Abstract
Key points
In the adult turtle spinal cord, action potential generation in motoneurones is inhibited by spillover of serotonin to extrasynaptic serotonin 1A (5‐HT1A) receptors at the axon initial segment. We explored whether ingestion of the 5‐HT1A receptor partial agonist, buspirone, decreases motoneurone excitability in humans.
Following ingestion of buspirone, two tests of motoneurone excitability showed decreases. F‐wave areas and persistence in an intrinsic muscle of the hand were reduced, as was the area of cervicomedullary motor evoked potentials in biceps brachii.
Our findings suggest that activation of 5‐HT1A receptors depresses human motoneurone excitability. Such a depression could contribute to decreased motoneurone output during fatiguing exercise if there is high serotonergic drive to the motoneurones.
Abstract
Intense serotonergic drive in the turtle spinal cord results in serotonin spillover to the axon initial segment of the motoneurones where it activates serotonin 1A (5‐HT1A) receptors and inhibits generation of action potentials. We examined whether activation of 5‐HT1A receptors decreases motoneurone excitability in humans by determining the effects of a 5‐HT1A receptor partial agonist, buspirone, on F waves and cervicomedullary motor evoked potentials (CMEPs). In a placebo‐controlled double‐blind study, 10 participants were tested on two occasions where either placebo or 20 mg of buspirone was administered orally. The ulnar nerve was stimulated supramaximally to evoke F waves in abductor digiti minimi (ADM). CMEPs and the maximal M wave were elicited in biceps brachii by cervicomedullary stimulation and brachial plexus stimulation, respectively. Following buspirone intake, F‐wave area and persistence, as well as CMEP area, were significantly decreased. The mean post‐pill difference in normalized F‐wave areas and persistence between buspirone and placebo days was –27% (–42, –12; 95% confidence interval) and –9% (–16, –2), respectively. The mean post‐pill difference in normalized CMEP area between buspirone and placebo days showed greater variation and was –31% (–60, –2). In conclusion, buspirone reduces motoneurone excitability in humans probably via activation of 5‐HT1A receptors at the axon initial segment. This has implications for motor output during high drive to the motoneurones when serotonin may spill over to these inhibitory receptors and consequently inhibit motoneurone output. Such a mechanism could potentially contribute to fatigue with exercise.
Keywords: 5HT1A receptors, central fatigue, cervicomedullary motor evoked potentials, F waves, motoneurones, muscle fatigue, serotonin
Key points
In the adult turtle spinal cord, action potential generation in motoneurones is inhibited by spillover of serotonin to extrasynaptic serotonin 1A (5‐HT1A) receptors at the axon initial segment. We explored whether ingestion of the 5‐HT1A receptor partial agonist, buspirone, decreases motoneurone excitability in humans.
Following ingestion of buspirone, two tests of motoneurone excitability showed decreases. F‐wave areas and persistence in an intrinsic muscle of the hand were reduced, as was the area of cervicomedullary motor evoked potentials in biceps brachii.
Our findings suggest that activation of 5‐HT1A receptors depresses human motoneurone excitability. Such a depression could contribute to decreased motoneurone output during fatiguing exercise if there is high serotonergic drive to the motoneurones.
Abbreviations
- 5‐HT
serotonin
- 5‐HT1A
serotonin 1A receptor subtype
- ADM
abductor digiti mini
- CI
confidence interval
- CMEP
cervicomedullary motor evoked potential
- EMG
electromyography
- FDI
first dorsal interosseous
- Mmax
maximal compound muscle action potential
Introduction
Serotonin (5‐HT) is a neuromodulator that can alter motoneurone output in humans and animal models. Spinal motoneurones receive direct, dense serotonergic innervation of the soma and dendrites from neurones with cell bodies in the medullary raphe nuclei (Carlsson et al. 1963; Carlsson et al. 1964; Steinbusch 1981; Kiehn et al. 1992; Alvarez et al. 1998; Schmidt & Jordan, 2000; Hornung, 2003). Increased firing of serotonergic raphe neurons suggests that the release of 5‐HT onto motoneurones increases during periods of motor activity (Fornal et al. 1985; Veasey et al. 1995; Jacobs & Fornal, 1997; Jacobs et al. 2002). In various animal models and in humans, 5‐HT has been shown to have excitatory effects on intrinsic motoneurone excitability via the activation of G‐q coupled 5‐HT2B/C receptors that are distributed on the dendrites and the soma of the motoneurones (Perrier & Hounsgaard, 2003; Harvey et al. 2006 a; Harvey et al. 2006 b; D'Amico et al. 2013 b). However, in some animal models, 5‐HT also directly inhibits motoneurone excitability via G‐i coupled 5‐HT1A receptors located at the axon initial segment (Innis et al. 1988; Jackson & White, 1990; Pennington & Kelly, 1990; Cotel et al. 2013). The axon initial segment is the site of action potential generation as a result of the high density of voltage‐gated sodium channels that act to lower the threshold for action potential initiation (Coombs et al. 1957; Duflocq et al. 2008; Duflocq et al. 2011). Activation of 5‐HT1A receptors at the axon initial segment inhibits the sodium current responsible for spike initiation, with a consequent decrease in motoneurone output (Cotel et al. 2013).
It is difficult to discern the functional implications of serotonin release on motoneurone excitability given the direct opposing actions of 5‐HT at the motoneurones. However, recent work in the adult turtle spinal cord has shed light on the mechanisms that allow dual modulation of motoneurone excitability by 5‐HT. In the adult turtle spinal cord, brief (∼ 1 s) stimulation of the serotonergic dorsolateral funiculus tract produced excitation of the motoneurones through activation of 5‐HT2B/C receptors, despite the higher affinity of 5‐HT for inhibitory 5‐HT1A receptors (Cotel et al. 2013). Unlike motoneurone excitation via the 5‐HT2B/C receptors, which are in close proximity to serotonergic synapses, 5‐HT1A mediated inhibition only occurred during prolonged stimulation of the dorsolateral funiculus (3 min) and appears to depend on the spillover of 5‐HT to the axon initial segment, which does not receive serotonergic innervation (Cotel et al. 2013; Montague et al. 2013; Maratta et al. 2015). Therefore, it appears that serotonergic inhibition of the motoneurones only occurs during high serotonergic drive to the motoneurones, whereas overall excitation occurs at lower levels of drive. This novel finding has identified these 5‐HT1A receptors, which are present in the human spinal cord (Laporte et al. 1996), as a potential contributor to central fatigue (Cotel et al. 2013). Central fatigue represents the contribution of the nervous system to muscle fatigue (Gandevia, 2001). That is, the progressive decline in the ability to produce voluntary muscle force as exercise continues. In humans, large reductions in the excitability and firing rates of motoneurones occur during and following fatiguing maximal contractions (Bellemare et al. 1983; Bigland‐Ritchie et al. 1983; Gandevia et al. 1999; Butler et al. 2003; Khan et al. 2012). The proposition that findings in the adult turtle spinal cord translate to effects on human motor performance relies on two assumptions: (i) activation of 5‐HT1A receptors located on human motoneurones depresses motoneurone output and (ii) there is a high level of serotonergic drive to the motoneurones during fatiguing, maximal contractions. In the present study, we aimed to confirm the first assumption, that is, whether activation of 5‐HT1A receptors with the selective partial agonist, buspirone (Eison & Temple, 1986; Mahmood & Sahajwalla, 1999; Loane & Politis, 2012), depresses motoneurone excitability in healthy individuals. An abstract of our results has been published previously (D'Amico et al. 2015).
