Abstract
Key points
A high‐fat diet (60% kcal from fat) is associated with motility disorders inducing constipation and loss of nitrergic myenteric neurons in the proximal colon. Gut microbiota dysbiosis, which occurs in response to HFD, contributes to endotoxaemia. High levels of lipopolysaccharide lead to apoptosis in cultured myenteric neurons that express Toll‐like receptor 4 (TLR4).
Consumption of a Western diet (WD) (35% kcal from fat) for 6 weeks leads to gut microbiota dysbiosis associated with altered bacterial metabolites and increased levels of plasma free fatty acids. These disorders precede the nitrergic myenteric cell loss observed in the proximal colon.
Mice lacking TLR4 did not exhibit WD‐induced myenteric cell loss and dysmotility. Lipopolysaccharide‐induced in vitro enteric neurodegeneration requires the presence of palmitate and may be a result of enhanced NO production.
The present study highlights the critical role of plasma saturated free fatty acids that are abundant in the WD with respect to driving enteric neuropathy and colonic dysmotility.
Abstract
The consumption of a high‐fat diet (HFD) is associated with myenteric neurodegeneration, which in turn is associated with delayed colonic transit and constipation. We examined the hypothesis that an inherent increase in plasma free fatty acids (FFA) in the HFD together with an HFD‐induced alteration in gut microbiota contributes to the pathophysiology of these disorders. C57BL/6 mice were fed a Western diet (WD) (35% kcal from fat enriched in palmitate) or a purified regular diet (16.9% kcal from fat) for 3, 6, 9 and 12 weeks. Gut microbiota dysbiosis was investigated by fecal lipopolysaccharide (LPS) measurement and metabolomics (linear trap quadrupole‐Fourier transform mass spectrometer) analysis. Plasma FFA and LPS levels were assessed, in addition to colonic and ileal nitrergic myenteric neuron quantifications and motility. Compared to regular diet‐fed control mice, WD‐fed mice gained significantly more weight without blood glucose alteration. Dysbiosis was exhibited after 6 weeks of feeding, as reflected by increased fecal LPS and bacterial metabolites and concomitant higher plasma FFA. The numbers of nitrergic myenteric neurons were reduced in the proximal colon after 9 and 12 weeks of WD and this was also associated with delayed colonic transit. WD‐fed Toll‐like receptor 4 (TLR4)−/− mice did not exhibit myenteric cell loss or dysmotility. Finally, LPS (0.5–2 ng·ml–1) and palmitate (20 and 30 μm) acted synergistically to induce neuronal cell death in vitro, which was prevented by the nitric oxide synthase inhibitor NG‐nitro‐l‐arginine methyl ester. In conclusion, WD‐feeding results in increased levels of FFA and microbiota that, even in absence of hyperglycaemia or overt endotoxaemia, synergistically induce TLR4‐mediated neurodegeneration and dysmotility.
Keywords: LPS, myenteric neurons, nitrergic, palmitate, TLR4, western‐diet
Key points
A high‐fat diet (60% kcal from fat) is associated with motility disorders inducing constipation and loss of nitrergic myenteric neurons in the proximal colon. Gut microbiota dysbiosis, which occurs in response to HFD, contributes to endotoxaemia. High levels of lipopolysaccharide lead to apoptosis in cultured myenteric neurons that express Toll‐like receptor 4 (TLR4).
Consumption of a Western diet (WD) (35% kcal from fat) for 6 weeks leads to gut microbiota dysbiosis associated with altered bacterial metabolites and increased levels of plasma free fatty acids. These disorders precede the nitrergic myenteric cell loss observed in the proximal colon.
Mice lacking TLR4 did not exhibit WD‐induced myenteric cell loss and dysmotility. Lipopolysaccharide‐induced in vitro enteric neurodegeneration requires the presence of palmitate and may be a result of enhanced NO production.
The present study highlights the critical role of plasma saturated free fatty acids that are abundant in the WD with respect to driving enteric neuropathy and colonic dysmotility.
Abbreviations
- BW
body weight
- FFA
free fatty acids
- GF
germ‐free
- GE
gastric emptying
- GI
gastrointestinal
- HEK
human embryonic kidney
- HFD
high‐fat diet
- IFN
interferon
- IL
interleukin
- KC
keratinocyte‐derived chemokine
- Lcn2
lipocalin2
- l‐NAME
n G‐nitro‐l‐arginine methyl ester
- LPS
lipopolysaccharide
- NADPH
nicotinamide adenine dinucleotide phosphate
- nNOS
neuronal nitric oxide synthase
- RD
regular diet
- TLR4
Toll‐like receptor 4
- WD
Western diet
- WT
wild‐type
Introduction
Obesity and high‐fat diet (HFD) consumption are associated with enteric neuronal dysfunction and gastrointestinal (GI) motility disorders (Taba Taba Vakili et al., 2015; Mushref & Srinivasan, 2013). Myenteric neurons that play a major role in the regulation of motor functions appear to be particularly sensitive to HFD consumption (Stenkamp‐Strahm et al., 2013; Reichardt et al., 2013; Rivera et al., 2014; Beraldi et al., 2014; Stenkamp‐Strahm et al., 2015; Soares et al., 2015). We previously have shown that HFD‐fed mice (60% kcal from fat) exhibited a reduced number of colonic myenteric neurons expressing neuronal nitric oxide synthase (nNOS), which in turn was associated with delayed colonic transit (Nezami et al., 2014). We recently demonstrated the important role of HFD‐induced gut microbiota dysbiosis in driving enteric neurodegeneration via Toll‐like receptor 4 (TLR4) in mice exhibiting increased plasma lipopolysaccharide (LPS) concentrations (Anitha et al., 2016). High levels of LPS induce proinflammatory cytokines release and apoptosis in cultured myenteric neurons (Arciszewski et al., 2005; Voss & Ekbald, 2014; Coquenlorge et al., 2014). This neurodegenerative process could be also mediated by saturated free fatty acids (FFA) such as palmitate (C16:0) that are abundant in HFD (Voss et al., 2013). Because endotoxaemia and hyperlipidaemia have been described in HFD‐fed mice (Sumiyoshi et al., 2006), both LPS and palmitate may simultaneously be present at the level of the myenteric plexus. Both LPS and palmitate can activate TLR4 expressed by enteric neurons (Barajon et al., 2009). However, the relative roles of LPS and palmitate in HFD‐induced enteric nitrergic neuronal loss have not yet been defined.
To address this question, we used a Western diet (WD) containing less fat than routinely used in a HFD (35% kcal from fat for WD vs. 60% kcal from fat for HFD) that has been designed to represent the typical Western dietary practices in all aspects, including caloric value and macro‐ and micronutrient composition (Hintze et al., 2012). With this diet, our goal was to assess enteric neuronal damage and determine how LPS and saturated FFA contribute to this disorder. Therefore, the present study aimed to: (i) evaluate the impact of WD consumption on myenteric nitrergic neurons and motility; (ii) characterize the importance of gut microbiota dysbiosis, TLR4 signalling, and plasma LPS and FFA levels in the development of WD‐induced neuropathy; and (iii) evaluate the synergic effects of LPS and palmitate in vitro on enteric NOS neuronal cell survival.
Methods
Ethical approval
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Emory and Georgia State Universities. Animals were killed by CO2 inhalation in accordance with IACUC guidelines.
Animals
Six‐week‐old male C57Bl/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA) and placed after acclimation on WD (34.5% calories from fat: TD.140304; Harlan Laboratories, Madison, WI, USA) or regular diet (RD) (16.9% calories from fat: TD.140305) for 3, 6, 9 and 12 weeks. Age‐matched TLR4−/− mice on a BL/10 background, also purchased from Jackson Laboratories, were fed RD or WD for 12 weeks. Female Germ‐Free (GF) wild‐type (WT) C57BL/6 mice maintained under GF conditions in the Georgia State University gnobiotic facility using Park Bioservices isolators were fed for 6 weeks. The WD was designed as described by Hintze et al. (2012), RD is the control of WD. Diet compositions provided by the manufacturer (Table 1) suggest that the relative palmitate content in WD and RD is ∼4:1. Mice were monitored for body weight (BW) and stool indices throughout the experiments. Blood glucose was measured in 6 h fasted RD and WD mice fed for 12 weeks with a Performa Accu‐Check glucose meter (Roche, Basel, Switzerland). Mice were killed after 4 h of fasting.
Table 1.
