Abstract
Key points
To swallow food and liquid safely, airway protection is essential.
Upward and forward movements of the hyoid and larynx in the neck during swallowing vary in magnitude between individuals.
In healthy human adults, hyoid and laryngeal movements during swallowing were scaled by differences in initial upper airway area before swallowing.
Individuals increased laryngeal elevation during swallowing in response to increased airway opening before swallowing.
We show that when upper airway protection requirements change, individuals use an internal sensorimotor scaling system to adapt movements to maintain swallow safety.
Abstract
Hyoid and laryngeal movements contribute to laryngeal vestibule closure and upper oesophageal sphincter opening during swallowing. Evidence of an internal sensorimotor scaling system allowing individuals to achieve these functional goals is lacking. In speech, speakers adjust their articulatory movement magnitude according to the movement distance required to reach an articulatory target for intelligible speech. We investigated if swallowing is similar in that movement amplitude may be scaled by the functional goal for airway protection during swallowing, rather than by head and neck size. We hypothesized that healthy individuals adapt to their own anatomy by adjusting hyo‐laryngeal movements to achieve closure of the upper airway. We also investigated if individuals would automatically compensate for changes in their initial hyo‐laryngeal positions and area when head position was changed prior to swallowing. Videofluoroscopy was performed in 31 healthy adults. Using frame‐by‐frame motion analysis, anterior and superior hyoid and laryngeal displacement, and hyo‐laryngeal area were measured prior to and during swallowing. Kinematic measurements during swallowing were examined for relationships with pharyngeal neck length, and initial hyo‐laryngeal positions, length and area before swallowing. During swallowing, individuals altered laryngeal elevation magnitude to exceed hyoid elevation based on hyo‐laryngeal length before swallowing. Anterior laryngeal displacement was related to initial larynx distance from the spine, while hyoid elevation was predicted by pharyngeal neck length and initial hyoid distance from the mandible prior to the swallow. In conclusion, individuals automatically adapt hyo‐laryngeal movement during swallowing based on targets required for closing the hyo‐laryngeal area for safe swallowing.
Keywords: airway protection, deglutition, hyoid elevation, kinematics, laryngeal elevation, sensorimotor feedback, upper esophageal sphincter, vestibule closure
Key points
To swallow food and liquid safely, airway protection is essential.
Upward and forward movements of the hyoid and larynx in the neck during swallowing vary in magnitude between individuals.
In healthy human adults, hyoid and laryngeal movements during swallowing were scaled by differences in initial upper airway area before swallowing.
Individuals increased laryngeal elevation during swallowing in response to increased airway opening before swallowing.
We show that when upper airway protection requirements change, individuals use an internal sensorimotor scaling system to adapt movements to maintain swallow safety.
Abbreviations
- AIC
Akaike information criterion
- AP
antero‐posterior
- C
cervical vertebra
- ICC
intra‐class correlation coefficient
- PAS
penetration aspiration scale
- SI
supero‐inferior
- SP(EXP)
spatial (exponential) covariance structure
- UOS
upper oesophageal sphincter
Introduction
Swallowing or deglutition is initiated by a brainstem patterned motor response that is influenced by cortical input and sensory feedback (Jean, 2001). The cortex exerts volitional control over the onset and magnitude of neural activity for swallowing (Martin et al. 1997, 1999, 2001). Sensory feedback from the oral cavity, pharynx and larynx is crucial for initiating the brainstem swallowing response and modulating cortical activity (Miller, 1972; Jean & Car, 1979; Murray & Sessle, 1992; Martin et al. 1997; Teismann et al. 2007; Lowell et al. 2008; Soros et al. 2008). Deprivation of sensory input can be detrimental to swallow safety by altering airway protection during swallowing (Jafari et al. 2003).
When peripheral and cortical inputs exceed an activation threshold, the brainstem swallow response is triggered, which synchronizes oropharyngeal and laryngeal movements with upper oesophageal sphincter (UOS) relaxation for bolus transfer from the mouth into the oesophagus (Jean, 2001). Upper airway closure during swallowing occurs at the level of the vocal folds, ventricular folds and aryepiglottic folds (Shaker et al. 1990; Logemann et al. 1992; Ohmae et al. 1995; Kawasaki et al. 2001; Inamoto et al. 2011). The three levels constrict and close the laryngeal vestibule to protect the airway. Upward and forward displacements of the hyoid and larynx contribute to two important functions for safe swallowing: laryngeal vestibule closure (Shaker et al. 1990; Logemann et al. 1992; Ohmae et al. 1995; Kahrilas et al. 1997; Kawasaki et al. 2001; Inamoto et al. 2011) and UOS opening (Cook et al. 1989; Jacob et al. 1989; Kahrilas et al. 1991; Yokoyama et al. 2000; Williams et al. 2001).
Swallowing movements in humans may adapt to changes in anatomy and swallowing conditions throughout development. During gestation, swallows are triggered in the fetus and exhibit increasing oropharyngeal movement coordination, but they do not fully resemble adult swallows (Miller et al. 2003). Infants weaning from a liquid to semi‐solid diet develop retroflexion of the epiglottis over the laryngeal vestibule during swallowing, suggesting implicit adaptation to increases in the need for airway protection (Crompton et al. 1997, 2008). The epiglottis in the infant overlaps with the soft palate and appears to enable a strong oral cavity seal during suction build‐up associated with suck–swallow–breathe sequences (Delaney & Arvedson, 2008). As craniofacial development and laryngeal descent occurs, the epiglottis no longer overlaps with the soft palate and the biomechanical dynamics shift toward what we see in adults (Delaney & Arvedson, 2008). Despite facial growth and laryngeal descent between infancy and adolescence, the spatial relationships among the mandible, hyoid and larynx are maintained (Lieberman et al. 2001). This may facilitate adaptation of swallow‐related hyo‐laryngeal movements to anatomical changes, and maintain safe deglutition throughout growth and development (Lieberman et al. 2001). In a mature swallowing system, healthy individuals automatically adjust swallowing movement magnitudes when eating and drinking food and liquid of different volumes and textures (Kahrilas et al. 1996; Ishida et al. 2002), and when swallowing with different head positions (Leigh et al. 2015). These findings suggest that a central swallowing system may adapt hyoid and laryngeal movements to changes in anatomy and swallowing conditions, based on sensory feedback over years of swallowing experience.
It is unclear how swallowing movements are scaled by the sensorimotor system, given considerable variation in hyoid and laryngeal movements observed during swallowing (Molfenter & Steele, 2011). Do individuals adapt swallowing movements to body size, or to the spatial relationships among individual structures required for upper airway closure and UOS opening? Either of these scaling mechanisms may be subject to gradual changes associated with ontogeny, allowing the sensorimotor system for swallowing to slowly adjust. However, the spatial configuration among swallowing structures may also be altered, in which case the system may compensate immediately (Gay et al. 1994) or require sufficient repeated episodes to adapt (Humbert et al. 2013). In walking, stride length can be scaled by leg length (Hof, 1996; Carty & Bennett, 2009). Swallowing may be similarly scaled by overall size, as 14% of the variance in hyoid elevation magnitudes across healthy individuals could be explained by differences in pharyngeal neck length (Molfenter & Steele, 2014), or by differences in height between individuals (Leonard et al. 2000).
Despite the vital role of laryngeal excursion and laryngeal vestibule closure in airway protection during swallowing, factors determining how laryngeal movement and vestibule closure are scaled by the sensorimotor system are unknown. The relative ease in measuring the hyoid in motion analysis of swallowing may have obliterated the importance of examining movement adaptation for laryngeal vestibule closure. Past studies have found body height to be unrelated to the extent of laryngeal approximation to the hyoid during swallowing (Leonard et al. 2000), and the distance between the mandibular symphysis and the larynx did not predict anterior laryngeal displacement (Kern et al. 1999).
An alternative to the scaling of swallowing movement by overall size is that swallowing may resemble other forms of skilled motor control such as speech production. In this case, movement magnitude may be predicted by the travel distance required to reach the movement target. Jaw displacement during speech was similar between children and adults, and among adults of varying orofacial sizes (Riely & Smith, 2003). This suggested that speech movement amplitudes may be independent of overall facial size, but instead depended upon the distance required to approximate the articulators for intelligible speech. As movement targets may not vary despite differences in facial size, few differences in movement magnitudes are seen. On the other hand, when perturbation was applied to the lower lip, abruptly increasing the distance between the lower and upper lip during bilabial closure, individuals immediately compensated by increasing upper lip lowering to achieve bilabial closure (Gracco & Abbs, 1985). Thus altering distance away from a functional movement target resulted in adjustments in movement to maintain speech intelligibility.
We conducted two studies to examine if the adaptation of hyoid and laryngeal displacements in normal swallowing is driven predominantly by differences in overall body size (i.e. size dependent), or by differences in movement targets (i.e. goal directed). Hyoid and laryngeal displacements may be scaled by the extent of movement required to move the larynx to the hyoid to close the vestibule. In the first study, we examined if differences in movement requirements among healthy individuals due to differences in anatomy would predict hyoid and laryngeal displacement magnitudes required for airway protection during swallowing. We hypothesized that individual differences in the positions among the hyoid, larynx, mandible and cervical spine at rest would better predict the extent of hyoid and laryngeal movements required for safe swallowing than pharyngeal neck length. If so, then we would expect less variation across individuals after normalizing hyo‐laryngeal displacements by the resting dimensions of these structures prior to swallowing. Sex differences in hyo‐laryngeal displacements due to size would also be expected to diminish after spatial normalization.
In the second study, we manipulated hyo‐laryngeal spatial configurations at rest by changing head positions to determine if healthy individuals would immediately compensate by altering hyo‐laryngeal displacement magnitudes during swallowing. We hypothesized that changes in head position, which alter the spatial relationships among structures, would be compensated for by changes in movement magnitudes to maintain safe swallowing.
Methods
Study 1
Subjects and ethical approval
Healthy adult volunteers between 20 and 80 years old gave written informed consent to participate in protocols approved by the Institutional Review Boards (IRBs) at James Madison University (14‐0288) and Sentara Rockingham Memorial Hospital (RMH) Medical Centre (14‐04). Volunteers were excluded based on a screening questionnaire if they had swallowing difficulty, history of neurological disorder affecting swallowing function, acid reflux diagnosed by a physician, or history of head and neck cancer. De‐identified archived video recordings of healthy volunteers gathered under an IRB approved protocol (99‐N‐0178) from the National Institute of Neurological Disorders and Stroke were also used. The study conformed to the standards set by the Declaration of Helsinki.
Procedure
A radio‐opaque ball with a 19 mm diameter was taped on the subject's neck posterior to the spine for calibration of pixels into millimetres. A digital Siemens fluoroscope (Model AXIOM Luminos TF) captured a lateral view of the anterior neck from the trachea and below the UOS, to the posterior spine from the first (C1) to the 6th (C6) cervical vertebra, and superiorly to the floor of the nasal cavity (Fig. 1). The examiner delivered 5 ml of thin liquid barium (Varibar; 40% w/v) orally by syringe to the subject. This liquid consistency and volume are consistent with that used for clinical videofluoroscopic examinations of swallowing in patients with dysphagia (Martin‐Harris et al. 2008), which will allow future comparisons to be made with a clinical population. The fluoroscope was turned on and the examiner instructed the subject to swallow the entire bolus in one swallow, on command to ‘swallow now’. Each subject completed a least one swallow trial of 5 ml thin liquid. Magnification was unchanged throughout the swallow. Each fluoroscopic swallow trial was captured at 30 frames s−1 to synchronize with the digital fluoroscopic pulsing rate of 30 frames s−1, and saved in .avi format using a D‐scope System (D‐scope Systems, Brooklyn, NY, USA).
