTN). We hypothesized that (i) whole‐body cooling would elicit greater cutaneous vasoconstriction and greater increases in skin sympathetic nervous system activity (SSNA) in HTN (n = 14; 56 ± 2 years) compared to age‐matched normotensive adults (NTN; n = 14; 55 ± 2 years) and (ii) augmented reflex vasoconstriction in HTN would be mediated by an increase in cutaneous vascular adrenergic sensitivity and a greater contribution of non‐adrenergic sympathetic co‐transmitters. SSNA (peroneal microneurography) and red cell flux (laser Doppler flowmetry; dorsum of foot) were measured during whole‐body cooling (water‐perfused suit). Sympathetic adrenergic‐ and non‐adrenergic‐dependent contributions to reflex cutaneous vasoconstriction and vascular adrenergic sensitivity were assessed pharmacologically using intradermal microdialysis. Cooling elicited greater increases in SSNA (NTN: +64 ± 13%baseline vs. HTN: +194 ± 26%baseline; P < 0.01) and greater reductions in skin blood flow (NTN: −16 ± 2%baseline vs. HTN: −28 ± 3%baseline; P < 0.01) in HTN compared to NTN, reflecting an increased response range for sympathetic reflex control of cutaneous vasoconstriction in HTN. Norepinephrine dose–response curves showed no HTN‐related difference in cutaneous adrenergic sensitivity (logEC50; NTN: −7.4 ± 0.3 log M vs. HTN: −7.5 ± 0.3 log M; P = 0.84); however, non‐adrenergic sympathetic co‐transmitters mediated a significant portion of the vasoconstrictor response to cold stress in HTN. Collectively, these findings indicate that hypertension increases the peripheral cutaneous vasoconstrictor response to cold via greater increases in skin sympathetic outflow coupled with an increased reliance on non‐adrenergic neurotransmitters.
Keywords: cold stress, skin blood flow, sympathetic nerve activity, vascular sensitivity
Key points
In hypertensive adults (HTN), cardiovascular risk increases disproportionately during environmental cold exposure.
Despite ample evidence of dysregulated sympathetic control of the peripheral vasculature in hypertension, no studies have examined integrated neurovascular function during cold stress in HTN.
The findings of the present study show that whole‐body cold stress elicits greater increases in sympathetic outflow directed to the cutaneous vasculature and, correspondingly, greater reductions in skin blood flow in HTN.
We further demonstrate an important role for non‐adrenergic sympathetic co‐transmitters in mediating the vasoconstrictor response to cold stress in hypertension.
In the context of thermoregulation and the maintenance of core temperature, sympathetically‐mediated control of the cutaneous vasculature is not only preserved, but also exaggerated in hypertension. Given the increasing prevalence of hypertension, clarifying the mechanistic underpinnings of hypertension‐induced alterations in neurovascular function during cold exposure is clinically relevant.
Abbreviations
- BP
blood pressure
- CVC
cutaneous vascular conductance
- HR
heart rate
- HTN
hypertensive adult
- NE
norepinephrine
- NTN
normotensive adult
- PU
perfusion unit
- ROCK
Rho‐kinase
- SSNA
skin sympathetic nervous system activity
- Tsk
skin temperature
- Y+P
yohimbine + propranolol
Introduction
Hypertension is characterized, in part, by pervasive impairments in neurovascular function, resulting in a shift toward a proconstrictor mediator profile (Cardillo & Panza, 1998). In hypertensive adults (HTN), cardiovascular‐related morbidity and mortality increase disproportionately during environmental cold exposure (Woodhouse et al. 1993; Minami et al. 1996); this increase in risk is assumed to result from the untoward consequences of vascular dysfunction, sympathetic overactivity and impaired blood pressure (BP) regulation (Liu et al. 2015; Grassi & Ram, 2016). Given the critical role for sympathetic control of vascular function in mediating the physiological responses to cooling (Thompson‐Torgerson et al. 2008), as well as the increasing prevalence of hypertension (Writing Group et al. 2016), clarifying the mechanistic underpinnings of hypertension‐induced alterations in neurovascular function during cold exposure is clinically relevant.
Whole‐body cold stress (i.e. decreased mean skin and/or core temperature) elicits reflex increases in efferent skin sympathetic nerve activity (SSNA), evoking cutaneous vasoconstriction and subsequent reductions in skin blood flow to minimize heat loss (Charkoudian, 2010; Holowatz & Kenney, 2010; Greaney et al. 2015 a). Human cutaneous vasoconstriction is mediated by both norepinephrine (NE) and non‐adrenergic sympathetic co‐transmitters [e.g. neuropeptide Y (NPY) and ATP] (Stephens et al. 2001; Stephens et al. 2004; Thompson & Kenney, 2004). Functional differences at multiple points along the sympathetic reflex axis could conceivably contribute to alterations in neurovascular function during cold stress in HTN. Surrogate measures of sympathetic activity [e.g. heart rate (HR) and BP variability] provide indirect support that sympathetic hyper‐reactivity to cold exposure is characteristic of hypertension (Hintsala et al. 2014 b; Hintsala et al. 2016). Cutaneous vasoconstriction in response to a local cooling stimulus is greater in HTN compared to normotensive adults (NTN), partly as a result of greater adrenergic‐dependent vasoconstriction (Smith et al. 2013); however, no studies have measured the mechanistically distinct sympathetic reflex effector response to whole‐body cooling in human hypertension. Non‐adrenergic co‐transmitters NPY and ATP are co‐localized in, and co‐released with NE from, sympathetic nerve endings, contributing to non‐adrenergic vasoconstriction (Lundberg, 1996; Stephens et al. 2001). Interestingly, higher neural stimulation frequencies are necessary to facilitate the release of the large dense core vesicles containing non‐adrenergic co‐transmitters (Sawasaki et al. 2001). Although co‐transmitter release appears to be increased in hypertension (Kahan et al. 1992; Han et al. 1998), whether a portion of reflex cutaneous vasoconstriction is mediated by non‐adrenergic mechanisms in HTN remains unclear.
The present study aimed to investigate integrated sympathetic control of reflex cutaneous vasoconstriction during whole‐body cooling‐induced reductions in mean skin temperature (T sk) in otherwise healthy middle‐aged adults with essential hypertension by directly measuring efferent skin sympathetic outflow, as well as pharmacologically interrogating the neural control of cutaneous vasoconstriction. We hypothesized that mild whole‐body cold stress would elicit exaggerated increases in SSNA and greater reductions in skin blood flow in HTN. We further hypothesized that augmented reflex cutaneous vasoconstriction in HTN would be mediated by both an increase in vascular adrenergic sensitivity and a greater contribution of non‐adrenergic sympathetic co‐transmitters.
Methods
Subjects
All procedures and protocols were approved by The Pennsylvania State University Institutional Review Board and conformed to the guidelines set forth in the Declaration of Helsinki. Informed verbal and written consent were obtained voluntarily from all subjects prior to participation. Fourteen NTN and 14 HTN participated in the study (Table 1). All participants underwent a complete medical screening, including physical examination, resting 12 lead electrocardiogram and 12 h fasting blood chemistry (Quest Diagnostics; Pittsburgh, PA, USA). Subjects were non‐obese (body mass index <30 kg m−2), did not use tobacco products and were recreationally active.
Table 1.