Diet compositions
| Composition | RD | WD |
|---|---|---|
| (g kg–1) | TD.140305 | TD.140304 |
| Casein | 200 | 190 |
| l‐Cystine | 3 | 2.85 |
| Corn starch | 420.086 | 230 |
| Maltodextrin | 132 | 70 |
| Sucrose | 100 | 260.322 |
| Olive oil | 0 | 28 |
| Soybean oil | 70 | 31.4 |
| Corn oil | 0 | 16.5 |
| Lard | 0 | 28 |
| Beef tallow | 0 | 24.8 |
| Anhydrous milkfat | 0 | 36.3 |
| Cholesterol | 0 | 0.4 |
| Pectin | 7.5 | 8.5 |
| Cellulose | 19.9 | 22.5 |
| Mineral mix | 35 | 35 |
| AIN‐93G‐MX (94046) | nTWD (110422) | |
| Sodium chloride | 0 | 4 |
| Vitamin mix | 10 | 10 |
| AIN93‐VX (94047) | nTWS (110423) | |
| Choline bitartrate | 2.5 | 1.4 |
| tert‐Butylhydroquinone (TBHQ), anti‐oxidant | 0.014 | 0.028 |
Plasma measurements
Blood was collected by intracardiac puncture and plasma separated by centrifugation (3000 g for 15 min at room temperature). Endotoxin was measured using an LAL assay (QCL‐1000; Lonza, Basel, Switzerland). Plasma and colonic mucosa FFA ( ≥ 16 carbons chain) were measured using a free fatty acid Quantification Kit (Abcam, Cambridge, UK). Plasma citrulline, a marker of metabolic disorder development (Sailer et al. 2013), was determined by an enzyme‐linked immunosorbent assay (BlueGene Biotech, Shanghai, China).
Adipose tissue inflammatory markers
Interferon (IFN)γ, interleukin (IL)‐6, keratinocyte‐derived chemokine (KC) (or CXCL1) and MCP1 expressions were analysed by quantitative RT‐PCR as described previously (Etienne‐Mesmin et al., 2016).Total RNA was isolated from mesenteric (fat pads surrounding the proximal colon) and epididymal adipose tissue using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) and purified using a RNeasy mini kit (Qiagen, Hilden, Germany). Quantitative RT‐PCR was performed in a CFX96 apparatus Touch Real‐Time PCR Detection System (Bio‐Rad, Hercules, CA, USA) using the iScriptTM One‐Step RT‐PCR Kit with SYBR Green (Bio‐Rad) and gene‐specific oligonucleotides primers: IFNγ (forward) 5′‐ AGCAAGGCGAAAAAGGATGC‐3′ and (reverse) 5′‐TCATTGAATGCTTGGCGCTG‐3′; IL‐6 (forward) 5′‐GTGGCTAAGGACCAAGACCA‐3′ and (reverse) 5′‐GGTTTGCCGAGTAGACCTCA‐3′; KC (forward) 5′‐TTGTGCGAAAAGAAGTGCAG‐3′ and (reverse) 5′‐TACAAACACAGCCTCCCACA‐3′; MCP1 (forward) 5′‐GCTGGAGCATCCACGTGTT‐3′ and (reverse) 5′‐TGGGATCATCTTGCTGGTGAA‐3′. Results were normalized to 36B4 (housekeeping gene).
Fecal LPS and flagellin
Fecal LPS and flagellin (TLR5 agonist) levels were quantified using human embryonic kidney (HEK)‐Blue‐mTLR4 and (HEK)‐Blue‐mTLR5 cells, respectively (InvivoGen, San Diego, CA, USA), as previously described (Chassaing et al., 2014 a).
Fecal metabolomics
Feces sampled at 6 weeks were extracted in an acetonitrile/water solution (2:1) containing a mixture of internal standards and analysed as described previously (Uppal et al., 2015). Mass spectrometry was performed using a LTQ‐Velos‐Orbitrap mass spectrometer (Thermo Fisher, San Diego, CA, USA). Metabolites were characterized according their mass/charge ratio with associated retention time using bioinformatics databases (METLIN: https://metlin.scripps.edu; HMDB: http://www.hmdb.ca). Peaks intensities analyses and heatmap were made using MetaboAnalyst, version 3.0 (www.metaboanalyst.ca).
Fecal lipocalin2
Fecal levels of lipocalin2 (Lcn2) were investigated to evaluate intestinal inflammation using Duoset murine Lcn‐2 ELISA kit (R&D Systems, Minneapolis, MN, USA), as described previously (Chassaing et al., 2012).
Enteric neuron staining
Proximal colon and distal ileum (1 cm above the cecum) were freshly dissected to isolate myenteric ganglia and longitudinal muscle layers. Proximal colon mucosa was used to measure FFA content. Myenteric neurons were stained for the neuronal marker peripherin (Millipore, Billerica, MA, USA) or nicotinamide adenine dinucleotide phosphate (NADPH)‐diaphorase (1 h of incubation with β‐NADPH diaphorase, 1 mg ml–1; nitroblue tetrazolium, 0.1 mg ml–1; and 0.3% Triton‐X 100 in PBS at 37 °C; all from Sigma‐Aldrich, St Louis, MO, USA) that colocalizes with nNOS (Belai et al., 1992). Neuronal quantifications were performed blinded on 10 images per mouse randomly chosen with the same magnification (20×) and expressed by number of neurons/field, as described recently (Carbone et al., 2016). Cryosections from 6‐week fed mice colons were stained for cleaved‐caspase3 (Cell Signaling, Beverly, MA, USA) and PGP, version 9.5 (Abcam) and five sections per mouse were used to score the cleaved‐caspase3 area within the myenteric tissue using Photoshop (Adobe Systems, San Jose, CA, USA).
GI transit
GI and colonic transit were measured in all mice 3 days and 4 h, respectively, before death, using dye‐propagation and bead expulsion techniques, as reported previously (Anitha et al., 2016). Gastric emptying (GE) and small intestinal transit were investigated in 9‐week fed mice, as described previously (Anitha et al. 2006).
Cell culture
IM‐FEN cells were cultured with modified N2 medium (33 °C, 5% СO2) as described previously (Anitha et al., 2008). Differentiation was induced by changing medium to Neurobasal‐A Medium (Gibco, Thermo Fisher, CA, USA) supplemented with 1% FBS, 2 mm l‐glutamine, 100 mg ml–1 streptomycin (all from Sigma‐Aldrich), B27 (0.1x; Gibco) and glial cell‐derived neurotrophic factor (GDNF) (10 ng ml–1; Shenandoah Biotechnology Inc., Warwick, PA, USA) for 5 days at 39 °C before treatment. Palmitate (100 mm stock solution in isopropanol), LPS (in ng ml–1, 1 ng ≈ 10 EU) and N G‐nitro‐l‐arginine methyl ester (l‐NAME; NOS inhibitor) were provided by Sigma‐Aldrich and added to the medium for an additional 24 h. Cell survival was then investigated using the MTS [3‐(4,5‐dimethylthiazol‐2‐yl)‐5‐(3‐carboxymethoxyphenyl)‐2‐(4‐sulfophenyl)‐2H‐tetrazolium] assay (Promega, Madison, WI, USA) as reported previously (Nezami et al., 2014). Data from five or more experiments were used in the statistical analysis.
Western blotting
Western blot analysis was performed as described previously (Anitha et al., 2016). Lysates obtained from remaining cells after 24 h of treatment were used to probe for nNOS and PGP9.5 sing nNOS (BD Biosciences, San Jose, CA, USA) and PGP9.5 specific antibodies (Abcam). Β‐actin (Cell Signaling) was used as a loading control. Data from three or more experiments were used for the statistical analysis.
Statistical analysis
Statistical analyses were performed using Student's t or Mann–Whitney tests with Prism software (GraphPad Inc, La Jolla, CA, USA) as Pearson's correlations. Data numbers are reported as appropriate. For clarity, only RD and WD animals fed for similar durations were compared together. Results from parametric data are presented as the mean ± SEM. P < 0.05 was considered statistically significant (* P < 0.05, ** P < 0.01, *** P < 0.001).