Figure 1. Left lateral view of a fluoroscopic video frame with points showing structures tracked in the x and y dimensions during motion analysis.
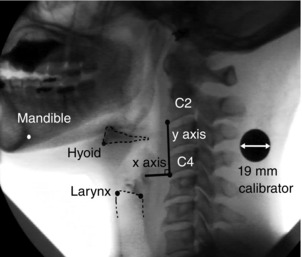
Points shown are: antero‐inferior corner of the 2nd cervical vertebra (C2); antero‐inferior corner of the 4th cervical vertebra (C4); antero‐inferior corner of the hyoid bone (representing the hyoid); antero‐superior corner of the subglottic air column (representing the larynx); postero‐superior corner of the subglottic air column; and postero‐inferior corner of the mandibular symphysis (representing the mandible). The y‐axis intersects the antero‐inferior corners of C2 and C4. The x‐axis is at 90 deg to y and intersects the origin at the antero‐inferior corner of C4. The hyoid bone and the superior aspect of the subglottic air column are outlined.
Data processing
Recordings were imported into Peak Motus 8.5 (Vicon Denver, Centennial, CO, USA) for distance calibration and two‐dimensional motion analysis. Whenever archived recordings comprised more than one 5 ml thin liquid trial per subject, the first of these trials was analysed to be consistent with other subjects who completed only one swallow trial.
Measurement of airway protection
Each de‐identified videofluoroscopic swallow was rated by the first investigator on the penetration–aspiration scale (PAS) (Rosenbek et al. 1996) to assess the integrity of airway protection.
Conversion into millimetres
The diameter of the calibration ball was measured for each swallow trial when the head of the bolus reached the angle of the mandible. As fluoroscopic magnification remained unchanged, the same scaling factor was automatically applied to all other frames in the same recording.
Spatial analysis
The antero‐inferior corner of the 4th cervical vertebra (C4) was the origin for the x‐ and y‐axes in the horizontal and vertical dimensions, respectively. The y‐axis connected the origin to the antero‐inferior corner of the 2nd cervical vertebra (C2), while the x‐axis was perpendicular to the y‐axis at the origin (Fig. 1). A spatial model of the measurement points for motion analysis was set up in Peak Motus 8.5, where the position of each point was tracked manually frame‐by‐frame using a cursor. Points tracked on each frame of the video recording were: (i) antero‐inferior corner of C2; (ii) antero‐inferior corner of C4; (iii) antero‐inferior corner of the hyoid bone (representing the hyoid); (iv) antero‐superior corner of the subglottic air column (representing the larynx); (v) postero‐superior corner of the subglottic air column; and (vi) postero‐inferior corner of the mandibular symphysis (Fig. 1). Segmental distances among these measurement points, to be explained further, were also derived from each frame.
Motion analysis began before swallowing on the frame 1 s before the head of the bolus reached the angle of the mandible, and continued until 1 s after the tail of the bolus passed the antero‐inferior corner of C6 (Fig. 2). If hyoid and larynx movement had already begun 1 s before the bolus head reached the mandibular angle, then motion analysis began closer to the start of the fluoroscopic recording to capture the resting positions of the hyoid and larynx while the bolus was held in the oral cavity. The rationale for measuring positions prior to the bolus reaching the mandible was that motor planning for hyoid and laryngeal motion probably depends upon oral sensation of the bolus in the mouth and the spatial configuration of the pharyngeal and laryngeal structures prior to swallowing (Humbert et al. 2012). Furthermore, the bolus‐hold position prior to swallowing was found to be a stable reference across videofluoroscopic studies for kinematic measurements of swallowing (Leonard et al. 2004).
Figure 2. Time series plot of hyoid x (anterior) displacement across time in a subject.
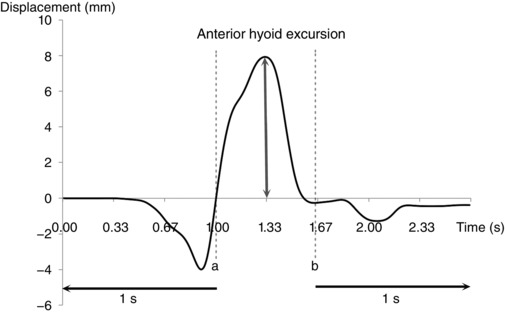
Dotted line (a) is the time when the bolus head reached the angle of the mandible. Dotted line (b) represents the time when the tail of the bolus passed the level of the 6th cervical vertebra (C6). Motion tracking began 1 s before time (a) and ended 1 s after time (b). Initial x position at the first data point was transposed to a displacement of 0 mm. Anterior hyoid excursion was the difference between the maximum and initial x positions.
Filtering the kinematic time series data
A fourth‐order zero time lag Butterworth low‐pass filter with a cutoff frequency of 4 Hz was applied within Peak Motus 8.5 to smooth the time series kinematic data for x and y over time. As recursive forward and backward passes were made during filtering, no time lag occurred. The smoothed position and segmental distance time series data were exported into MATLAB R2013a (The Mathworks, Inc., Natick, MA, USA).
Anatomical measurements made at rest before swallowing
Pharyngeal neck length between C2 and C4 was previously found to be highly correlated with body height (Molfenter & Steele, 2014) and therefore represented body size (Table 1, Fig. 3). At rest, measurements were made of distances between the hyoid and larynx. First, the distance between the antero‐inferior hyoid and the postero‐superior corner of the subglottic air column was measured as the hyo‐laryngeal length (Table 1, Fig. 4 A). Previous studies have measured laryngeal vestibule opening by the width between the epiglottic base and the arytenoids (Welch et al. 1993; Inamoto et al. 2011), or the height between the hyoid and the antero‐superior subglottic air column before swallowing (Bülow et al. 1999; Leonard et al. 2000; Kuhl et al. 2003). In contrast with resting position (Fig. 4 B), the undersurface of the epiglottis cannot be reliably identified during vestibule closure when the epiglottis approximates the aryepiglottic folds and the arytenoids (Fig. 4 C, and Kahrilas et al. 1997). Therefore we chose to measure the hyo‐laryngeal area from the antero‐inferior hyoid to the postero‐superior and the anterior dimensions of the subglottic air column (Table 1, Fig. 4 A). At rest, measurements of hyo‐laryngeal length, hyo‐laryngeal area, and hyoid and laryngeal positions were made from the first data point in the time series (i.e. the first frame of motion analysis; Table 1, and Figs 3 and 4 A).
Table 1.
Anatomical measurements at rest before swallowing, and kinematic measurements during swallowing obtained from motion analysis of each videofluoroscopic recording
| Anatomical measurements before swallowing (units) | Definition |
|---|---|
| Hyo‐laryngeal length at rest (mm) a | Distance between: antero‐inferior corner of the hyoid bone (representing the hyoid) and postero‐superior corner of the subglottic air column. |
| Hyo‐laryngeal area at rest (mm2) a | Area of the triangle bounded by: hyoid, antero‐superior corner of the subglottic air column (representing the larynx) and postero‐superior corner of the subglottic air column. Calculated using Heron's formula (Veljan, 2000) on the segmental distances between these 3 points. |
| Hyoid AP position (mm) a | Horizontal distance between: hyoid and y‐axis (cervical spine) where they intersect at 90 deg. |
| Hyoid to mandible distance (mm) a | Vertical distance between: hyoid and mandible. Obtained by subtracting the y coordinate of the hyoid from the y coordinate of the postero‐inferior corner of the mandibular symphysis. |
| Hyoid AP and SI position (mm2) | Area of the rectangle bounded by: hyoid AP position and hyoid to mandible distance. |
| Larynx AP position (mm) a | Horizontal distance between: larynx and y‐axis (cervical spine) where they intersect at 90 deg. |
| Pharyngeal neck length (mm) | Distance between: antero‐inferior corner of the 2nd (C2) and 4th (C4) cervical vertebrae. |
| Kinematic measurements during swallowing (units) | Definition |
|---|---|
| Laryngeal elevation (mm) a | Maximum − Initial y coordinates of the antero‐superior subglottic air column (representing the larynx). |
| Anterior laryngeal excursion (mm) a | Maximum − Initial x coordinates of the larynx. |
| Hyoid elevation (mm) a | Maximum − Initial y coordinates of the antero‐inferior corner of the hyoid bone (representing the hyoid). |
| Anterior hyoid excursion (mm) a | Maximum − Initial x coordinates of the hyoid. |
| Hyoid AP and SI excursion (mm2) | Area of the right angle triangle bounded by hyoid elevation and anterior hyoid excursion ((Hyoid elevation × Anterior hyoid excursion)/2). |
| Laryngeal–hyoid elevation difference (mm) a | Represents the extent to which laryngeal elevation exceeds hyoid elevation during swallowing. |
| Minimal hyo‐laryngeal area during swallowing (mm2) a | Minimum area of the triangle bounded by: the hyoid, larynx and the postero‐superior corner of the subglottic air column during swallowing. Obtained by applying Heron's formula (Veljan, 2000) to the time series of the segmental distances between the 3 points, and identifying the minimum value. |
AP, antero‐posterior; SI, supero‐inferior. aMeasurements used in Study 2.
Figure 3. Left lateral view of a fluoroscopic video frame showing anatomical distance and area measurements before swallowing, obtained from the first frame tracked in each video.
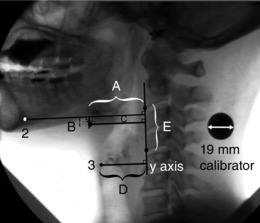
Hyoid antero‐posterior (AP) position (A, horizontal distance between the antero‐inferior corner of the hyoid bone (1) and the y‐axis); hyoid to mandible distance (B, vertical distance between the antero‐inferior corner of the hyoid bone (1) and the horizontal line connecting the postero‐inferior corner of the mandibular symphysis (2) perpendicularly to the y‐axis); hyoid AP and supero‐inferior (SI) position (C, area of the rectangle bounded by length A and height B); larynx AP position (D, horizontal distance between the antero‐superior corner of the subglottic air column (3) and the y‐axis); and pharyngeal neck length (E, distance between the antero‐inferior corners of the 2nd (C2) and 4th (C4) cervical vertebrae).
Figure 4. Measurements of the hyo‐laryngeal region.
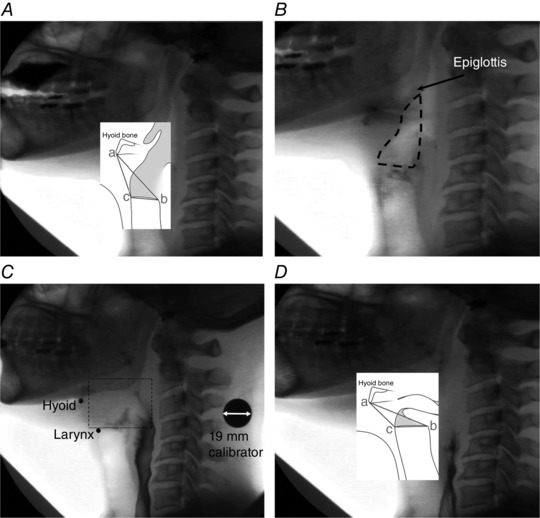
A left lateral view illustrating measurements made at rest. Hyo‐laryngeal length was from the antero‐inferior hyoid (a) to the postero‐superior corner of the subglottic air column (b). Hyo‐laryngeal area was from the antero‐inferior corner of the hyoid (a) to the postero‐superior corner of the subglottic air column (b) to the antero‐superior corner of the subglottic air column (c). B, outline of the laryngeal vestibule at rest. C, the first frame of laryngeal vestibule closure. The region bounded by the dotted rectangle shows the difficulty in frame by frame tracking of the positions of the undersurface and tip of epiglottis due to loss of airspace contrast between the epiglottis and the arytenoids, and bolus flow over the epiglottal tip. D, minimal hyo‐laryngeal area during swallowing, obtained from the minimum area of the triangle bounded by the hyoid (a), anterior larynx (c), and the postero‐superior corner of the subglottic air column (b) during swallowing.