Methods
In a double‐blind, placebo‐controlled study, participants attended the laboratory on two days to determine the effects of buspirone (a 5‐HT1A receptor partial agonist) (Eison & Temple, 1986; Mahmood & Sahajwalla, 1999; Loane & Politis, 2012) on motoneurone excitability tested with F waves and cervicomedullary motor evoked potentials (CMEPs).
Participants and ethics
All experiments were approved by the Human Research Ethics Committee at the University of New South Wales and conformed with the Declaration of Helsinki. All participants provided their written, informed consent prior to participating in the study. Participants visited the laboratory on a screening/familiarization day to ensure that responses could be elicited in the muscles of interest by cervicomedullary stimulation and to determine whether the different types of electrical stimulation were tolerated. During this visit, a medical screening questionnaire was carried out to ensure participant safety and eligibility. Following this initial visit, two individuals were not eligible to participate because CMEPs could not be elicited. In total, 10 healthy individuals participated in the study (age 33 ± 12 years, four females). All participants received remuneration for their time.
Drug administration
In experimental sessions, participants received either placebo or a 20 mg dose of the anxiolytic drug, buspirone, which was administered orally. The usual therapeutic dose of buspirone is 20–30 mg daily in two to three divided doses (Eison & Temple, 1986). All participants were not taking buspirone or any other medication. The drug and placebo were housed in a capsule to blind participants and the experimenters. The order of drug delivery was randomized by one of the investigators who did not take part in the experimental sessions or initial data analysis. Participants were asked to report any side effects during both visits to the laboratory.
Set‐up
In each session, participants sat with the right arm resting on a table. The hand was pronated and held in place with Velcro straps across the fingers, the wrist and the forearm. Participants’ fingers were loosely taped together to prevent a change in finger position. Additionally, the elbow and proximal forearm were clamped in place by foam blocks to prevent movement of the forearm during brachial plexus stimulation.
Surface electromyography (EMG) recordings were obtained from right biceps brachii (biceps), abductor digiti minimi (ADM) and first dorsal interosseus (FDI) muscles. EMG signals from the biceps were recorded through adhesive Ag‐AgCl electrodes (Cleartrace; ConMed Corporation, Utica, NY, USA) placed over the motor point and distal tendon. EMG signals from ADM and FDI were obtained through adhesive EMG Triode electrodes (T3402M; Thought Technology Ltd, Montréal‐Ouest, QC, Canada) placed over the muscle belly and the fifth and second metacarpo‐phalangeal joints, respectively. EMG signals were amplified (× 300) and bandpass filtered (16–1000 Hz). In addition, highly filtered (200–1000 Hz) and amplified (× 3000) ADM and FDI EMG signals were collected in separate channels for identification and measurement of F waves (Fig. 1) (Khan et al. 2012). All EMG signals were processed using CED1902 amplifiers (Cambridge Electronic Design, Cambridge, UK) and sampled at 5 kHz using a 16‐bit A/D converter (CED1401) and Spike 2, version 7 (Cambridge Electronic Design).
Figure 1. Measurement of F waves.
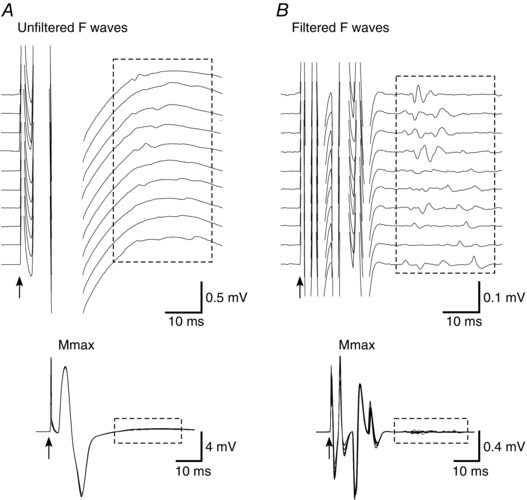
Raw traces of the same ADM F waves collected using 16–1000 Hz filtering (A) and 200–1000 Hz filtering (B) (highly‐filtered). Top: raster of 10 consecutive F‐wave traces. Bottom: superimposed traces and maximal M waves. The boxed rectangles encompass the F waves.
Stimulation
Three types of stimulation were used in the present study and these were all performed at rest. These included stimulation of the corticospinal tract axons at the level of the cervicomedullary junction, stimulation of the brachial plexus and stimulation of the ulnar nerve. All types of stimulation were well tolerated, albeit uncomfortable.
Cervicomedullary stimulation
Corticospinal tract axons were stimulated by single electrical pulses (0.2 ms pulse width) delivered by a constant current stimulator (DS7AH; Digitimer, Welwyn Garden City, UK) through two surface electrodes positioned posterior and superior to the tips of the mastoid processes. The cathode was placed on the left to reduce the likelihood of spread of the stimulus to the ventral roots supplying the right biceps. Responses were recorded from the biceps and the stimulus intensity (105–240 mA) was adjusted to evoke a CMEP with an amplitude of ∼10% of the maximal M wave.
Brachial plexus stimulation
Maximal M waves were evoked in biceps by electrical stimulation of the brachial plexus through surface electrodes positioned in the supraclavicular fossa (Erb's point: cathode) and on the acromion (anode) (DS7AH constant current stimulator; pulse width 0.2 ms). Stimulus intensity was set 20% above the level required to produce a maximal compound muscle action potential (M max) in the biceps (24–160 mA).
