Abstract
Key points
Maternal high‐fat diet impairs brown adipocyte function and correlates with obesity in offspring.
Maternal resveratrol administration recovers metabolic activity of offspring brown adipose tissue.
Maternal resveratrol promotes beige adipocyte development in offspring white adipose tissue.
Maternal resveratrol intervention protects offspring against high‐fat diet‐induced obesity.
Abstract
Promoting beige/brite adipogenesis and thermogenic activity is considered as a promising therapeutic approach to reduce obesity and metabolic syndrome. Maternal obesity impairs offspring brown adipocyte function and correlates with obesity in offspring. We previously found that dietary resveratrol (RES) induces beige adipocyte formation in adult mice. Here, we evaluated further the effect of resveratrol supplementation of pregnant mice on offspring thermogenesis and energy expenditure. Female C57BL/6 J mice were fed a control diet (CON) or a high‐fat diet (HFD) with or without 0.2% (w/w) RES during pregnancy and lactation. Male offspring were weaned onto a HFD and maintained on this diet for 11 weeks. The offspring thermogenesis and related regulatory factors in adipose tissue were evaluated. At weaning, HFD offspring had lower thermogenesis in brown and white adipose tissues compared with CON offspring, which was recovered by maternal RES supplementation, along with the appearance of multilocular brown/beige adipocytes and elevated thermogenic gene expression. Adult offspring of RES‐treated mothers showed increased energy expenditure and insulin sensitivity when on an obesogenic diet compared with HFD offspring. The elevated metabolic activity was correlated with enhanced brown adipose function and white adipose tissue browning in HFD+RES compared with HFD offspring. In conclusion, RES supplementation of HFD‐fed dams during pregnancy and lactation promoted white adipose browning and thermogenesis in offspring at weaning accompanied by persistent beneficial effects in protecting against HFD‐induced obesity and metabolic disorders.
Keywords: adipose tissue, beige adipocyte, browning, obesity, offspring, resveratrol, thermogenesis
Key points
Maternal high‐fat diet impairs brown adipocyte function and correlates with obesity in offspring.
Maternal resveratrol administration recovers metabolic activity of offspring brown adipose tissue.
Maternal resveratrol promotes beige adipocyte development in offspring white adipose tissue.
Maternal resveratrol intervention protects offspring against high‐fat diet‐induced obesity.
Abbreviations
- AMPK
AMP‐activated protein kinase
- BAT
brown adipose tissue
- CD137
cluster of differentiation 137
- Cidea
cell death‐inducing DFFA‐like effector A
- CON
control diet
- Cox7a1
cytochrome c oxidase subunit Vlla polypeptide 1
- Cyto C
cytochrome c
- Elovl3
elongation of very long‐chain fatty acids protein 3
- EpiWAT
epididymal white adipose tissue
- FABP4
fatty‐acid binding protein 4
- GTT
glucose tolerance test
- H&E
Haemotoxylin and Eosin
- HFD
high‐fat diet
- IHC
immunohistochemical
- IngWAT
inguinal white adipose tissue
- PGC‐1α
peroxisomal proliferator‐activated receptor γ coactivator‐1
- PPARγ
peroxisomal proliferator‐activated receptor γ
- PRDM16
PR domain‐containing 16
- RES
resveratrol
- Sirt1
sirtuin 1
- Tbx1
T‐box 1
- Tmem26
transmembrane protein 26
- UCP1
uncoupling protein 1
Introduction
A growing body of evidence suggests that maternal nutrition and physiological conditions are important determinants of the health outcome of offspring (Borengasser et al. 2013; Nathanielsz et al. 2013; Giussani et al. 2014; Forhead et al. 2015). Maternal dietary intake during pregnancy and lactation affects the development of the fetus and neonate, resulting in developmental programming that alters offspring life‐course physiology and metabolism (Boekelheide et al. 2012; Poston, 2012). Epidemiological and experimental studies show that maternal obesity or high‐fat diet (HFD) consumption is associated with increased susceptibility to hepatic steatosis and inflammation (Bruce et al. 2009), hypertension and cardiovascular disorders (Samuelsson et al. 2008; Blackmore et al. 2014; Taylor et al. 2014), impaired hippocampal neurogenesis (Tozuka et al. 2009; Dearden & Ozanne, 2015), adrenal and thyroid dysfunction (Franco et al. 2012), as well as obesity and insulin resistance (Boyle et al. 2016) in offspring at different stages of development.