Kinematic measurements during swallowing
Initial positions of the hyoid and larynx were linearly transposed so that all first data points had a position of 0 mm (Fig. 2). Measurements of hyoid and laryngeal excursion and minimal hyo‐laryngeal area during swallowing are defined in Table 1. As stated earlier, the epiglottal tip and its lateral surface often cannot be distinguished and may be obscured by bolus flow during swallowing (Fig. 4 C, and Kahrilas et al. 1997), interfering with measuring laryngeal vestibule closure. Therefore, we chose the antero‐inferior hyoid instead of the epiglottis as the rostral limit of the hyo‐laryngeal space in the time interval between bolus‐hold and swallow completion. The spatial measure of minimal hyo‐laryngeal area during swallowing was defined in this study as the minimum area of the triangle between the antero‐inferior hyoid and the anterior to posterior aspect of the superior subglottic air column during swallowing (Table 1, Fig. 4 D). The boundaries of this area were consistent with those of the hyo‐laryngeal area measured before swallowing (Fig. 4 A). When tracked frame‐by‐frame, they represent dynamic change in space between the hyoid and larynx from resting to the end of swallowing. Our area measurement also quantified the antero‐posterior and supero‐inferior dimensions of the space, in contrast to previous studies using a one‐dimensional measure of larynx to hyoid approximation to represent vestibule closure (Leonard et al. 2000; Kuhl et al. 2003).
Statistical analyses
Statistical analyses were conducted using IBM SPSS Statistics for Macintosh, Version 22.0 (IBM Corp, Armonk, NY, USA).
Measurement reliability
The first investigator reanalysed all videos to replicate all 14 anatomical and swallowing kinematic measurements in Table 1 for intra‐rater reliability. A second rater independently analysed 30% of the videos for inter‐rater reliability. For each measure, a single‐measure intra‐class correlation coefficient (ICC) was computed based on a two‐way random effects model (assuming the effects of subject and swallow trial were random). The indications of intra‐ and inter‐rater agreement, respectively, were determined by: (1) the absolute and percentage differences of the second data set relative to the first data set processed by the first investigator, and (2) the absolute and percentage differences between the second rater and the first data set processed by the first investigator. The first data set processed by the first investigator was used in subsequent analyses.
Relationships between anatomical and kinematic measurements
Simple linear regressions were conducted to determine if the anatomical measurements at rest predicted the kinematic measurements made for each swallow (Table 2). Linear equations were derived in the form of Y = bX + c, where b was the slope and c was the intercept. To correct for multiple predictors, α = 0.05/the number of anatomical measurements examined for movement prediction. For laryngeal elevation, three predictors were tested (α = 0.017): hyo‐laryngeal length, hyo‐laryngeal area and pharyngeal neck length at rest. For anterior laryngeal excursion, two predictors were tested (α = 0.025): larynx antero‐posterior (AP) position and pharyngeal neck length at rest. For hyoid elevation, two predictors were tested (α = 0.025): hyoid to mandible distance and pharyngeal neck length at rest. For anterior hyoid excursion, two predictors were tested (α = 0.025): hyoid AP position and pharyngeal neck length at rest. The relationship between hyoid AP and supero‐inferior (SI) excursion and hyoid AP and SI position at rest was tested (α = 0.05). The relationship between laryngeal–hyoid elevation difference and hyo‐laryngeal length at rest was tested (α = 0.05). Lastly, the relationship between minimal hyo‐laryngeal area during swallowing and hyo‐laryngeal area at rest was tested (α = 0.05). If more than one anatomical measure predicted a particular kinematic measure, a multiple regression with simultaneous entry of the predictors was conducted to examine which anatomical measure(s) contributed to predicting movement magnitude (α = 0.05). Effect sizes were determined using r 2 values.
Table 2.
Results of simple linear regressions of anatomical measurements before swallowing on movements during swallowing in 21 healthy volunteers
| Movement measurement during swallow (Y) | Anatomical measurement before swallowing (X) | r (r 2) | Equation Y = bX + c | SEb | F (1,19) | P |
|---|---|---|---|---|---|---|
| Laryngeal elevation | Hyo‐laryngeal length at rest | 0.88 (0.77) | Y = 0.86X − 9.5† | 0.11 | 62.6 | <0.001* |
| Hyo‐laryngeal area at rest | 0.85 (0.71) | Y = 0.04X + 10.0† | 0.006 | 47.3 | <0.001* | |
| Pharyngeal neck length | 0.64 (0.41) | Y = 1.3X − 22.9 | 0.37 | 13.1 | 0.002* | |
| Anterior laryngeal excursion | Larynx AP position | −0.67 (0.45) | Y = −0.46X + 21.7† | 0.12 | 15.6 | 0.001** |
| Pharyngeal neck length | 0.19 (0.04) | Y = 0.18X − 0.1 | 0.22 | 0.7 | 0.42 | |
| Hyoid elevation | Hyoid to mandible distance | 0.62 (0.38) | Y = 0.31X + 8.7† | 0.09 | 11.7 | 0.003** |
| Pharyngeal neck length | 0.66 (0.44) | Y = 0.88X − 18.3† | 0.23 | 14.7 | 0.001** | |
| Anterior hyoid excursion | Hyoid AP position | −0.28 (0.08) | Y = −0.18X + 19.8† | 0.14 | 1.6 | 0.23 |
| Pharyngeal neck length | 0.23 (0.05) | Y = 0.17X + 6.6 | 0.17 | 1.0 | 0.33 | |
| Hyoid AP and SI excursion | Hyoid AP and SI position | 0.59 (0.35) | Y = 0.06X + 46.0† | 0.02 | 10.3 | 0.005*** |
| Laryngeal–hyoid elevation difference | Hyo‐laryngeal length at rest | 0.72 (0.51) | Y = 0.42X − 5.5 | 0.09 | 19.9 | <0.001*** |
| Minimal hyo‐laryngeal area during swallowing | Hyo‐laryngeal area at rest | 0.93 (0.86) | Y = 0.62X − 4.0 | 0.06 | 113.1 | <0.001*** |
Significant relationships in multiple regression analyses are in bold. r, Pearson correlation coefficient; r 2, proportion of variance explained by the regression model; b, slope of the regression line; c, y‐intercept of the regression line; SEb, standard error of slope b; F, F ratio; P, P value; AP, antero‐posterior; SI, supero‐inferior. †Intercept significantly different from 0, P < 0.05. *Significant using corrected α = 0.017. **Significant using corrected α = 0.025. ***Significant using corrected α = 0.05.
Non‐linear exponential relationships between the above anatomical and movement measurements were also tested. This verified if linearity was a sufficient and most parsimonious representation of each relationship. Exponential relationships were expressed by the equation , where C was the y‐intercept and k was the approximate percentage change in Y for every unit increase in x. The linear and exponential relationships were compared using the goodness of fit statistic, R 2 = (1 − Residual sum of squares)/Corrected sum of squares, with larger R 2 values indicating a better fit.
Relationship between hyo‐laryngeal area reduction and hyoid and laryngeal displacements
Simple linear regressions were conducted between reduction in hyo‐laryngeal area from rest to swallowing (Hyo‐laryngeal area at rest – Minimal hyo‐laryngeal area during swallowing) and laryngeal elevation, anterior laryngeal excursion, hyoid elevation, anterior hyoid excursion, and laryngeal–hyoid elevation difference. A Bonferroni‐corrected α of 0.01 was used to correct for multiple analyses.
Effect of spatial normalization
To determine if correcting for individual differences in anatomy reduced variability in kinematic measurements of swallowing between subjects, we compared the coefficient of variation (standard deviation divided by the mean) of raw kinematic measurements of hyoid and laryngeal displacement and minimal hyo‐laryngeal area during swallowing with spatially normalized kinematic measurements. Spatially normalized measurements were derived using the formula:
However, if linear regression of the raw kinematic measurement on an anatomical measurement produced an intercept that was significantly different from 0, then this intercept was accounted for in spatial normalization. For example,
If a relationship was better represented by an exponential than a linear equation, then
To study the effect of spatial normalization on sex, differences between males and females in raw and normalized kinematic measurements were determined using independent t tests (α = 0.05).
Study 2
In Study 2, we determined if hyoid and laryngeal movement adaptation for safe swallowing changed in response to direct manipulation of the starting head position within individuals.
Subjects and ethical approval
Healthy adults between 20 and 80 years old were recruited as volunteers separately from Study 1, and gave written informed consent to participate in protocols approved by the IRBs at James Madison University (14‐0288) and Sentara RMH Medical Centre (14‐04). The same inclusion/exclusion criteria as Study 1 were followed. The study conformed to the standards set by the Declaration of Helsinki.
Procedure
The same fluoroscopic and recording equipment as those in Study 1 were used. Figure 5 shows the experiment setup. A straight metal strip was taped to the left side of the subject's face between the tragus of the left ear and the lower border of the left eye orbit. A 6 cm long straight metal rod was attached along the left side of the neck. The position of this rod was adjusted under fluoroscopy so that it was parallel to the cervical spine between C2 and C4. Each subject also wore a headband with a laser pointer attached to it just above the left ear, to project the laser beam onto a wall about 2.5 m opposite. The subject was instructed to keep the head in a comfortable position while seated on the fluoroscopy chair. To measure head tilt angle relative to the cervical spine, a digital goniometer (iGaging, St Clemente, CA, USA) was placed over the opening of the left external ear canal. The angle between the metal strip at the orbit and the metal rod on the neck was measured. This was the head tilt angle in neutral position (‘neutral angle’). A target circle 7.5 cm in diameter was attached to the wall where the laser beam projected while the subject maintained neutral head position. The subject was instructed to maintain this head position by keeping the laser beam within the boundary of the circle. A syringe containing 5 ml of thin liquid barium (Varibar, 40% w/v) was delivered orally by the examiner. The subject was reminded to keep the laser beam within the circle while holding the bolus in the mouth and throughout the swallow. The fluoroscope was then turned on and the examiner instructed the subject to swallow the entire bolus in one swallow, on command to ‘swallow now’.
Figure 5. Schematic drawing (not to scale) of the experimental setup for swallow trials with the head in a neutral position.
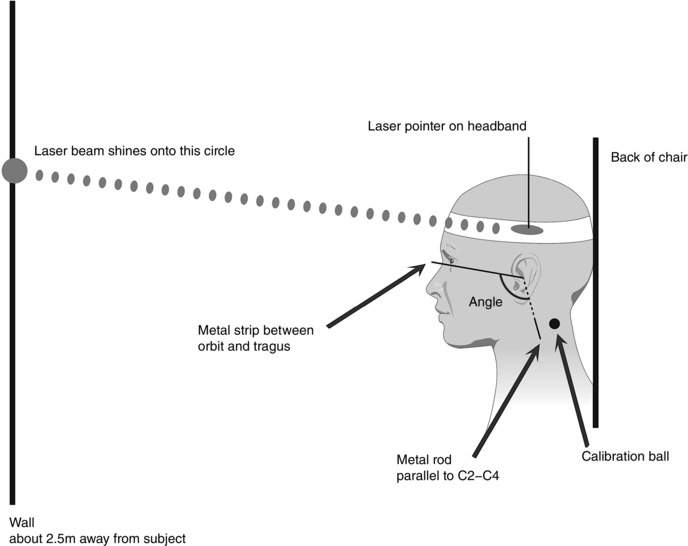
Head tilt angle was measured between the metal strip between the orbit and tragus, and the metal rod parallel to the 2nd (C2) and 4th (C4) cervical vertebrae. Head position was stabilized using visual feedback from the laser beam shining onto the circle on the wall.