Results
WD increased body‐weight and plasma FFA but had no effect on LPS and glucose levels
Our first step was to characterize whether WD consumption results in obesity and diabetes analogous to that previously observed in response to HFD (60% kcal from fat). Therefore, we monitored BW gain and blood glucose in RD and WD mice fed for 3, 6, 9 and 12 weeks. Compared to RD, WD mice gained 21% and 42%, respectively, after 6 and 12 weeks (Fig. 1 A). Increased mesenteric adiposity contributed to the BW increase at 12 weeks (Fig. 1 B), although this was not associated with a significant elevation in blood glucose or plasma citrulline (Fig. 1 C and D). Next, we measured levels of LPS and FFA, both of which can potentially activate TLR4. Plasma LPS was not altered in all groups of WD‐fed mice (Fig. 1 E); on the other hand, plasma FFA were found to be increased only in 6‐week WD‐fed mice (+18%) (Fig. 1 F). In addition, colonic mucosal/submucosal FFA had a tendency to be increased after 6 weeks (Fig. 1 G). To investigate whether adipose tissue inflammation is responsible for this FFA increase, we measured proinflammatory gene expression in both mesenteric and epididymal tissues from 6‐week fed RD and WD mice. No significant changes were observed in IFNγ, IL‐6, KC and MCP1 expression (Fig. 1 H), suggesting together that 6 weeks of WD feeding induced neither metabolic endotoxaemia, nor adipose tissue inflammation, as observed previously in HFD‐fed mice (60% kcal from fat) (Kim et al., 2012; Anitha et al., 2016).
Figure 1. WD increased body‐weight gain and induced transitory increase of FFA after 6 weeks of feeding.
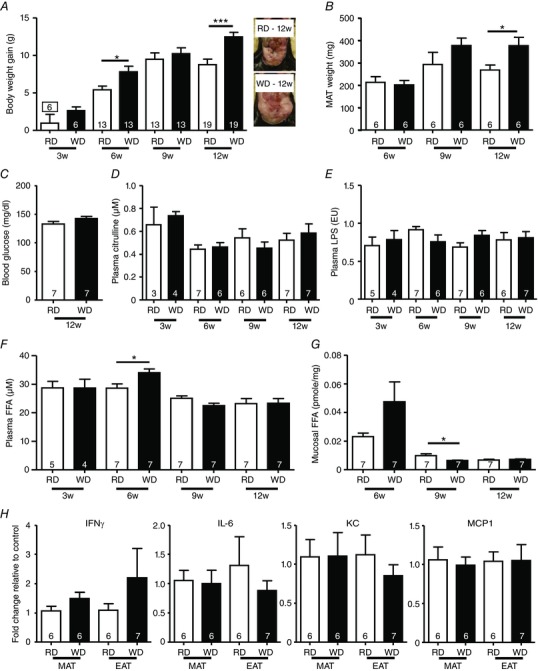
A, body weight gain of RD or WD mice fed for 3, 6, 9 and 12 weeks and representative pictures of visceral fat from 12‐week fed‐mice. B, mesenteric fat pads from 6, 9 and 12 weeks. C, blood glucose from 12‐week fed mice. Plasma levels of citrulline (D), LPS (E) and FFA (F) in RD and WD mice fed for 3, 6, 9 and 12 weeks. G, FFA levels from proximal colon mucosa/submucosa from RD or WD mice fed for 6, 9 and 12 weeks. H, mesenteric (MAT) and epididymal adipose tissue (EAT) mRNAs expression of IFNγ, IL‐6, KC and MCP1 from WT mice fed for 6 weeks with a RD or a WD. [Color figure can be viewed at wileyonlinelibrary.com]
WD induced gut microbiota dysbiosis without intestinal inflammation
Because diet plays a key role in microbial ecology, we assessed the concentration of two bacterial products, LPS and flagellin, which are increasingly being used to reflect microbiota dysbiosis. Fecal LPS, but not flagellin, was significantly increased (+160%) in feces after 6 weeks of WD consumption (Fig. 2 A and B). We next subjected fecal samples of 6‐week fed RD and WD mice to linear trap quadrupole‐Fourier transform mass spectrometer metabolomics analysis from which 3370 metabolites were quantified. Among the metabolites increased in WD feces compared to RD that we identified, there were compounds related to bacterial wall metabolism (muramic acid and diaminopimelic acid); metabolites common to microbiota and host such as N‐acetyl glucosamine, tetraethylammonium, glucuronic acid, citrulline, 5‐hydroxytryptamine and hypoxanthine; and those resulting from diet consumption (palmitate, triglycerides) (Fig. 2 C). Taurocholate levels were decreased in WD‐fed mice. Finally, no alteration in fecal Lcn2 was observed, suggesting that WD‐fed mice did not exhibit intestinal inflammation (Fig. 2 D).
Figure 2. WD for 6 weeks induced gut microbiota dysbiosis.
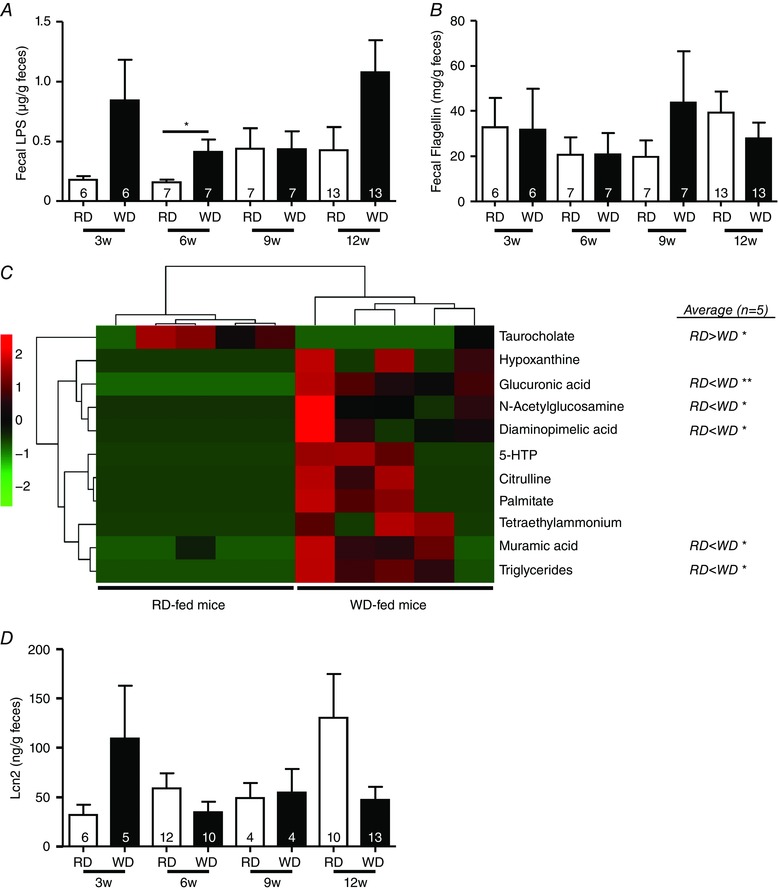
Fecal levels of LPS (A) and flagellin (B) in RD and WD mice fed for 3, 6, 9 and 12 weeks. C, heatmap showing fecal metabolites from RD and WD mice fed for 6 weeks. Statistics after data normalization and averaging (n = 5 per group) are shown on the right. D, fecal Lcn2 in RD and WD mice fed for 3, 6, 9 and 12 weeks.
WD increases plasma FFA in a microbiota‐dependent manner
We found a positive correlation between fecal LPS and plasma FFA levels in 6‐week fed WD‐mice (Fig. 3 A) and used germ‐free mice to investigate this phenomenon. In these animals, we observed a slight increase of BW gain in the 6‐week WD‐fed group (Fig. 3 B), although no change in FFA was observed (Fig. 3 C).
Figure 3. WD‐induced plasma FFA increase is microbiota‐dependent.
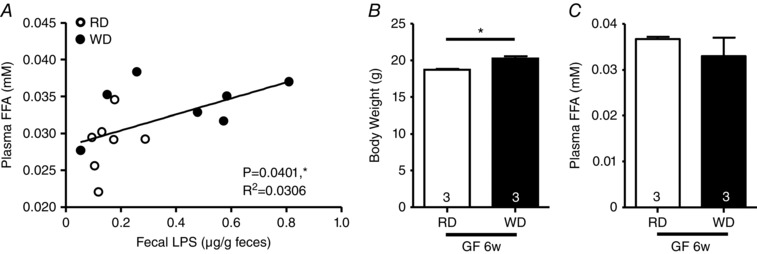
A, correlation between fecal LPS levels and plasma FFA concentrations in 6‐week fed WT mice (RD, n = 7; WD, n = 7). B, body weight gain and plasma FFA (C) in GF mice fed for 6 weeks with a RD or a WD.
