Abstract
Key points
We report that the small molecule CK‐2066260 selectively slows the off‐rate of Ca2 + from fast skeletal muscle troponin, leading to increased myofibrillar Ca2 + sensitivity in fast skeletal muscle.
Rodents dosed with CK‐2066260 show increased hindlimb muscle force and power in response to submaximal rates of nerve stimulation in situ.
CK‐2066260 has no effect on free cytosolic [Ca2 +] during contractions of isolated muscle fibres.
We conclude that fast skeletal muscle troponin sensitizers constitute a potential therapy to address an unmet need of improving muscle function in conditions of weakness and premature muscle fatigue.
Abstract
Skeletal muscle dysfunction occurs in many diseases and can lead to muscle weakness and premature muscle fatigue. Here we show that the fast skeletal troponin activator, CK‐2066260, counteracts muscle weakness by increasing troponin Ca2+ affinity, thereby increasing myofibrillar Ca2+ sensitivity. Exposure to CK‐2066260 resulted in a concentration‐dependent increase in the Ca2+ sensitivity of ATPase activity in isolated myofibrils and reconstituted hybrid sarcomeres containing fast skeletal muscle troponin C. Stopped‐flow experiments revealed a ∼2.7‐fold decrease in the Ca2+ off‐rate of isolated troponin complexes in the presence of CK‐2066260 (6 vs. 17 s−1 under control conditions). Isolated mouse flexor digitorum brevis fibres showed a rapidly developing, reversible and concentration‐dependent force increase at submaximal stimulation frequencies. This force increase was not accompanied by any changes in the free cytosolic [Ca2+] or its kinetics. CK‐2066260 induced a slowing of relaxation, which was markedly larger at 26°C than at 31°C and could be linked to the decreased Ca2+ off‐rate of troponin C. Rats dosed with CK‐2066260 showed increased hindlimb isometric and isokinetic force in response to submaximal rates of nerve stimulation in situ producing significantly higher absolute forces at low isokinetic velocities, whereas there was no difference in force at the highest velocities. Overall muscle power was increased and the findings are consistent with a lack of effect on crossbridge kinetics. In conclusion, CK‐2066260 acts as a fast skeletal troponin activator that may be used to increase muscle force and power in conditions of muscle weakness.
Keywords: Ca2+ sensitivity, skeletal muscle, troponin C
Key points
We report that the small molecule CK‐2066260 selectively slows the off‐rate of Ca2 + from fast skeletal muscle troponin, leading to increased myofibrillar Ca2 + sensitivity in fast skeletal muscle.
Rodents dosed with CK‐2066260 show increased hindlimb muscle force and power in response to submaximal rates of nerve stimulation in situ.
CK‐2066260 has no effect on free cytosolic [Ca2 +] during contractions of isolated muscle fibres.
We conclude that fast skeletal muscle troponin sensitizers constitute a potential therapy to address an unmet need of improving muscle function in conditions of weakness and premature muscle fatigue.
Abbreviations
- EDL
extensor digitorum longus
- FDB
flexor digitorum brevis
- SR
sarcoplasmic reticulum
Introduction
Within the sarcomere, calcium ions (Ca2+) act as a signalling moiety that bind to troponin C and modulate the conformation of the troponin–tropomyosin regulatory complex on the actin filament, leading to actin–myosin cross‐bridge formation and subsequent muscle contraction (Gordon et al. 2000). Nerve input at the neuromuscular junction triggers action potentials, which propagate along the surface membrane and into the transverse tubules where they stimulate finely controlled Ca2+ release from the sarcoplasmic reticulum (SR). At low to mid frequencies of neuromuscular activation, intracellular SR Ca2+ release and the resulting force production increase proportionally, whereas at higher frequencies the contractile machinery is saturated with Ca2+ and increased SR Ca2+ release no longer increases force (Gordon et al. 2000; Chin, 2010).
Skeletal muscle weakness is a common symptom in numerous diseases (MacIntosh, 2003; Ochala, 2010), including chronic obstructive pulmonary disease (COPD) (Ottenheijm et al. 2005), long term bed rest (Mounier et al. 2009), heart failure (Pina et al. 2003) and spinal muscular atrophy (Stevens et al. 2008). In principle, reduced SR Ca2+ release, decreased myofibrillar Ca2+ sensitivity, and/or impaired force production intrinsic to cross‐bridges are potential causes of muscle weakness (Westerblad et al. 2010). Thus, one potential therapeutic approach to counteract muscle weakness is to directly modulate myofibrillar function. Accordingly, myofibrillar Ca2+ sensitizers have been shown to increase force production in chemically skinned muscle fibres from muscle biopsies of COPD patients and muscle tissue from a preclinical model of ventilator‐induced diaphragm dysfunction (van Hees et al. 2009; Ochala et al. 2010). However, these previously described Ca2+ sensitizers have undesirable off‐target effects, including changes in cardiac contractility (Ochala, 2010).
Previously, we have described tirasemtiv, a fast skeletal troponin activator that is currently under clinical investigation in amyotrophic lateral sclerosis (Russell et al. 2012; Shefner et al. 2012). In the present study, we characterized the specificity and potency of CK‐2066260, a structural analogue of tirasemtiv, to increase Ca2+ sensitivity in skeletal and cardiac myofibrils and reconstituted sarcomere complexes. We utilized CK‐2066260 to study the effect of troponin activation on the mechanics and kinetics of muscle contraction and further to demonstrate that changes in force output are independent of changes in free cytosolic [Ca2+] ([Ca2+]i) using muscle in situ and intact single muscle fibres. The results show that CK‐2066260 induces a concentration‐ and temperature‐dependent increase in myofibrillar Ca2+ sensitivity specifically in fast‐twitch skeletal muscle fibres. Conversely, CK‐2066260 does not affect SR Ca2+ release or intrinsic cross‐bridge force production. CK‐2066260 also caused a marked, temperature dependent slowing of relaxation at the end of tetanic contractions. Given this observation, we utilized CK‐2066260 as a tool to investigate the relative importance of factors that affect the speed of relaxation, which is a complex process depending on the rate of [Ca2+]i decrease and the subsequent return of the troponin–tropomyosin complex to its off state leading to cross‐bridge detachment (Gordon et al. 2000). Our results show that the rate of Ca2+ dissociation from the troponin complex has a major influence on the speed of force relaxation.
Methods
Ethical approval
Female Sprague–Dawley rats and female C57BL/6 mice were obtained from Charles River Laboratories (Hollister, CA, USA) and acclimated for at least 3 days before being used in experiments. They were maintained on standard chow and water and kept on a 12 h light–dark cycle. All rat experiments were performed at Cytokinetics (San Francisco, CA, USA) and conducted in accordance with approved protocols by the Cytokinetics Institutional Animal Care and Use Committee (IACUC). For in situ muscle experiments, rats were anaesthetized with 1–5% inhaled isoflurane (vaporized in 100% O2), reached a stable anaesthetic plane (consistent breath rate and unresponsive toe pinch), and were dosed intravenously in a 10% dimethylacetamide (DMA):50% PEG:16% Cavitron solution. At the end of the experiment, rats were killed by a carbon dioxide overdose. A total of 18 rats were used. All mouse experiments were performed at the Karolinska Institute and complied with the Swedish Animal Welfare Act, the Swedish Welfare ordinance, and applicable regulations and recommendations from Swedish authorities. The study was approved by the Stockholm North Ethical Committee on Animal Experiments. Mice were killed by rapid neck disarticulation and muscles were subsequently isolated. A total of 11 C57BL/6 mice were used. Rabbit back muscle, bovine masseter muscle and cardiac muscle tissues were purchased from Pel‐Freez Biologicals (Rogers, AR, USA). The investigators understand the ethical principles under which The Journal of Physiology operates and the experiments comply with the principles and regulations as described in the editorial by Grundy (2015).