Five more 5 ml thin liquid barium swallows trials used the same procedures as described, each in a different head tilt position from neutral, totalling six swallow trials per subject. For Trials 2–6, the subject was instructed to tilt the head backward, or forward and downward relative to the neutral angle measured in Trial 1, to produce head tilt angles at +5, +10, −5, −10 and −15 deg from the neutral angle. These were presented in randomized order for each subject. The order was randomized instead of counter‐balanced across subjects, as the possible number of permutations to counter‐balance five different head positions (120 combinations) far exceeded the number of subjects that could be recruited. The head tilt downward angles chosen were consistent with the angles of −11 to −19 deg from neutral as previously reported (Shanahan et al. 1993; Welch et al. 1993; Steele et al. 2011). The upper limit for head backward position was +10 deg from neutral, as head extension of more than 15 deg from neutral may affect UOS relaxation (Castell et al. 1993). With each change in head tilt angle, the examiner moved the circle target up or down the wall according to where the laser beam projected, and the subject used the laser light within the circle as visual feedback to minimize extraneous head movement during a trial.
Data processing
Six swallow trials were analysed per subject. Recordings were imported into Peak Motus 8.5 (Vicon Denver, Centennial, CO, USA) for distance calibration, two‐dimensional motion analysis and data smoothing following the same procedures as Study 1.
Measurement of airway protection
The videofluoroscopic recording of each swallow trial was rated by the first investigator on the penetration–aspiration scale (PAS) (Rosenbek et al. 1996) to assess the integrity of airway protection, without knowledge of the experimental conditions.
Anatomical and kinematic measurements
Anatomical measurements of hyo‐laryngeal positions before swallowing were a subset of those in Study 1 (Table 1) including: (a) hyo‐laryngeal length at rest; (b) hyo‐laryngeal area at rest; (c) hyoid AP position; (d) hyoid to mandible distance; and (e) larynx AP position. Additionally, two measurements were extracted from the same video frame: (f) hyoid y position, and (g) larynx y position relative to the origin at C4.
The following measurements of displacement and minimal hyo‐laryngeal area were derived during swallowing (Table 1): laryngeal elevation, anterior laryngeal excursion, hyoid elevation, anterior hyoid excursion, laryngeal–hyoid elevation difference and minimal hyo‐laryngeal area. In addition, reduction in hyo‐laryngeal area from resting to swallowing was calculated (Hyo‐laryngeal area at rest – Minimal hyo‐laryngeal area during swallowing).
Change in head tilt angle
For each swallow trial at a different head tilt position including neutral, head tilt angle relative to the cervical spine was derived from Peak Motus 8.5. The angle between the line connecting C2 to C4 and the line connecting the orbit to the tragus was measured on every frame during motion tracking on every trial. Each angle time series was smoothed using a fourth‐order zero‐phase Butterworth low‐pass filter with a cutoff frequency of 4 Hz in Peak Motus 8.5. The mean head tilt angle across all frames for each trial was then computed. For each of the six swallows produced by each subject, Change in head tilt angle relative to neutral angle = Mean head tilt angle − Neutral angle. Thus neutral head position had a change in angle of 0 deg, while positive angles greater than 0 deg indicated higher back tilt of the head position relative to neutral head position, and negative angles less than 0 deg represented lower head down position relative to neutral. These angles varied on a continuous scale rather than in stepwise increments, as the subjects were unable to produce the same degree of head tilt as targeted despite visual feedback with the laser light.
Statistical analyses
Analyses were conducted using the proc mixed command in SAS (SAS software Version 9.4 of the SAS System for Windows; SAS Institute Inc., Cary, NC, USA).
Effect of head position on hyo‐laryngeal position, length and area at rest
A linear mixed model was used to examine the relationship between change in head tilt angle relative to neutral angle and each of the following seven measurements at rest (Table 4): hyoid AP position, hyoid y position, larynx AP position, larynx y position, hyo‐laryngeal length, hyo‐laryngeal area and hyoid to mandible distance (Table 4). Change in head tilt angle was entered as a fixed effect predictor. Five mixed effects model specifications were tested in each of the seven analyses for goodness of fit based on the Akaike information criterion (AIC). The model specifications were: (1) random intercept only; (2) random intercept and random slope, with unstructured covariance between subjects; (3) no random intercept or slope, with continuous first‐order autoregressive (AR1) within‐subjects covariance structure; (4) random intercept only with continuous AR1 within‐subjects covariance structure; and (5) random intercept and random slope with unstructured between‐subjects covariance and continuous AR1 within‐subjects covariance structure. The most parsimonious model with the lowest AIC was selected for null hypothesis testing and derivation of estimates for fixed and random effects. A Bonferroni‐corrected α of 0.007 (0.05/7) was used to correct for multiple analyses.
Table 4.
Effect of change in head tilt angle from neutral on hyo‐laryngeal spatial configuration measurements at rest in 10 healthy volunteers
| Fixed effects of tilt angle on spatial configuration at rest | Random effects covariance estimate; P value | |||||||
|---|---|---|---|---|---|---|---|---|
| Spatial measurement at rest | Slope (SE) | t (d.f.) | P | Intercept (SE) | t (d.f.) | P | Slope | Intercept |
| Hyoid AP position | 0.12 (0.04) | 2.6 (9.0) | 0.03 | 39.3 (1.5) | 26.3 (9.0) | <0.0001 | 0.01; 0.06 a | 22.1; 0.02† |
| Hyoid y position | 1.11 (0.1) | 10.6 (8.8) | <0.0001* | 10.5 (3.4) | 3.1 (9.0) | 0.013 | 0.08; 0.06 a | 114; 0.02† |
| Larynx AP position | −0.02 (0.07) | −0.3 (9.5) | 0.75 | 31.1 (1.5) | 20.5 (9.0) | <0.0001 | 0.03; 0.06 a | 22.6; 0.02† |
| Larynx y position | 0.77 (0.1) | 7.5 (9.2) | <0.0001* | −19.1 (3.3) | −5.8 (9) | 0.0003 | 0.07; 0.06 a | 107; 0.02† |
| Hyo‐laryngeal length at rest | 0.26 (0.04) | 6.6 (48.2) | <0.0001* | 37.7 (2.1) | 17.9 (9) | <0.0001 | — b | 44.1; 0.02† |
| Hyo‐laryngeal area at rest | 3.39 (0.5) | 6.7 (39.6) | <0.0001* | 277.0 (36.0) | 7.7 (9) | <0.0001 | — c | 12,840; 0.02† |
| Hyoid to mandible distance | 1.03 (0.1) | 8.4 (9.1) | <0.0001* | 19.6 (4.1) | 4.7 (9) | 0.001 | 0.09; 0.09 a | 169; 0.02† |
Significant relationships are in bold. SE, standard error; t, t statistic; d.f., degrees of freedom; P, P value; AIC, Akaike information criterion; AR1, continuous first‐order autoregressive covariance structure; SP(EXP), spatial (exponential) covariance structure; AP, antero‐posterior. *Effect of change in tilt angle significantly different from 0 using corrected α = 0.007. †Intercepts differed significantly across individuals, P < 0.05. aLowest AIC in random intercept and random slope model with unstructured between‐subjects covariance. bLowest AIC in random intercept‐only model. cLowest AIC in random intercept‐only model with continuous AR1 within‐subjects covariance (covariance estimate SP(EXP) = 0.4, P = 0.05).
Effect of initial hyo‐laryngeal position, length and area on hyo‐laryngeal displacements during swallowing
Measurements of initial hyo‐laryngeal positions, length and area that were significantly predicted by change in head tilt angle, were then tested for whether they predicted changes in hyo‐laryngeal movements and minimal hyo‐laryngeal area during swallowing. For laryngeal elevation, the three possible predictors were: hyo‐laryngeal length, hyo‐laryngeal area, and/or initial y position of the larynx at rest (α = 0.017). For anterior laryngeal excursion, a possible predictor was larynx AP position (α = 0.05). For hyoid elevation, two possible predictors were: initial y position of the hyoid and/or hyoid to mandible distance (α = 0.025). For anterior hyoid excursion, a possible predictor was hyoid AP position (α = 0.05). For laryngeal–hyoid elevation difference, a possible predictor was hyo‐laryngeal length at rest (α = 0.05). For minimal hyo‐laryngeal area during swallowing, a possible predictor was hyo‐laryngeal area at rest (α = 0.05). Each predictor was entered univariately into a linear mixed effects model as a fixed effect. For each relationship, the five model specifications described above were tested for goodness of fit, and the one with the lowest AIC statistic and greatest parsimony was selected for null hypothesis testing and derivation of fixed and random effects estimates.
Relationship between hyo‐laryngeal area reduction and difference between laryngeal and hyoid elevation
We investigated if the reduction in hyo‐laryngeal area from its initial to minimum area during swallowing was predicted by laryngeal–hyoid elevation difference during swallowing at different head tilt positions (α = 0.05). Five linear mixed model specifications, as described above, were also examined for goodness of fit before null hypothesis testing based on the lowest AIC and greatest parsimony.
Results
Study 1
Subject and swallow characteristics
Swallows of 21 adults (9 males) between the ages of 20 and 69 years (mean = 39 years) were analysed in Study 1. No penetration or aspiration occurred in 15 of the swallows (PAS score 1). In six swallows, transient penetration into the vestibule above the level of the vocal folds was seen during swallowing, which was cleared spontaneously on swallow completion (PAS score 2).
Measurement reliability
Intra‐rater agreement was excellent (mean ICC coefficient = 0.95, range 0.89–0.98), and inter‐rater was good to excellent (mean ICC coefficient = 0.87, range 0.66–0.99) on the 14 measurements listed in Table 1. Laryngeal–hyoid elevation difference had the lowest inter‐rater ICC of 0.66, as inter‐rater measurement differences in both laryngeal elevation and hyoid elevation would have contributed to inter‐rater difference in this composite measurement. The average absolute difference (average percentage difference in parentheses) between measurements replicated by the same rater was 1.2 mm (7.1%) for distance or length measurements, and 33.7 mm2 (10.0%) for area measurements. The average absolute difference (average percentage difference in parentheses) between measurements derived by two different raters was 1.6 mm (9.1%) for distance or length measurements, and 32.7 mm2 (11.1%) for area measurements. Only measurements by the first investigator from the first dataset were used for all subsequent data analyses.
Predictors of hyoid and laryngeal displacements during swallowing
Simple linear regression analyses showed that laryngeal elevation during swallowing was significantly predicted by: hyo‐laryngeal length, hyo‐laryngeal area and pharyngeal neck length at rest (Table 2). However, multiple linear regression of these anatomical measurements on laryngeal elevation showed that only hyo‐laryngeal length at rest significantly predicted laryngeal elevation (slope b = 0.62, t = 2.29, P = 0.035), semipartial r 2 (sr 2)representing the unique proportion of variance explained in the multiple regression model = 0.07. Neither hyo‐laryngeal area (b = 0.01, t = 0.65, P = 0.52, sr 2 = 0.005), nor pharyngeal neck length at rest (b = 0.20, t = 0.59, P = 0.56, sr 2 = 0.004) had a unique contribution in predicting laryngeal elevation, as sr 2 was negligible in both. Exponential relationships did not perform better than simple linear models in representing these relationships; change in R 2 between the exponential and linear models (R 2 change) ≤0.02.