Subject characteristics
| Baseline characteristic | NTN | HTN |
|---|---|---|
| n (male/female) | 14 (7/7) | 14 (6/8) |
| Age (years) | 55 ± 2 | 56 ± 2 |
| Height (cm) | 172 ± 3 | 170 ± 3 |
| Mass (kg) | 80 ± 3 | 82 ± 5 |
| BMI (kg m–2) | 26.9 ± 0.8 | 28.0 ± 0.8 |
| HR (beats min–1) | 68 ± 3 | 68 ± 3 |
| Screening SBP (mmHg)ǂ | 123 ± 3‡ | 139 ± 3* |
| Screening DBP (mmHg)ǂ | 75 ± 2 | 89 ± 2* |
| 24 h SBP (mmHg)ǂ | 112 ± 2 | 134 ± 3* |
| 24 h DBP (mmHg)ǂ | 74 ± 1 | 84 ± 2* |
| Experimental SBP (mmHg) | 124 ± 2‡ | 145 ± 4*‡ |
| Experimental DBP (mmHg) | 75 ± 1 | 86 ± 2* |
| Blood biochemistry | ||
| HbA1c (%) | 5.6 ± 0.1 | 5.7 ± 0.1 |
| Fasting total cholesterol (mg dl–1) | 187 ± 8 | 199 ± 8 |
| Fasting HDL (mg dl–1) | 53 ± 5 | 57 ± 6 |
| Fasting LDL (mg dl–1) | 105 ± 11 | 106 ± 11 |
| Fasting triglycerides (mg dl–1) | 111 ± 12 | 123 ± 25 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein. Experimental SBP and DBP are the mean values calculated from each protocol visit. Values are the mean ± SE.
* P < 0.001 vs. NTN.
‡ P < 0.05 vs. 24 h.
ǂHTN were not withdrawn from antihypertensive medications for these blood pressure (BP) measurements.
Consistent with JNC7 guidelines (Chobanian et al. 2003), HTN had a resting seated systolic BP >140 mmHg or a diastolic BP >90 mmHg, as assessed in accordance with American Heart Association guidelines (Pickering et al. 2005), or were on anti‐hypertensive medication. Ambulatory BP monitoring (Ambulo 2400; Mortara Instrument Inc, Milwaukee, WI, USA) was used to confirm the diagnosis of hypertension because of its greater accuracy for describing the BP profile in the daily routine and avoiding ‘white coat’ hypertension (O'Brien et al. 2013). The average BP values obtained in 24 h ambulatory monitoring are typically lower than those obtained by office measurements as a result of nocturnal dipping; thus, the consensus values for the diagnosis of hypertension using 24 h ambulatory measures are systolic BP >130 mmHg or diastolic BP >80 mmHg (Mancia et al. 2013; O'Brien et al. 2013). These diagnostic thresholds for ambulatory BP monitoring yielded 10 year cardiovascular risks similar to those using diagnostic values obtained on office measurement (Kikuya et al. 2007). Ambulatory measures were obtained every 30 min when awake and every 60 min when asleep. Participants taking anti‐hypertensive medication (n = 5; angiotensin receptor blocker, n = 1; angiotensin‐converting enzyme inhibitor, n = 4; diuretic, n = 1) discontinued treatment for two full days before each experimental visit to minimize the confound of BP‐lowering medication on data interpretation (Delaney et al. 2010; Greaney et al. 2014). For those participants who discontinued anti‐hypertensive treatment for two full days before each experimental visit, BP was monitored three times per day with a portable automatic BP monitor (BP742N; Omron Healthcare, Lake Forest, IL, USA) to ensure subject safety during this medication‐free period. Other medications used by both NTN and HTN adults were not discontinued (cholesterol‐lowering, n = 5; asthma, n = 1; gastroesophageal reflux disease, n = 2; thyroid, n = 1). No subjects had any evidence of overt cardiovascular disease, apart from hypertension, or any evidence or diagnosis of associated comorbidities, including renal, pulmonary, neurological or dermatological disease. Women taking, or who had recently taken, hormone replacement therapy were excluded. Protocols were conducted on separate visits; before each visit, participants abstained from eating (4 h), caffeinated and alcoholic beverages (12 h), and strenuous physical activity (24 h). All protocols were performed in a thermoneutral laboratory (22°C).
Protocol 1: SSNA responsiveness during whole‐body cooling
To control T sk, subjects wore a water‐perfused suit that covered the entire body except for the face, hands, feet and lower left leg. Copper‐constantan thermocouples were affixed to the skin (calf, thigh, abdomen, chest, back and upper arm); the unweighted mean provided a continuous measurement of mean T sk. Cutaneous blood flow was estimated on the dorsum of the foot, within the area of neural innervation of the peroneal nerve, using an integrated laser Doppler flowmeter probe placed in a local heating unit (moorVMS‐LDF2; Moor Instruments Inc., Axminster, UK). The local heater was clamped at 33°C for the duration of the experiment to specifically isolate reflex mechanisms mediating cutaneous vasoconstriction (Lang et al. 2009; Greaney et al. 2015 b). Multifibre recordings of postganglionic SSNA were obtained by a tungsten microelectrode in the peroneal nerve, as previously described in detail (Greaney et al. 2015 b; Gagnon et al. 2016). A reference electrode was inserted 2–3 cm from the recording electrode. The position of the recording electrode was adjusted until bursts of SSNA were identified based upon: (i) responsiveness to arousal (loud noise) stimuli or deep inspiration but not during respiratory apnea; (ii) responsiveness to somatosensory stimulation in the innervated region; and (iii) lack of synchronicity of discharges with pulse rate (Hagbarth et al. 1972). Nerve signals were amplified, bandpass filtered (700–2000 Hz), rectified and integrated (time constant 0.1 s) (Nerve Traffic Analyser; University of Iowa Bioengineering, Iowa City, IA, USA). Mean voltage neurograms were visually displayed and routed to a loudspeaker for continuous monitoring. SSNA responsiveness to an auditory stimulus was confirmed at the conclusion of the protocol to ensure a consistent recording site. Beat‐to‐beat BP was obtained using finger photoplethysomography (BMEYE; Nexfin, St Louis, MO, USA). Automated brachial artery BP (Cardiocap; GE Healthcare, Milwaukee, WI, USA) was obtained every 3 min throughout the experiment to confirm absolute finger BP measurements. HR was measured using an electrocardiogram (Cardiocap; GE Healthcare). Respiratory movements were monitored (but not quantified) using a strain‐gauge pneumograph (Pneumotrace; UFI, Morro Bay, CA, USA) to ensure that subjects did not inadvertently perform Valsalva manoeuvres or breath‐holds during the protocol because these abnormal breathing patterns are known to influence sympathetic outflow. Qualitatively, respiratory pattern did not appear to differ between groups.