Obesity, characterized by excess adipose tissue accumulation, is a major risk factor leading to metabolic syndrome and type 2 diabetes. In mammals, white adipose tissue (WAT) stores excess energy in the form of triglycerides (Giralt & Villarroya, 2013). In contrast, two populations of uncoupling protein 1 (UCP1)‐positive thermogenic adipocytes, referred to as classical brown adipocytes in brown adipose tissue (BAT; Cannon & Nedergaard, 2004) and brite/beige (brown in white) adipocytes of WAT (Giralt & Villarroya, 2013), dissipate energy directly as heat. Thus, pharmacological and nutritional strategies for increasing BAT thermogenic activity and browning of WAT promote energy dissipation and combat obesity and type 2 diabetes (Bonet et al. 2013).
Together with dietary macro‐ and/or micronutrients, provision of bioactive food components, such as polyphenols, to mothers can programme offspring growth and metabolic pathways, which can further alter lifelong susceptibility to obesity and its related complications (Santangelo et al. 2014; Vega et al. 2016). Several reports have indicated the metabolic programming effects of maternal polyphenol intake on offspring. For example, grape skin extract was effective in protecting adult offspring against obesity, hypertension and insulin resistance when administered during lactation in rats (Resende et al. 2013). Maternal dietary genistein supplementation throughout pregnancy and lactation prevented obesity in mouse offspring (Dolinoy et al. 2006). The maternal administration of quercetin during gestation and lactation decreased offspring body weight, and improved insulin sensitivity and lipid metabolism in adipose tissue (Wu et al. 2014). Moreover, although offspring from rat dams fed HFD with low doses of grape seed procyanidin during pregnancy and lactation exhibited an increase in adiposity, these animals possessed healthier adipose tissue than the offspring of dams fed HFD (Del Bas et al. 2015).
As a nutraceutical dietary supplement, resveratrol (RES), a natural polyphenolic compound commonly found in grape skins and other fruits, has been shown to protect against HFD‐induced obesity in mammals (Kim et al. 2011; Jeon et al. 2012) and exerts health benefits in obese persons (Timmers et al. 2011). In addition, maternal RES intake improves fetal outcomes impaired as a result of to hypoxia (Bourque et al. 2012), ethanol exposure (Kumar et al. 2011), protein restriction (Vega et al. 2016), hypertension (Care et al. 2016) and valproic acid‐induced neurological disorder (Bambini‐Junior et al. 2014). However, to our knowledge, the effect of RES supplementation of HFD mothers on brown and beige adipose development and thermogenesis in offspring has not been examined.
Proposed anti‐obesity mechanisms of RES include decreased adipogenesis (Rayalam et al. 2008) and lipogenesis (Li et al. 2016), increased lipolysis (Lasa et al. 2012), adipocyte apoptosis (Rayalam et al. 2008), increased thermogenesis (Wang et al. 2015) and enhanced mitochondrial function (Lagouge et al. 2006). One plausible explanation for these beneficial effects is the induction of beige adipogenesis, which promotes lipid oxidation to generate heat (Wang et al. 2015). Thus, we hypothesized that maternal resveratrol administration could facilitate brown and beige adipogenesis during early offspring development, and exert beneficial effects on energy metabolism and adaptive thermogenesis in adult offspring.
Methods
Ethical approval
All animal experimental and care procedures were performed in accordance with the guidelines of the National Institutes of Health and approved by the Institutional Animal Use and Care Committee of Washington State University.