Pharyngeal neck length did not predict anterior laryngeal excursion (Table 2; R 2 change from linear to exponential model = 0), but larynx AP position did (Table 2). An exponential equation represented this relationship better than the linear
where C = 101.9 and k = 0.087 (R 2 change = 0.18). For every 1 mm that the larynx was closer to the cervical spine before swallowing, anterior laryngeal excursion increased by about 9% during swallowing (Fig. 6).
Figure 6. Exponential relationship between larynx antero‐posterior (AP) position before swallowing and anterior laryngeal excursion during swallowing.
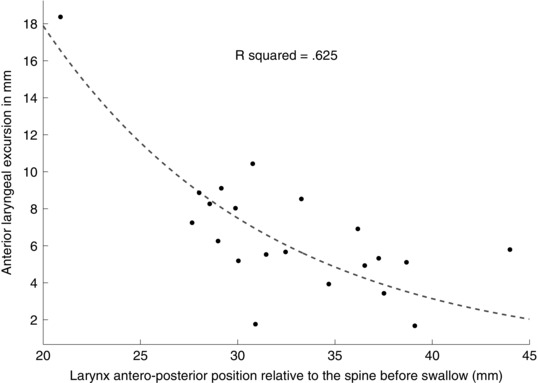
The farther forward the larynx is anterior to the cervical spine, the less anterior movement occurs during swallowing. Each dot represents one subject in Study 1 (n = 21).
Hyoid elevation was significantly related to pharyngeal neck length as well as hyoid to mandible distance (Table 2). Based on multiple linear regression, both had significant unique contributions in predicting hyoid elevation: pharyngeal neck length: b = 0.71, t = 3.60, P = 0.002, sr 2 = 0.26; and hyoid to mandible distance: b = 0.24, t = 3.20, P = 0.005, sr 2 = 0.20. Hyoid AP and SI excursion was significantly related to hyoid AP and SI position before swallowing (Table 2). Anterior hyoid excursion was unrelated to either of the anatomical measures examined (Table 2). The exponential relationships were comparable to the simple linear relationships (change in R 2 ≤ 0.02).
Laryngeal–hyoid elevation difference was significantly predicted by hyo‐laryngeal length at rest, while minimal hyo‐laryngeal area during swallowing was significantly related to hyo‐laryngeal area before swallowing (Table 2). Exponential relationships were also comparable to the linear relationships (change in R 2 ≤ 0.01).
Relationship between hyo‐laryngeal area reduction and hyoid and laryngeal displacements
Laryngeal–hyoid elevation difference significantly predicted reduction in hyo‐laryngeal area (Fig. 7), F(1,19) = 45.8, P < 0.001, b = 12.7, standard error of the slope (SEb) = 1.9, r 2 = 0.71. Laryngeal elevation also predicted the reduction in hyo‐laryngeal area (F(1,19) = 38.6, P < 0.001, b = 7.4, SEb = 1.2, r 2 = 0.67), but not anterior laryngeal excursion (F(1,19) = 3.3, P = 0.09, r 2 = 0.15), hyoid elevation (F(1,19) = 6.1, P = 0.02, r 2 = 0.24), or anterior hyoid excursion (F(1,19) = 1.3, P = 0.26, r 2 = 0.07) using a corrected α of 0.01.
Figure 7. Relationship between laryngeal‐hyoid elevation difference and hyo‐laryngeal closure during swallowing.
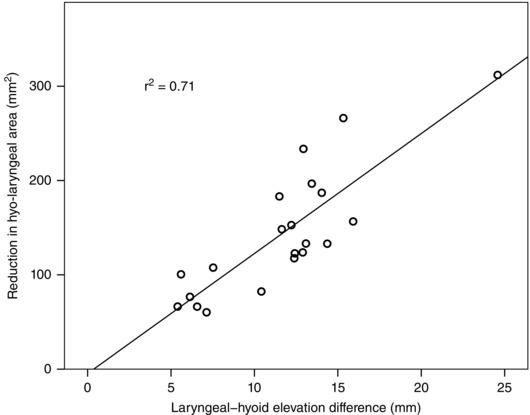
The greater the magnitude of laryngeal elevation exceeding that of hyoid elevation during swallowing, the greater the reduction in hyo‐laryngeal area from resting to swallowing. Each circle represents one subject in Study 1 (n = 21).
Effect of spatial normalization
As laryngeal elevation, anterior laryngeal excursion, hyoid elevation, hyoid AP and SI excursion, laryngeal–hyoid elevation difference, and minimal hyo‐laryngeal area during swallowing were each predicted by at least one anatomical measure, their corresponding normalized measurements were computed to examine variability after correcting for individual differences in anatomy. Normalized anterior laryngeal excursion was computed using the equation:
Normalizing laryngeal elevation as a percentage of hyo‐laryngeal length at rest reduced individual variability substantially compared to raw laryngeal elevation (Table 3). This was not the case when normalizing by hyo‐laryngeal area at rest, or by pharyngeal neck length (Table 3). Normalizing anterior laryngeal excursion by larynx AP position also reduced variability compared to raw anterior laryngeal excursion (Table 3). Normalizing hyoid elevation as a percentage of pharyngeal neck length reduced individual variability (Table 3). However, normalizing hyoid elevation by hyoid to mandible distance, and normalizing hyoid AP and SI excursion by hyoid AP and SI position increased variability (Table 3). Variability reduced when laryngeal–hyoid elevation difference was normalized by hyo‐laryngeal length at rest. Further, when minimal hyo‐laryngeal area during swallowing was normalized by hyo‐laryngeal area at rest, variability was reduced (Table 3).
Table 3.
Mean (M), standard deviation (SD) and coefficient of variation (CV) of raw and spatially normalized kinematic measurements of swallowing in 21 healthy volunteers
| Measurement | M | SD | CV | % change in CV compared to raw measurement |
|---|---|---|---|---|
| Raw laryngeal elevation (mm) | 25.7 | 7.4 | 0.29 | — |
| Laryngeal elevation as % of hyo‐laryngeal length at rest | 86.0 | 8.4 | 0.10 | −66 |
| Laryngeal elevation as % of hyo‐laryngeal area at rest | 4.3 | 1.1 | 0.25 | −14 |
| Laryngeal elevation as % of pharyngeal neck length | 69.4 | 16.5 | 0.24 | −17 |
| Raw anterior laryngeal excursion (mm) | 6.7 | 3.5 | 0.53 | — |
| Anterior laryngeal excursion relative to larynx AP position | 110.8 | 49.6 | 0.45 | −15 |
| Raw hyoid elevation (mm) | 14.0 | 4.8 | 0.34 | — |
| Hyoid elevation as % of pharyngeal neck length | 88.0 | 9.5 | 0.11 | −68 |
| Hyoid elevation as % of hyoid to mandible distance | 27.7 | 27.2 | 0.98 | +188 |
| Raw hyoid AP and SI excursion (mm2) | 89.7 | 42.7 | 0.48 | — |
| Hyoid AP and SI excursion as % of hyoid AP and SI position | 6.6 | 5.7 | 0.87 | +81 |
| Raw laryngeal–hyoid elevation difference (mm) | 11.7 | 4.4 | 0.38 | — |
| Laryngeal–hyoid elevation difference as % of hyo‐laryngeal length at rest | 28.1 | 7.7 | 0.27 | −29 |
| Raw minimal hyo‐laryngeal area during swallow (mm2) | 222.2 | 97.6 | 0.44 | — |
| Minimal hyo‐laryngeal area during swallow as % of hyo‐laryngeal area at rest | 60.4 | 9.72 | 0.16 | −64 |
AP, antero‐posterior; SI, supero‐inferior.
As expected, males had greater hyo‐laryngeal length (t = 6.1, P < 0.001, Cohen's d = 2.7), hyo‐laryngeal area (t = 10.6, P < 0.001, Cohen's d = 4.5) and pharyngeal neck length (t = 3.7, P = 0.002, Cohen's d = 1.6) than females. Sex differences in laryngeal elevation (t = 3.9, P = 0.001, Cohen's d = 1.7) diminished after correcting for hyo‐laryngeal length (t = −0.5, P = 0.6, Cohen's d = 0.2) and hyo‐laryngeal area at rest (t = −0.9, P = 0.4, Cohen's d = 0.4). However, males and females still differed after correcting laryngeal elevation by pharyngeal neck length (t = 2.7, P = 0.01, Cohen's d = 1.2). Males had greater hyoid elevation than females (t = 2.5, P = 0.02, Cohen's d = 1.0), but this difference was non‐significant after normalizing by pharyngeal neck length (t = 0.3, P = 0.8, Cohen's d = 0.1).
Study 2
Subject and swallow characteristics
Ten adults (3 males) between the ages of 21 and 66 years (mean = 47 years) participated, two of whom also participated in Study 1. Sixty swallow trials were analysed, six from each subject. Of the 60, no penetration or aspiration (PAS score 1) was observed in 46 swallows; 13 swallows had penetration into the vestibule above the level of the vocal folds with spontaneous clearance on swallow completion (PAS score 2); and one swallow received a PAS score of 4 (penetration down to the level of the vocal folds with clearance). The anterior and postero‐superior corners of the subglottic air column at rest before the swallow were obscured by the shoulders in one trial of a subject. Therefore 59 data points were analysed on the following measurements: larynx AP position, initial y position of the larynx, hyo‐laryngeal length at rest, hyo‐laryngeal area at rest, laryngeal elevation, anterior laryngeal excursion, laryngeal–hyoid elevation difference, and minimal hyo‐laryngeal area during each swallow.
Effect of head position on hyo‐laryngeal position, length and area at rest
Change in head tilt angle from neutral position significantly (P < 0.007) altered the following anatomical measurements before swallowing (Table 4): hyoid y position (Fig. 8 A); larynx y position (Fig. 8 B); hyo‐laryngeal length (Fig. 8 C); hyo‐laryngeal area (Fig. 8 D); and hyoid to mandible distance (Fig. 8 E). Change in head tilt angle did not alter hyoid x position or larynx x position before a swallow (Table 4).
Figure 8. Effect of head position on spatial configuration measurements at rest.
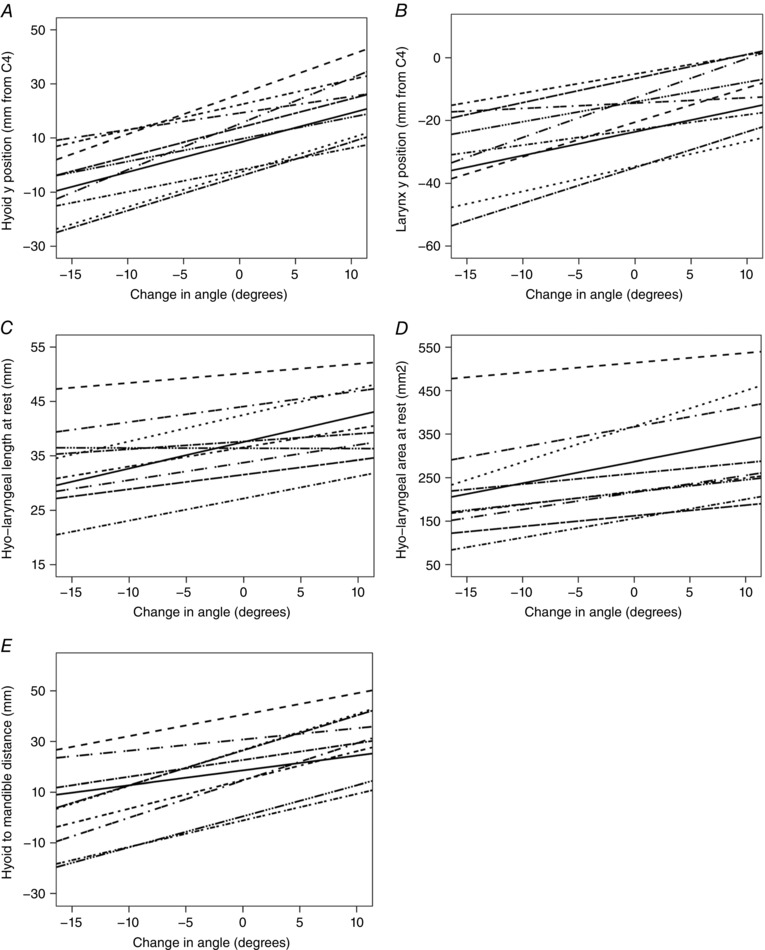
Greater change in head tilt angle relative to neutral angle (on x‐axis of each figure) was significantly (P < 0.007) associated with increase in: hyoid y position at rest (A), larynx y position at rest (B), hyo‐laryngeal length at rest (C), hyo‐laryngeal area at rest (D), and hyoid to mandible distance at rest (E). Measurements were made from 10 subjects who each swallowed in six different head tilt positions. The trendline for each subject is shown on each plot.