Ulnar nerve stimulation
To evoke F waves in the ADM, supramaximal electrical stimuli were delivered to the ulnar nerve at the wrist (DS7AH constant current stimulator; pulse width 0.2 ms). Electrodes were placed ∼2 cm apart over the nerve with the cathode positioned distal to the anode and ∼2 cm proximal to the wrist. Stimulus intensity was set at 20% above the level required to produce a maximal compound muscle action potential in ADM (30–54 mA). Potentials were also recorded from ulnar‐innervated FDI, although supramaximal stimulation for this muscle was not ensured. If all motor axons were not stimulated, then the possibility cannot be ruled out that some component of the response at F‐wave latency could occur via activation of motoneurones through the synaptic influence of Ia afferent input. During the present study, stimuli were delivered in sets of 30 at a rate of 2 Hz.
Protocol
Participants were tested on two occasions separated by at least one week. Once the experimental set‐up was complete, baseline measurements of F waves, CMEPs and M max were obtained. In each measurement set, participants received two blocks of 30 ulnar nerve stimuli (2 Hz) with a 1 min break between blocks. After a brief break, these were followed by five cervicomedullary stimuli (0.1 Hz) and then two brachial plexus stimuli (0.1 Hz). Up to five baseline measurement sets were performed at 10 min intervals. If CMEPs were not close to the target size, stimulus intensity was adjusted and two or three sets at the new intensity were performed. If CMEPs showed a progressive increase or decrease across sets, a fourth set was performed and the first set discarded from the analysis. After pill intake, measurements resumed at 25 min post intake and again every 10 min up to 105 min post‐pill intake. This timing was chosen to ensure that measurements were performed at peak plasma levels of buspirone, which typically occurs between 50 and 90 min following ingestion (Mahmood & Sahajwalla, 1999; Loane & Politis, 2012). By completion of the experiment, eight of the 10 participants had fully recovered from any side effects and motoneurone excitability had recovered.
Data analysis
All data analysis was performed offline using Signal, version 4 (Cambridge Electronic Design) and Excel (Microsoft Corp., Redmond, WA, USA). Root mean square values were calculated for 100 ms of the EMG signal prior to each stimulus. The areas of the compound muscle action potentials (F waves, M waves and CMEPs) were calculated using built‐in software functions. F waves in ADM and FDI were measured from the highly filtered channels (Khan et al. 2012). Their areas were first normalized to their corresponding M max, also from the highly filtered channel, and then averaged over the 60 sweeps for each time point. F‐wave persistence (%) for ADM and FDI was calculated as the percentage of stimuli (out of 60) that evoked an F wave greater than 20 μV in amplitude (Khan et al. 2012). To determine changes in M‐wave areas in ADM and FDI, maximal M‐wave areas were measured from the non‐highly‐filtered channels and averaged over the 60 sweeps in each measurement set. Biceps CMEPs were measured individually, averaged for the five stimuli in each measurement set and then normalized to the average of the two maximal M waves obtained in the biceps during the same set. Pre‐ and post‐drug measurements were normalized to the averaged baseline values and are expressed as a percentage of baseline (mean ± SD). F‐wave persistence was not normalized and is reported as a raw value (mean ± SD). Lastly, differences in measurements between days were calculated by subtracting the buspirone measurements from the placebo measurements at each time point and these are expressed as the mean ± 95% confidence interval (CI).
Statistical analysis
Results are presented as the mean ± SD in the text and figures. Differences between days are presented as the mean (95% CI). All statistical analyses were performed with SPSS, version 22 (IBM Corp., Armonk, NY, USA). Two‐way repeated measures ANOVAs were carried out to determine the effects of drug and time on the F waves, CMEPs and maximal M waves. Mauchly's test of sphericity was performed and, for data that were not spherical, Greenhouse–Geisser correction was used. When a significant drug × time interaction was found, a post hoc Bonferroni test was used to determine at which time points buspirone values differed from placebo values. One‐way repeated measures ANOVAs were used to determine whether the three baseline measurements differed within days and whether background EMG differed within days. Paired t tests were used to determine whether baseline measurements and background EMG differed between days. P < 0.05 was considered statistically significant.
Results
Ingestion of buspirone decreased motoneurone excitability as indicated by reductions in F waves measured in two intrinsic hand muscles and CMEPs measured in biceps brachii. Side effects were reported by eight participants on the buspirone day. These varied between participants and included light‐headedness, drowsiness, blurry vision, nausea and sweating. These side effects were short‐lasting with recovery within 40 min of onset and occurred between 35 and 105 min following pill ingestion. Three of these same participants also reported side effects on the placebo day, which included feeling cold, feeling strange and experiencing chills.
F waves
Raw F‐wave traces from a single participant are shown in Fig. 2 A. Compared to the placebo, F waves were reduced following buspirone intake.
Figure 2. Individual subject F waves and CMEPs on placebo and buspirone days.
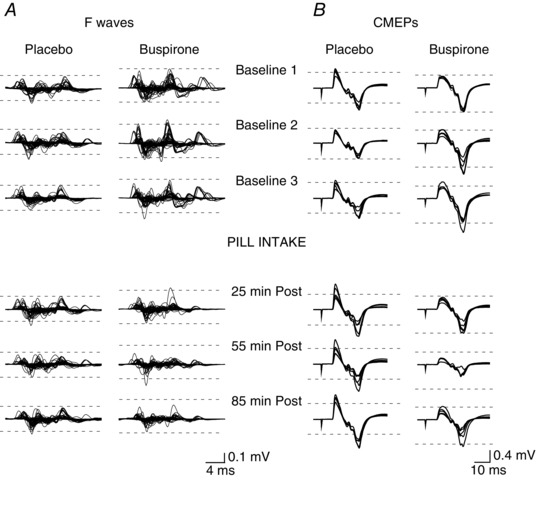
Raw EMG traces of ADM F waves (60 traces overlaid) (A) and biceps CMEPs (five traces overlaid) (B) from the same individual subject on the placebo (left) and buspirone days (right) before and at selected times after pill ingestion. F waves are shown without the preceding M waves. Supramaximal ulnar nerve stimuli were delivered at 22 ms prior to the start of each trace. For CMEPs, stimulus artefacts indicate timing of cervicomedullary stimulation. Dotted lines are drawn to aid comparison between times and encompass the smallest set of potentials recorded at baseline on each day. Both F waves and CMEPs are reduced after ingestion of buspirone.