Purification of myofibrils and sarcomere components
Fast skeletal myofibrils were prepared from rabbit back muscle (Pel‐Freez Biologicals) based upon the method of Herrmann et al. (1993). Minced muscle was homogenized in 10 volumes of ice‐cold detergent buffer (50 mm Tris, 100 mm potassium acetate, 5 mm KCl, 5 mm EDTA, 0.5 mm NaN3, 2 mm DTT, 1 mm benzamidine‐HCl, 0.2 mm phenylmethylsulfonyl fluoride (PMSF), 1 μg ml−1 leupeptin, 1 μg ml−1 pepstatin and 0.5% (v/v) Triton X‐100, final pH 7.4 at 4°C) using an Omni‐Macro homogenizer. Myofibrils were recovered by low speed centrifugation in a swinging bucket rotor (3000 g, 10 min, 4°C) and washed two more times in the detergent‐containing buffer to ensure removal of cellular membranes. Following the detergent washes, myofibrils were washed 3 times in wash buffer (50 mm Tris, 100 mm potassium acetate, 5 mm KCl, 2 mm magnesium acetate, 0.5 mm NaN3, 2 mm DTT, 0.2 mm PMSF, final pH 7.4 at 4°C) and once in storage buffer (12 mm Pipes, 60 mm KCl, 2 mm MgCl2, 1 mm DTT, 0.2 mm PMSF, 1 μg ml−1 leupeptin, 1 μg ml−1 pepstatin, final pH 6.8). The final pellet was resuspended in storage buffer and solid sucrose was added (10% w/v) prior to flash freezing in liquid nitrogen and storage at −80°C. Slow skeletal myofibrils were prepared from bovine masseter tissue (Pel‐Freez Biologicals) using the same fast skeletal myofibril procedure.
Cardiac myofibrils were prepared from bovine left ventricle tissue (Pel‐Freez Biologicals). Minced muscle was homogenized using an Omni‐Macro homogenizer in 10 volumes of ice‐cold relaxation buffer (10 mm imidazole, pH 7.2, 75 mm KCl, 2 mm MgCl2, 2 mm EGTA, 1 mm NaN3, 1 mm DTT, 10 mm EDTA, 4 mm phosphocreatine, 1 mm ATP, 50 mm butadione monoxime, 1 mm benzamidine hydrochloride, 0.1 mm PMSF, 1 μg ml−1 leupeptin, 1 μg ml−1 pepstatin, and 1% Triton X‐100, final pH 7.2). Myofibrils were recovered by low speed centrifugation using a swinging‐bucket rotor (3000 g, 10 min, 4°C) and washed 4 times in detergent‐containing wash buffer (10 mm imidazole, pH 7.2, 75 mm KCl, 2 mm MgCl2, 2 mm EGTA, and 1 mm NaN3, 1 mm DTT, 0.1 mm PMSF, and 1% Triton X‐100, final pH 7.2) by repeated pelleting and resuspension to ensure removal of cellular membranes and mitochondria. Myofibrils were then washed 4 times in detergent‐free wash buffer (10 mm imidazole, pH 7.2, 75 mm KCl, 2 mm MgCl2, 2 mm EGTA, 1 mm NaN3, and 1 mm DTT, final pH 7.2) to remove residual Triton X‐100. Myofibrils were washed once with 10 volumes of storage buffer (12 mm Pipes, pH 6.8, 60 mm KCl, 2 mm MgCl2, and 1 mm DTT, pH 6.8), resuspended in storage buffer, and sucrose was added (10% w/v) prior to flash freezing in liquid nitrogen and storage at −80°C.
Cardiac myosin was purified from bovine left ventricle tissue (Pel‐Freez Biologicals) based on the method of Margossian & Lowey (1982). Cardiac and skeletal tropomyosins were purified from muscle acetone powders using a method based from Smillie (Smillie, 1982). Skeletal and cardiac muscle troponin complexes were purified from muscle acetone powders using the method of Potter (1982). Actin was prepared from bovine cardiac acetone powder based on the method of Pardee & Spudich (1982).
ATPase assays
Steady‐state ATPase activity was measured using a pyruvate kinase and lactate dehydrogenase‐coupled enzyme system that regenerates myosin‐produced ADP into ATP by oxidizing NADH, producing an absorbance change at 340 nm. Reconstituted sarcomere ATPase assays were performed in PM12 buffer (12 mm Pipes, 2 mm MgCl2, 1 mm DTT, pH 6.8) containing 1 mm ATP. Myofibril ATPase assays were performed in PM12 buffer supplemented with 60 mm KCl and ATP at 3–10 times the K M for the particular myofibril system (0.5 mm ATP for fast skeletal, 0.05 mm ATP for slow skeletal and cardiac). Myofibrils were present at 0.25 mg ml−1 (fast skeletal) or 1 mg ml−1 (slow skeletal, cardiac). Absorbance measurements (340 nm) were carried out at 25°C using a SpectraMax plate reader (Molecular Devices, Sunnyvale, CA, USA). Data analysis was performed with GraphPad Prism (GraphPad Software, San Diego, CA, USA).
Isothermal titration calorimetry
Isothermal titration calorimetry experiments were carried out using a Micro‐Cal Auto ITC HT microcalorimeter (Microcal Inc., now Malvern, Inc. Westborough, MA, USA) at 25°C. A solution of 300 μm CK‐2066260 in 12 mm Pipes (pH 6.8), 100 mm KCl, 250 μm CaCl2, 5 mm β‐mercaptoethanol, and 3% DMSO (pH 6.8) was titrated into the sample cell, which contained 15 μm rabbit fast skeletal muscle troponin complex in the same buffer. The troponin concentration was determined by UV absorbance (280 nm) in 6 m guanidine‐HCl using a calculated extinction coefficient of 30,000 m −1 cm−1 based on the sequence of rabbit skeletal troponin subunits. Injections (10 μl) were made every 300 s. To correct for the heats of dilution and slight buffer mismatches between the titrant and sample, the average heat signal from the last three injections at the end of the experiment (when binding was saturated) was subtracted from all values. Data collection and analysis was performed using the modified Origin software included with the instrument, using a single binding site model.
Measurement of Ca2+ release from troponin
Ca2+ release from the troponin complex was measured with the fluorescent Ca2+ chelator Quin‐2, using a variation on the method of Rosenfeld & Taylor (1985). Quin‐2 (Life Technologies, Carlsbad, CA, USA) was dissolved in N,N‐dimethylformamide (DMF) and diluted in PM12 buffer (12 mm K‐Pipes, 2 mm MgCl2, pH 6.8) just prior to use. Rabbit skeletal muscle troponin (20 μm) in PM12 buffer with 1 mm DTT was pre‐incubated with DMSO (0.5%) or 40 μm CK‐2066260 in DMSO. The troponin concentration was determined by UV absorbance (278 nm) in 6 m guanidine‐HCl using an extinction coefficient of 0.45 ml mg−1 cm−1 for a 1 mg ml−1 solution. Troponin solutions were not supplemented with Ca2+, as sufficient Ca2+ was present in PM12 buffer to occupy the Ca2+ binding sites of troponin in the absence of chelators such as EGTA or EDTA. Troponin and Quin‐2 were rapidly mixed at 25°C using an SF‐61DX stopped‐flow fluorimeter (TgK Scientific, Bradford‐on‐Avon, UK) with excitation provided by a monochromator (337 nm, 10 mm slit width), and emission was measured through a 495 nm long pass filter. The full scale was adjusted using Ca2+‐saturated Quin‐2, and initial fluorescence was subtracted from curves before fitting.
In situ muscle contractile characteristics
For in situ extensor digitorum longus (EDL) experiments, the anaesthetized rat was placed on a heated platform maintained at 37°C and the right hindlimb was secured to a metal frame by metal screws at the knee joint. The right EDL was exposed and the distal tendon was attached to the lever arm of a force transducer (Aurora Scientific, Aurora, Ontario, Canada) with a 4‐0 silk suture. The exposed muscle was coated with Aquasonic ultrasound gel (Parker Laboratories, Fairield, NJ, USA) to insulate and prevent drying of the muscle. The muscle was activated by electrical stimulation of the peroneal nerve and the optimal length (L o) was determined by adjusting the muscle length until maximum twitch force was produced. Once L o had been established, the nerve was stimulated every 2 min with a 30 Hz train (1 ms pulse width, 350 ms duration) for the entire duration of the experiment. After five baseline 30 Hz stimulations, CK‐2066260 was administered intravenously in a sequential dose regimen of 2, 2, 2 and 4 mg kg−1, for a total dose of 10 mg kg−1. Doses were spaced 15 min apart, and force measurements were continued for 30 min after the final dose. Blood was collected from either the femoral vein or carotid artery, and the resulting plasma was analysed to determine the CK‐2066260 concentration by liquid chromatography–mass spectrometry.