Animals and experimental design
Female C57BL/6 J mice (3 months old) were maintained in controlled conditions (23 ± 2°C, 12 h–12 h light–dark cycle) with free access to food and water. The day of mating was determined by examination of vaginal smears. This day was designated as day 0 of gestation. Pregnant mice were housed individually and randomly divided into four dietary groups (n = 10 per group). Two groups were fed with a control diet (CON; 10% energy from fat, D12450H; Research Diets, New Brunswick, NJ, USA) or an identical diet supplemented with resveratrol (CON+RES). The other two groups were fed with an HFD (45% energy from fat, D12451; Research Diets) or an identical diet supplemented with resveratrol (HFD+RES). Resveratrol (Orchid Pharma, Inc., Princeton, NJ, USA) was mixed with either powdered CON or HFD diet at a concentration of 0.2% (dry feed, w/w), and pellets were then reconstituted. All dams were fed their respective diets from day 0 of gestation until weaning (postnatal day 21). In previous studies, dietary supplementation of 0.1–0.4% RES effectively ameliorated HFD‐induced adiposity (Lagouge et al. 2006; Kim et al. 2011; Wang et al. 2015). Thus, we chose an average, 0.2% RES, supplementation for our study, which was equivalent to ∼200 mg (kg body weight)−1 day−1. As discussed previously, similar doses demonstrated beneficial effects to the health of mothers and their fetuses (Vega et al. 2016). Weight gain and food intake were monitored. Litters were culled to six pups within 24 h after birth to ensure adequate and uniform maternal nutrition to pups. Litters with fewer than six pups were removed from the study. On postnatal day 21, pups were weaned, and dams and one male pup from each litter were killed by carbon dioxide inhalation and cervical dislocation. Blood samples were collected by cardiac puncture and centrifuged at 4°C to collect serum. The interscapular BAT, inguinal WAT (IngWAT) and epididymal WAT (EpiWAT) were rapidly isolated and weighed. Tissues from one side were fixed in 4% paraformaldehyde for sectioning and staining, and from the other side they were rapidly frozen in liquid nitrogen and stored at −80°C until further analyses. After weaning, the remaining male pups were weaned onto HFD (D12451) to mimic a postweaning obesogenic environment. The offspring body weights and food intake were recorded weekly. After 11 weeks of HFD challenge, mice were killed by CO2 inhalation and cervical dislocation, and blood was collected by cardiac puncture. The liver, BAT, IngWAT and EpiWAT were harvested and weighed.
Glucose tolerance test (GTT) and blood characteristics
At 1 week before the mice were killed, following an overnight fast, mice were injected i.p. with d‐glucose (1 g kg−1). Blood samples were collected from the tail vein at 0, 15, 30, 60 and 90 min after injection, and glucose concentrations were measured using a glucometer (Bayer Contour, Tarrytown, NY, USA), and the area under the curve was quantified (Um et al. 2010; Fu et al. 2015). Serum insulin concentration was measured using a Mouse Ultrasensitive Insulin ELISA Kit (no. 80‐INSMSU‐E10; ALPCO Diagnostics, Salem, NH, USA), and triglycerides were determined using the triglyceride colorimetric assay kit from Cayman (no. 10010303; Ann Arbor, MI, USA).
Whole‐body metabolic analysis
The whole‐body metabolic rate [oxygen consumption (), carbon dioxide production (), respiratory exchange ratio (RER) and heat production] was measured using an Oxymax indirect open‐circuit calorimetry system (Columbus Instruments, Columbus, OH, USA) installed in a constant environmental temperature and light regimen (12 h light and 12 h dark). Mice in each chamber had free access to food and water.
Acute cold tolerance test
Mice were individually housed in precooled cages and exposed to a cold temperature (4°C) for 6 h with free access to food and water. Their rectal temperature was measured hourly using a digital thermometer (Thermalert TH‐5; Physitemp, Clifton, NJ, USA).
Histological analysis
Fresh adipose tissues were fixed for 24 h at room temperature in PBS containing 4% paraformaldehyde, and embedded in paraffin. Then, 5‐μm‐thick tissue sections were deparaffinized, rehydrated and either stained with Hematoxylin and Eosin (H&E) or used for UCP1 immunohistochemical (IHC) staining as previously described (Wang et al. 2015). At least four images per section and four sections from each individual mouse were analysed. Adipocyte diameters were measured by Image‐Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA; Liang et al. 2016).