For the group, the area of F waves in the ADM was ∼7% of the corresponding M max during both visits (placebo: 6.8 ± 2.6%, buspirone: 7.1 ± 2.8%; P = 0.49) and had high persistence (placebo: 92 ± 7%, buspirone: 93 ± 7%, P = 0.46). Following pill intake, mean F‐wave area and persistence were decreased after buspirone ingestion but not placebo (Fig. 3). For F‐wave area, a two‐way repeated measures ANOVA showed significant effects of drug (F 1,9 = 16.3, P = 0.003) and time (F 9,81 = 3.49, P = 0.001) and a drug × time interaction (F 9,81 = 2.52, P = 0.013) with post hoc tests showing a significant reduction on the buspirone day compared to the placebo day from 35 to 75 min (Fig. 3 A). Persistence of F waves showed drug and time effects (drug: F 1, 9 = 7.91, P = 0.02; time: F 2.50, 22.22 = 6.82, P = 0.003) with no interaction effect (F 2.48, 22.38 = 2.27, P = 0.11) (Fig. 3 B). The differences in ADM F‐wave area and persistence between the buspirone and placebo day for each individual participant are shown in Figs 3 C and D. The mean difference in ADM F‐wave area post pill intake was –27% (–42, –12) and the mean difference in persistence was –9% (–16, –2).
Figure 3. Changes in ADM F‐wave area and persistence following buspirone intake.
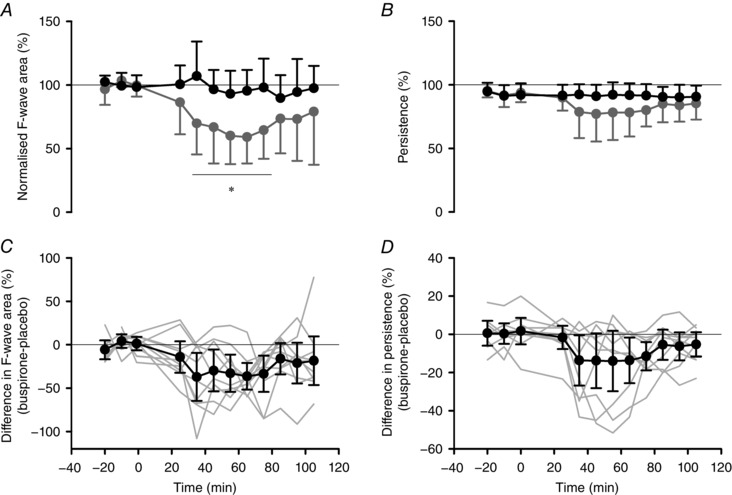
Group mean ± SD of normalized ADM F‐wave area (A) and ADM F‐wave persistence (B) on placebo (black circles) and buspirone (grey circles) days. F‐wave area was first expressed relative to the area of corresponding M waves and then as a percentage of the mean of the baseline trials on each day. Group differences between buspirone and placebo days (±95% CI: black circles) for normalized F‐wave area (C) and persistence (D). Grey lines indicate individual subject data. Pill ingestion occurred at time 0. Significant differences for drug × time interaction effect identified by post hoc tests: * P < 0.05.
Maximal M waves in ADM remained constant throughout both visits and did not differ between days (all P > 0.45). Because participants were relaxed throughout the protocol, levels of incidental EMG were very low and were similar between days and throughout days (placebo: 1.3 ± 0.5 μV, buspirone: 1.2 ± 0.4 μV, all P > 0.23).
F waves recorded from first dorsal interosseous (FDI) displayed a similar pattern to those in ADM with decreases in both the F‐wave area and persistence after buspirone intake (not shown).
CMEPs
CMEP raw traces recorded from biceps from an individual subject are shown in Fig. 2 B. Similar to the F waves in this subject (Fig. 2 A), CMEPs declined dramatically following intake of buspirone but not placebo.
For the group, baseline CMEPs were 12 ± 6% of maximal biceps M‐waves during both visits (P = 0.45). Although normalized CMEP area decreased on both days following pill intake (Fig. 4 A), this decrease was greater following buspirone. Two‐way repeated measures ANOVA showed a significant drug effect (F 1,9 = 5.86, P = 0.039) but no time effect (F 2.12,19.06 = 2.153, P = 0.14), nor a drug × time interaction (F 1.43,12.88 = 1.235, P = 0.31). The time course of differences in CMEP area for individual participants varied (Fig. 4 B, grey lines) with the mean difference in CMEP area post pill intake –31% (–60, –2). Maximal biceps M waves did not differ within days or between days (all P > 0.05). Incidental biceps EMG was very low and similar between and within days (placebo: 2.7 ± 0.7 μV, buspirone: 3.1 ± 1.1 μV, all P > 0.38).
Figure 4. Changes in biceps CMEP area following buspirone intake.
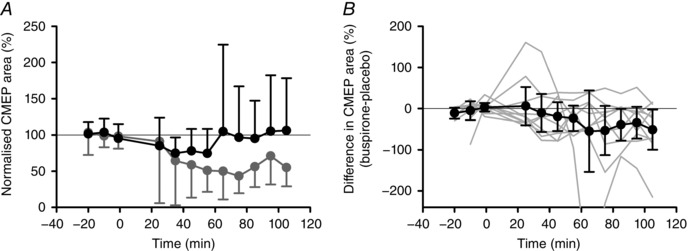
A, group mean ± SD of normalized biceps CMEP area. CMEP area was expressed relative to the corresponding M wave and then as a percentage of the mean of the baseline trials on each day. B, group differences between buspirone and placebo days (± 95% CI: black circles) for normalized biceps CMEP area. Grey lines indicate individual subject data. For one subject, one point (−432%) at 65 min is outside the graph. Pill ingestion occurred at time 0.
Discussion
The key novel finding of the present study is that ingestion of a 5‐HT1A receptor agonist (buspirone) depresses motoneurone excitability in healthy humans. Reduction of motoneurone excitability by activation of 5‐HT1A receptors is consistent with previous work on the turtle spinal cord (Cotel et al. 2013; see Introduction), and supports the concept that activation of these receptors during fatiguing exercise could reduce motoneurone output.
Two measures were used to assess whether buspirone intake altered motoneurone excitability. Cervicomedullary stimulation activates motoneurones synaptically and has a large monosynaptic component when measured in biceps brachii (Petersen et al. 2002; Taylor & Gandevia, 2004). Thus, the decrease in CMEPs following buspirone intake could be explained by decreases in (i) the excitability of spinal motoneurones; (ii) the efficacy of corticomotoneuronal synapses or (iii) the excitability of corticospinal tract axons at the site of stimulation. By contrast, F waves represent recurrent discharge of motoneurones after invasion of their soma by the antidromic action potentials evoked by stimulation of the peripheral nerve (Eccles, 1955; McLeod & Wray, 1966). Because stimulation of the motor axons remained supramaximal throughout the experiments, F‐wave size and persistence depended only on the excitability of the motoneurones. In general, although not always, a decrease in recurrent discharges reflects a decrease in motoneurone excitability (Schiller & Stalberg, 1978). Taken together, the decreases in both F waves and CMEPs are a strong indication of decreased motoneurone excitability following buspirone intake. Methodological limitations required us to elicit CMEPs and F waves in different muscles. Discomfort associated with the high stimulus intensities that are required makes it difficult to elicit CMEPs in hand muscles, whereas F waves cannot be measured in proximal muscles because of the short latency of the responses. However, because oral buspirone produced systemic effects, there is no reason to expect different actions on the two motoneurone pools tested in the present study.