An isometric force–frequency profile was obtained by applying stimulation frequencies between 10 and 200 Hz in rats given vehicle or CK‐2066260 (10 mg kg−1, i.v.). After completion of contractile testing, the length and mass of the EDL was measured. The physiological cross‐sectional area (PCSA) was calculated based on the following equation: PCSA = muscle mass (g)/(muscle length (cm) × 1.056 g cm−3 (tissue density)). Specific tension (N cm−2) was determined by dividing muscle force by the PCSA.
In a separate set of experiments, the muscle isokinetic force–velocity relationship was measured in the hindlimb ankle plantar flexor muscles (gastrocnemius and soleus complex) in situ. In anaesthetized rats, an incision was made on the lateral side of the hindlimb to expose and isolate the sciatic nerve. Distal to the sciatic nerve, the peroneal nerve was severed to prevent co‐contraction of the antagonist dorsiflexor muscles. The right leg was fixed at the knee with screws and the foot was attached to a footplate force transducer (Aurora Scientific). The force output at different isokinetic velocities (0–25 rad s−1) was measured after treatment with vehicle or CK‐2066260 (10 mg kg−1, i.v.), given as a bolus injection over 2 min.
Single fibre force and [Ca2+]i measurements
Intact single muscle fibres with tendons were mechanically dissected from mouse flexor digitorum brevis (FDB) muscles. Aluminium clips were attached to the tendons, and the fibre was mounted in a chamber between an Akers 801 force transducer (Kronex Technologies, Oakland, CA, USA) and an adjustable holder. The fibre length was adjusted to obtain maximum tetanic force, and the diameter of the fibre at this length was used to calculate the cross‐sectional area. The fibre was stimulated with supramaximal electrical pulses (0.5 ms in duration) delivered via platinum electrodes placed along the long axis of the fibre.
Fibres were superfused with a Tyrode solution containing (mm): 121 NaCl, 5.0 KCl, 1.8 CaCl2, 0.5 MgCl2, 0.4 NaH2PO4, 24.0 NaHCO3, 0.1 EDTA, and 5.5 glucose. The solution was bubbled with 95% O2–5% CO2, giving a pH of 7.4. Fetal calf serum (0.2%) was added to the solution. Experiments were performed at room temperature (∼26°C), or 31°C, which is the physiological temperature for rested FDB muscles in vivo (Bruton et al. 1998).
Fibres were microinjected with the fluorescent Ca2+ indicator indo‐1 (Molecular Probes/Invitrogen, Carlsbad, CA, USA). The emitted fluorescence of indo‐1 was measured with a system consisting of a xenon lamp, a monochromator, and two photomultiplier tubes (Photon Technology International, Wedel, Germany). The excitation light was set to 360 nm, and the light emitted at 405 ± 5 and 495 ± 5 nm was measured by the photomultipliers. The ratio of the light emitted at 405 nm to that at 495 nm (R) was converted to [Ca2+]i using the following equation:
where K d is the apparent dissociation constant of indo‐1, β is the ratio of the 495 nm signals at very low and saturating [Ca2+]i, R min is the ratio at very low [Ca2+]i, and R max is the ratio at saturating [Ca2+]i (Andrade et al. 1998). Fluorescence and force signals were sampled online and stored on a computer for subsequent data analysis.
The steady‐state force–[Ca2+]i relationship under control conditions was obtained at 26°C by stimulating fibres for 350 ms at 15–150 Hz every 1 min and fitting the resulting force and [Ca2+]i values to the following Hill equation:
where P is the relative force, P max is the force at saturating [Ca2+], Ca50 is the [Ca2+]i giving 50% of P max, and N is a constant related to the steepness of the relation. Ca50 was also assessed in the presence of CK‐2066260 by using measurements from two contractions: one on the steepest portion of the force–[Ca2+]i relationship (15–30 Hz) and one close to P max (120 Hz; see Fig. 7). Ca50 was then obtained by fitting these measurements to the Hill equation assuming that the steepness of the relationship (N) remained constant.
Figure 7. The slowing of relaxation during CK‐2066260 exposure cannot be explained by the increased steady‐state myofibrillar Ca2+ sensitivity per se .
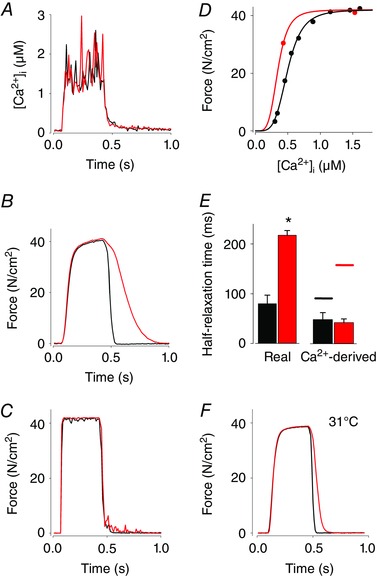
A–D, representative [Ca2+]i and force data obtained in a single FDB fibre stimulated at 26°C and used for analysis of relaxation in the absence (black lines) and presence of 10 μm CK‐2066260 (red lines). [Ca2+]i (A) and force (B) records obtained during 120 Hz tetani produced before and after 5 min exposure to CK‐2066260. C, modelled Ca2+‐derived force records obtained from the [Ca2+]i records (A) assuming that force instantly responds to the change in [Ca2+]i according to the respective steady‐state force–[Ca2+]i relationship obtained in the absence and presence of CK‐2066260 (D). E, mean (±SEM; n = 4) half‐relaxation times before (black bars) and during (red bars) CK‐2066260 exposure of real force (left panel) and modelled Ca2+‐derived force (right panel). Horizontal lines in the right panel show predicted mean half‐relaxation times also taking into account the time required for Ca2+ release from the troponin complex. * P < 0.05. F, representative force records obtained during 120 Hz tetani produced before and after 5 min exposure to CK‐2066260 at 31°C. Note that the slowing of relaxation is modest at this physiological temperature.
Fibres were exposed to 1, 5 and 10 μm CK‐2066260 for 30 min and stimulated at different time points to assess the time course for changes of [Ca2+]i and force. We used a high stimulation frequency (120 Hz at both 26 and 31°C), which produced close to maximal force, and a submaximal frequency (15–30 Hz at 26°C and 30–40 Hz at 31°C), which produced ∼30% of the maximal force in the control solution, i.e. before adding CK‐2066260.
We observed a markedly slowed force relaxation at the end of tetani in the presence of CK‐2066260, especially at the lower temperature studied (26°C). To determine the relation between this slowing and the altered steady‐state force–[Ca2+]i relation in CK‐2066260, real force records were compared with [Ca2+]i‐derived forces, which were generated by converting [Ca2+]i records obtained during tetani to force using the force–[Ca2+]i relationship (Westerblad & Allen, 1993).
Statistical analysis
Statistical evaluation employing unpaired t tests was performed using Graphpad Prism Software. Data are presented as mean ± SD or SEM. Significance was set at a P value < 0.05.
Results
CK‐2066260 increases the Ca2+ sensitivity of myofibrillar ATPase activity of fast skeletal myofibrils
CK‐2066260 added to rabbit skinned fast skeletal myofibrils caused a concentration‐dependent leftward shift in the ATPase activity–Ca2+ relationship, reaching a maximum decrease in Ca50 from 2.04 μm without CK‐2066260 to 0.26 μm with 40 μm CK‐2066260 (Fig. 1 A). CK‐2066260 did not affect the maximum myofibrillar ATPase activity.