WD‐fed mice display myenteric neuronal cell loss in the proximal colon but not in distal ileum
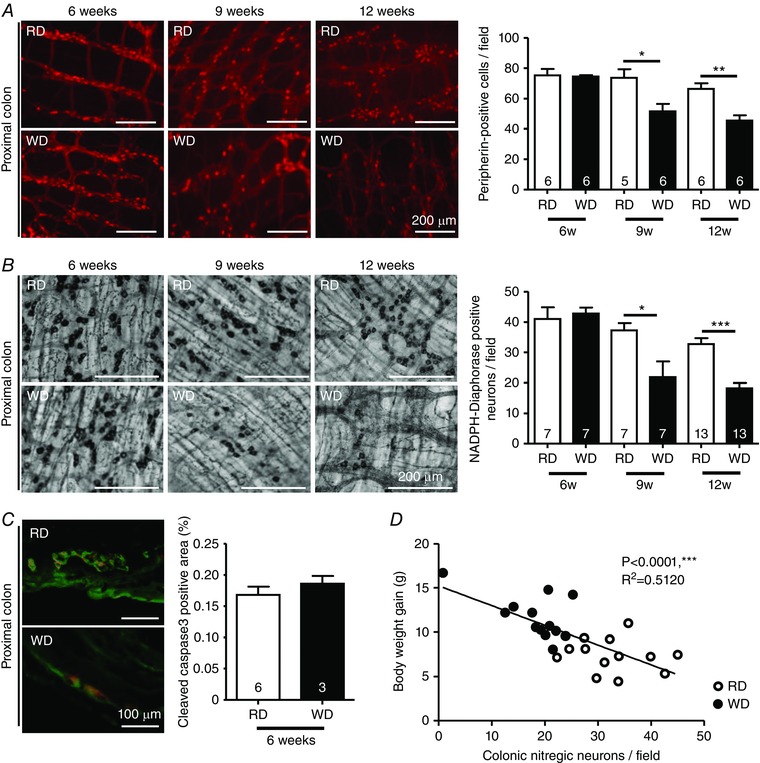
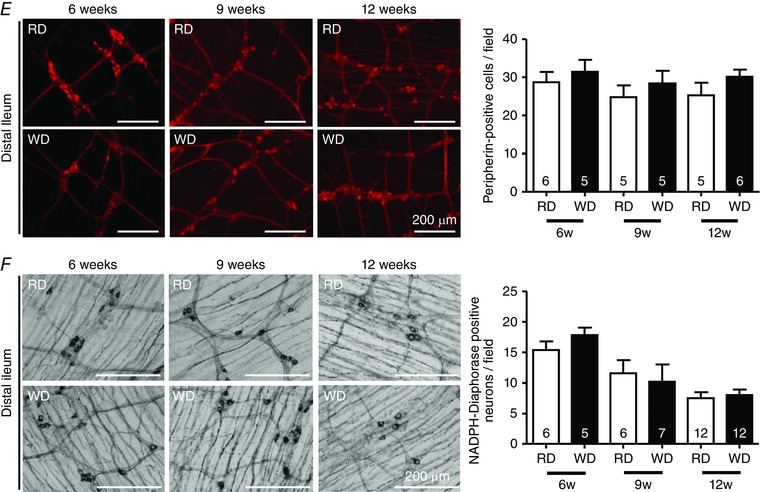
Next, we investigated the effects of WD consumption on colonic and ileal myenteric neurons after 6, 9 and 12 weeks. The total number of myenteric neurons in the proximal colon, as shown by peripherin staining, was reduced by 30% and 31%, respectively, after 9 and 12 weeks of WD (Fig. 4 A). This loss was also observed for NADPH‐diaphorase stained neurons that were reduced by 41% and 51%, respectively, after 9 and 12 weeks of WD (Fig. 4 B), although it was not preceded by increased myenteric cleaved‐caspase3 staining at 6 weeks (Fig. 4 C). Moreover, we observed that the decreased number of nitrergic neurons correlated with a greater BW gain in 12‐week fed mice (Fig. 4 D). By contrast, neither total, nor nitrergic neurons in the ileum were affected by WD (Fig. 4 E and F).
Figure 4. WD reduced the numbers of nitrergic myenteric neurons in the proximal colon.
Proximal colon myenteric neurons stained for peripherin (A) and NADPH‐diaphorase (B) in RD and WD mice fed for 6, 9 and 12 weeks. C, cleaved‐caspase3/PGP9.5 costaining in colonic cryosections from 6‐week fed RD or WD mice. D, correlation between colonic nitrergic neurons and body weight gain in mice fed for 12 weeks (n = 13 for each group). Distal ileum myenteric neurons stained for peripherin (E) and NADPH‐diaphorase (F) in RD and WD mice fed for 6, 9 and 12 weeks.
WD‐induced colonic neuronal cell loss is associated with dysmotility
We next investigated how intestinal motor functions were impacted by the WD‐induced nitrergic neurodegeneration. We observed delayed colonic transit in 12‐week WD‐fed mice (+ 144%) but not at other time points (Fig. 5 A). This was not associated with a delay of the whole GI transit at 12 weeks (Fig. 5 B). At 6 weeks, there was a delay in whole gut transit (+33%) followed by a faster transit at 9 weeks (–31%). In 12‐week fed mice, a reduced of number of nitrergic neurons was associated with a longer colonic transit time (Fig. 5 C). In addition, a delayed colonic transit correlated with an increased BW gain (Fig. 5 D). Next, we assessed the GE in 9‐week fed mice to investigate the faster GI transit; although GE was similar between RD‐fed and WD‐fed mice (Fig. 5 E), the dye propagation from the stomach tended to be faster, suggesting upper small intestinal acceleration (Fig. 5 F). Finally, we observed that WD for 6 weeks did not alter GI transit in GF mice (Fig. 5 G), indicating that gut microbiota may play a role in the delay observed in WT WD‐fed mice.
Figure 5. WD altered GI motility.
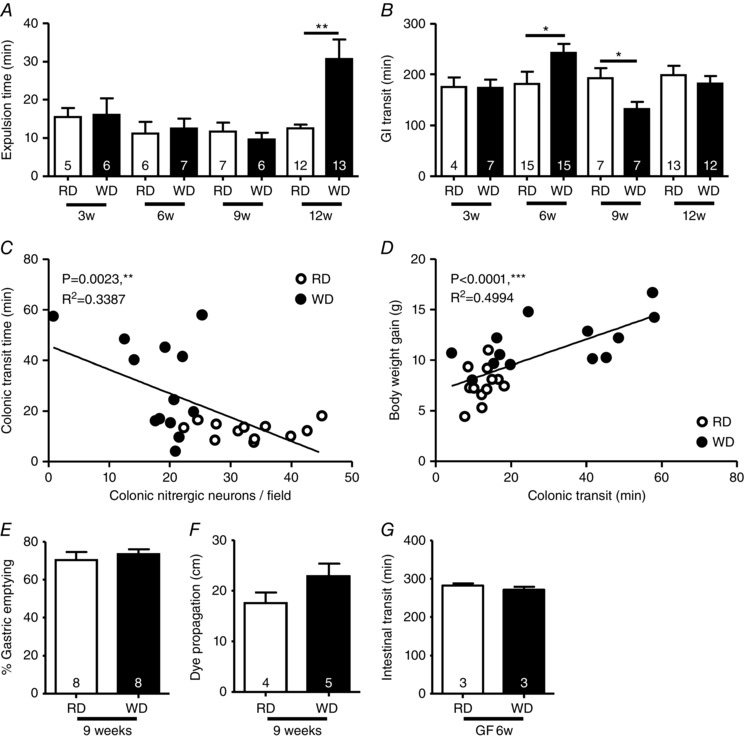
Assessment of GI in mice fed a RD or WD for 3, 6, 9 and 12 weeks, bead expulsion time (A) and dye transit (B) after oral gavage with Evans blue dye/methyl cellulose solution. Correlations between colonic nitrergic neurons and colonic transit (bead expulsion time) (RD, n = 12; WD, n = 13) (C) and between colonic transit and body weight gain (RD, n = 13, WD, n = 12) (D). GE (E) and dye propagation (F) in upper small intestine in WD‐fed mice for 9 weeks. G, GI transit in GF mice fed with a RD or a WD for 6 weeks.