We argue that the depression of motoneurone excitability after ingestion of buspirone is a result of the activation of 5‐HT1A receptors on the motoneurones, probably at the axon initial segment of the motoneurones. First, electrophysiological work carried out in animal models supports a decrease in motoneurone excitability following activation of 5‐HT1A receptors located on the motoneurones (Jackson & White, 1990; Rasmussen & Aghajanian, 1990; Cotel et al. 2013). Recent work on the turtle spinal cord has confirmed that, when 8‐OH‐DPAT (a 5‐HT1A /5‐HT7 receptor agonist) is focally applied at the axon initial segment of the motoneurone in the presence of a 5‐HT7 receptor antagonist, action potential generation is inhibited (Cotel et al. 2013). Application of high concentrations of 8‐OH‐DPAT to spinal motoneurones in the rat inhibits glutamate‐evoked motoneurone firing, although low concentrations have little or no effect (Jackson & White, 1990). Second, labelling has demonstrated 5‐HT1A receptors in the ventral horn of the aged human spinal cord with greater numbers of receptors in thoracic and lumbar regions than in cervical regions (Laporte et al. 1996). Similar distributions are seen in the rat (Marlier et al. 1991). Third, buspirone behaves as a selective partial agonist to the 5‐HT1A receptors with no affinity for other 5‐HT receptors, although it does display some affinity for dopamine (D2‐like) receptors (McMillen et al. 1983; Eison & Temple, 1986; Loane & Politis, 2012). Although mammalian spinal motoneurones express both the D1‐like and D2‐like receptors (Han et al. 2007; Han & Whelan, 2009; Schwarz & Peever, 2011), to date, most of the effects of dopamine on motoneurone excitability have been attributed to activation of D1‐like receptors, with no effect of antagonists or agonists to D2‐like receptors (Han & Whelan, 2009; Schwarz & Peever, 2011; Sharples et al. 2014). Thus, the reduction in CMEPs and F waves following buspirone intake is probably mediated by activation of 5‐HT1A receptors located directly on the motoneurones.
Despite the probable actions of buspirone at the motoneurones, we cannot exclude the possibility that buspirone ingestion may have indirectly affected motoneurone excitability through a change in descending monoaminergic drive to the spinal cord (Sotelo et al. 1990; Fornal et al. 1994; Azmitia et al. 1996; Dalley et al. 1996; Gobert et al. 1999; Reader et al. 2000; Silverstone et al. 2012). However, because buspirone and its metabolite have opposing effects on the serotonergic and noradrenergic systems, indirect effects are difficult to predict. Moreover, such effects may be small in the present study because all measurements were made at rest. Although the strong electrical stimuli used to elicit CMEPs and F‐waves may produce arousal, studies in behaving cats suggest that nociceptive stimulation does not increase firing of descending serotonergic neurones (Auerbach et al, 1985; Jacobs et al, 2002). Rather, firing is best associated with motor output (Jacobs et al, 2002).
Buspirone has been shown to inhibit the release of 5‐HT from the raphe nuclei via 5‐HT1A autoreceptors (Sotelo et al. 1990; Fornal et al. 1994; Azmitia et al. 1996). By contrast, via the actions of its metabolite on NA‐α2 receptors, it has also been shown to increase the release of noradrenaline from the locus coeruleus (Dalley et al. 1996; Gobert et al. 1999; Silverstone et al. 2012), from which neurones innervate multiple spinal and cortical areas. 5‐HT and noradrenaline can alter motoneurone excitability via the 5‐HT2B/C and noradrenaline NA‐α1 receptors, respectively, located on the motoneurones. Both the serotonergic and noradrenergic receptors are G‐q coupled excitatory receptors and increase motoneurone excitability through the activation of large persistent inward currents, as well as by decreasing leak conductances and consequently altering the resting membrane potential of the motoneurones (Perrier & Delgado‐Lezama, 2005; Harvey et al. 2006; Li et al. 2007; D'Amico et al. 2013 b). Any changes in the activation of persistent inward currents would not affect the measurements used in the present study because these slowly‐activating currents require long‐duration inputs to be fully activated (Murray et al. 2011; D'Amico et al. 2013 a) and therefore would not be activated in response to brief electrical stimuli. However, both CMEPs and F waves could be affected by changes in resting membrane potential. Because buspirone has opposing actions on descending serotonergic and noradrenergic drive to the motoneurones, any decrease in excitability as a result of the removal of serotonergic drive to the motoneurones would probably be negated by the concomitant increase in noradrenergic drive to the spinal cord. Additionally, for rat motoneurones, the basal levels of serotonin, as recorded within the serotonergic ventral funiculus in rats at rest (Gerin et al. 1995), are considerably lower than the concentrations of 5‐HT required to alter motoneurone resting membrane potential and the threshold for action potential spike initiation (Harvey et al. 2006). Finally, indirect evidence from humans also suggests that there are low levels of 5‐HT and noradrenaline in the spinal cord at rest. Namely, the selective 5‐HT reuptake inhibitor citalopram and the selective noradrenaline reuptake inhibitor reboxetine both fail to alter motoneurone excitability tested at rest with F‐wave responses (citalopram and reboxetine) and H reflexes (reboxetine) (Plewnia et al. 2002; Robol et al. 2004). Furthermore, blockade of 5‐HT2 and NA‐α1 receptors with chlorpromazine also has no effect on resting H‐reflexes (Metz et al. 1982). Measurements in the present study were made at rest and, when taken together with the arguments above, it is difficult to attribute the large depression (∼30% decrease in responses) in excitability of spinal motoneurones after buspirone intake to the effects of buspirone on monoaminergic release.
Could depression of motoneurones through activation of 5‐HT1A receptors contribute to central fatigue in human voluntary contractions?