Figure 1. CK‐2066260 specifically increases the Ca2+ sensitivity of fast skeletal myofibrils.
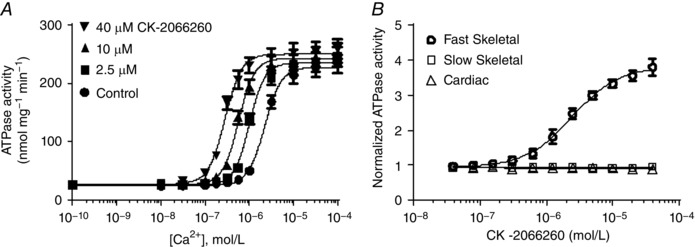
A, activation of the ATPase activity in rabbit fast skeletal myofibrils at different [Ca2+] in the absence and presence of 2.5–40 μm CK‐2066260 (mean ± SEM; n = 6). DMSO (2%) was present in all reactions. Curve fitting gave a Ca50 of 2.2 μm under control conditions (●), which was decreased to 1.0, 0.57 and 0.28 μm with 2.5 μm (■), 10 μm (▲) and 40 μm (▼) CK‐2066260, respectively. B, activation of the ATPase activity (mean ± SD; n = 6) of rabbit fast skeletal (open circles), bovine slow skeletal (open squares) and bovine cardiac (open triangles) myofibril ATPase activity at a fixed [Ca2+] of 1 μm, approximately the pCa25 for each myofibril type. ATPase activity was normalized to control reactions containing 2% DMSO.
CK‐2066260 selectively activates fast skeletal myofibril ATPase
Fast skeletal, slow skeletal and cardiac muscle contractile characteristics are distinct in part due to their respective troponin isoforms (Brotto et al. 2006). The muscle‐type selectivity of CK‐2066260 was examined using myofibrils prepared from different muscles: rabbit psoas (fast skeletal), bovine masseter (slow skeletal) and bovine heart (cardiac); muscle types were confirmed by myosin heavy chain isoform analysis (data not shown). The ATPase activity at a fixed Ca2+ concentration (Ca25, i.e. giving 25% of the maximal activity) was measured in response to increasing concentrations of CK‐2066260. Slow skeletal and cardiac myofibrils demonstrated minimal ATPase activation in response to increasing concentrations of CK‐2066260 (Fig. 1 B). In contrast, fast skeletal myofibrils exhibited a 3.8‐fold increase in ATPase activity and an EC50 (concentration at 50% ATPase activity) of 2.4 μm (Fig. 1 B), thus demonstrating CK‐2066260 specificity for fast skeletal muscle fibres.
CK‐2066260 specifically activates the fast skeletal muscle troponin complex
To identify the sarcomere protein interacting with CK‐2066260, purified sarcomere components (actin, troponin, tropomyosin, soluble S1 fragment of myosin) from cardiac bovine and fast skeletal rabbit muscle were reconstituted into hybrid sarcomere complexes. These complexes hydrolyse ATP with Ca2+ dependence similar to skinned fibres and myofibrils. A concentration‐dependent increase in ATPase was observed when CK‐2066260 was titrated into a reconstituted hybrid sarcomere containing fast skeletal muscle troponin and tropomyosin, cardiac myosin and cardiac actin that was partially activated at Ca25 (Fig. 2 A). Given that intact cardiac myofibrils are not affected by CK‐2066260 (see Fig. 1 B), the activation of this hybrid sarcomere by CK‐2066260 must be due to one of the fast skeletal components present. To determine what component CK‐2066260 acts on, sarcomere hybrid complexes where either the fast skeletal muscle troponin or tropomyosin isoform was replaced and tested with its analogus cardiac isoform. Only complexes that contained the fast skeletal muscle troponin isoform demonstrated increased ATPase activity in the presence of CK‐2066260 (Fig. 2 B).
Figure 2. CK‐2066260 specifically activates fast skeletal muscle troponin.
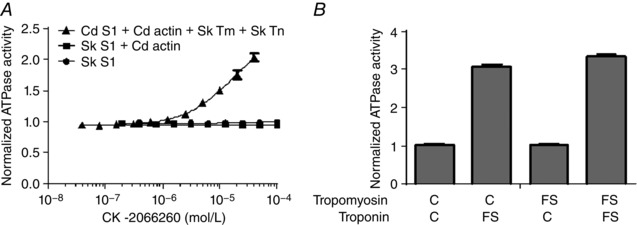
A, activation of the ATPase activity (mean ± SD, n = 4) of rabbit fast skeletal myosin subfragment 1 (circles), rabbit fast skeletal myosin subfragment 1 stimulated with cardiac actin (squares) and bovine cardiac myosin subfragment 1 stimulated with cardiac actin combined with fast skeletal troponin and tropomyosin (triangles). The latter was tested at a free calcium concentration of 0.32 μm (approximately the pCa25). ATPase activity was normalized to control reactions containing 2% DMSO. B, activation of the ATPase activity (mean ± SD, n = 2) of reconstituted hybrid sarcomeres treated with a 40 μm CK‐2066260 at a fixed [Ca2+] of 0.17 μm (approximately the pCa25). Assay mixtures contained bovine cardiac myosin (S1 fragment) and thin filaments assembled from bovine cardiac actin and either cardiac (C) or fast skeletal (FS) tropomyosin and troponin. ATPase activity was normalized to control reactions containing 2% DMSO.
A direct interaction of CK‐2066260 with fast skeletal muscle troponin (Tn) was further demonstrated using isothermal titration calorimetry (ITC). Titration of CK‐2066260 into a solution of purified rabbit fast skeletal muscle troponin (TnT/TnI/TnC) produced a robust heat signal that fitted well to a single site binding model (Fig. 3 A). Pooled data from six binding reactions indicated that CK‐2066260 bound exothermically (heat of enthalpy reaction (ΔH) = −19 ± 0.6 kcal mol−1) to fast skeletal muscle troponin with high affinity (K d = 1.5 ± 0.12 μm) and a stoichiometry consistent with a single binding site per troponin complex (n = 1.06±0.0013).
Figure 3. CK‐2066260 binds directly to fast skeletal muscle troponin complex and slows the Ca2+ release from troponin.
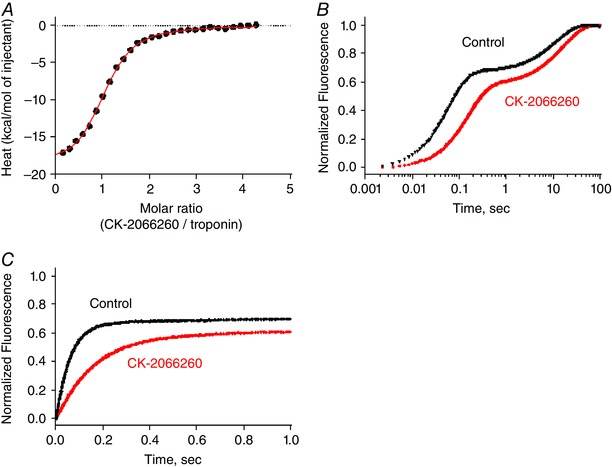
A, exothermic binding of CK‐2066260 to rabbit fast skeletal muscle troponin complex as measured by isothermal titration calorimetry. A representative binding reaction is shown; pooled data from six reactions (mean ± SD) indicated that CK‐2066260 bound to fast skeletal muscle troponin with high affinity (K d = 1.5 ± 0.12 μm) and a stoichiometry consistent with a single binding site per troponin complex (n = 1.06 ± 0.013). Thermodynamic parameters: ΔH = −19 ± 0.6 kcal mol−1, change in entropy (ΔS) = −37 ± 2 cal mol−1 deg−1. B and C, slowed Ca2+ dissociation from rabbit fast skeletal muscle troponin (10 μm) as measured by stopped‐flow using the fluorescent Ca2+ chelator Quin‐2 (75 μm) under control conditions (black) or with 20 μm CK‐2066260 (red). B, mean data from 4 reactions were baseline subtracted and normalized before fitting to a double exponential function, which yielded rates of 6 s−1 and 0.06 s−1 for CK‐2066260 vs. 17 s−1 and 0.09 s−1 for control. The first 1 s of the reaction is expanded and plotted on a linear time scale in C to show more clearly the effect of CK‐2066260 on the rapidly exchanging regulatory Ca2+‐binding sites of fast skeletal troponin.