TLR4−/− mice did not exhibit WD‐induced neuronal cell loss and dysmotility
Because we hypothesized that TLR4 is essential in HFD‐induced neuronal loss, we assessed the effect of WD feeding on NOS neurons and GI motility in mice lacking this receptor. Compared to WT, 12‐week WD‐fed TLR4−/− mice did not exhibit any increase in BW gain (Fig. 6 A). Fecal levels of Lcn2 were not affected by the lack of TLR4 (Fig. 6 B). However, increased levels of fecal LPS and flagellin were observed in WD‐fed TLR4−/− mice compared to RD‐fed TLR4−/− and WD‐fed WT mice (Fig. 6 C and D). Importantly, no change was seen in NADPH‐diaphorase stained myenteric neurons in the proximal colon between RD‐fed or WD‐fed TLR4−/− mice (Fig. 6 B), although both exhibited less neurons than control WT mice. The colonic transit was similar between the two groups of TLR4−/− mice (Fig. 6 F). By contrast, WD‐fed TLR4−/− mice exhibited more NADPH‐diaphorase stained cells in the distal ileum than their controls (Fig. 6 G), without an alteration in GI transit (Fig. 6 H).
Figure 6. TLR4−/− mice were protected from WD induced neuronal loss and dysmotility.
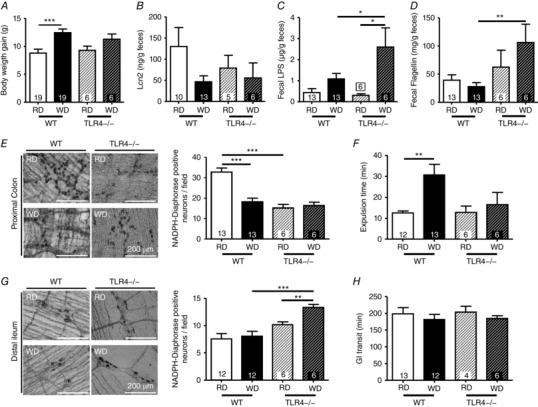
Comparisons of wild type and TLR4−/− fed a WD or RD for 12 weeks. A, body weight gain. Fecal levels of Lcn2 (B), LPS (C) and flagellin (D). E, proximal colon NADPH‐diaphorase staining of WT and TLR4−/− mice myenteric neurons. F, colonic transit measured by expulsion time (bead test). G, distal ileum NADPH‐diaphorase staining of wild‐type and TLR4−/− mice myenteric neurons. H, GI transit measurement in WT and TLR4−/− fed a RD or WD.
Synergistic effects of LPS and palmitate in inducing enteric neuronal loss are TLR4‐dependent
Having noted in vivo changes in myenteric neurons, we cultured enteric neurons in vitro in the presence of palmitate and LPS aiming to clarify the mechanisms leading to neurodegeneration. Incubation for 24 h with palmitate ≥ 30 μm induced enteric neuronal loss (Fig. 7 A). By contrast, LPS (0.5–2 ng ml–1) alone had no effect on neuronal survival (Fig. 7 B). When incubated with palmitate at 20 and 30 μm, LPS 0.5 ng ml–1 exacerbated the neuronal loss induced by palmitate alone compared to the vehicle (Fig. 7 C). This cell loss was substantially mitigated in the presence of the NOS‐inhibitor l‐NAME (200 μm). Next, we measured nNOS and PGP9.5 expression by Western blotting to estimate nNOS reduction. nNOS expression was not altered by LPS alone 0.5 ng ml–1 but significantly reduced after incubation with palmitate 0.02 or 0.03 μm ± LPS (Fig. 7 D) and this decrease was countered by the addition of L‐NAME in the milieu. PGP9.5 expression was slightly reduced after incubation with LPS 0.5 ng ml–1 alone and this reduction was even more pronounced in presence of palmitate 20 μm (Fig. 7 E), Together, these results suggest that LPS and palmitate act synergistically to damage enteric neurons via nNOS activity.
Figure 7. Synergic action of palmitate and LPS on the survival of primary enteric neuronal cells from WT mice.
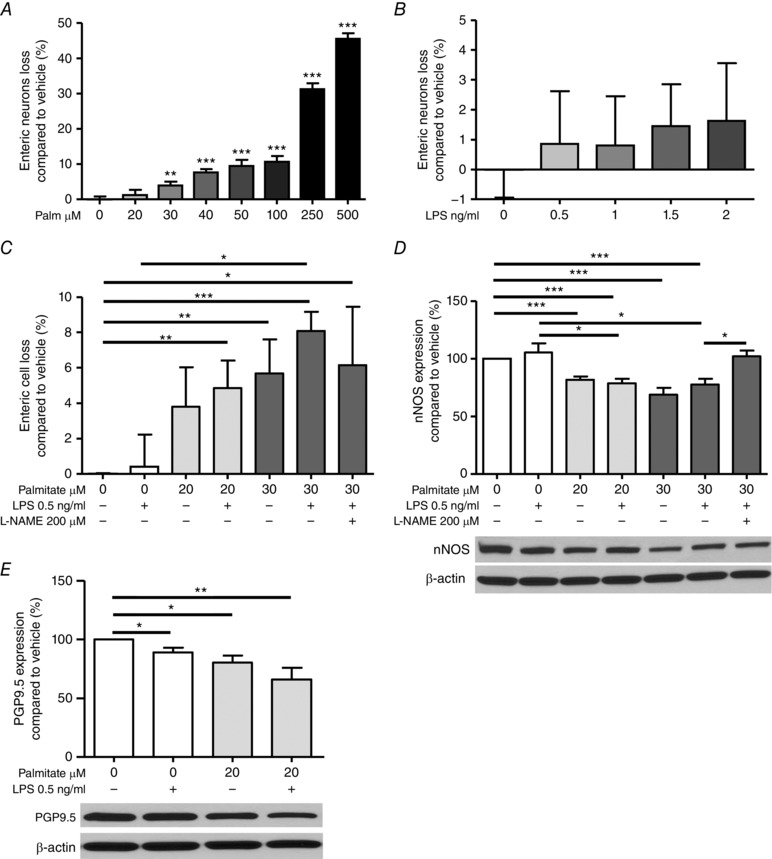
A, incubation for 24 h of an enteric neuronal cell line with palmitate (from 20 to 500 μm) induced cell loss. Results are expressed as the percentage increase in cell viability over vehicle‐treated cells. B, incubations with LPS (from 0 to 2 ng ml–1). C, co‐incubations of low LPS (0.5 ng ml–1) with palmitate 20 or 30 μm for 24 h in presence or absence of the NOS inhibitor l‐NAME (200 μm). Western blot analyses and representative gels showing nNOS (D) and PGP9.5 (E) expression after incubation for 24 h with palmitate 20 or 30 μm, +/– LPS 0.5 ng ml–1 and + l‐NAME (200 μm).
Discussion
In the present study, we report that, in mice, the consumption of a WD induces nitrergic myenteric neurodegeneration in the proximal colon via TLR4 signalling. This alteration is consequent to gut microbiota dysbiosis and plasma FFA increases after 6 weeks of WD feeding and leads to delayed colonic transit. Finally, cell culture experiments highlight that palmitate potentiates the LPS‐neurodegenerative action in enteric neurons via NO production.
One major finding is that, within 9 weeks, WD leads to colonic nitrergic myenteric neuronal loss in the absence of overt hyperglycemia, intestinal inflammation or endotoxaemia, which are three possible causes of the initiation of neuronal loss (Lakhan & Kirchgessner, 2010; Chandrasekharan et al., 2011; Anitha et al., 2016). RD and WD mice fed for 12 weeks exhibited not only similar blood glucose levels, but also no alteration of plasma citrulline, a marker of metabolic syndrome development in HFD‐fed mice (Sailer et al., 2013). Enteric cell loss in the absence of glucose intolerance has been recently reported in mice fed a high‐fat high‐cholesterol diet (21% fat, 2% cholesterol); however, this was after 33 weeks of feeding (Rivera et al., 2014). Intestinal inflammation was investigated through fecal Lcn2 measurement and was similar between RD and WD mice. Finally, we observed no variation of plasma LPS in all the groups of WD mice, although endotoxaemia has been suggested after 3 weeks of HFD containing more fat (45–60% kcal) and reported to be increased in mice exhibiting NOS neurodegeneration after 12 weeks (Hamilton et al., 2015; Anitha et al., 2016).
Fecal LPS, in contrast, was increased in WD‐fed mice, although only after 6 weeks, suggesting gut microbial alteration (Chassaing et al., 2014 b).Moreover, this alteration was associated with a FFA increase in the plasma of those mice. We measured adipose proinflammatory genes to determine adipose tissue expansion or inflammation (Bjorndal et al., 2011; Cullberg et al., 2014). However, mesenteric fat pads were not heavier after 6 weeks of WD and no alteration of genes of interest was observed in mesenteric and epididymal fat pads. In addition, a positive trend in FFA content in proximal colonic mucosa/submucosa was observed after 6 weeks. We found a positive correlation between fecal LPS and plasma FFA and tested the hypothesis that the plasma FFA increase may be related to the gut microbiota dysbiosis. GF mice fed a RD or a WD for 6 weeks did not exhibit hyperlipidaemia despite a slight body weight increase. Future studies will examine the underlying mechanisms that may involve modulation of lipoprotein lipase activity because it is known that microbiota controls the intestinal expression of a lipoprotein lipase circulating inhibitor (Bäckhed et al., 2004). We will investigate further to what extent the microbial dysbiosis might be related to dyslipidemia in WD‐fed mice.