Following 10 min of baseline data collection at thermoneutrality, cool water (∼16°C) was perfused through the suit to gradually lower mean T sk from 34°C to 30.5°C (∼30 min), where it was clamped for an additional 5 min. This cooling stimulus elicits progressive reductions in T sk without effecting core temperature (Thompson & Kenney, 2004; Greaney et al. 2015 b). During cooling, acceptable SSNA recordings were maintained in 14 NTN and 10 HTN. To more specifically isolate the cooling stimulus, a non‐thermoregulatory sympathoexcitatory stimulus (mental stress) was applied at thermoneutrality and was repeated at a mean T sk of 30.5°C. For mental stress, each participant performed 1 min of mental arithmetic (Muller et al. 2013; Greaney et al. 2015 b), in which they continuously subtracted ‘7’ from a randomly selected three‐digit number. A new number was provided by an investigator every 10 s. Subjects answered verbally and were encouraged to answer as quickly as possible. At the conclusion of arithmetic, subjects rated their perceived stress using a standard scale (0 = not stressful; 1 = somewhat stressful; 2 = stressful; 3 = very stressful; 4 = very, very stressful) (Callister et al. 1992). Importantly, the SSNA response to mental stress is reproducible within a subject both between successive trials during the same experimental visit as well as between trials during different experimental visits (Muller et al. 2013). During mental stress, acceptable SSNA recordings were maintained in 10 NTN and 10 HTN.
Protocol 2: Cutaneous vascular adrenergic sensitivity
Using a sterile technique, one intradermal microdialysis probe (CMA Linear 30 probe, 6 kDa; Harvard Apparatus, Holliston, MA, USA) was inserted in the dermal layer of the lateral calf, also within the dermatome of peroneal nerve innervation. Sites were perfused with lactated Ringer solution for 60–90 min following probe placement to allow for the resolution of local hyperaemia (Hodges et al. 2009; Lang et al. 2009; Stanhewicz et al. 2013; Greaney et al. 2015 b). As described above, an index of cutaneous blood flow was obtained directly over the microdialysis site during perfusion (2 μl min−1; Hive controller and microinfusion pumps; BASi, West Lafayette, IN, USA) of progressively increasing doses of NE (10−12 to 10−2 mol l−1; Sigma, St Louis, MO, USA) (Wilson et al. 2004; Greaney et al. 2015 b). Brachial BP (Cardiocap; GE Healthcare) was measured every 5 min during the protocol.
Protocol 3: Adrenergic‐ and non‐adrenergic‐dependent contributions to reflex cutaneous vasoconstriction
In a subset of participants (6 NTN, 8 HTN), two microdialysis probes were placed in the lateral calf. Microdialysis fibres were perfused with either lactated Ringer solution (control) or 5 mmol l−1 yohimbine + 1 mmol l−1 propranolol (Y+P; antagonism of α‐ and β‐adrenergic receptors) (USP, Rockville, MD, USA). Following baseline measurements, whole‐body cooling was initiated, as described in Protocol 1, wherein skin blood flow was continuously recorded over each site (Thompson & Kenney, 2004; Smith et al. 2013). Local T sk at each microdialysis site was clamped at 33°C for the duration of the experiment to ensure that changes in cutaneous blood flow were reflex in origin (Lang et al. 2009; Greaney et al. 2015 b). HR was monitored continuously and brachial BP was obtained every 3 min throughout the protocol (Cardiocap; GE Healthcare). At the conclusion of the protocol, exogenous NE (10−6 mmol l−1) was perfused at the Y+P site to test the efficacy of adrenergic receptor blockade, as described previously (Thompson & Kenney, 2004; Lang et al. 2009; Smith et al. 2013).
Pharmacological agents
Pharmacological agents for each intradermal microdialysis protocol were prepared immediately prior to use and dissolved in lactated Ringer solution. Ascorbic acid (Sigma; 1 mg ml−1; 5.7 mmol l−1) was added to NE as a preservative to extend the half‐life (Hughes & Smith, 1978; Thompson & Kenney, 2004; Greaney et al. 2015 b) because prolonged infusion of NE at higher concentrations causes uncoupling and desensitization of G protein‐coupled receptors (Seasholtz et al. 1997; Cabrera‐Wrooman et al. 2010; Akinaga et al. 2013). Local administration of higher concentrations of ascorbate (10 mmol l−1) has been demonstrated to blunt adrenergic‐mediated vasoconstriction in response to local skin cooling in young adults (Yamazaki, 2010) and to improve reflex cutaneous vasodilatation in HTN (Holowatz & Kenney, 2007). However, in pilot testing, cutaneous adrenergic sensitivity was not different between NTN and HTN at sites perfused with NE with and without ascorbic acid. Therefore, the addition of ascorbic acid to NE likely did not contribute to the cutaneous vascular responses to NE in the present study. All solutions were filtered (Acrodisc; Pall, Ann Arbor, MI, USA) and wrapped in foil to prevent degradation as a result of light exposure.
Statistical analysis
All data were recorded at 40–1000 Hz (Powerlab and LabChart; ADInstruments, Bella Vista, NSW, Australia) and subsequently converted into 1 min averages for data analysis. Cutaneous vascular conductance (CVC) was calculated as red blood cell flux (perfusion units; PU) divided by mean arterial pressure and expressed as both a percentage change and absolute change from baseline. As previously described in detail (Hagbarth et al. 1972; Young et al. 2009 b), integrated bursts of SSNA can occur with varying widths and can contain multiple peaks, making the calculation of burst frequency difficult. We therefore quantified total integrated SSNA activity as the sum of the area of all bursts detected within the 1 min period of interest (Greaney et al. 2015 b; Gagnon et al. 2016). To account for differences in microelectrode position within the nerve fascicle, which greatly influences the strength of the integrated signal and cannot be controlled for, SSNA data were analysed as the relative change from the baseline value. Accordingly, a 1 min segment of SSNA at baseline was assigned a value of 100, and 1 min segments during cooling were normalized to and expressed as a percentage of baseline to provide an estimate of relative changes in total activity, which is an analytical methodology consistent with previous studies examining SSNA (Young et al. 2009 a; Muller et al. 2013; Greaney et al. 2015 b; Gagnon et al. 2016). During whole‐body cooling, data were calculated as mean values over an initial thermoneutral baseline and at each 0.5°C decrease in mean T sk. For each mental stress trial (Protocol 1), data were averaged during baseline immediately preceding the onset of the stressor and during the full 1 min of mental arithmetic (Muller et al. 2013). In Protocol 2, data were averaged during baseline and the last min of each NE dose (Wilson et al. 2004; Greaney et al. 2015 b).
Data were analysed using two‐way (group × temperature) or three‐way (group × temperature × condition) mixed model repeated measures ANOVA in SAS, version 9.1.3 (SAS Institute Inc., Cary, NC, USA). When appropriate, post hoc Bonferroni corrections were applied to correct for multiple comparisons. Pearson correlations were used to examine the relation between SSNA and CVC, and linear regression analysis was used to probe group differences in the slope of the SSNA:CVC relation. NE sigmoidal dose–response curves with variable slope were generated using four parameter non‐linear regression modelling in Prism, version 5.0 (GraphPad, San Diego, CA, USA), as previously described in detail (Wenner et al. 2011; Greaney et al. 2015 b). Constraints were set for the top (baseline CVC = 100%) to best fit parameters of the model and differences between groups in the logEC50 (sensitivity) and E max (maximal vasoconstrictor capacity) were analysed using an F test for repeated measures comparisons (Greaney et al. 2015 b), which takes into account all points over the entire curve as opposed to each specific dose (Cook & Bielkiewicz, 1984). Data are reported as the mean ± SE. P < 0.05 was considered statistically significant.
Results
Participants were well‐matched for age, anthropometric characteristics and blood biochemistry (Table 1). By study design, resting screening systolic and diastolic BP and 24 h systolic and diastolic BP were significantly elevated in HTN (P < 0.01). Systolic and diastolic BP were also elevated in HTN at the experimental visits (P < 0.001).