CK‐2066260 slows release of Ca2+ from skeletal muscle troponin
One potential mechanism for sensitizing the sarcomere to Ca2+ is by stabilizing the Ca2+–troponin complex. This slows the rate of Ca2+ dissociation from the complex, prolonging the time troponin spends in the active (open) conformation (Gordon et al. 2000). The rate of Ca2+ dissociation from troponin was monitored by rapidly mixing Ca2+‐saturated fast skeletal muscle troponin complex with Quin‐2, a fluorescent Ca2+ buffer whose fluorescence intensity increases when it binds Ca2+. Fast skeletal muscle troponin contains four divalent cation binding sites: two high affinity sites (K d ∼50 nm) that exchange slowly, and two low affinity Ca2+‐specific sites (K d ∼5 μm) that exchange more rapidly and control muscle contraction (Potter & Gergely, 1975; Gordon et al. 2000). As shown in Fig. 3 B and C, CK‐2066260 causes a marked slowing of troponin Ca2+ release. Fitting Ca2+ release data to a single exponential function gives a rate constant under control conditions of 17 s−1, which corresponds well with previously reported values for the Ca2+‐specific sites (Rosenfeld & Taylor, 1985), and the rate constant is decreased to 6 s−1 in the presence of CK‐2066260. Thus, these data are consistent with a CK‐2066260‐induced stabilization of the Ca2+‐bound, ‘active’ conformation of troponin.
CK‐2066260 increases in situ force output at submaximal frequency nerve stimulation
The observed increases in fast skeletal muscle troponin Ca2+ sensitivity with CK‐2066260 treatment should result in an increase of muscle force in situ. To test this hypothesis, the isometric force output of the rat EDL (a muscle composed almost exclusively of fast‐twitch fibres) produced by stimulation of the peroneal nerve was measured in situ. CK‐2066260 was dosed intravenously in a sequential regimen of 2, 2, 2 and 4 mg kg−1, for a total dose of 10 mg kg−1. In response to 30 Hz stimulations, EDL force output increased with increasing CK‐2066260 accumulation that correlated closely with increasing plasma concentrations of CK‐2066260 (Fig. 4 A). The isometric force–frequency relationship was investigated from 10 to 200 Hz under control (pre‐dose) conditions and after CK‐2066260 (10 mg kg−1, i.v.) administration. Force output in response to stimulations between 30 and 60 Hz was significantly higher after CK‐2066260 administration, whereas there was no difference at the higher frequencies where force approached its maximum (Fig. 4 B). Resting tension did not increase after CK‐2066260 administration.
Figure 4. CK‐2066260 increases muscle force in situ at submaximal stimulations.
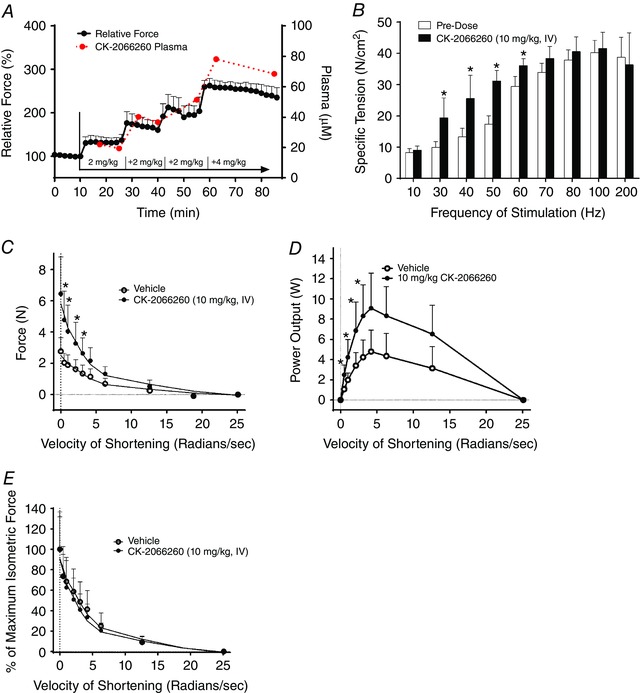
A, rat EDL muscles were stimulated in situ every 2 min with a 30 Hz stimulation train (350 ms duration) and doses of CK‐2066260 were injected intravenously as indicated up to a total of 10 mg (kg body weight)−1. Force is expressed relative to the level before CK‐2066260 administration (n = 4). The plasma level of CK‐2066260 was measured every 2 min over a 90 min period (n = 4). B, force of rat EDL muscles stimulated in situ at 10–200 Hz (350 ms duration) before and after administration of 10 mg kg−1 CK‐2066260 (n = 4). The force–velocity relationship (C) and power output (D) of the gastrocnemius complex stimulated in situ with 30 Hz pulse trains (Vehicle, n = 4; CK2066260 (10 mg kg−1, i.v.), n = 5). E, the force–velocity relationship of the gastrocnemius complex stimulated in situ with 30 Hz pulse trains (Vehicle, n = 4; CK2066260 (10 mg kg−1, i.v.), n = 5) plotted as isometric force set to 100%. Data are mean ± SD; * P < 0.05.
CK‐2066260 increases isokinetic force and power at submaximal nerve stimulation
In situ muscle force was further characterized by measuring the ankle plantarflexor muscle group (primarily fast fibre gastrocnemius complex) force response to sciatic nerve stimulation at different isokinetic velocities after vehicle or CK‐2066260 (10 mg kg−1, i.v.) treatment. In response to 30 Hz stimulation, rats treated with CK‐2066260 produced significantly higher absolute forces at low isokinetic velocities than vehicle‐treated animals, whereas there was no difference at the highest velocities (Fig. 4 C). Muscle power plotted as a function of velocity also demonstrated higher power output with CK‐2066260 treatment over a range of lower isokinetic velocities (Fig. 4 D). When the isometric force was set to 100%, the shape of the force–velocity relationships was very similar between vehicle‐ and CK‐2066260 treatment (Fig. 4 E). Thus, CK‐2066260 increased force and power without demonstrating an effect on the rate of cross‐bridge kinetics.
CK‐2066260 increases submaximal force in single muscle fibres without affecting [Ca2+]i
A final set of experiments were performed at 26 and 31°C to study how CK‐2066260 affects the relation between force and [Ca2+]i in intact muscle fibres and whether CK‐2066260 affected [Ca2+]i during contractions. Force and [Ca2+]i were then recorded in intact single muscle fibres isolated from mouse FDB muscles. At 26°C, application of 5 or 10 μm, but not 1 μm, CK‐2066260 induced a rapid increase in submaximal force (stimulation frequency set to achieve ∼30% of the maximal force before application of CK‐2066260) reaching close to its maximum after only 1 min of CK‐2066260 exposure (Fig. 5). The increase in force with CK‐2066260 was rapidly reversed upon washout reaching the control level after 5 min. At 31°C (i.e. the physiological temperature for rested FDB muscle in vivo; Bruton et al. 1998), application of 5 or 10 μm CK‐2066260 induced a major increase of submaximal force with a time course similar to that at 26°C and with a magnitude that was about half of that at 26°C (Fig. 6). During submaximal contractions, [Ca2+]i tended to decrease (∼10%) in fibres treated with 5 and 10 μm CK‐2066260, but the decrease did not reach statistical significance (P = 0.09); at 31°C 10 μm CK‐2066260 had no effect on [Ca2+]i during contractions (Fig. 6 B). Application of CK‐2066260 had no significant effect on resting [Ca2+]i; for instance, resting [Ca2+]i at 26°C was 65 ± 6 nm before CK‐2066260 exposure, 66 ± 5 nm after 30 min in 10 μm CK‐2066260, and 72 ± 7 nm after a 30 min washout. We never observed any effect of CK‐2066260 on resting force before or after contractions.