We next analysed fecal metabolites to investigate the gut microbiota alteration suggested by the increased fecal LPS levels in WD‐mice fed for 6 weeks. Among those with a bacterial origin, we found increased levels of muramic acid reflecting increased bacterial fermentation and excretion (Sepehr et al., 2003), diaminopimelic acid produced by Gram negative and some Gram‐positive bacteria during peptidoglycan synthesis (Gillner et al., 2009) and triamethylammonium associated with gut bacterial ecology disruption (Hong et al., 2010). Moreover, a decreased concentration of the primary bile acid taurocholate in WD‐mice feces, compared to control, highlights changes in gut bacterial metabolism. We also found metabolites in WD feces related to the host metabolism: N‐acetylglucosamine, hypoxanthine produced by intestinal cells during endotoxemia (Schmidt et al., 1997), 5‐HTP suggesting higher serotoninergic metabolism as already reported in diet‐induce obese mice (Reichardt et al., 2013), citrulline and, finally, glucuronic acid, reflecting an enhanced elimination of excessive metabolites from plasma to feces via the bile (Karanam et al., 2007). Finally, we characterized metabolites related to WD consumption such as palmitate and triglycerides, and similar increases in feces have been already reported not only in HFD‐fed WT mice, but also GF mice (Rabot et al., 2010). Certain fecal metabolites, in particular bacterial compounds such as diaminopimelic acid with N‐acetylglucosamine or muramic acid with triglycerides, were simultaneously increased in a majority of WD‐fed mice compared to RD‐fed mice.
Focusing next on myenteric neurons, we observed that their total numbers were reduced in WD‐fed mice by 30% and 31% in the proximal colon after 9 and 12 weeks, respectively. These losses included NOS neurons that were concomitantly reduced by 41% and 51%. Representing 40% of the total colonic myenteric cells (Murphy et al., 2007), nitrergic neurons are more important in the proximal colon where they are essential to induce greater relaxation for fecal storage and excess fluid absorption (Takahashi & Owyang, 1998). We previously reported that they represent ∼50% in the murine proximal colon (Nezami et al., 2014; Anitha et al., 2016). By contrast, no changes were observed in the distal ileum of WD‐fed mice. Neuronal counts have all been reported as counts of nerve cells per microscope field. This method could be affected by changes in intestinal length (e.g. during inflammation); however, we did not observe any evidence of macroscopic inflammation in the intestine.
Colonic nitrergic neurodegeneration is associated with changes in motor function, as illustrated by the correlation between colonic myenteric NOS neuronal loss after 12 weeks of WD and delayed colonic transit. Moreover, a delayed colonic transit correlates with an increased body weight gain, suggesting that colonic dysmotility could be associated with obesity development. We recently highlighted a relationship between constipation and a high saturated fat diet in humans (Taba Taba Vakili et al., 2015).
By contrast to the colonic transit, the whole GI transit was delayed after 6 weeks and accelerated after 9 weeks, but not altered after 12 weeks. The transit delay after 6 weeks was not observed in WD‐fed GF mice, highlighting the interactions among microbiota and GI transit (Kashyap et al., 2013). This suggests that microbiota are critical in the delayed GI transit noted at 6 weeks. This is not neurodegenerative because cleaved‐caspase3 staining in colonic myenteric neurons was not increased. Enhanced NOS activity, however, may lead to NO overproduction within cells and oxidative stress, explaining the neuronal loss occurring in WD‐fed mice between 6 and 9 weeks of feeding (Rivera et al., 2011). The faster GI transit observed in 9‐week WD‐fed mice was first considered to be the consequence of an acceleration of the GE because such dysmotility is reported in pre‐diabetic HFD‐fed mice (Baudry et al., 2012). RD and WD mice fed for 9 weeks exhibited similar GE; however, the proximal intestine transit had a tendency to be accelerated. Enteric neuronal plasticity leading to changes in excitatory neurotransmission may contribute to this accelerated motility phenotype and this needs to be investigated further. Future studies will aim to clarify the impact of HFD on upper intestine motility, in particular the GE that is accelerated in non‐diabetic HFD‐fed models but delayed during diabetes.
Another major point is that mice lacking TLR4 do not exhibit WD colonic NOS neuron loss and the resulting colonic delay. In the ENS, both neurons and glial cells express TLR4 (Di Liddo et al., 2015). This expression is mainly found on inhibitory nerves and is stronger in the distal large bowel than in the proximal (Arciszewski et al., 2005; Barajon et al., 2009), suggesting why ileal neurons could be less sensitive to WD‐induced neurodegeneration. In addition, this receptor is important for NOS neurons development because we showed that TLR4−/− mice possessed reduced numbers of colonic nitrergic neurons (Anitha et al., 2014). This was confirmed in the present study, where NADPH‐diaphorase neurons were reduced in TLR4−/−. This may indicate that there are two populations of myenteric NOS neurons, expressing or not expressing TLR4, and further experiments will help to characterize this point.
Finally, our in vitro experiments highlight that low palmitate, similar to circulating values reported in healthy patients (Normand‐Lauziere et al., 2010), potentiates LPS‐induced enteric neurodegeneration. We observed first that increasing doses of palmitate induce neuronal loss but not LPS. Next, we saw that a low dose of LPS (0.5 ng·ml–1) amplifies the neurodegeneration cell loss initiated by palmitate (20 or 30 μm). This synergic action was confirmed by the increased reduction of PGP9.5 expression after co‐treatment with palmitate (20 μm) and LPS. This suggested that ingested saturated fat, once in the plasma, may enhance nitrergic myenteric neuron TLR4 activation by basal LPS. We next confirmed that an enhancement of NOS activity is responsible for cultured neuronal loss because this phenomenon was prevented by the addition of l‐NAME (200 μm). nNOS expression was reduced after treatment with palmitate, without further reduction when LPS was also added.
A similar synergic action between LPS and palmitate has been already reported in macrophages where they trigger inflammation (Schilling et al., 2013). Future studies will focus on mechanisms by which palmitate enhances enteric TLR4 signalling. Saturated fatty acids, in addition to being activators, also facilitate TLR4 dimerization (Wong et al., 2009). Variation at a genetic level may be also involved because we showed that TLR4 expression is increased within myenteric ganglia from HFD‐fed mice (Anitha et al., 2016). In addition, these new data demonstrate that enteric TLR4 signalling controls nNOS activity. LPS‐induced nNOS activation has been already reported in a cultured primary oligodendrocyte precursor (Yao et al., 2010). The question remains as to whether enteric TLR4 induction not only leads to nNOS over activity, but also inducible nitric oxide synthase activation as shown in macrophages, resulting in NO production and oxidative stress (Lee et al., 2005).
Taken together, our data support the idea that WD‐induced neuronal loss is initiated by a plasma FFA increase and is dependent on TLR4 signalling. We propose that the transient plasma FFA increase observed after 6 weeks of WD feeding, along with gut microbiota dysbiosis, enhances LPS action and TLR4 signalling within nitrergic myenteric neurons. This initiates nNOS overactivity, leading to neurodegeneration and colonic transit delay, respectively, as observed after 9 and 12 weeks (Fig. 8). Therefore, hyperlipidaemia is essential in HFD‐induced neuropathies. The key findings of the present study are the development of a new model representing a typical WD not associated with diabetes or inflammation, but by dysbiosis as illustrated by fecal metabolomics. There was evidence of colonic neuronal loss in this model that is prevented by lack of TLR4 and requires the synergic action of LPS and saturated fatty acids. Further studies from our laboratory will evaluate the proportion of vasoactive intestinal peptide neurons affected by WD feeding and also other neuronal phenotypes such as intrinsic primary afferent neurons, excitatory motor neurons, interneurons or interstitial cells of Cajal. Moreover, we plan to investigate liver physiology in WD‐fed mice because it was recently suggested by Rivera et al. (2014) that enteric neuron damage may contribute to the GI complications of fatty liver disease. In conclusion, the results of the present study suggest that a WD induces colonic dysmotility through myenteric neurodegeneration. Understanding these mechanisms can lead to new strategies to prevent colonic dysmotility in countries where HFDs are widespread.