Effect of initial hyo‐laryngeal position, length and area on hyo‐laryngeal displacements during swallowing
Laryngeal elevation was significantly predicted by hyo‐laryngeal length (Fig. 9 A) and hyo‐laryngeal area at rest (Fig. 9 B), but not by the initial y position of the larynx (Table 5). Hyoid elevation was significantly predicted by hyoid to mandible distance (Fig. 9 C), but not by hyoid y position before swallowing (Table 4). Hyo‐laryngeal length at rest significantly predicted laryngeal–hyoid elevation difference during swallowing (Fig. 9 D, Table 5), while hyo‐laryngeal area at rest significantly predicted minimal hyo‐laryngeal area during swallowing (Fig. 9 E, Table 5). As anterior laryngeal and hyoid excursions did not have any predictors altered by change in head position, no further analyses were conducted.
Figure 9. Significant relationships (P < 0.001) between measurements during swallowing and anatomical measurements before swallowing across 6 trials in 10 subjects.
The trendline for each subject is shown on each plot. A, relationship between laryngeal elevation during swallowing and hyo‐laryngeal length at rest. B, relationship between laryngeal elevation during swallowing and hyo‐laryngeal area at rest. C, relationship between hyoid elevation and hyoid to mandible distance at rest. D, relationship between laryngeal–hyoid elevation difference and hyo‐laryngeal length at rest. E, relationship between minimal hyo‐laryngeal area during swallowing and hyo‐laryngeal area at rest. F, significant (P < 0.0001) relationship between reduction in hyo‐laryngeal area from resting to swallowing and laryngeal–hyoid elevation difference.
Table 5.
Effect of change in hyo‐laryngeal spatial configuration measurements at rest on swallowing movement
| Fixed effects of spatial configuration measurements on movement | Random effects covariance estimate; P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Movement measurement | Spatial configuration at rest before swallow | Slope (SE) | t (d.f.) | P | Intercept (SE) | t (d.f.) | P | Slope | Intercept |
| Laryngeal elevation | Larynx y position | −0.04 (0.1) | −0.3 (9.0) | 0.75 | 21.0 (3.1) | 6.8 (7.4) | 0.0002 | 0.07; 0.09 a | 68.6; 0.06 |
| Hyo‐laryngeal length at rest | 0.62 (0.1) | 4.3 (50.7) | <0.0001* | −1.1 (5.6) | −0.2 (43.9) | 0.85 | — b | 24.7; 0.03† | |
| Hyo‐laryngeal area at rest | 0.05 (0.01) | 4.5 (26.8) | 0.0001* | 9.1 (3.3) | 2.8 (20.8) | 0.01 | — c | 21.5; 0.03† | |
| Hyoid elevation | Hyoid y position | −0.00 (0.09) | −0.1 (7.9) | 0.96 | 11.5 (2.1) | 5.6 (7.8) | 0.0006 | 0.06; 0.07 a | 38.6; 0.03† |
| Hyoid to mandible distance | 0.19 (0.05) | 4.1 (56.8) | 0.0002* | 8.4 (1.4) | 5.8 (15.1) | <0.0001 | — b | 11.9; 0.03† | |
| Laryngeal‐hyoid elevation difference | Hyo‐laryngeal length at rest | 0.39 (0.08) | 4.7 (46.6) | <0.0001* | −4.2 (3.2) | −1.3 (41.3) | 0.21 | — b | 7.0; 0.02† |
| Minimal hyo‐laryngeal area during swallowing | Hyo‐laryngeal area at rest | 0.57 (0.06) | 9.6 (22) | <0.0001* | −2.0 (18.1) | −0.1 (17.7) | 0.91 | — b | 531; 0.03† |
Significant relationships are in bold. SE, standard error; t, t statistic; d.f., degrees of freedom; P, P value; AIC, Akaike information criterion; AR1, continuous first‐order autoregressive covariance structure; SP(EXP), spatial (exponential) covariance structure. *Fixed effect of slope significantly different from 0. †Intercepts differed significantly across individuals, P < 0.05. aLowest AIC in random intercept and random slope model with unstructured between‐subjects covariance. bLowest AIC in random intercept‐only model. cLowest AIC in random intercept‐only model with continuous AR1 within‐subjects covariance (covariance estimate SP(EXP) = 5.0, P = 0.07).
Relationship between hyo‐laryngeal area reduction and difference between laryngeal and hyoid elevation
Based on the ‘random intercept‐only’ model that yielded the lowest AIC, laryngeal–hyoid elevation difference was significantly related to the reduction in hyo‐laryngeal area during swallowing (fixed effect of slope = 8.2, SE = 1.3, d.f. = 56.8, t = 6.5, P < 0.0001; random intercept covariance estimate = 1107, SE = 567, Z = 2.0, P = 0.03) (Fig. 9 F).
Discussion
Two studies were conducted to examine if movement during swallowing depends upon overall size, or is driven by movement targets required for laryngeal airway protection for safe swallowing. If swallowing movements were scaled by body size, then in the first study, stronger relationships would have been found between pharyngeal neck length and swallowing movement across healthy individuals of different body size. Individual variability and sex differences in movement magnitudes would also reduce substantially after controlling for body size. Conversely, if swallowing movements were goal directed, movement magnitudes would be more related to the hyo‐laryngeal area before swallowing. These relationships would be found across individuals in the first study, as well as within individuals in the second study when the hyo‐laryngeal length and area changed with different head positions for swallowing. Correcting for individual differences in the location of movement targets would reduce variability in movement magnitudes across individuals. Given a typically lower larynx position in males than females, controlling for larynx position relative to the hyoid would also reduce differences in movement magnitudes between males and females.
Results of both studies supported the hypothesis that swallowing movement magnitudes for airway protection were more goal directed than dependent upon the overall size of the pharyngeal cavity. In Study 1, the extent of laryngeal elevation during swallowing was not scaled by pharyngeal neck length, but by the extent of hyo‐laryngeal distance before swallowing. Greater hyo‐laryngeal distance before swallowing predicted that the larynx travelled upward a greater distance relative to the distance that the hyoid travelled upward during the swallow. In addition, maximum anterior laryngeal displacement was exponentially related to individual differences in laryngeal position relative to the cervical spine at rest. Scaling laryngeal elevation and anterior movement magnitudes by their movement targets reduced variation among healthy individuals of different age and sex. Study 2 also supported goal‐directed movement scaling in swallowing, by demonstrating that healthy individuals compensated for changes in hyo‐laryngeal distance at rest induced by changes in head position, by altering their magnitude of laryngeal elevation.
Anatomical predictors of laryngeal displacement during swallowing have not been previously identified. Here, we found that individuals whose larynx was close to the spine (<35 mm) before swallowing used much greater anterior laryngeal displacements during swallowing. This pattern of movement scaling may be necessary to augment UOS opening and widen the pyriform sinuses during swallowing. On the other hand, the larynx might not need to be pulled forward by as much during swallowing if it was already greater than 35 mm away from the spine and further forward movement was not required for UOS opening. In addition, we found that healthy adults with greater hyo‐laryngeal distance between the hyoid and the larynx before swallowing adapted to the need for hyo‐laryngeal closure in two ways: by elevating their larynx more, and/or by increasing laryngeal elevation distance over hyoid elevation distance. This was necessary because laryngeal elevation had to overcome hyoid elevation in order to reduce the hyo‐laryngeal area for airway protection.
Difference in initial positions of the hyoid and larynx may contribute to variation in hyo‐laryngeal area reduction across individuals. The larynx is lower in the neck in adult males than females and may descend with ageing, thus increasing the hyo‐laryngeal area in males and older individuals (Fitch & Giedd, 1999; Leonard et al. 2004; Vorperian et al. 2005). As hyo‐laryngeal area reduction for airway protection is an invariant requirement of swallowing, individuals displaced the larynx to the extent needed to close the area based on their own anatomy. This may explain the large variation in laryngeal elevation magnitude among healthy individuals across age and sex (Molfenter & Steele, 2011). Correcting for hyo‐laryngeal length before swallowing alleviated 66% of variability in laryngeal elevation, while normalizing by pharyngeal neck length only reduced 17% of the variability (Table 3). Males and females adapted laryngeal elevation to their respective differences in hyo‐laryngeal length and area, such that sex differences in laryngeal elevation disappeared after correcting for hyo‐laryngeal length and area. Conversely, sex differences were still found after normalizing laryngeal elevation by pharyngeal neck length. These findings suggest that in contrast to hyoid elevation (Molfenter & Steele, 2014), the control of laryngeal elevation in normal swallowing is better determined by requirements for the goal of airway protection than overall body size.
In Study 2, hyo‐laryngeal length at rest was increased by tilting the head backward relative to neutral head position during swallowing. Here, individuals compensated by increasing the magnitude of laryngeal elevation to overcome the initial distance between the hyoid and larynx before swallowing. By raising the larynx to a greater degree than the hyoid, they reduced the distance between the hyoid and larynx during the swallow. Conversely, when hyo‐laryngeal length was reduced as the head tilted forward and down relative to neutral head position, the magnitude of laryngeal elevation did not exceed that of hyoid elevation to the same degree. This is consistent with previous reports of chin down positions reducing hyoid to larynx distance at rest (Bülow et al. 1999, 2001; Leigh et al. 2015), and reducing laryngeal elevation during swallowing (Leigh et al. 2015). Greater approximation between the hyoid and larynx in chin down position may help the larynx reach its movement target for airway protection earlier (Young et al. 2015). This may reduce the risk of airway penetration in dysphagic patients with difficulty achieving adequate and/or timely larynx to hyoid approximation for airway protection (Kahrilas et al. 1997; Bülow et al. 2001).
The immediate compensation for an altered functional movement target that we found in swallowing concurs with previous observations in speech production (Gracco & Abbs, 1985; Houde & Jordan, 1998; Villacorta et al. 2007). Similarly, Gay et al. (1994) examined the effect of bite block placement in the mouth prior to swallowing. Some of their subjects altered tongue movement to maintain the same degree of palatal contact for swallowing after the bite block was inserted. Thus our results and those of Gay et al. (1994) both suggest that swallowing may be goal directed, and feed‐forward control may prime the individual to plan the range of movement needed to complete the task safely and effectively. The predictability of the bite block and prior similar sensorimotor experiences (e.g. tilting the head back to drink) may explain why movement adjustments were immediate in subsequent swallows in our Study 2 and in Gay et al. (1994). However, they contrast with the gradual compensation of hyoid elevation to unexpected lowering of the hyoid with electrical stimulation of the sternohyoid muscle (Humbert et al. 2006, 2013). We postulate that this may be due to differences with prior sensorimotor experiences. We modulated hyo‐laryngeal length and area by changing head position, which our adult subjects would have experienced previously such as when tilting the head back to finish drinking water from a tall glass. This prior experience, together with ongoing proprioceptive feedback as the bolus was held in the mouth in preparation to swallow, probably facilitated an adjustment of the swallowing motor pattern in response to changes in hyo‐laryngeal length and area due to head posture. On the other hand, subjects in the Humbert et al. (2013) study encountered a novel unexpected resistance to hyoid elevation that they might not have had experience with and thus had no internal sensorimotor schema for immediate accommodation. As Humbert et al. (2006) previously demonstrated that surface electrical stimulation primarily lowers the hyoid while the larynx is not lowered to the same degree, the subjects studied by Humbert et al. (2013) may have had to assemble a novel motor response based on sensory feedback and predictive learning over several trials. Perhaps feed‐forward control that is dependent upon the sensation of the bolus and position of the upper airway may facilitate swallowing airway protection.