Neurocardiovascular responses to whole‐body cooling
Baseline mean T sk was not different between groups (NTN: 34.2 ± 0.03°C vs. HTN: 34.2 ± 0.08°C; P = 0.85). Cooling decreased mean T sk to 30.5°C in all subjects with no group differences in the rate of cooling (NTN: 0.15 ± 0.01°C min−1 vs. HTN: 0.14 ± 0.01°C min−1; P = 0.21). At thermoneutrality, neither red cell flux (NTN: 9.4 ± 1.6 PU vs. HTN: 8.2 ± 0.4 PU; P = 0.45), nor absolute CVC (NTN: 0.10 ± 0.01 flux mmHg−1 vs. HTN: 0.09 ± 0.04 flux mmHg−1; P = 0.14) were different between groups.
Throughout cooling, HTN exhibited greater increases in SSNA compared to NTN (Fig. 1 A) and this was accompanied by augmented reflex cutaneous vasoconstriction when expressed both as a relative change (Fig. 1 B) and as an absolute change from baseline (NTN: −0.02 ± 0.003 flux mmHg−1 vs. HTN: −0.03 ± 0.003 flux mmHg−1; P = 0.04). Despite a greater range of response, the slope of the ΔSSNA:ΔCVC relation was not different between groups (Fig. 1 C; NTN: −0.167 ± 0.05 vs. HTN: −0.136 ± 0.02; P = 0.56). BP was greater throughout cooling in HTN with no group difference in the change in BP (Table 2). Similarly, although HR was elevated in HTN, the response to cooling was not different (Table 2).
Figure 1. The increase in skin sympathetic outflow and cutaneous vasoconstriction during whole‐body cooling.
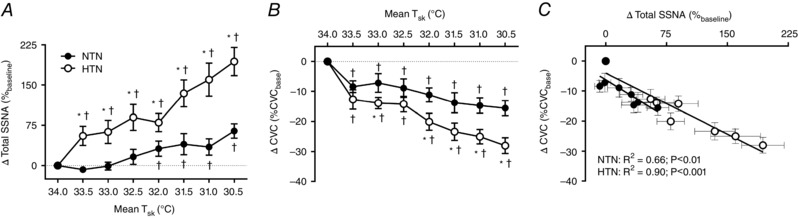
Group summary data for the change in total skin sympathetic nerve activity (ΔTotal SSNA) (A) and the change in cutaneous vascular conductance (ΔCVC) (B) at each 0.5°C decrease in skin temperature (T sk) during whole‐body cooling, as well as the ΔSSNA:ΔCVC relation (C), in normotensive (NTN; filled symbols) and hypertensive adults (HTN; open symbols). * P < 0.05 vs. NTN. † P < 0.05 vs. mean T sk 34°C.
Table 2.
Cardiovascular responses to whole‐body cooling
| Mean T sk 34.0°C | Mean T sk 30.5°C | Δ | |
|---|---|---|---|
| SBP (mmHg) | |||
| NTN | 118 ± 2 | 12 6 ± 2† | 8 ± 2 |
| HTN | 132 ± 4* | 145 ± 4*,† | 13 ± 2 |
| DBP (mmHg) | |||
| NTN | 72 ± 1 | 78 ± 2† | 7 ± 1 |
| HTN | 8 5 ± 3* | 91 ± 3*,† | 7 ± 1 |
| MAP (mmHg) | |||
| NTN | 87 ± 1 | 94 ± 2† | 7 ± 1 |
| HTN | 100 ± 3* | 109 ± 3*,† | 9 ± 1 |
| HR (beats min–1) | |||
| NTN | 59 ± 2 | 59 ± 2 | −1 ± 1 |
| HTN | 66 ± 2* | 66 ± 2* | 0 ± 1 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate. Values are the mean ± SE.
* P < 0.05 vs. NTN.
† P < 0.05 vs. mean T sk 34.0°C.
The reflex SSNA and haemodynamic responses to mental stress are presented in Table 3. SSNA increased during mental stress at a mean T sk of 34.0 and 30.5°C in both groups (P < 0.05) and the magnitude of the SSNA response to mental stress was not different between NTN and HTN at either temperature condition (Table 3). In response to mental stress, the increase in BP was attenuated in HTN (Table 3); however, there was no effect of temperature on either SSNA or cardiovascular responses to mental stress in either group (Table 3). There were no temperature‐ or group‐related differences in perceived stress (NTN: 2 ± 0.2 units at a T sk of 34.0°C vs. 2 ± 0.3 units at a T sk of 30.5°C; HTN: 1 ± 0.3 units at a T sk of 34.0°C vs. 2 ± 0.2 units at a T sk of 30.5°C; P > 0.05 for all comparisons).
Table 3.
Neurocardiovascular responses to mental stress
| Mean T sk 34.0°C | Mean T sk 30.5°C | |
|---|---|---|
| Δ SSNA (%baseline) | ||
| NTN | 316 ± 129 | 235 ± 75 |
| HTN | 312 ± 112 | 161 ± 67 |
| Δ SBP (mmHg) | ||
| NTN | 9 ± 3 | 7 ± 3 |
| HTN | 5 ± 1* | 2 ± 2* |
| Δ DBP (mmHg) | ||
| NTN | 5 ± 2 | 6 ± 2 |
| HTN | 4 ± 1 | 3 ± 2* |
| Δ MAP (mmHg) | ||
| NTN | 7 ± 2 | 6 ± 2 |
| HTN | 4 ± 1* | 2 ± 2* |
| Δ HR (beats min–1) | ||
| NTN | 9 ± 2 | 7 ± 2 |
| HTN | 8 ± 2 | 8 ± 2 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate. Values are the mean ± SE.
* P < 0.05 vs. NTN.
Cutaneous vascular responsiveness to exogenous NE
Baseline CVC was not different between groups (NTN: 0.14 ± 0.02 flux mmHg−1 vs. HTN: 0.23 ± 0.07 flux mmHg−1; P = 0.13). There were no differences in cutaneous vascular adrenergic sensitivity, assessed as the logEC50 of the NE dose–response curve, whether expressed as a percentage change (Fig. 2) (NTN: −7.4 ± 0.3 log M vs. HTN: −7.5 ± 0.3 log M; P = 0.84) or an absolute change from baseline (NTN: −7.7 ± 0.4 log M vs. HTN: −7.4 ± 0.7 log M; P = 0.82). Maximal NE‐induced cutaneous vasoconstriction (10−2 mmol l−1) was greater in HTN adults (NTN: −0.09 ± 0.01 flux mmHg−1 vs. HTN: −0.19 ± 0.03 flux mmHg−1; P = 0.057).
Figure 2. Cutaneous vascular adrenergic sensitivity.
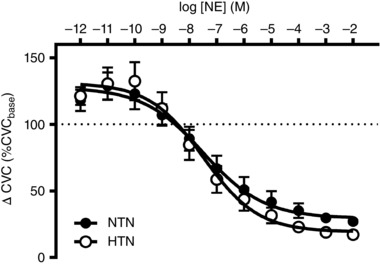
Group summary data for exogenous norepinephrine (NE)‐induced cutaneous vasoconstriction in normotensive (NTN; filled symbols) and hypertensive adults (HTN; open symbols). Data are expressed as percentage change from baseline in cutaneous vascular conductance (ΔCVC).