Figure 5. CK‐2066260 increases force in submaximal contractions without affecting [Ca2+]i in single mouse FDB fibres.
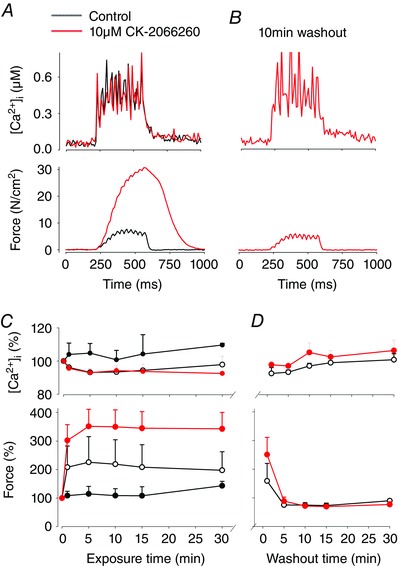
Typical records of [Ca2+]i and force obtained during 30 Hz contractions elicited at 26°C in a single fibre (A) before (black line) and after 5 min exposure to 10 μm CK‐2066260 (red line), and (B) after 10 min washout. Mean data (±SEM) of [Ca2+]i and force during CK‐2066260 exposure (C) and during washout (D). CK‐2066260: black circles, 1 μm (n = 4); open circles, 5 μm (n = 3); red circles, 10 μm (n = 4).
Figure 6. CK‐2066260 increases force in submaximal contractions in single mouse FDB fibres at 31°C without affecting [Ca2+]i .
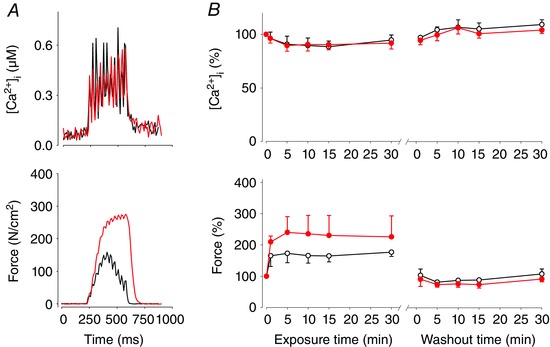
A, typical data of [Ca2+]i and force during 30 Hz contractions of a single fibre (A) before (black line) and after 5 min exposure to 10 μm CK‐2066260 (red line). B, mean data (±SEM) of [Ca2+]i and force during exposure to CK‐2066260: open circles, 5 μm (n = 3); filled circles, 10 μm (n = 4).
CK‐2066260 induced a marked slowing of force relaxation in submaximal contractions at 26°C (Figs 5 A and 6 A). To further assess the slowed relaxation, we studied CK‐2066260 at tetanic (120 Hz) stimulations, where CK‐2066260 addition does not further increase force. CK‐2066260 (10 μm) induced a slowing of force relaxation also in maximal contractions, whereas there was no effect on [Ca2+]i (Fig. 7 A and B). Compared to 26°C conditions, the slowing of relaxation was less pronounced at 31°C both at submaximal (cf. Figs 5 A and 6 A) and maximal stimulation frequencies (cf. Fig. 7 A and E). Further analyses of the mechanism behind the slowing of force relaxation during CK‐2066260 exposure were therefore performed at 26°C. One tentative mechanism for the slowing of relaxation is that it is a direct consequence of increased myofibrillar Ca2+ sensitivity (i.e. the leftward shift of the steady‐state force–[Ca2+]i relationship). To study this possibility, fibres were stimulated at different frequencies and the force–[Ca2+]i relationship was obtained by curve fitting using the resulting force and [Ca2+]i values, and eqn (1). Under control conditions the Ca50 value was 0.49 ± 0.03 μm, the P max was 392 ± 22 kN m−2, and the steepness of the relationship (N) was 5.3 ± 0.7 (n = 4). Application of CK‐2066260 did not affect the maximum force. On the assumption that the steepness of the force–[Ca2+]i relationship stays constant (see Fig. 1 A), application of CK‐2066260 decreased Ca50 from 0.49 ± 0.03 μm to 0.30 ± 0.03 μm (n = 4). The force–[Ca2+]i relationships and the measured tetanic [Ca2+]i records without and with CK‐2066260 were used to construct Ca2+‐derived force records, i.e. the force that would occur if force instantly followed the steady‐state force–[Ca2+]i relationship (Westerblad & Allen, 1993). A typical example of this analysis shows that the unaltered tetanic [Ca2+]i (Fig. 7 A) is accompanied by a markedly slowed relaxation of real force with CK‐2066260 (Fig. 7 B) and this slowing was not present in the Ca2+‐derived force records (Fig. 7 C), which were constructed from the respective [Ca2+]i records and the steady‐state force–[Ca2+]i relationships (Fig. 7 D). Accordingly, the mean value for real force half‐relaxation times is more than two times higher with CK‐2066260 (Fig. 7 E, left panel), whereas the half‐relaxation time of [Ca2+]i‐derived force is similar with and without CK‐2066260 (Fig. 7 E, right panel).
During relaxation, the dissociation of Ca2+ from troponin provides a delay between the instant force–[Ca2+]i relationship and the real force. The Ca2+ off‐rate (k off) of the fast skeletal muscle troponin complex was reduced from 17 s−1 in control to 6 s−1 at 25°C in the presence of CK‐2066260 (see Fig. 3), which translates to half‐relaxation times (t 1/2 = ln(2)/k off) of 41 and 116 ms, respectively. Adding this delay to that obtained in the [Ca2+]i‐derived force records gives a mean half‐relaxation time of 90 ms without CK‐2066260 (horizontal lines in Fig. 7 E), which is similar to the half‐relaxation time of real force (80 ms). In the presence of CK‐2066260, addition of the delay gives a mean half‐relaxation time of 159 ms. This time is still somewhat shorter than the mean half‐relaxation time of real force (218 ms), which might indicate that steps in the actin filament deactivation following Ca2+ dissociation from troponin were also slowed in the presence of CK‐2066260. Thus, these analyses indicate that the CK‐2066260‐induced slowing of relaxation is not due to the increase in steady‐state myofibrillar Ca2+ sensitivity per se, but rather caused by a decreased rate of Ca2+ dissociation from troponin and slowed actin filament deactivation.
Discussion
In this study we characterized properties of a novel, small‐molecule fast skeletal troponin activator, CK‐2066260. CK‐2066260 increases the Ca2+ sensitivity of the fast skeletal muscle troponin complex by decreasing the off‐rate of Ca2+ release from the regulatory fast troponin C. Due to the increased myofibrillar Ca2+ sensitivity, the force produced at submaximal stimulation frequencies in the presence of CK‐2066260 was markedly higher in whole fast muscles in situ and isolated fast muscle fibres ex vivo. Because motor units operate at submaximal frequencies during most daily living activities of rats (Hennig & Lomo, 1985; Jasmin & Gardiner, 1987) as well as in humans (Marsden et al. 1971; Grimby & Hannerz, 1977), CK‐2066260 and other fast skeletal troponin activators could potentially ease the difficulty of these tasks in patient populations with muscle weakness.
In an initial set of experiments, we used isolated myofibrils and hybrid sarcomere complexes to study the fibre‐type selectivity of CK‐2066260. The results show that CK‐2066260 induces a concentration‐dependent increase in the Ca2+ sensitivity of myosin ATPase activity in myofibrils of fast skeletal muscle, whereas the myosin ATPase activity of slow skeletal and cardiac myofibrils was not affected (see Fig. 1). Subsequent experiments that mixed cardiac and skeletal tropomyosin, actin and myosin components into hybrid sarcomere complexes revealed that CK‐2066260 increased Ca2+‐dependent ATPase activity only when fast skeletal muscle troponin was part of the complex (Fig. 2). Thus, CK‐2066260 is selective for fast skeletal troponin, which is a marked improvement compared to previously described small‐molecule sensitizers that, for instance, also affect cardiac contractility (Ochala, 2010).