Depression of motoneurone excitability and slowing of motoneurone firing rates are commonly reported in association with fatiguing exercise, and are proposed to contribute to central fatigue (Bellemare et al. 1983; Bigland‐Ritchie et al. 1983; Gandevia et al. 1999; Butler et al. 2003; Khan et al. 2012). The findings of the present study are consistent with the proposal that activation of 5‐HT1A receptors could be a mechanism that contributes to such changes. However, physiological activation of inhibitory 5‐HT1A receptors may require extrasynaptic spread of 5‐HT from the synapses on the soma and dendrites. In the turtle spinal cord, Cotel et al. (2013) observed inhibition of motoneurone output only after sustained stimulation of the serotonergic dorsolateral funiculus. Thus, this mechanism probably operates only when serotonergic drive to the motoneurones is high, and it is not known when high serotonergic drive occurs in humans. Studies in intact, freely‐behaving animals have shown a link between descending serotonergic drive and motor activity (Fornal et al. 1985; Fornal et al. 1996; Jacobs & Fornal, 1997; Jacobs et al. 2002). In humans, a recent study that examined the effects of serotonin as a spinal gain modulator suggest that 5‐HT release is graded with the strength of voluntary contractions, and that this release is quite diffuse (Wei et al. 2014). Thus, 5‐HT release could be high during strong voluntary contractions in humans and this may explain the parallels between resting motoneurone behaviour during and following 2 min of maximal voluntary contraction in humans and 3 min of stimulation of the dorsolateral funiculus in the turtle spinal cord (McNeil et al. 2009; Cotel et al. 2013). The proposition that high serotonergic drive reduces motoneurone excitability runs counter to the idea that motoneurone excitability is enhanced because of high descending monoaminergic drive with stress or arousal (Heckman et al. 2003). One possible explanation may be that stress‐related descending monoaminergic drive may be more noradrenergic than serotonergic (Auerbach et al. 1985; Abercrombie & Jacobs, 1987; Jacobs et al. 2002).
In the present study, we have examined the effects of buspirone on motoneurones at rest. It still remains unclear how activation of 5‐HT1A receptors may affect task performance. A few studies have demonstrated that oral intake of buspirone can impair performance during motor tasks in normal and high ambient temperatures (Marvin et al. 1997; Bridge et al. 2001; Bridge et al. 2003). These effects were attributed without direct evidence to the non‐serotonergic effects of buspirone at the supraspinal level (Bridge et al. 2003). However, none of the above studies examined changes at the spinal cord level. Our results indicate that buspirone intake leads to a decrease in motoneurone excitability and this may have contributed to the reduced motor performance noted in previous studies. Specifically, there was an increase in ratings of perceived exertion (Marvin et al. 1997) along with a reduction in time to task failure following buspirone intake (Marvin et al. 1997; Bridge et al. 2001; Bridge et al. 2003), which may reflect the increased central drive required to overcome inhibition of the motoneurones. It would be ideal to determine whether administration of a 5‐HT1A receptor antagonist reduces central fatigue during motor performance; however, a suitable 5‐HT1A receptor antagonist for human use is not yet available. Therefore, further studies are required to determine whether the effects of buspirone on motor performance are in part mediated via its actions at the spinal level.
To conclude, in individuals at rest, ingestion of a selective 5‐HT1A receptor partial agonist reduces motoneurone excitability. This reduction is consistent with the activation of inhibitory 5‐HT1A receptors on the axon initial segment of the motoneurones and the proposal that this mechanism could contribute to the reduction in motoneurone output during fatiguing exercise.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
This work was carried out at Neuroscience Research Australia, in Sydney, Australia. JMD, AAB, MEH, FC, JFMP, JEB, SCG and JLT contributed to the conception and design of the study. JMD and AAB collected the data. JMD analysed the data. JMD, AAB, MEH, JEB, SCG and JLT were involved in interpretation of the analysed data. JMD and JLT drafted the manuscript. All authors approved the final version of the manuscript and all persons who qualify for authorship are listed.
Funding
This work was funded by a National Health and Medical Research Council (NHMRC) program grant (no. 1055084). Additionally, JEB, SCG and JLT are supported by NHMRC research fellowships.
References
- Abercrombie ED & Jacobs BL (1987). Single‐unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. I. Acutely presented stressful and nonstressful stimuli. J Neurosci 7, 2837–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Pearson JC, Harrington D, Dewey D, Torbeck L & Fyffe RE (1998). Distribution of 5‐hydroxytryptamine‐immunoreactive boutons on alpha‐motoneurons in the lumbar spinal cord of adult cats. J Comp Neurol 393, 69–83. [PubMed] [Google Scholar]
- Auerbach S, Fornal C & Jacobs BL (1985). Response of serotonin‐containing neurons in nucleus raphe magnus to morphine, noxious stimuli, and periaqueductal gray stimulation in freely moving cats. Exp Neurol 88, 609–628. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Gannon PJ, Kheck NM & Whitaker‐Azmitia PM (1996). Cellular localization of the 5‐HT1A receptor in primate brain neurons and glial cells. Neuropsychopharmacology 14, 35–46. [DOI] [PubMed] [Google Scholar]
- Bellemare F, Woods JJ, Johansson R & Bigland‐Ritchie B (1983). Motor‐unit discharge rates in maximal voluntary contractions of three human muscles. J Neurophysiol 50, 1380–1392. [DOI] [PubMed] [Google Scholar]
- Bigland‐Ritchie B, Johansson R, Lippold OC, Smith S & Woods JJ (1983). Changes in motoneurone firing rates during sustained maximal voluntary contractions. J Physiol 340, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge MW, Marvin G, Thompson CE, Sharma A, Jones DA & Kendall MJ (2001). Quantifying the 5‐HT1A agonist action of buspirone in man. Psychopharmacology 158, 224–229. [DOI] [PubMed] [Google Scholar]
- Bridge MW, Weller AS, Rayson M & Jones DA (2003). Responses to exercise in the heat related to measures of hypothalamic serotonergic and dopaminergic function. Eur J Appl Physiol 89, 451–459. [DOI] [PubMed] [Google Scholar]