Reflex cutaneous vasoconstriction during adrenergic blockade
Baseline CVC (Ringer solution site) was again not different between groups (NTN: 0.18 ± 0.04 flux mmHg−1 vs. HTN: 0.17 ± 0.06 flux mmHg−1; P = 0.79). Y+P elicited small, but significant, increases in baseline CVC (NTN: Ringer solution: 0.13 ± 0.01 flux mmHg−1 vs. Y+P: 0.21 ± 0.03 flux mmHg−1; P < 0.01; HTN: Ringer solution: 0.15 ± 0.03 flux mmHg−1 vs. Y+P: 0.33 ± 0.06 flux mmHg−1; P < 0.01), but there were no differences between groups (P = 0.28). Reflex cutaneous vasoconstriction was blunted in HTN at the Y+P site, whereas in NTN, Y+P completely abolished reflex cutaneous vasoconstriction (Fig. 3). The integrity of the adrenergic blockade was confirmed in both groups by the lack of further vasoconstriction in response to 10−6 mmol l−1 exogenous NE (NTN: Δ0.03 ± 0.02 flux mmHg−1; HTN: Δ0.02 ± 0.02 flux mmHg−1).
Figure 3. Reflex cutaneous vasoconstriction during adrenergic receptor antagonism.
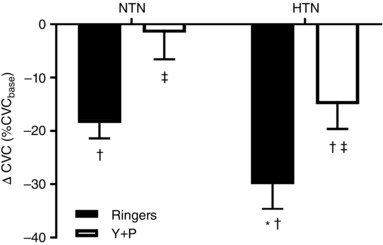
Reflex cutaneous vasoconstriction during whole‐body cooling, expressed as a percentage change from baseline in cutaneous vascular conductance (ΔCVC), at a Ringer solution‐treated microdialysis site (control; filled bars) and a yohimbine+propranolol (Y+P)‐treated microdialysis site (adrenergic blockade; open bars) in normotensive (NTN) and hypertensive adults (HTN). * P < 0.05 v. NTN at the Ringers‐treated site; † P < 0.05 v. baseline (i.e., mean T sk 34°C); ‡ P < 0.05 v. Ringer solution‐treated site.
Discussion
In the present study, we demonstrated that whole‐body cooling elicits greater increases in SSNA and, relatedly, augmented cutaneous vasoconstriction in HTN. Contrary to our hypothesis, cutaneous adrenergic sensitivity was similar between NTN and HTN, suggesting that increased vascular responsiveness to NE does not contribute to the greater reflex vasoconstriction in HTN. Rather, our findings suggest that this is a result of (i) enhanced SSNA and (ii) a greater dependency on non‐adrenergic sympathetic co‐transmitters in mediating the vasoconstrictor response to cold stress in HTN.
In response to whole‐body cooling‐induced decreases in mean T sk (30.5°C), both NTN and HTN exhibited increases in SSNA. The increase in neural outflow directed to the cutaneous vasculature of the innervated dermatome was linearly related to reductions in skin blood flow in both subject groups, reflective of preserved signal transduction (Greaney et al. 2015 b). However, the SSNA response to cooling was three times greater in HTN compared to NTN and comparable to that previously observed in young adults in our laboratory (Greaney et al. 2015 b), as well as that reported in other studies (Sawasaki et al. 2001; Cui et al. 2006). Our findings are also in agreement with exaggerated sympathetic responses to cold stress in rat models of hypertension (Morley et al. 1990; Tkachenko & Kozyreva, 2010) and with reports of sympathetic hyper‐reactivity to cold exposure (albeit indirectly assessed) in human hypertension (Hintsala et al. 2014 b; Hintsala et al. 2016). The results of the present study build on and extend previous findings by demonstrating that the augmented increase in SSNA during cooling in HTN directly correlates with enhanced vasoconstriction in the area of neural innervation. Although the sensitivity of the reflex response (the slope of the linear relation between the change in SSNA and the change in skin blood flow during cooling) was not different between subject groups, the response range of the ΔSSNA:ΔCVC relation was extended in HTN. Accordingly, these findings suggest that greater cooling‐induced reflex vasoconstriction in HTN is mediated by alterations in the neural reflex arc and not by enhanced responsiveness of the vasculature to sympathetic stimuli.
Does the exaggerated SSNA response to cold exposure in HTN simply reflect an augmented generalized neural responsiveness to any sympathoexcitatory stimulus? To address this possibility, we superimposed a non‐thermoregulatory stimulus (mental stress) on whole‐body cooling (Greaney et al. 2015 b). SSNA is highly responsive to arousal stimuli (Wallin & Charkoudian, 2007) and sustained and reproducible increases in SSNA are observed during mental stress (Muller et al. 2013; Greaney et al. 2015 b). We saw similar increases in SSNA during mental stress at thermoneutrality and during cold stress in both subject groups, arguing against the premise that generalized sympathetic hyper‐reactivity explains the exaggerated increase in SSNA during whole‐body cooling in HTN. Although absolute BP remained higher during mental stress in HTN, the pressor response to mental arithmetic was blunted relative to NTN at each temperature condition. Previous results have been equivocal, with reports of both similar (Tsai et al. 2003; Khan et al. 2015) and exaggerated (Kohler et al. 1997; Palatini et al. 2011) BP responsiveness to mental stress in HTN. These disparate findings may be related to the different mental stress paradigms utilized, the large interindividual variability in responsiveness to mental stress (Carter & Goldstein, 2015) or the potential dissociation between neural and BP reactivity to mental stress (Carter & Ray, 2009). Nevertheless, when considered collectively, our findings indicate that generalized sympathetic hyper‐reactivity does not explain greater SSNA responsiveness to cold stress in hypertension.
In response to rapid body cooling, skin vasoconstriction was greater in rats with arterial hypertension compared to normotensive controls (Lomakina et al. 2002); however, because thermoregulatory control of the human cutaneous circulation is unique (Johnson et al. 2014), extrapolation of data from animal models has limited utility. Surprisingly few studies have examined the mechanisms mediating cold stress‐induced cutaneous vasoconstriction in human hypertension. Smith et al. (2013) reported greater adrenergically‐mediated cutaneous vasoconstriction during local cooling of the skin in adults with essential hypertension, suggesting that increased vascular responsiveness to sympathetic adrenergic neurotransmitters may mediate greater vasoconstriction in HTN. In contrast to that hypothesis, we found that cutaneous vascular adrenergic sensitivity, assessed as the logEC50 of the NE dose‐response curve, was not different between groups. Maximal NE‐induced vasoconstriction was greater in HTN; however, this only occurred at supraphysiological doses of NE. Although enhanced α‐adrenergic constriction contributes to increased total peripheral resistance in hypertension via basal vascular smooth muscle cell hyper‐contractility, the present findings are consistent with those reported in isolated human resistance arteries dissected from gluteal skin biopsies (Angus et al. 1992). Because the skin plays little role in BP regulation but rather subserves a thermoregulatory function, the uniqueness of the present findings is perhaps not surprising.