Figure 8. A proposed model where the hyperlipidemia observed after 6 weeks of WD could amplify the TLR4 activation by LPS at the level of the nitrergic myenteric neurons and leads to apoptosis after 9 and 12 weeks of feeding.
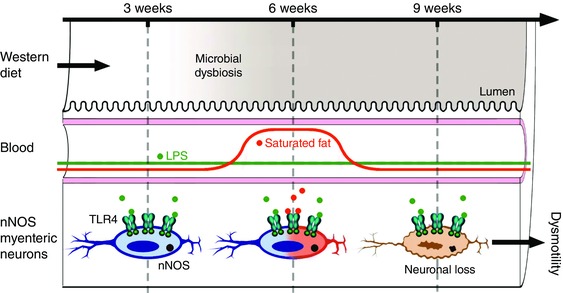
We propose that WD‐induced gut microbiota dysbiosis is accompanied by an increase of plasma saturated fat as observed after 6 weeks of feeding, initiating LPS‐induced NOS activity within TLR‐4 expressing enteric neurons. This leads to specific nitrergic neurodegeneration, as observed in WD‐fed mice for 9 and 12 weeks, and delayed colonic transit.
Additional information
Conflict of interest
The authors declare that they have no competing interests.
Authors contributions
FR, BC, DJ, ATG and SS designed the experiments, analysed and interpreted the data, and wrote the article. FR, BC, BGN, GL, ST, BL and KU collected and analysed the data. BGN, SM and M‐K revised the article critically. All authors contributed to the final version of the manuscript and approved the version submitted for publication.
Funding
This research was funded by NIH grant number NIH‐RO1‐DK080684 and a VA‐Merit Award.
Translational perspective
High‐fat diet (HFD) consumption is associated with colonic motility disorders inducing constipation and nitrergic myenteric neuronal loss in the proximal colon. The pathophysiology of these disorders may involve the bacterial component lipopolysaccharide (LPS) and the saturated free fatty acids (both increase during HFD) that activate the receptor Toll‐like receptor 4 (TLR4) expressed by enteric neurons. To understand the underlying mechanisms of HFD induced neurodegeneration and dysmotility, mice were fed a Western‐diet (WD) (35% Cal from fat) containing excessive saturated fat such as palmitate vs. a regular diet (16.9% Cal from fat) for 3, 6, 9 and 12 weeks. We observed that WD mice fed for 6 weeks exhibit higher fecal bacterial metabolites such as muramic acid as shown by high‐resolution metabolomics suggesting dysbiosis. They also had increased plasma FFA levels but no changes in plasma LPS. After 9 weeks, we found neurodegeneration of nitrergic myenteric neurons in the proximal colon and this was amplified after 12 weeks where it was associated with a delayed colonic transit. We finally highlighted the importance of TLR4 in this process: first by showing that mice lacking this receptor did not exhibit WD‐induced disorders, and then by inducing in vitro cell death in cultured neurons incubated with LPS and palmitate. Together, these data demonstrate that the WD‐induced loss of the nitrergic myenteric neurons is dependent of both gut microbial dysbiosis‐associated LPS increase and the plasma FFA increase, and this specific neurodegeneration contributes to the delayed colonic transit. The present study will help to clarify the underlying mechanisms leading to HFD‐induced motility disorders.
Acknowledgements
The authors would like to thank the animal facilities at Emory University, Atlanta VA Medical Center and Georgia State University.
Linked articles This article is highlighted by a Perspective by Nyavor & Balemba. To read this Perspective, visit http://dx.doi.org/10.1113/JP273888.
This is an Editor's Choice article from the 1 March 2017 issue.
References
- Anitha M, Gondha C, Sutliff R, Parsadanian A, Mwangi S, Sitaraman SV & Srinivasan S (2006). GDNF rescues hyperglycemia‐induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest 116, 344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitha M, Joseph I, Ding X, Torre ER, Sawchuck MA, Mwangi S, Hochmann S, Sitaraman SV, Anania F & Srinivasan S (2008). Characterization of fetal and postnatal enteric neuronal cell lines with improvement in intestinal neural function. Gastroenterology 134, 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitha M, Vijay‐Kumar M, Sitaraman SV, Gewirtz AT & Srinivasan S (2012). Gut microbial products regulate murine gastrointestinal motility via Toll‐like receptor 4 signaling. Gastroenterology 143, 1006–1016.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitha M, Reichardt F, Tabatabavakili S, Nezami BG, Chassaing B, Mwangi S, Vijay‐Kumar M, Gewirtz AT & Srinivasan S (2016). Intestinal dysbiosis contributes to the delayed gastrointestinal transit in high‐fat diet fed mice. Cell Mol Gastroenterol Hepatol 2, 328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciszewski M, Pierzynowski S & Ekblad E (2005). Lipopolysaccharide induces cell death in cultured porcine myenteric neurons. Dig Dis Sci 50, 1661–1668. [DOI] [PubMed] [Google Scholar]
- Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF & Gordon JI (2004). The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 101, 15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barajon I, Serrao G, Arnaboldi F, Opizzi E, Ripamonti G, Balsari A & Rumio C (2009). Toll‐like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia. J Histochem Cytochem 57, 1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry C, Reichardt F, Marchix J, Bado A, Schemann M, des Varannes SB, Neunlist M & Moriez R (2012). Diet‐induced obesity has neuroprotective effects in murine gastric enteric nervous system: involvement of leptin and glial cell line‐derived neurotrophic factor. J Physiol 590, 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belai A, Schmidt HH, Hoyle CH, Hassall CJ, Saffrey MJ, Moss J, Fostermann U, Murad F & Burnstock G (1992). Colocalization of nitric oxide synthase and NADPH‐diaphorase in the myenteric plexus of the rat gut. Neurosci Lett 143, 60–64. [DOI] [PubMed] [Google Scholar]
- Beraldi EJ, Soares A, Borges SC, de Souza AC, Natali MR, Bazotte RB & Buttow NC (2014). High‐fat diet promotes neuronal loss in the myenteric plexus of the large intestine in mice. Dig Dig Sci 60, 841–849. [DOI] [PubMed] [Google Scholar]
- Bjorndal B, Burri L, Staalesen V, Skorve J & Berge RK (2011). Different adipose depots: their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J Obes 2011, 490–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone SE, Jovanovska V, Brookes SJ, Nurgali K (2016). Electrophysiological and morphological changes in colonic myenteric neurons from chemotherapy‐treated patients: a pilot study. Neurogastroenterol Motil 8, 975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekharan B, Anitha M, Blatt R, Shahnavaz N, Kooby D, Staley C, Mwangi S, Jones DP, Sitaraman SV & Srinivasan S (2011). Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil 23, 131–138, e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT & Vijay‐Kumar M (2012). Fecal lipocalin 2, a sensitive and broadly dynamic non‐invasive biomarker for intestinal inflammation. PloS one 7, e44328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B, Koren O, Carvalho FA, Ley Re & Gewirtz AT (2014. a). AIEC pathobiont instigates chronic colitis in susceptible hosts by altering microbiota composition. Gut 63, 1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B, Ley RE & Gewirtz AT (2014. b). Intestinal epithelial cell toll‐like receptor 5 regulates the intestinal microbiota to prevent low‐grade inflammation and metabolic syndrome in mice. Gastroenterology 147, 1363–1377.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquenlorge S, Duchalais E, Chevalier J, Cossais F, Rolli‐Derkinderen M & Neunlist M (2014). Modulation of lipopolysaccharide‐induced neuronal response by activation of the enteric nervous system. J Neuroinflammation 11, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullberg KB, Larsen JO, Pedersen SB & Richelsen B (2014). Effects of LPS and dietary free fatty acids on MCP‐1 in 3T3‐L1 adipocytes and macrophages in vitro. Nutr Diabetes 4, e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Liddo R, Bertalot T, Schuster A, Schrenck S, Tasso A, Zanusso I, Conconi MT & Schafer KH (2015). Anti‐inflammatory activity of Wnt signaling in enteric nervous system: in vitro preliminary evidences in rat primary cultures. J Neuroinflammation 12, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne‐Mesmin L, Vijay‐Kumar M, Gewirtz, AT & Chassaing B (2016). Hepatocyte toll‐like receptor 5 promotes bacterial clearance and protects mice against high‐fat diet‐induced liver disease. Cell Mol Gastroenterol Hepatol 2, 584–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillner D, Armoush N, Holz RC, & Becker DP (2009). Inhibitors of bacterial N‐succinyl‐L,L‐diaminopimelic acid desuccinylase (DapE) and demonstration of in vitro antimicrobial activity. Bioorg Med Chem Lett 19, 6350–6352. [DOI] [PubMed] [Google Scholar]