- Butler JE, Taylor JL & Gandevia SC (2003). Responses of human motoneurons to corticospinal stimulation during maximal voluntary contractions and ischemia. J Neurosci 23, 10224–10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A, Magnusson T & Rosengren E (1963). 5‐Hydroxytryptamine of the spinal cord normally and after transection. Experientia 19, 359. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Falk B, Fuxe K & Hillarp NA (1964). Cellular localization of monoamines in the spinal cord. Acta Physiol Scand 60, 112–119. [DOI] [PubMed] [Google Scholar]
- Coombs JS, Curtis DR & Eccles JC (1957). The generation of impulses in motoneurones. J Physiol 139, 232–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotel F, Exley R, Cragg SJ & Perrier JF (2013). Serotonin spillover onto the axon initial segment of motoneurons induces central fatigue by inhibiting action potential initiation. Proc Natl Acad Sci USA 110, 4774–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Mason K & Stanford SC (1996). Increased levels of extracellular noradrenaline in the frontal cortex of rats exposed to naturalistic environmental stimuli: modulation by acute systemic administration of diazepam or buspirone. Psychopharmacology 127, 47–54. [DOI] [PubMed] [Google Scholar]
- D'Amico JM, Li Y, Bennett DJ & Gorassini MA (2013. a). Reduction of spinal sensory transmission by facilitation of 5‐HT1B/D receptors in noninjured and spinal cord‐injured humans. J Neurophysiol 109, 1485–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico JM, Murray KC, Li Y, Chan KM, Finlay MG, Bennett DJ & Gorassini MA (2013. b). Constitutively active 5‐HT2/alpha1 receptors facilitate muscle spasms after human spinal cord injury. J Neurophysiol 109, 1473–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico JM, Butler AA, Butler JE, Gandevia SC & Taylor JL (2015). Activation of 5HT1A receptors: a plausible contributor to central fatigue? 20th Annual European College of Sport Science Congress, Malmo, Sweden, 20‐0939. From the European Database of Sport Science (EDSS), European College of Sport Science, Cologne. [Google Scholar]
- Duflocq A, Le Bras B, Bullier E, Couraud F & Davenne M (2008). Nav1.1 is predominantly expressed in nodes of Ranvier and axon initial segments. Mol Cell Neurosci 39, 180–192. [DOI] [PubMed] [Google Scholar]
- Duflocq A, Chareyre F, Giovannini M, Couraud F & Davenne M (2011). Characterization of the axon initial segment (AIS) of motor neurons and identification of a para‐AIS and a juxtapara‐AIS, organized by protein 4.1B. BMC Biol 9, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC (1955). The central action of antidromic impulses in motor nerve fibres. Pflügers Arch 260, 385–415. [DOI] [PubMed] [Google Scholar]
- Eison AS & Temple DL, Jr (1986). Buspirone: review of its pharmacology and current perspectives on its mechanism of action. Am J Med 80, 1–9. [DOI] [PubMed] [Google Scholar]
- Fornal C, Auerbach S & Jacobs BL (1985). Activity of serotonin‐containing neurons in nucleus raphe magnus in freely moving cats. Exp Neurol 88, 590–608. [DOI] [PubMed] [Google Scholar]
- Fornal CA, Litto WJ, Metzler CW, Marrosu F, Tada K & Jacobs BL (1994). Single‐unit responses of serotonergic dorsal raphe neurons to 5‐HT1A agonist and antagonist drug administration in behaving cats. J Pharmacol Exp Ther 270, 1345–1358. [PubMed] [Google Scholar]
- Fornal CA, Metzler CW, Marrosu F, Ribiero‐do‐Valle LE & Jacobs BL (1996). A subgroup of dorsal raphe serotonergic neurons in the cat is strongly activated during oral‐buccal movements. Brain Res 716, 123–133. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Petersen N, Butler JE & Taylor JL (1999). Impaired response of human motoneurones to corticospinal stimulation after voluntary exercise. J Physiol 521, 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC (2001). Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 4, 1725–1789. [DOI] [PubMed] [Google Scholar]
- Gerin C, Becquet D & Privat A (1995). Direct evidence for the link between monoaminergic descending pathways and motor activity. I. A study with microdialysis probes implanted in the ventral funiculus of the spinal cord. Brain Res 704, 191–201. [DOI] [PubMed] [Google Scholar]
- Gobert A, Rivet JM, Cistarelli L, Melon C & Millan MJ (1999). Buspirone modulates basal and fluoxetine‐stimulated dialysate levels of dopamine, noradrenaline and serotonin in the frontal cortex of freely moving rats: activation of serotonin 1A receptors and blocked of α2‐adrenergic receptors underlie its actions. Neuroscience 93, 1251–1262. [DOI] [PubMed] [Google Scholar]
- Han P, Nakanishi ST, Tran MA & Whelan PJ (2007). Dopaminergic modulation of spinal neuronal excitability. J Neurosci 27, 13192–13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P & Whelan PJ (2009). Modulation of AMPA currents by D(1)‐like but not D(2)‐like receptors in spinal motoneurons. Neuroscience 158, 1699–1707. [DOI] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y & Bennett DJ (2006. a). 5HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 96, 1158–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y & Bennett DJ (2006. b). Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol 96, 1171–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Lee RH & Brownstone RM (2003). Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci 26, 688–695. [DOI] [PubMed] [Google Scholar]
- Hornung JP (2003). The human raphe nuclei and the serotonergic system. J Chem Neuroanat 26, 331–343. [DOI] [PubMed] [Google Scholar]
- Innis RB, Nestler EJ & Aghajanian GK (1988). Evidence for G protein mediation of serotonin‐ and GABA B induced hyperpolarization of rat dorsal raphe neurons. Brain Res 459, 27–36. [DOI] [PubMed] [Google Scholar]
- Jackson DA & White SR (1990). Receptor subtypes mediating facilitation by serotonin of excitability of spinal motoneurons. Neuropharmacology 29, 787–797. [DOI] [PubMed] [Google Scholar]
- Jacobs BL & Fornal CA (1997). Serotonin and motor activity. Curr Opin Neurobiol 7, 820–825. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Martin‐Cora FJ & Fornal CA (2002). Activity of medullary serotonergic neurons in freely moving animals. Brain Res Rev 40, 45–52. [DOI] [PubMed] [Google Scholar]
- Khan SI, Giesebrecht S, Gandevia SC & Taylor JL (2012). Activity‐dependent depression of the recurrent discharge of human motoneurones after maximal voluntary contractions. J Physiol 590, 4957–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O, Rostrup E & Moller M (1992). Monoaminergic systems in the brainstem and spinal cord of the turtle Pseudemys scripta elegans as revealed by antibodies against serotonin and tyrosine hydroxylase. J Comp Neurol 325, 527–547. [DOI] [PubMed] [Google Scholar]