Sensory feedback is crucial for the implicit modulation of the swallow motor response (Jafari et al. 2003; Humbert et al. 2012). The laryngeal vestibule and the laryngeal surface of the epiglottis contain high densities of slowly and rapidly adapting afferents of the internal branch of the superior laryngeal nerve (iSLN) (Davis & Nail, 1987; Mu & Sanders, 2000; Yoshida et al. 2000). Discharges from the iSLN increase during laryngeal elevation and thyrohyoid muscle contraction (Shin et al. 1988). Sensory feedback probably contributes to gradual adaptation of the swallowing motor pattern to laryngeal posture changes during development, to facilitate rapid responses to increased risk of material entering the vestibule. Although our studies were not designed to isolate the effect of feedback control on movement magnitude in swallowing, we postulate that if sensory feedback were impaired, then feed‐forward control might be compromised. In the absence of laryngeal sensory feedback after anaesthesia to the iSLN bilaterally, the frequency of penetration increased, and resulted in aspiration in 25% of the swallows in healthy volunteers (Jafari et al. 2003). Most subjects could not adapt airway protection to mitigate the detrimental effect of sensory loss, despite the fact that anaesthesia was consistent and predictable throughout the experiment (Jafari et al. 2003). However, 30% of the subjects with initial penetration were able to resume normal airway protection by the end of the experiment (Jafari et al. 2003). These findings suggest the importance of an intact afferent system for hyo‐laryngeal movement scaling to maintain safe swallowing, and the possible influence of altered sensory experience on subsequent feed‐forward motor planning. The co‐influence of feed‐forward and feedback control on each other may explain why different types of aetiologies may impair swallowing airway protection.
Our results demonstrated that the minimal area between the hyoid and the larynx during swallowing scaled to about 60% of the hyo‐laryngeal area before the swallow (see Table 3) with a regression slope = 0.62 (Table 2), and when individuals swallowed in different head positions (regression slope = 0.57, Table 5). This supports the existence of an internal target or scaling factor that determines the extent of larynx to hyoid approximation in normal swallowing needed for airway protection. Perhaps the swallowing sensorimotor system allows individuals to implicitly learn, through numerous swallows during development and thereafter, how to adjust the magnitude of hyoid and laryngeal displacement to maintain airway protection. Some have found laryngeal penetration occurs with increasing frequency in normal ageing adults without swallowing complaints (McCullough et al. 2007; Butler et al. 2010). Our results may suggest that the feed‐forward mechanism of compensation shown here may become reduced in ageing and lead to increased frequency of penetration in older adults.
We also examined if the amount of approximation between the hyoid and the larynx during swallowing was associated with how much the laryngeal elevation magnitude exceeded that of hyoid elevation. This relationship was significant both between and within individuals (Figs 7 and 9 F). A greater extent of laryngeal elevation relative to hyoid elevation predicted greater reduction in the area between the hyoid and larynx during swallowing. On the other hand, neither anterior hyoid, anterior laryngeal excursion nor hyoid elevation was associated with the extent of area reduction between the hyoid and larynx in Study 1. This suggests that adaptation by the extent of laryngeal elevation may be more important than hyoid elevation to ensure adequate larynx to hyoid approximation for vestibule closure.
We found that individual differences in hyoid elevation magnitude in normal swallowing were related to variation in pharyngeal neck length and hyoid position. However, neither of these measurements predicted the extent of anterior hyoid excursion, a finding that agrees with previous reports (Kang et al. 2010; Molfenter & Steele, 2014). The extent of anterior hyoid displacement required for UOS opening is proposed to be consistent across individuals and bolus volumes and textures, and thus may contribute to its relative invariance in normal swallowing compared to hyo‐laryngeal movements in other directions (Jacob et al. 1989; Kahrilas et al. 1991; Ishida et al. 2002). Although this study and Molfenter & Steele (2014) found that body size might co‐contribute to hyoid elevation magnitude in swallowing and explain sex differences in non‐normalized measurements of hyoid elevation, this study did not find sufficient evidence of body size scaling in regulating laryngeal elevation magnitude.
A limitation in Study 1 was that only one bolus size and texture in a single swallow was studied. We do not know if the relationships between spatial measurements at rest and displacement/area measurements during swallowing are specific to swallowing 5 ml of thin liquid in healthy individuals. These relationships may change with different bolus volumes and textures and subjects, altering the mathematical computation of the normalized displacement measurements. Another limitation was our measurement of hyo‐laryngeal area rather than vestibule opening and closure areas by defining the rostral boundary by the hyoid rather than the epiglottis. Two‐dimensional quantification of changes in the hyo‐laryngeal space over time was relatively novel compared to established ways of measuring vestibule width or height, or identifying a single time point when vestibule closure occurs. The surrogate measure of hyo‐laryngeal area in this study, although unconventional, still served to quantify spatial changes during hyo‐laryngeal approximation for upper airway closure. A further limitation was identified in Study 2 – as larynx and hyoid positions in the antero‐posterior plane were not systematically altered by different degrees of head tilt, their effects on anterior hyoid and laryngeal displacement during swallowing could not be determined. This effect may be observed by comparing swallowing in upright vs. supine positions (Perry et al. 2012). A previous report demonstrated no change in the coordination of the pharyngeal swallow or the onset of vocal fold closure for airway protection between upright and supine positions during swallowing (Barkmeier et al. 2002). These findings suggest that hyo‐laryngeal movement amplitude rather than timing may adapt to swallowing in different body positions.
Kinematic measurements of swallowing are frequently derived in deglutition literature, but how movements are scaled to maintain the same goal for safe and efficient swallowing across and within individuals is largely unknown. We found that goal‐directed movement scaling, which has been reported in other areas of skilled motor control such as speech, was predominant in explaining the extent of hyo‐laryngeal displacement in normal swallowing. Larynx to hyoid approximation for vestibule closure and anterior laryngeal displacement away from the cervical spine for UOS opening, are two important movement goals for swallowing. These movement goals probably explain how healthy individuals differed in their extent of hyo‐laryngeal displacement produced during swallowing based on their vestibule size and hyo‐laryngeal positions at rest prior to swallowing. Under swallowing conditions that altered the hyo‐laryngeal length and area before swallowing, individuals also adjusted the extent of hyoid and laryngeal elevation so that laryngeal elevation could override the extent of hyoid elevation to meet an internal target for hyo‐laryngeal closure. This adaptation may be possible with years of continued swallowing experience and sensorimotor integration that allow individuals to implicitly employ an internal model of swallowing. This internalized schema is evidence of a feed‐forward system that probably depends on sensory feedback of initial positions prior to swallowing to determine the extent of hyo‐laryngeal movement required for airway protection during swallowing.
Additional information
Competing interests
None declared.
Author contributions
S.M.W., C.L.L. and S.F. designed the studies; S.M.W. collected the data; S.M.W., R.J.D. and C.L.L. analysed and interpreted the data; S.M.W. wrote the manuscript with C.L.L. All authors critically revised the manuscript and have approved the final version. All authors agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed. The experiments and analyses took place at James Madison University and Sentara RMH Medical Center and one set of anonymized recordings took place at the National Institutes of Health (NIH) Clinical Center.
Funding
S.M.W. received sponsorship from the Singapore General Hospital for her doctoral studies. Funding for this study came from the American Speech‐Language‐Hearing Foundation Graduate Student Scholarship awarded to S.M.W. in 2013.
Acknowledgements
The authors thank Dr Erin Kamarunas for assistance with videofluoroscopy and Ms Hui Hui Gan for assistance with data analysis.