In young adults, NE mediates ∼60% of the vasoconstrictor response during graded whole‐body cooling because selective postsynaptic antagonism of cutaneous adrenergic receptors diminished, but did not eliminate, vasoconstriction (Kellogg et al. 1989; Stephens et al. 2001; Stephens et al. 2004; Thompson & Kenney, 2004). The remainder of the vasoconstrictor response is mediated by non‐adrenergic sympathetic co‐transmitters. NPY and ATP, which are the two most probable candidates, are produced, co‐localized and co‐released with NE from perivascular nerve endings in multiple vascular beds including the skin (Lundberg, 1996; Stephens et al. 2001). In the cutaneous circulation, NPY directly mediates vasoconstriction and also potentiates NE‐mediated vasoconstriction via postsynaptic Y1 receptors (Racchi et al. 1999; Stephens et al. 2004), whereas ATP mediates vasoconstriction via P2X receptors (Flavahan & Vanhoutte, 1986; Ralevic & Dunn, 2015). NPY release appears to be increased in general in hypertension, specifically in the cutaneous circulation (Kahan et al. 1992; Han et al. 1998). Interestingly, higher stimulation frequencies are required to facilitate the release of the large dense core vesicles that contain sympathetic co‐transmitters (Sawasaki et al. 2001). Therefore, it is plausible to suggest that the substantially greater increases in efferent SSNA during cooling in HTN compared to NTN reflect a greater relative contribution of sympathetic co‐transmitters to reflex cutaneous vasoconstriction in hypertension. To address this possibility, reflex vasoconstriction was assessed in a subset of participants during concurrent cutaneous vascular adrenergic receptor antagonism. In NTN, adrenergic receptor antagonism abolished reflex cutaneous vasoconstriction, a finding consistent with that reported in healthy older (69 ± 2 years) individuals (Thompson & Kenney, 2004). By contrast, in HTN, adrenergic receptor antagonism blunted, but did not eliminate, the vasoconstrictor response to cooling, suggesting that non‐adrenergic co‐transmitters mediate a larger portion of reflex cutaneous vasoconstriction in hypertension.
Both BP and HR were elevated in HTN at thermoneutrality and remained higher throughout cooling. Despite our finding that the SSNA and end‐organ responses to whole‐body cooling were exaggerated in HTN, the BP and HR responses to cooling‐induced reductions in T sk were similar between groups, results consistent with the few previous studies examining the haemodynamic responses to whole‐body cooling in hypertension (Hintsala et al. 2014 a; Hintsala et al. 2014 b; Hintsala et al. 2016). It is possible that a more severe cooling stimulus, including reductions in core temperature, would elicit a greater pressor response in HTN, thus contributing to increased cardiovascular risk; this warrants future examination. Our laboratory recently examined the sympathetic control of reflex vasoconstriction in healthy older (57 ± 2 years) compared to young adults, reporting that blunted increases in SSNA directly contribute to age‐related impairments in cutaneous vasoconstriction (Greaney et al. 2015 b). The increases in SSNA reported in NTN in the present study (+65%baseline) are remarkably consistent with the increases demonstrated previously (+51%baseline), further validating our assessment of SSNA as a means of quantifying sympathetic control of cutaneous vasoconstriction during cooling.
Limitations
The mechanisms mediating cutaneous vascular function likely differ between glabrous and non‐glabrous skin (Johnson et al. 2014). During cooling, CVC was measured on the dorsum of the foot (mixed glabrous/non‐glabrous skin) in Protocol 1 and on the lateral calf (non‐glabrous skin) in Protocol 3; the lateral calf was utilized for intradermal microdialysis protocols because it provided a larger surface area for fibre insertion and is also within the area of neural innervation of the peroneal nerve. Although the specific mechanistic mediators of cutaneous vasoconstriction may differ between these two anatomical locations, reflex vasoconstriction was greater in HTN during both protocols, indicating significant functional alterations in neurovascular function during cold exposure in hypertension. In addition, we were unable to delineate the specific non‐adrenergic co‐transmitters involved in reflex vasoconstriction, nor was it possible to definitively establish the degree to which receptor number or downstream co‐transmitter signal transduction mechanisms contributed to the altered responses observed in hypertension. Moreover, we cannot exclude a role for alterations in second‐messenger signalling pathways in contributing to the enhanced reflex cutaneous vasoconstrictor response in hypertension. For example, the Rho‐kinase (ROCK) pathway is a proconstrictor mechanism that mediates reflex vasoconstriction during whole‐body cooling (Lang et al. 2009). ROCK activity is upregulated in hypertension (Seko et al. 2003) and ROCK‐dependent vasoconstriction in response to local cooling is increased in HTN (Smith et al. 2013), making it a likely candidate. Nonetheless, it is clear that non‐adrenergic sympathetic co‐transmitters play a substantial role in mediating the full reflex cutaneous vasoconstrictor response to cold exposure in HTN adults.
Perspectives
There is increasing evidence suggesting that dysregulated sympathetic control of vascular function contributes to cardiovascular disease development in hypertension (Grassi & Ram, 2016). Thus, understanding the neural mechanisms mediating reflex control of peripheral vasoconstriction during cold stress in HTN may provide novel insights into hypertension‐associated neurovascular dysfunction and its pathological sequelae. Although hypertension magnifies cold exposure‐related cardiovascular risk (Woodhouse et al. 1993; Minami et al. 1996), the evidence reported in the present study suggests that, in the context of thermoregulation and the maintenance of core temperature during cold stress, sympathetically‐mediated control of the cutaneous vasculature is not only preserved in HTN, but also exaggerated. The greater range of responsiveness of sympathetic reflex control of cutaneous vasoconstriction is mediated by both adrenergic and non‐adrenergic neurotransmitters, a functional mechanism of peripheral vasoconstriction noted in young, but not older, adults (Stephens et al. 2001; Stephens et al. 2004; Thompson & Kenney, 2004). Future studies designed to pharmacologically target cutaneous vascular signalling mechanisms, including specific non‐adrenergic sympathetic co‐transmitters, as well as downstream second‐messenger pathways (e.g. ROCK, angiotensin II), are warranted.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
JLG, WLK and LMA contributed to the conception and design of the research. JLG contributed to data acquisition and analysis. JLG, WLK and LMA contributed to data interpretation. JLG drafted the manuscript. JLG, WLK and LMA critically revised the manuscript. All authors approved the final version of the manuscript, agree to be accountable for all aspects of the work, and qualify for authorship. This study was conducted in the Department of Kinesiology at The Pennsylvania State University.
Funding
This work was supported by National Institutes of Health grant HL093238 (LMA). J. L. Greaney was supported by a postdoctoral fellowship from the National Institutes of Health (F32 HL120471).
Acknowledgements
We greatly appreciate the effort expended by the volunteer participants. We also thank Susan Slimak, R.N., Anna Stanhewicz, Ph.D., and Jane Pierzga, M.S. for their laboratory assistance.