- Hamilton MK, Boudry G, Lemay DG & Raybould HE (2015). Changes in intestinal barrier function and gut microbiota in high‐fat diet‐fed rats are dynamic and region dependent. Am J Physiol Gastrointest Liver Physiol 308, G840–G851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintze KJ, Benninghoff AD & Ward RE (2012). Formulation of the Total Western Diet (TWD) as a basal diet for rodent cancer studies. J Agric Food Chem 60, 6736–6742. [DOI] [PubMed] [Google Scholar]
- Hong YS, Ahn YT, Park JC, Lee JH, Lee H, Huh CS, Kim DH, Ryu do H & Hwang GS (2010). 1H NMR‐based metabonomic assessment of probiotic effects in a colitis mouse model. Arch Pharm Res 33, 1091–1101. [DOI] [PubMed] [Google Scholar]
- Karanam B, Madeira M, Bradley S, Wenning L, Desai R, Soli E, Schenk D, Jones A, Dean B, Doss G, Garrett G, Crumbley T, Nirula A & Lai E (2007). Absorption, metabolism, and excretion of [(14)C]MK‐0524, a prostaglandin D(2) receptor antagonist, in humans. Drug Metab Dispos 35, 1196–1202. [DOI] [PubMed] [Google Scholar]
- Kashyap PC, Marcobal A, Ursell LK, Larauche M, Duboc H, Earle KA, Sonnenburg ED, Ferraya JA, Higginbottom SK, Million M, Tache Y, Pasricha PJ, Knight R, Farrugia G & Sonnenburg JL (2013). Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology 144, 967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KA, Gu W, Lee IA, Joh EH & Kim DH (2012). High fat diet‐induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PloS one 7, e47713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhan SE & Kirchgessner A (2010). Neuroinflammation in inflammatory bowel disease. J Neuroinflammation 7, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Lowell CA, Lemay DG, Youn HS, Rhee SH, Sohn KH, Jang B, Ye J, Chung JH & Hwang DH (2005). The regulation of the expression of inducible nitric oxide synthase by Src‐family tyrosine kinases mediated through MyD88‐independent signaling pathways of Toll‐like receptor 4. Biochem Pharm 70, 1231–1240. [DOI] [PubMed] [Google Scholar]
- Murphy EM, Defontgalland D, Costa M, Brookes SJ & Wattchow DA (2007). Quantification of subclasses of human colonic myenteric neurons by immunoreactivity to Hu, choline acetyltransferase and nitric oxide synthase. Neurogastroenterol Motil 19, 126–134. [DOI] [PubMed] [Google Scholar]
- Mushref MA & Srinivasan S (2013). Effect of high fat‐diet and obesity on gastrointestinal motility. Ann Transl Med 1, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezami BG, Mwangi SM, Lee JE, Jeppsson S, Anitha M, Yarandi SS, Faris AB, 3 & Srinivasan S (2014). MicroRNA 375 mediates palmitate‐induced enteric neuronal damage and high‐fat diet‐induced delayed intestinal transit in mice. Gastroenterology 146, 473–483 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normand‐Lauziere F, Frisch F, Labbe SM, Behrer P, Gagnon R, Cunnane SC & Carpentier AC (2010). Increased postprandial nonesterified fatty acid appearance and oxidation in type 2 diabetes is not fully established in offspring of diabetic subjects. PloS ONE 5, e10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabot S, Membrez M, Bruneau A, Gerard P, Harach T, Moser M, Raymond F, Mansourian R & Chou CJ (2010). Germ‐free C57BL/6J mice are resistant to high‐fat‐diet‐induced insulin resistance and have altered cholesterol metabolism. FASEB J 24, 4948–4959. [DOI] [PubMed] [Google Scholar]
- Reichardt F, Baudry C, Gruber L, Mazzuoli G, Moriez R, Scherling C, Kollmann P, Daniel H, Kisling S, Haller D, Neunlist M & Schemann M (2013). Properties of myenteric neurones and mucosal functions in the distal colon of diet‐induced obese mice. J Physiol 591, 5125–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera LR, Poole DP, Thacker M & Furness JB (2011). The involvement of nitric oxide synthase neurons in enteric neuropathies. Neurogastroenterol Motil 23, 980–988. [DOI] [PubMed] [Google Scholar]
- Rivera LR, Leung C, Pustovit RV, Hunne BL, Andrikopoulos S, Herath C, Testro A, Angus PW & Furness JB (2014). Damage to enteric neurons occurs in mice that develop fatty liver disease but not diabetes in response to a high‐fat diet. Neurogastroenterol Motil 26, 1188–1199. [DOI] [PubMed] [Google Scholar]
- Sailer M, Dahlhoff C, Giesbertz P, Eidesn MK, de Wit N, Rubio‐Aliaga I, Boeckschoten MV, Muller M & Daniel H (2013). Increased plasma citrulline in mice marks diet‐induced obesity and may predict the development of the metabolic syndrome. PloS ONE 8, e63950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling JD, Machkovech HM, He L, Sidhu R, Fujiwara H, Weber K, Ory DS & Schaffer JE (2013). Palmitate and lipopolysaccharide trigger synergistic ceramide production in primary macrophages. J Biol Chem 288, 2923–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Weigand MA, Li C, Schmidt W, Martin E & Bardenheuer HJ (1997). Intestinal formation of hypoxanthine and uric acid during endotoxemia. J Surg Res 71, 61–66. [DOI] [PubMed] [Google Scholar]
- Sepehr E, Peace RW, Storey KB, Jee P, Lampi BJ & Brooks SP (2003). Folate derived from cecal bacterial fermentation does not increase liver folate stores in 28‐d folate‐depleted male Sprague–Dawley rats. J Nutr 133, 1347–1354. [DOI] [PubMed] [Google Scholar]
- Soares A, Beraldi EJ, Ferreira PE, Bazotte RB & Buttow NC (2015). Intestinal and neuronal myenteric adaptations in the small intestine induced by a high‐fat diet in mice. BMC Gastroenterol 15, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenkamp‐Strahm CM, Kappmeyer AJ, Schmalz JT, Gericke M & Balemba O (2013). High‐fat diet ingestion correlates with neuropathy in the duodenum myenteric plexus of obese mice with symptoms of type 2 diabetes. Cell Tissue Res 354, 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenkamp‐Strahm CM, Nyavor YE, Kappmeyer AJ, Horton S, Gericke M & Balema O (2015). Prolonged high fat diet ingestion, obesity, and type 2 diabetes symptoms correlate with phenotypic plasticity in myenteric neurons and nerve damage in the mouse duodenum. Cell Tissue Res 361, 411–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumiyoshi M, Sakanaka M & Kimura Y (2006). Chronic intake of high‐fat and high‐sucrose diets differentially affects glucose intolerance in mice. J Nutr 136, 582–587. [DOI] [PubMed] [Google Scholar]
- Taba Taba Vakili S, Nezami BG, Shetty A, Chetty VK & Srinivasan S (2015). Association of high dietary saturated fat intake and uncontrolled diabetes with constipation: evidence from the National Health and Nutrition Examination Survey. Neurogastroenterol Motil 27, 1389–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T & Owyang C (1998). Regional differences in the nitrergic innervation between the proximal and the distal colon in rats. Gastroenterology 115, 1504–1512. [DOI] [PubMed] [Google Scholar]
- Uppal K, Soltow QA, Promislow DE, Watchman LM, Quyyumi AA & Jones DP (2015). MetabNet: an R package for metabolic association analysis of high‐resolution metabolomics data. Front Bioeng Biotechnol 3, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss U & Ekblad E (2014). Lipopolysaccharide‐induced loss of cultured rat myenteric neurons ‐ role of AMP‐activated protein kinase. PloS ONE 9, e114044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss U, Sand E, Olde B & Ekblad E (2013). Enteric neuropathy can be induced by high fat diet in vivo and palmitic acid exposure in vitro. PloS ONE 8, e81413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K & Hwang DH (2009). Fatty acids modulate Toll‐like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species‐dependent manner. J Biol Chem 284, 27384–27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao SY, Ljunggren‐Rose A, Chandramohan N, Whetsell WO, Jr & Sriram S (2010). In vitro and in vivo induction and activation of nNOS by LPS in oligodendrocytes. J Neuroimmunol 229, 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]