References
- Barkmeier JM, Bielamowicz S, Takeda N & Ludlow CL (2002). Laryngeal activity during upright vs. supine swallowing. J Appl Physiol (1985) 93, 740–745. [DOI] [PubMed] [Google Scholar]
- Bülow M, Olsson R & Ekberg O (1999). Videomanometric analysis of supraglottic swallow, effortful swallow, and chin tuck in healthy volunteers. Dysphagia 14, 67–72. [DOI] [PubMed] [Google Scholar]
- Bülow M, Olsson R & Ekberg O (2001). Videomanometric analysis of supraglottic swallow, effortful swallow, and chin tuck in patients with pharyngeal dysfunction. Dysphagia 16, 190–195. [DOI] [PubMed] [Google Scholar]
- Butler SG, Stuart A, Leng X, Rees C, Williamson J & Kritchevsky SB (2010). Factors influencing aspiration during swallowing in healthy older adults. Laryngoscope 120, 2147–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty CP & Bennett MB (2009). The use of dimensionless scaling strategies in gait analysis. Hum Mov Sci 28, 218–225. [DOI] [PubMed] [Google Scholar]
- Castell JA, Castell DO, Schultz AR & Georgeson S (1993). Effect of head position on the dynamics of the upper esophageal sphincter and pharynx. Dysphagia 8, 1–6. [DOI] [PubMed] [Google Scholar]
- Cook IJ, Dodds WJ, Dantas RO, Massey B, Kern MK, Lang IM, Brasseur JG & Hogan WJ (1989). Opening mechanisms of the human upper esophageal sphincter. Am J Physiol 257, G748–G759. [DOI] [PubMed] [Google Scholar]
- Crompton AW, German RZ & Thexton AJ (1997). Mechanisms of swallowing and airway protection in infant mammals (Sus domesticus and Macaca fascicularis). J Zool 241, 89–102. [Google Scholar]
- Crompton AW, German RZ & Thexton AJ (2008). Development of the movement of the epiglottis in infant and juvenile pigs. Zoology (Jena) 111, 339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PJ & Nail BS (1987). Quantitative analysis of laryngeal mechanosensitivity in the cat and rabbit. J Physiol 388, 467–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney AL & Arvedson JC (2008). Development of swallowing and feeding: prenatal through first year of life. Dev Disabil Res Rev 14, 105–117. [DOI] [PubMed] [Google Scholar]
- Fitch WT & Giedd J (1999). Morphology and development of the human vocal tract: A study using magnetic resonance imaging. J Acoust Soc Am 106, 1511–1522. [DOI] [PubMed] [Google Scholar]
- Gay T, Rendell JK, Spiro J, Mosier K & Lurie AG (1994). Coordination of oral cavity and laryngeal movements during swallowing. J Appl Physiol (1985) 77, 357–365. [DOI] [PubMed] [Google Scholar]
- Gracco VL & Abbs JH (1985). Dynamic control of the perioral system during speech: kinematic analyses of autogenic and nonautogenic sensorimotor processes. J Neurophysiol 54, 418–432. [DOI] [PubMed] [Google Scholar]
- Hof AL (1996). Scaling gait data to body size. Gait Posture 4, 222–223. [DOI] [PubMed] [Google Scholar]
- Houde JF & Jordan MI (1998). Sensorimotor adaptation in speech production. Science 279, 1213–1216. [DOI] [PubMed] [Google Scholar]
- Humbert IA, Christopherson H, Lokhande A, German R, Gonzalez‐Fernandez M & Celnik P (2013). Human hyolaryngeal movements show adaptive motor learning during swallowing. Dysphagia 28, 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert IA, Lokhande A, Christopherson H, German R & Stone A (2012). Adaptation of swallowing hyo‐laryngeal kinematics is distinct in oral vs. pharyngeal sensory processing. J Appl Physiol (1985) 112, 1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert IA, Poletto CJ, Saxon KG, Kearney PR, Crujido L, Wright‐Harp W, Payne J, Jeffries N, Sonies BC & Ludlow CL (2006). The effect of surface electrical stimulation on hyolaryngeal movement in normal individuals at rest and during swallowing. J Appl Physiol (1985) 101, 1657–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamoto Y, Fujii N, Saitoh E, Baba M, Okada S, Katada K, Ozeki Y, Kanamori D & Palmer JB (2011). Evaluation of swallowing using 320‐detector‐row multislice CT. Part II: kinematic analysis of laryngeal closure during normal swallowing. Dysphagia 26, 209–217. [DOI] [PubMed] [Google Scholar]
- Ishida R, Palmer JB & Hiiemae KM (2002). Hyoid motion during swallowing: factors affecting forward and upward displacement. Dysphagia 17, 262–272. [DOI] [PubMed] [Google Scholar]
- Jacob P, Kahrilas PJ, Logemann JA, Shah V & Ha T (1989). Upper esophageal sphincter opening and modulation during swallowing. Gastroenterology 97, 1469–1478. [DOI] [PubMed] [Google Scholar]
- Jafari S, Prince RA, Kim DY & Paydarfar D (2003). Sensory regulation of swallowing and airway protection: a role for the internal superior laryngeal nerve in humans. J Physiol 550, 287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean A (2001). Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev 81, 929–969. [DOI] [PubMed] [Google Scholar]
- Jean A & Car A (1979). Inputs to the swallowing medullary neurons from the peripheral afferent fibres and the swallowing cortical area. Brain Res 178, 567–572. [DOI] [PubMed] [Google Scholar]
- Kahrilas PJ, Lin S, Chen J & Logemann JA (1996). Oropharyngeal accommodation to swallow volume. Gastroenterology 111, 297–306. [DOI] [PubMed] [Google Scholar]
- Kahrilas PJ, Lin S, Rademaker AW & Logemann JA (1997). Impaired deglutitive airway protection: a videofluoroscopic analysis of severity and mechanism. Gastroenterology 113, 1457–1464. [DOI] [PubMed] [Google Scholar]
- Kahrilas PJ, Logemann JA, Krugler C & Flanagan E (1991). Volitional augmentation of upper esophageal sphincter opening during swallowing. Am J Physiol 260, G450–G456. [DOI] [PubMed] [Google Scholar]
- Kang BS, Oh BM, Kim IS, Chung SG, Kim SJ & Han TR (2010). Influence of aging on movement of the hyoid bone and epiglottis during normal swallowing: a motion analysis. Gerontology 56, 474–482. [DOI] [PubMed] [Google Scholar]
- Kawasaki A, Fukuda H, Shiotani A & Kanzaki J (2001). Study of movements of individual structures of the larynx during swallowing. Auris Nasus Larynx 28, 75–84. [DOI] [PubMed] [Google Scholar]
- Kern M, Bardan E, Arndorfer R, Hofmann C, Ren J & Shaker R (1999). Comparison of upper esophageal sphincter opening in healthy asymptomatic young and elderly volunteers. Ann Otol Rhinol Laryngol 108, 982–989. [DOI] [PubMed] [Google Scholar]
- Kuhl V, Eicke BM, Dieterich M & Urban PP (2003). Sonographic analysis of laryngeal elevation during swallowing. J Neurol 250, 333–337. [DOI] [PubMed] [Google Scholar]
- Leigh JH, Oh BM, Seo HG, Lee GJ, Min Y, Kim K, Lee JC & Han TR (2015). Influence of the chin‐down and chin‐tuck maneuver on the swallowing kinematics of healthy adults. Dysphagia 30, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R, Kendall KA & McKenzie S (2004). Structural displacements affecting pharyngeal constriction in nondysphagic elderly and nonelderly adults. Dysphagia 19, 133–141. [DOI] [PubMed] [Google Scholar]
- Leonard RJ, Kendall KA, McKenzie S, Goncalves MI & Walker A (2000). Structural displacements in normal swallowing: a videofluoroscopic study. Dysphagia 15, 146–152. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, McCarthy RC, Hiiemae KM & Palmer JB (2001). Ontogeny of postnatal hyoid and larynx descent in humans. Arch Oral Biol 46, 117–128. [DOI] [PubMed] [Google Scholar]
- Logemann JA, Kahrilas PJ, Cheng J, Pauloski BR, Gibbons PJ, Rademaker AW & Lin S (1992). Closure mechanisms of laryngeal vestibule during swallow. Am J Physiol 262, G338–G344. [DOI] [PubMed] [Google Scholar]
- Lowell SY, Poletto CJ, Knorr‐Chung BR, Reynolds RC, Simonyan K & Ludlow CL (2008). Sensory stimulation activates both motor and sensory components of the swallowing system. Neuroimage 42, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough GH, Rosenbek JC, Wertz RT, Suiter D & McCoy SC (2007). Defining swallowing function by age: promises and pitfalls of pigeonholing. Top Geriatr Rehabil 23, 290–307. [Google Scholar]
- Martin RE, Goodyear BG, Gati JS & Menon RS (2001). Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol 85, 938–950. [DOI] [PubMed] [Google Scholar]
- Martin RE, Kemppainen P, Masuda Y, Yao D, Murray GM & Sessle BJ (1999). Features of cortically evoked swallowing in the awake primate (Macaca fascicularis). J Neurophysiol 82, 1529–1541. [DOI] [PubMed] [Google Scholar]
- Martin RE, Murray GM, Kemppainen P, Masuda Y & Sessle BJ (1997). Functional properties of neurons in the primate tongue primary motor cortex during swallowing. J Neurophysiol 78, 1516–1530. [DOI] [PubMed] [Google Scholar]
- Martin‐Harris B, Brodsky MB, Michel Y, Castell DO, Schleicher M, Sandidge J, Maxwell R & Blair J (2008). MBS measurement tool for swallow impairment – MBSImp: Establishing a standard. Dysphagia 23, 392–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AJ (1972). Characteristics of the swallowing reflex induced by peripheral nerve and brain stem stimulation. Exp Neurol 34, 210–222. [DOI] [PubMed] [Google Scholar]
- Miller JL, Sonies BC & Macedonia C (2003). Emergence of oropharyngeal, laryngeal and swallowing activity in the developing fetal upper aerodigestive tract: an ultrasound evaluation. Early Hum Dev 71, 61–87. [DOI] [PubMed] [Google Scholar]
- Molfenter SM & Steele CM (2011). Physiological variability in the deglutition literature: hyoid and laryngeal kinematics. Dysphagia 26, 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfenter SM & Steele CM (2014). Use of an anatomical scalar to control for sex‐based size differences in measures of hyoid excursion during swallowing. J Speech Lang Hear Res 57, 768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu L & Sanders I (2000). Sensory nerve supply of the human oro‐ and laryngopharynx: a preliminary study. Anat Rec 258, 406–420. [DOI] [PubMed] [Google Scholar]
- Murray GM & Sessle BJ (1992). Functional properties of single neurons in the face primary motor cortex of the primate. I. Input and output features of tongue motor cortex. J Neurophysiol 67, 747–758. [DOI] [PubMed] [Google Scholar]
- Ohmae Y, Logemann JA, Kaiser P, Hanson DG & Kahrilas PJ (1995). Timing of glottic closure during normal swallow. Head Neck 17, 394–402. [DOI] [PubMed] [Google Scholar]
- Perry JL, Bae Y & Kuehn DP (2012). Effect of posture on deglutitive biomechanics in healthy individuals. Dysphagia 27, 70–80. [DOI] [PubMed] [Google Scholar]
- Riely RR & Smith A (2003). Speech movements do not scale by orofacial structure size. J Appl Physiol (1985) 94, 2119–2126. [DOI] [PubMed] [Google Scholar]
- Rosenbek JC, Robbins JA, Roecker EB, Coyle JL & Wood JL (1996). A penetration‐aspiration scale. Dysphagia 11, 93–98. [DOI] [PubMed] [Google Scholar]
- Shaker R, Dodds WJ, Dantas RO, Hogan WJ & Arndorfer RC (1990). Coordination of deglutitive glottic closure with oropharyngeal swallowing. Gastroenterology 98, 1478–1484. [DOI] [PubMed] [Google Scholar]
- Shanahan TK, Logemann JA, Rademaker AW, Pauloski BR & Kahrilas PJ (1993). Chin‐down posture effect on aspiration in dysphagic patients. Arch Phys Med Rehabil 74, 736–739. [DOI] [PubMed] [Google Scholar]
- Shin T, Maeyama T, Morikawa I & Umezaki T (1988). Laryngeal reflex mechanism during deglutition – observation of subglottal pressure and afferent discharge. Otolaryngol Head Neck Surg 99, 465–471. [DOI] [PubMed] [Google Scholar]
- Soros P, Lalone E, Smith R, Stevens T, Theurer J, Menon RS & Martin RE (2008). Functional MRI of oropharyngeal air‐pulse stimulation. Neuroscience 153, 1300–1308. [DOI] [PubMed] [Google Scholar]
- Steele CM, Hung D, Sejdić E, Chau T & Fraser S (2011). Variability in execution of the chin‐down maneuver by healthy adults. Folia Phoniatr Logop 63, 36–42. [DOI] [PubMed] [Google Scholar]
- Teismann IK, Steinstraeter O, Stoeckigt K, Suntrup S, Wollbrink A, Pantev C & Dziewas R (2007). Functional oropharyngeal sensory disruption interferes with the cortical control of swallowing. BMC Neurosci 8, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veljan D (2000). The 2500‐year‐old Pythagorean theorem. Mathematics Magazine 73, 259–272. [Google Scholar]
- Villacorta VM, Perkell JS & Guenther FH (2007). Sensorimotor adaptation to feedback perturbations of vowel acoustics and its relation to perception. J Acoust Soc Am 122, 2306–2319. [DOI] [PubMed] [Google Scholar]
- Vorperian HK, Kent RD, Lindstrom MJ, Kalina CM, Gentry LR & Yandell BS (2005). Development of vocal tract length during early childhood: a magnetic resonance imaging study. J Acoust Soc Am 117, 338–350. [DOI] [PubMed] [Google Scholar]
- Welch MV, Logemann JA, Rademaker AW & Kahrilas PJ (1993). Changes in pharyngeal dimensions effected by chin tuck. Arch Phys Med Rehabil 74, 178–181. [PubMed] [Google Scholar]
- Williams RB, Pal A, Brasseur JG & Cook IJ (2001). Space‐time pressure structure of pharyngo‐esophageal segment during swallowing. Am J Physiol Gastrointest Liver Physiol 281, G1290–G1300. [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Mitomi N, Tetsuka K, Tayama N & Niimi S (2000). Role of laryngeal movement and effect of aging on swallowing pressure in the pharynx and upper esophageal sphincter. Laryngoscope 110, 434–439. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Tanaka Y, Hirano M & Nakashima T (2000). Sensory innervation of the pharynx and larynx. Am J Med 108, Suppl. 4a, 51S–61S. [DOI] [PubMed] [Google Scholar]
- Young JL, Macrae P, Anderson C, Taylor‐Kamara I & Humbert IA (2015). The sequence of swallowing events during the chin‐down posture. Am J Speech Lang Pathol 24, 659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]