- Laporte AM, Doyen C, Nevo IT, Chauveau J, Hauw JJ & Hamon M (1996). Autoradiographic mapping of serotonin 5‐HT1A, 5‐HT1D, 5‐HT2A and 5‐HT3 receptors in the aged human spinal cord. J Chem Neuroanat 11, 67–75. [DOI] [PubMed] [Google Scholar]
- Li X, Murray K, Harvey PJ, Ballou EW & Bennett DJ (2007). Serotonin facilitates a persistent calcium current in motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 97, 1236–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane C & Politis M (2012). Buspirone: what is it all about? Brain Res 1461, 111–118. [DOI] [PubMed] [Google Scholar]
- Mahmood I & Sahajwalla C. (1999). Clinical pharmacokinetics and pharmacodynamics of buspirone, an anxiolytic drug. Clin Pharmacokinet 36, 277–287. [DOI] [PubMed] [Google Scholar]
- Maratta R, Fenrich KK, Zhao E, Neuber‐Hess MS & Rose PK (2015). Distribution and density of contacts from noradrenergic and serotonergic boutons on the dendrites of neck flexor motoneurons in the adult cat. J Comp Neurol 523, 1701–1716. [DOI] [PubMed] [Google Scholar]
- Marlier L, Teilhac JR, Cerruti C & Privat A (1991). Autoradiographic mapping of 5‐HT1, 5‐HT1A, 5‐HT1B and 5‐HT2 receptors in the rat spinal cord. Brain Res 550, 15–23. [DOI] [PubMed] [Google Scholar]
- Marvin G, Sharma A, Aston W, Field C, Kendall MJ & Jones DA (1997). The effects of buspirone on perceived exertion and time to fatigue in man. Exp Physiol 82, 1057–1060. [DOI] [PubMed] [Google Scholar]
- McLeod JG & Wray SH (1966). An experimental study of the F wave in the baboon. J Neurol Neurosurg Psychiatry 29, 196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen BA, Matthews RT, Sanghera MK, Shepard PD & German DC (1983). Dopamine receptor antagonism by the novel antianxiety drug, buspirone. J Neurosci 3, 733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Martin PG, Gandevia SC & Taylor JL (2009). The response to paired motor cortical stimuli is abolished at a spinal level during human muscle fatigue. J Physiol 587, 5601–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz J, Holcomb HH & Meltzer HY (1982). Effect of chlorpromazine on H‐reflex recovery curves in normal subjects and schizophrenic patients. Psychopharmacology 78, 342–345. [DOI] [PubMed] [Google Scholar]
- Montague SJ, Fenrich KK, Mayer‐Macaulay C, Maratta R, Neuber‐Hess MS & Rose PK (2013). Nonuniform distribution of contacts from noradrenergic and serotonergic boutons on the dendrites of cat splenius motoneurons. J Comp Neurol 521, 638–656. [DOI] [PubMed] [Google Scholar]
- Murray KC, Stephens MJ, Rank M, D'Amico J, Gorassini MA & Bennett DJ (2011). Polysynaptic excitatory postsynaptic potentials that trigger spasms after spinal cord injury in rats are inhibited by 5‐HT1B and 5‐HT1F receptors. J Neurophysiol 106, 925–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington NJ & Kelly JS (1990). Serotonin receptor activation reduces calcium current in an acutely dissociated adult central neuron. Neuron 4, 751–758. [DOI] [PubMed] [Google Scholar]
- Perrier JF & Hounsgaard J (2003). 5‐HT2 receptors promote plateau potentials in turtle spinal motoneurones by facilitating an L‐type calcium current. J Neurophysiol 89, 954–959. [DOI] [PubMed] [Google Scholar]
- Perrier JF & Delgado‐Lezama R (2005). Synaptic release of serotonin induced by stimulation of the raphe nucleus promotes plateau potentials in spinal motoneurons of the adult turtle. J Neurosci 25, 7993–7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen NT, Taylor JL & Gandevia SC (2002). The effect of electrical stimulation of the corticospinal tract on motor units of the human biceps brachii. J Physiol 544, 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plewnia C, Hoppe J, Hiemke C, Bartels M, Cohen LG & Gerloff C (2002). Enhancement of human cortico‐motoneuronal excitability by the selective norepinephrine reuptake inhibitor reboxetine. Neurosci Lett 330, 231–234. [DOI] [PubMed] [Google Scholar]
- Rasmussen K & Aghajanian GK (1990). Serotonin excitation of facial motoneurons: receptor subtype characterization. Synapse 5, 324–332. [DOI] [PubMed] [Google Scholar]
- Reader TA, Ase AR, Le Marec N & Lalonde R (2000). Effects of buspirone on indoleamines and catecholamines in wild‐type mice and Lurcher mutants. Eur J Pharmacol 398, 41–51. [DOI] [PubMed] [Google Scholar]
- Robol E, Fiaschi A & Manganotti P (2004). Effects of citalopram on the excitability of the human motor cortex: a paired magnetic stimulation study. J Neurol Sci 221, 41–46. [DOI] [PubMed] [Google Scholar]
- Schiller HH & Stalberg E (1978). F responses studied with single fibre EMG in normal subjects and spastic patients. J Neurol Neurosurg Psychiatry 41, 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BJ & Jordan LM (2000). The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull 53, 689–710. [DOI] [PubMed] [Google Scholar]
- Schwarz PB & Peever JH (2011). Dopamine triggers skeletal muscle tone by activating D1‐like receptors on somatic motoneurons. J Neurophysiol 106, 1299–1309. [DOI] [PubMed] [Google Scholar]
- Sharples SA, Koblinger K, Humphreys JM & Whelan PJ (2014). Dopamine: a parallel pathway for the modulation of spinal locomotor networks. Front Neural Circuits 8, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone PH, Lalies MD & Hudson AL (2012). Quetiapine and buspirone both elevate cortical levels of noradrenaline and dopamin in vivo, but do not have synergistic effects. Front Psychiatry 3, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo C, Cholley B, El Mestikawy S, Gozlan H & Hamon M (1990). Direct immunohistochemical evidence of the existence of 5‐HT1A autoreceptors on serotoninergic neurons in the midbrain raphe nuclei. Eur J Neurosci 2, 1144–1154. [DOI] [PubMed] [Google Scholar]
- Steinbusch HWM (1981). Distribution of serotonin‐immunoreactivity in the central nervous system of the rat – cell bodies and terminals. Neuroscience 6, 557–618. [DOI] [PubMed] [Google Scholar]
- Taylor JL & Gandevia SC (2004). Noninvasive stimulation of the human corticospinal tract. J Appl Physiol 96, 1496–1503. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW & Jacobs BL (1995). Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci 15, 5346–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei K, Glaser JI, Deng L, Thompson CK, Stevenson IH, Wang Q, Hornby TG, Heckman CJ & Kording KP (2014). Serotonin affects movement gain control in the spinal cord. J Neurosci 34, 12690–12700. [DOI] [PMC free article] [PubMed] [Google Scholar]