Quantitative real‐time PCR
Total RNA was extracted from different adipose tissues using TRIzol reagent (Sigma, Saint Louis, MO, USA) according to the manufacturer's instructions, and cDNA was synthesized from 500 ng of total RNA using the iScript™ cDNA Synthesis Kit (Bio‐Rad, Hercules, CA, USA). Real‐time quantitative PCR was carried out using the CFX RT‐PCR detection system (Bio‐Rad) as described previously with 18S rRNA used as a reference gene (Wang et al. 2015). The relative mRNA expression was determined using the method of (Livak & Schmittgen, 2001). The primer sequences are listed in Table 1.
Table 1.
Primer sequences used for real‐time quantitative PCR
| Gene | Forward (5′–3′) | Reverse (5′–3′) | Size (bp) | Accession no. |
|---|---|---|---|---|
| 18S | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG | 151 | NR_046233.2 |
| UCP1 | ACTGCCACACCTCCAGTCATT | CTTTGCCTCACTCAGGATTGG | 123 | NM_009463.3 |
| PRDM16 | CAGCACGGTGAAGCCATTC | GCGTGCATCCGCTTGTG | 87 | NM_001291029.1 |
| Cidea | ATCACAACTGGCCTGGTTACG | TACTACCCGGTGTCCATTTCT | 136 | NM_007702.2 |
| Elovl3 | GATGGTTCTGGGCACCATCTT | CGTTGTTGTGTGGCATCCTT | 73 | XM_006526624.1 |
| Cox7a1 | CAGCGTCATGGTCAGTCTGT | AGAAAACCGTGTGGCAGAGA | 112 | NM_009944.3 |
| PGC‐1α | CCCTGCCATTGTTAAGACC | TGCTGCTGTTCCTGTTTTC | 161 | XM_006503779.1 |
| CD137 | GTCGACCCTGGACGAACTGCTCT | CCTCTGGAGTCACAGAAATGGTGGTA | 132 | NM_001077590.1 |
| Tbx‐1 | TGAAGAAGAACCCGAAGGTGG | ACTTGGAACGTGGGGAACATT | 133 | XM_OO6536887.1 |
| TMEM26 | GAAACCAGTATTGCAGCACCCAAT | AATATTAGCAGGAGTGTTTGGTGGA | 205 | NM_177794.3 |
| PPARγ | AGCTCCAAGAATACCAAAGTGCGAT | AGGTTCTTCATGAGGCCTGTTGTAGA | 98 | XM_017321456.1 |
| FABP4 | CGACAGGAAGGTGAAGAGCATCATA | CATAAACTCTTGTGGAAGTCACGCCT | 158 | NM_024406.2 |
Immunoblotting analysis
Immunoblotting analysis was conducted as previously described using an Odyssey Infrared Image System (LI‐COR Biosciences, Lincoln, NE, USA; Wang et al. 2015). Antibodies against AMP‐activated protein kinase α (AMPKα; no. 2532), phospho‐AMPKα at Thr172 (no. 2535), cytochrome c (Cyto C; no. 4280), Sirtuin 1 (Sirt1; no. 2028), peroxisomal proliferator‐activated receptor γ (PPARγ; no. 2443) and β‐tubulin (no. 2146) were purchased from Cell Signaling (Danvers, MA, USA) and were diluted 1:1000. UCP1 polyclonal antibody (no. PA1‐24894) and PR domain‐containing 16 polyclonal antibody (PRDM16; no. PA5‐20872) were purchased from Thermo Scientific (Waltham, MA, USA) and were diluted 1:1000. Anti‐fatty‐acid binding protein 4 antibody (FABP4; no. sc‐18661) was purchased from Santa Cruz Biotechnology (Dallas, TX, USA) and was diluted 1:400. IRDye 800CW goat anti‐rabbit (no. 926‐32211) and IDRye 680RD goat anti‐mouse (no. 926‐68070) secondary antibodies for Western blotting were purchased from LI‐COR (Lincoln, NE, USA) and were diluted 1:15,000. Band density was quantified and then normalized to β‐tubulin content, because the levels of β‐tubulin did not differ between experimental groups.