The Ca2+ sensitivity of myofibrillar contraction depends on intricate interactions between Ca2+ activation of the troponin–tropomyosin complex and the kinetics of the resulting cross‐bridge formations (Gordon et al. 2000). Accordingly, increased myofibrillar Ca2+ sensitivity can, in principle, be due to augmented Ca2+ binding to troponin or altered cross‐bridge kinetics (Brenner, 1988; MacIntosh, 2003). To distinguish between these two mechanisms, two sets of experiments were performed on isolated fast skeletal muscle troponin complexes. First, isothermal titration calorimetry experiments provide results consistent with a direct binding of CK‐2066260 to the isolated troponin complexes (see Fig. 3 A). Second, stopped‐flow experiments show a ∼2.7‐fold slower rate of Ca2+ dissociation from the troponin complexes in the presence of CK‐2066260 (see Fig. 3 B), which is consistent with an increase in troponin Ca2+ affinity. On the other hand, the maximum rate of cross‐bridge cycling was not affected by CK‐2066260 in either isolated myofibrils (see Fig. 1 A) or gastrocnemius muscles stimulated in situ (see Fig. 4 C). Moreover, CK‐2066260 did not affect the maximum isometric force production in either rat EDL muscles stimulated in situ (see Fig. 4 B) or isolated mouse FDB fibres ex vivo (see Fig. 7 B). Although these measures of cross‐bridge function are not directly related to the cross‐bridge kinetics that influence myofibrillar Ca2+ sensitivity (Brenner, 1988), they still indicate that CK‐2066260 caused no major changes in cross‐bridge function. Thus, several lines of evidence indicate that the Ca2+ sensitizing effect of CK‐2066260 is due to a direct facilitation of Ca2+ binding to the troponin complex.
Troponin acts as a rapid myoplasmic Ca2+ buffer (Gordon et al. 2000; Baylor & Hollingworth, 2007). At the same stimulation frequency, this means that the CK‐2066260‐induced increase in troponin Ca2+ binding would tend to decrease [Ca2+]i during contractions. Accordingly, our mean data show a tendency for [Ca2+]i to decrease during submaximal contractions in the presence of 5 or 10 μm CK‐2066260 (see Figs 5 B and 6 B), but this decrease did not reach statistical significance. Thus from a quantitative perspective, the extra Ca2+ bound to troponin during CK‐2066260 exposure was too small to be detected as a decrease in tetanic [Ca2+]i, which is consistent with modelling of Ca2+ fluxes that show a net increase in the Ca2+ bound to troponin only for the first action potential of a tetanus (Baylor & Hollingworth, 2007).
CK‐2066260 markedly increased the force produced in response to submaximal stimulation both in rat EDL muscle in situ and isolated mouse FDB fibres ex vivo, whereas it had no effect on maximum force production. This difference between submaximal and maximal contractions is a consequence of the sigmoidal force–[Ca2+]i relationship (see Fig. 7 D): at the steepest portion of the relationship (i.e. corresponding to low submaximal stimulation frequencies), a small change in [Ca2+]i or Ca2+ sensitivity will have a large effect on force production. Importantly, CK‐2066260 did not increase [Ca2+]i, but rather it increased myofibrillar Ca2+ sensitivity, which then resulted in markedly increased force production. At high stimulation frequencies, on the other hand, the contractile machinery approaches Ca2+ saturation, the available cross‐bridges are already activated, and at this point increased [Ca2+]i or Ca2+ sensitivity will not affect maximal force production.
CK‐2066260 induced a slowing of relaxation in isolated single FDB fibres, especially when the experiments were performed at a lower temperature (26 vs. 31°C). To test whether this slowing was a direct consequence of the increased myofibrillar Ca2+ sensitivity, we translated tetanic [Ca2+]i records of single FDB fibres into force using their steady‐state force–[Ca2+]i relationship (see Fig. 7). The [Ca2+]i‐derived force records obtained in this way represent the situation that would occur if force responded to changes in [Ca2+]i without any delays. The relaxation of [Ca2+]i‐derived force was virtually identical in the absence and presence of CK‐2066260 when only taking into account the increase in Ca2+ sensitivity, which means that the CK‐2066260‐induced increase in myofibrillar Ca2+ sensitivity as such cannot explain the slowed relaxation. As discussed above, our results show no signs of markedly altered cross‐bridge kinetics in the presence of CK‐2066260. This implies that the slowing of relaxation with CK‐2066260 involves a step between the decrease in [Ca2+]i at the end of contraction and the subsequent cross‐bridge detachment. An obvious candidate is Ca2+ dissociation from the troponin complex, which occurred with a lower rate constant in the presence of CK‐2066260 (6 s−1) than in its absence (17 s−1). Adding the time required for Ca2+ dissociation from troponin to the modelled [Ca2+]i‐derived force records gave a half‐relaxation time similar to the real force measurements in the absence of CK‐2066260 (see Fig. 7 E). In the presence of CK‐2066260 this approach gave a half‐relaxation time that was markedly longer than in the absence of CK‐2066260, but it was still shorter than the half‐relaxation time of real force indicating the slowing of steps in the actin filament deactivation following Ca2+ dissociation from troponin. Thus, these data identify an important role of the rate of Ca2+ dissociation from troponin C and slowed actin filament deactivation for the speed of relaxation in fast‐twitch skeletal muscle fibres, which is in accordance with results obtained in myofibrils of fast‐twitch rabbit muscle with troponin C (Kreutziger et al. 2008). Nevertheless, it must be noted that this only has a minor functional consequence at temperatures within a physiological range (31°C, Bruton et al. 1998), where relaxation is fast even in the presence of CK‐2066260 (see Fig. 7 F).
One intriguing result of the above analyses using modelled [Ca2+]i‐derived force is that the relatively large increase in myofibrillar Ca2+ sensitivity induced by CK‐2066260 per se has little impact on the speed of force relaxation at the end of tetanic contractions. A similar picture emerges with pharmacological agents that affect SR Ca2+ handling. For instance, inhibition of the SR Ca2+ pumps with 2,5‐di(tert‐butyl)‐1,4‐benzohydroquinone (100 nm) or facilitation of SR Ca2+ release with caffeine (10 mm) resulted in a markedly delayed [Ca2+]i decline at the end of stimulation, but the rate of force relaxation was only marginally affected (Westerblad & Allen, 1994). Taken together these results implicate that rapid force relaxation is prioritized in mouse fast skeletal muscle fibres: the rate of Ca2+ clearance from the cytosol occurs with a safety margin and the force relaxation speed is not immediately affected by slightly delayed [Ca2+]i decline or increased myofibrillar Ca2+ sensitivity.
One potential risk with increased myofibrillar Ca2+ sensitivity is that resting [Ca2+]i may reach the concentration where a contraction is initiated and the muscle can no longer remain relaxed. This risk can be assessed by using the force–[Ca2+]i relationship measured in FDB fibres with and without CK‐2066260. The mean Ca50 decreased from 0.49 μm under control conditions to 0.30 μm in the presence of CK‐2066260. With these values, the calculated [Ca2+]i giving 1% of the maximum force decreased from 210 nm in control to 126 nm with CK‐2066260. Since the resting [Ca2+]i was ∼65 nm in both conditions, the risk of developing a contracture is increased in the presence of CK‐2066260 but the safety margin is relatively large and resting [Ca2+]i has to be at least doubled before causing any notable contractile activity (i.e. approaching 1% of the maximum force). Accordingly, we did not observe any CK‐2066260‐induced increase in resting force in either rat muscle studied in situ or isolated mouse muscle fibres.
Conclusions
The small molecule CK‐2066260 increases force production at submaximal stimulation frequencies by selectively increasing the myofibrillar Ca2+ sensitivity of fast skeletal muscle fibres. The increase in submaximal force occurs without any changes in SR Ca2+ handling, maximum cross‐bridge cycling rate, or tetanic force. This Ca2+ sensitizing effect would reduce the neural activation required for a given motor task. Thus, fast skeletal troponin activators like CK‐2066260 may be beneficial in conditions with muscle weakness, where it can facilitate physical activities and potentially counteract the development of muscle fatigue.