References
- Akinaga J, Lima V, Kiguti LR, Hebeler‐Barbosa F, Alcantara‐Hernandez R, Garcia‐Sainz JA & Pupo AS (2013). Differential phosphorylation, desensitization, and internalization of alpha1A‐adrenoceptors activated by norepinephrine and oxymetazoline. Mol Pharmacol 83, 870–881. [DOI] [PubMed] [Google Scholar]
- Angus JA, Jennings GL & Sudhir K (1992). Enhanced contraction to noradrenaline, serotonin and nerve stimulation but normal endothelium‐derived relaxing factor response in skin small arteries in human primary hypertension. Clin Exp Pharmacol Physiol Suppl 19, 39–47. [DOI] [PubMed] [Google Scholar]
- Cabrera‐Wrooman A, Romero‐Avila MT & Garcia‐Sainz JA (2010). Roles of the alpha1A‐adrenergic receptor carboxyl tail in protein kinase C‐induced phosphorylation and desensitization. Naunyn Schmiedebergs Arch Pharmacol 382, 499–510. [DOI] [PubMed] [Google Scholar]
- Callister R, Suwarno NO & Seals DR (1992). Sympathetic activity is influenced by task difficulty and stress perception during mental challenge in humans. J Physiol 454, 373–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardillo C & Panza JA (1998). Impaired endothelial regulation of vascular tone in patients with systemic arterial hypertension. Vasc Med 3, 138–144. [DOI] [PubMed] [Google Scholar]
- Carter JR & Goldstein DS (2015). Sympathoneural and adrenomedullary responses to mental stress. Compr Physiol 5, 119–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JR & Ray CA (2009). Sympathetic neural responses to mental stress: responders, nonresponders and sex differences. Am J Physiol Heart Circ Physiol 296, H847–H853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian N (2010). Mechanisms and modifiers of reflex induced cutaneous vasodilation and vasoconstriction in humans. J Appl Physiol (1985) 109, 1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr. , Jones DW, Materson BJ, Oparil S, Wright JT, Jr. , Roccella EJ, Joint National Committee on Prevention DE, Treatment of High Blood Pressure , National Heart L, Blood I & National High Blood Pressure Education Program Coordinating C (2003). Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42, 1206–1252. [DOI] [PubMed] [Google Scholar]
- Cook DA & Bielkiewicz B (1984). A computer‐assisted technique for analysis and comparison of dose‐response curves. J Pharmacol Methods 11, 77–89. [DOI] [PubMed] [Google Scholar]
- Cui J, Sathishkumar M, Wilson TE, Shibasaki M, Davis SL & Crandall CG (2006). Spectral characteristics of skin sympathetic nerve activity in heat‐stressed humans. Am J Physiol Heart Circ Physiol 290, H1601–H1609. [DOI] [PubMed] [Google Scholar]
- Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ & Farquhar WB (2010). Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 299, H1318–H1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavahan NA & Vanhoutte PM (1986). Sympathetic purinergic vasoconstriction and thermosensitivity in a canine cutaneous vein. J Pharmacol Exp Ther 239, 784–789. [PubMed] [Google Scholar]
- Gagnon D, Romero SA, Ngo H, Poh PY & Crandall CG (2016). Plasma hyperosmolality attenuates skin sympathetic nerve activity during passive heat stress in humans. J Physiol 594, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G & Ram VS (2016). Evidence for a critical role of the sympathetic nervous system in hypertension. J Am Soc Hypertens 10, 457–466. [DOI] [PubMed] [Google Scholar]
- Greaney JL, Alexander LM & Kenney WL (2015. a). Sympathetic control of reflex cutaneous vasoconstriction in human aging. J Appl Physiol (1985) 119, 771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney JL, Matthews EL, Boggs ME, Edwards DG, Duncan RL & Farquhar WB (2014). Exaggerated exercise pressor reflex in adults with moderately elevated systolic blood pressure: role of purinergic receptors. Am J Physiol Heart Circ Physiol 306, H132–H141. [DOI] [PubMed] [Google Scholar]
- Greaney JL, Stanhewicz AE, Kenney WL & Alexander LM (2015. b). Impaired increases in skin sympathetic nerve activity contribute to age‐related decrements in reflex cutaneous vasoconstriction. J Physiol 593, 2199–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth KE, Hallin RG, Hongell A, Torebjork HE & Wallin BG (1972). General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand 84, 164–176. [DOI] [PubMed] [Google Scholar]
- Han S, Chen X, Cox B, Yang CL, Wu YM, Naes L & Westfall T (1998). Role of neuropeptide Y in cold stress‐induced hypertension. Peptides 19, 351–358. [DOI] [PubMed] [Google Scholar]
- Hintsala H, Kandelberg A, Herzig KH, Rintamaki H, Mantysaari M, Rantala A, Antikainen R, Keinanen‐Kiukaanniemi S, Jaakkola JJ & Ikaheimo TM (2014. a). Central aortic blood pressure of hypertensive men during short‐term cold exposure. Am J Hypertens 27, 656–664. [DOI] [PubMed] [Google Scholar]
- Hintsala H, Kentta TV, Tulppo M, Kiviniemi A, Huikuri HV, Mantysaari M, Keinanen‐Kiukaannemi S, Bloigu R, Herzig KH, Antikainen R, Rintamaki H, Jaakkola JJ & Ikaheimo TM (2014. b). Cardiac repolarization and autonomic regulation during short‐term cold exposure in hypertensive men: an experimental study. PLoS ONE 9, e99973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintsala HE, Kiviniemi AM, Tulppo MP, Helakari H, Rintamaki H, Mantysaari M, Herzig KH, Keinanen‐Kiukaanniemi S, Jaakkola JJ & Ikaheimo TM (2016). Hypertension does not alter the increase in cardiac baroreflex sensitivity caused by moderate cold exposure. Front Physiol 7, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges GJ, Chiu C, Kosiba WA, Zhao K & Johnson JM (2009). The effect of microdialysis needle trauma on cutaneous vascular responses in humans. J Appl Physiol (1985) 106, 1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowatz LA & Kenney WL (2007). Local ascorbate administration augments NO‐ and non‐NO‐dependent reflex cutaneous vasodilation in hypertensive humans. Am J Physiol Heart Circ Physiol 293, H1090–H1096. [DOI] [PubMed] [Google Scholar]
- Holowatz LA & Kenney WL (2010). Peripheral mechanisms of thermoregulatory control of skin blood flow in aged humans. J Appl Physiol (1985) 109, 1538–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes IE & Smith JA. (1978). The stability of noradrenaline in physiological saline solutions. J Pharm Pharmacol 30, 124–126. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Minson CT & Kellogg DL, Jr. (2014). Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation. Compr Physiol 4, 33–89. [DOI] [PubMed] [Google Scholar]
- Kahan T, Taddei S, Pedrinelli R, Hjemdahl P & Salvetti A (1992). Nonadrenergic sympathetic vascular control of the human forearm in hypertension: possible involvement of neuropeptide Y. J Cardiovasc Pharmacol 19, 587–592. [DOI] [PubMed] [Google Scholar]
- Kellogg DL Jr, Johnson JM & Kosiba WA (1989). Selective abolition of adrenergic vasoconstrictor responses in skin by local iontophoresis of bretylium. Am J Physiol 257, H1599–H1606. [DOI] [PubMed] [Google Scholar]
- Khan SG, Geer A, Fok HW, Shabeeh H, Brett SE, Shah AM & Chowienczyk PJ (2015). Impaired neuronal nitric oxide synthase‐mediated vasodilator responses to mental stress in essential hypertension. Hypertension 65, 903–909. [DOI] [PubMed] [Google Scholar]