Additional information
Competing interests
D.T.H., J.J.H., A.C.H., K.L., N.D., A.J.R., F.I.M. and J.R.J. are current or former employees of Cytokinetics and were compensated financially for their work. The laboratory of A.J.C. and H.W. receives financial support from Cytokinetics.
Author contributions
All authors were involved in the study design. J.J.H.and K.L. performed the biochemical characterization experiments at Cytokinetics. The force and [Ca2+]i measurements of single fibres were performed by A.J.C at the Karolinska Institute. In situ experiments were performed by D.T.H., A.C.H. and N.D at Cytokinetics. The manuscript was drafted by D.T.H., A.J.C., F.I.M., H.W. and J.R.J. and all authors were involved in the revisions and approved the final, submitted version. All authors agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
The study was supported by grants from the Swedish Research Council (H.W.) and the Swedish National Centre for Research in Sports (A.J.C. and H.W.).
Acknowledgements
We would like to thank Raja Kawas, Lena Driscoll and Jessie Jia for their technical contributions.
D. T. Hwee and A. J. Cheng are both first authors.
References
- Andrade FH, Reid MB, Allen DG & Westerblad H (1998). Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol 509, 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor SM & Hollingworth S (2007). Simulation of Ca2+ movements within the sarcomere of fast‐twitch mouse fibers stimulated by action potentials. J Gen Physiol 130, 283–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B (1988). Effect of Ca2+ on cross‐bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci USA 85, 3265–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotto MA, Biesiadecki BJ, Brotto LS, Nosek TM & Jin JP (2006). Coupled expression of troponin T and troponin I isoforms in single skeletal muscle fibers correlates with contractility. Am J Physiol Cell Physiol 290, C567–C576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton JD, Lannergren J & Westerblad H (1998). Effects of CO2‐induced acidification on the fatigue resistance of single mouse muscle fibers at 28 degrees C. J Appl Physiol 85, 478–483. [DOI] [PubMed] [Google Scholar]
- Chin ER (2010). Intracellular Ca2+ signaling in skeletal muscle: decoding a complex message. Exerc Sport Sci Rev 38, 76–85. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Homsher E & Regnier M (2000). Regulation of contraction in striated muscle. Physiol Rev 80, 853–924. [DOI] [PubMed] [Google Scholar]
- Grimby L & Hannerz J (1977). Firing rate and recruitment order of toe extensor motor units in different modes of voluntary conraction. J Physiol 264, 865–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology . J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig R & Lomo T (1985). Firing patterns of motor units in normal rats. Nature 314, 164–166. [DOI] [PubMed] [Google Scholar]
- Herrmann C, Sleep J, Chaussepied P, Travers F & Barman T (1993). A structural and kinetic study on myofibrils prevented from shortening by chemical cross-linking. Biochemistry 32, 7255–7263. [DOI] [PubMed] [Google Scholar]
- Jasmin BJ & Gardiner PF (1987). Patterns of EMG activity of rat plantaris muscle during swimming and other locomotor activities. J Appl Physiol 63, 713–718. [DOI] [PubMed] [Google Scholar]
- Kreutziger KL, Piroddi N, Scellini B, Tesi C, Poggesi C & Regnier M (2008). Thin filament Ca2+ binding properties and regulatory unit interactions alter kinetics of tension development and relaxation in rabbit skeletal muscle. J Physiol 586, 3683–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntosh BR (2003). Role of calcium sensitivity modulation in skeletal muscle performance. News Physiol Sci 18, 222–225. [DOI] [PubMed] [Google Scholar]
- Margossian SS & Lowey S (1982). Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol 85 Pt B, 55–71. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Meadows JC & Merton PA (1971). Isolated single motor units in human muscle and their rate of discharge during maximal voluntary effort. J Physiol 217, 12–13P. [PubMed] [Google Scholar]
- Mounier Y, Tiffreau V, Montel V, Bastide B & Stevens L (2009). Phenotypical transitions and Ca2+ activation properties in human muscle fibers: effects of a 60‐day bed rest and countermeasures. J Appl Physiol 106, 1086–1099. [DOI] [PubMed] [Google Scholar]
- Ochala J (2010). Ca2+ sensitizers: An emerging class of agents for counterbalancing weakness in skeletal muscle diseases? Neuromuscul Disord 20, 98–101. [DOI] [PubMed] [Google Scholar]
- Ochala J, Radell PJ, Eriksson LI & Larsson L (2010). EMD 57033 partially reverses ventilator‐induced diaphragm muscle fibre calcium desensitisation. Pflugers Arch 459, 475–483. [DOI] [PubMed] [Google Scholar]
- Ottenheijm CA, Heunks LM, Sieck GC, Zhan WZ, Jansen SM, Degens H, de Boo T & Dekhuijzen PN (2005). Diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 172, 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee JD & Spudich JA (1982). Purification of muscle actin. Methods Enzymol 85 Pt B, 164–181. [DOI] [PubMed] [Google Scholar]
- Pina IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Myers JN & Sullivan MJ (2003). Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation 107, 1210–1225. [DOI] [PubMed] [Google Scholar]
- Potter JD (1982). Preparation of troponin and its subunits. Methods Enzymol 85 Pt B, 241–263. [DOI] [PubMed] [Google Scholar]
- Potter JD & Gergely J (1975). The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J Biol Chem 250, 4628–4633. [PubMed] [Google Scholar]
- Rosenfeld SS & Taylor EW (1985). Kinetic studies of calcium binding to regulatory complexes from skeletal muscle. J Biol Chem 260, 252–261. [PubMed] [Google Scholar]
- Russell AJ, Hartman JJ, Hinken AC, Muci AR, Kawas R, Driscoll L, Godinez G, Lee KH, Marquez D, Browne WF 4th, Chen MM, Clarke D, Collibee SE, Garard M, Hansen R, Jia Z, Lu PP, Rodriguez H, Saikali KG, Schaletzky J, Vijayakumar V, Albertus DL, Claflin DR, Morgans DJ, Morgan BP & Malik FI (2012). Activation of fast skeletal muscle troponin as a potential therapeutic approach for treating neuromuscular diseases. Nat Med 18, 452–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefner J, Cedarbaum JM, Cudkowicz ME, Maragakis N, Lee J, Jones D, Watson ML, Mahoney K, Chen M, Saikali K, Mao J, Russell AJ, Hansen RL, Malik F & Wolff AA (2012). Safety, tolerability and pharmacodynamics of a skeletal muscle activator in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 13, 430–438. [DOI] [PubMed] [Google Scholar]
- Smillie LB (1982). Preparation and identification of alpha‐ and beta‐tropomyosins. Methods Enzymol 85 Pt B, 234–241. [DOI] [PubMed] [Google Scholar]
- Stevens L, Bastide B, Maurage CA, Dupont E, Montel V, Cieniewski-Bernard C, Cuisset JM, Vallee L & Mounier Y (2008). Childhood spinal muscular atrophy induces alterations in contractile and regulatory protein isoform expressions. Neuropathol Appl Neurobiol 34, 659–670. [DOI] [PubMed] [Google Scholar]
- van Hees HW, Dekhuijzen PN & Heunks LM (2009). Levosimendan enhances force generation of diaphragm muscle from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 179, 41–47. [DOI] [PubMed] [Google Scholar]
- Westerblad H & Allen DG (1993). The contribution of [Ca2+]i to the slowing of relaxation in fatigued single fibres from mouse skeletal muscle. J Physiol 468, 729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H & Allen DG (1994). The role of sarcoplasmic reticulum in relaxation of mouse muscle; effects of 2,5‐di(tert‐butyl)‐1,4‐benzohydroquinone. J Physiol 474, 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Place N & Yamada T (2010). Mechanisms of skeletal muscle weakness. Adv Exp Med Biol 682, 279–296. [DOI] [PubMed] [Google Scholar]