- Kikuya M, Hansen TW, Thijs L, Bjorklund‐Bodegard K, Kuznetsova T, Ohkubo T, Richart T, Torp‐Pedersen C, Lind L, Ibsen H, Imai Y, Staessen JA & International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes I (2007). Diagnostic thresholds for ambulatory blood pressure monitoring based on 10‐year cardiovascular risk. Circulation 115, 2145–2152. [DOI] [PubMed] [Google Scholar]
- Kohler T, Fricke M, Ritz T & Scherbaum N (1997). Psychophysiological reactivity of borderline hypertensives and their recovery after mental stress. Psychother Psychosom 66, 261–267. [DOI] [PubMed] [Google Scholar]
- Lang JA, Jennings JD, Holowatz LA & Kenney WL (2009). Reflex vasoconstriction in aged human skin increasingly relies on Rho kinase‐dependent mechanisms during whole body cooling. Am J Physiol Heart Circ Physiol 297, H1792–H1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yavar Z & Sun Q (2015). Cardiovascular response to thermoregulatory challenges. Am J Physiol Heart Circ Physiol 309, H1793–H1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomakina SV, Tkachenko EY & Kozyreva TV (2002). Thermoregulatory reactions to cooling in rats with hereditary arterial hypertension. Bull Exp Biol Med 134, 432–435. [DOI] [PubMed] [Google Scholar]
- Lundberg JM (1996). Pharmacology of cotransmission in the autonomic nervous system: integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol Rev 48, 113–178. [PubMed] [Google Scholar]
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F & Task Force M (2013). 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 31, 1281–1357. [DOI] [PubMed] [Google Scholar]
- Minami J, Kawano Y, Ishimitsu T, Yoshimi H & Takishita S (1996). Seasonal variations in office, home and 24 h ambulatory blood pressure in patients with essential hypertension. J Hypertens 14, 1421–1425. [DOI] [PubMed] [Google Scholar]
- Morley RM, Conn CA, Kluger MJ & Vander AJ (1990). Temperature regulation in biotelemetered spontaneously hypertensive rats. Am J Physiol 258, R1064–1069. [DOI] [PubMed] [Google Scholar]
- Muller MD, Sauder CL & Ray CA (2013). Mental Stress Elicits Sustained and Reproducible Increases in Skin Sympathetic Nerve Activity. Physiol Rep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y & European Society of Hypertension Working Group on Blood Pressure M (2013). European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 31, 1731–1768. [DOI] [PubMed] [Google Scholar]
- Palatini P, Bratti P, Palomba D, Bonso E, Saladini F, Benetti E & Casiglia E (2011). BP reactivity to public speaking in stage 1 hypertension: influence of different task scenarios. Blood Press 20, 290–295. [DOI] [PubMed] [Google Scholar]
- Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ, Subcommittee of P & Public Education of the American Heart Association Council on High Blood Pressure R (2005). Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 45, 142–161. [DOI] [PubMed] [Google Scholar]
- Racchi H, Irarrazabal MJ, Howard M, Moran S, Zalaquett R & Huidobro‐Toro JP (1999). Adenosine 5‧‐triphosphate and neuropeptide Y are co‐transmitters in conjunction with noradrenaline in the human saphenous vein. Br J Pharmacol 126, 1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V & Dunn WR (2015). Purinergic transmission in blood vessels. Auton Neurosci 191, 48–66. [DOI] [PubMed] [Google Scholar]
- Sawasaki N, Iwase S & Mano T (2001). Effect of skin sympathetic response to local or systemic cold exposure on thermoregulatory functions in humans. Auton Neurosci 87, 274–281. [DOI] [PubMed] [Google Scholar]
- Seasholtz TM, Gurdal H, Wang HY, Johnson MD & Friedman E (1997). Desensitization of norepinephrine receptor function is associated with G protein uncoupling in the rat aorta. Am J Physiol 273, H279–H285. [DOI] [PubMed] [Google Scholar]
- Seko T, Ito M, Kureishi Y, Okamoto R, Moriki N, Onishi K, Isaka N, Hartshorne DJ & Nakano T (2003). Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ Res 92, 411–418. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Santhanam L & Alexander LM (2013). Rho‐Kinase activity and cutaneous vasoconstriction is upregulated in essential hypertensive humans. Microvasc Res 87, 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhewicz AE, Alexander LM & Kenney WL (2013). Oral sapropterin augments reflex vasoconstriction in aged human skin through noradrenergic mechanisms. J Appl Physiol (1985) 115, 1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DP, Aoki K, Kosiba WA & Johnson JM (2001). Nonnoradrenergic mechanism of reflex cutaneous vasoconstriction in men. Am J Physiol Heart Circ Physiol 280, H1496–H1504. [DOI] [PubMed] [Google Scholar]
- Stephens DP, Saad AR, Bennett LA, Kosiba WA & Johnson JM (2004). Neuropeptide Y antagonism reduces reflex cutaneous vasoconstriction in humans. Am J Physiol Heart Circ Physiol 287, H1404–H1409. [DOI] [PubMed] [Google Scholar]
- Thompson‐Torgerson CS, Holowatz LA & Kenney WL (2008). Altered mechanisms of thermoregulatory vasoconstriction in aged human skin. Exerc Sport Sci Rev 36, 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CS & Kenney WL (2004). Altered neurotransmitter control of reflex vasoconstriction in aged human skin. J Physiol 558, 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachenko EY & Kozyreva TV (2010). Mechanisms of modulation of thermoregulatory reactions during cooling in hypertensive rats by the sympathetic nervous system. Bull Exp Biol Med 149, 21–25. [DOI] [PubMed] [Google Scholar]
- Tsai PS, Yucha CB, Nichols WW & Yarandi H (2003). Hemodynamics and arterial properties in response to mental stress in individuals with mild hypertension. Psychosom Med 65, 613–619. [DOI] [PubMed] [Google Scholar]
- Wallin BG & Charkoudian N (2007). Sympathetic neural control of integrated cardiovascular function: insights from measurement of human sympathetic nerve activity. Muscle Nerve 36, 595–614. [DOI] [PubMed] [Google Scholar]
- Wenner MM, Wilson TE, Davis SL & Stachenfeld NS (2011). Pharmacological curve fitting to analyze cutaneous adrenergic responses. J Appl Physiol (1985) 111, 1703–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Monahan KD, Short DS & Ray CA (2004). Effect of age on cutaneous vasoconstrictor responses to norepinephrine in humans. Am J Physiol Regul Integr Comp Physiol 287, R1230–R1234. [DOI] [PubMed] [Google Scholar]
- Woodhouse PR, Khaw KT & Plummer M (1993). Seasonal variation of blood pressure and its relationship to ambient temperature in an elderly population. J Hypertens 11, 1267–1274. [PubMed] [Google Scholar]
- Writing Group M, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd , Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics C & Stroke Statistics S (2016). Heart disease and stroke statistics – 2016 update: a report from the American Heart Association. Circulation 133, e38–e360. [DOI] [PubMed] [Google Scholar]
- Yamazaki F (2010). Local ascorbate administration inhibits the adrenergic vasoconstrictor response to local cooling in the human skin. J Appl Physiol (1985) 108, 328–333. [DOI] [PubMed] [Google Scholar]
- Young CN, Fisher JP, Gallagher KM, Whaley‐Connell A, Chaudhary K, Victor RG, Thomas GD & Fadel PJ (2009. a). Inhibition of nitric oxide synthase evokes central sympatho‐excitation in healthy humans. J Physiol 587, 4977–4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CN, Keller DM, Crandall CG & Fadel PJ (2009. b). Comparing resting skin sympathetic nerve activity between groups: caution needed. J Appl Physiol (1985) 106, 1751–1752; author reply 1753. [DOI] [PubMed] [Google Scholar]