Statistical analysis
Data are presented as means ± SEM. The general linear model and Duncan's multiple range test (SAS Institute Inc., Cary, NC, USA) were used to analyse data and to determine the significance of differences among means of different treatments. A value of P < 0.05 was considered to be statistically significant.
Results
Maternal phenotype and metabolic parameters
The initial non‐pregnant female body weights were similar among groups (CON 19.8 ± 0.27 g, CON+RES 20.4 ± 0.44 g, HFD 19.6 ± 0.29 g and HFD ± RES 19.8 ± 0.44 g). At weaning, HFD‐fed dams had higher body weight (Fig. 1 A) and ratio of WAT mass to body weight (Fig. 1 B) than CON and CON+RES dams. Dietary RES protected dams against HFD‐induced body weight gain (Fig. 1 A) and fat accumulation (Fig. 1 B). Administration of RES did not change energy intake during pregnancy and lactation (Fig. 1 C). Moreover, no difference was observed in maternal blood glucose concentrations (Fig. 1 D). The HFD‐fed dams exhibited increased serum triglyceride and insulin concentrations, whereas RES supplementation to HFD dams significantly reduced the concentrations of triglycerides and insulin. There was no difference in triglyceride and insulin concentrations between CON and CON+RES dams (Fig. 1 E and F).
Figure 1. Maternal characteristics.
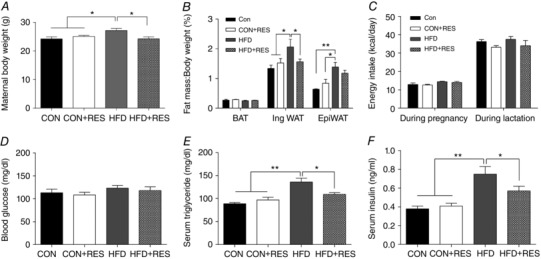
A, maternal body weight at the end of lactation. B, fat index (percentage of fat pad weight relative to the whole body weight) of BAT, inguinal WAT and epididymal WAT. C, maternal energy intake during pregnancy and lactation. D–F, blood glucose (D), serum triglyceride (E) and insulin concentrations (F) at the end of lactation. * P < 0.05 and ** P < 0.01. Data are shown as means + SEM. (n = 8). Abbreviations: BAT, brown adipose tissue; CON, control; EpiWAT, epididymal white adipose tissue; HFD, high‐fat diet; IngWAT, inguinal white adipose tissue; RES, resveratrol; WAT white adipose tissue.
Resveratrol increased metabolic activity in BAT of HFD offspring at weaning
After birth, HFD‐fed male offspring showed increased body weight, which was reduced by maternal RES treatment (CON 1.28 ± 0.03 g, CON+RES 1.22 ± 0.02 g, HFD 1.54 ± 0.05 g and HFD+RES 1.32 ± 0.05 g; P < 0.01 for CON vs. HFD, P < 0.05 for HFD vs. HFD+RES). Similar changes in offspring body weight were also observed at 7, 14 and 21 days (Fig. 2 A), as well as in WAT mass at weaning (Fig. 2 B). Adipose tissues of mice at postnatal day 21 were analysed further. Although BAT mass was unchanged, HFD offspring BAT became filled with large lipid droplets, a phenotype similar to white fat, which was recovered following RES supplementation (Fig. 2 C), consistent with changes in UCP1 immunohistochemical staining (Fig. 2 C). In agreement, the UCP1 protein level (Fig. 2 E and F) was reduced by the HFD but recovered by maternal RES supplementation. In addition, the mRNA levels of PPARγ coactivator‐1α (PGC‐1α), PRDM16, cell death‐inducing DFFA‐like effector A (Cidea), elongation of very long‐chain fatty acids protein 3 (Elovl3) and cytochrome c oxidase subunit Vlla polypeptide 1 (Cox7a1) were all decreased in HFD offspring compared with other treatments (Fig. 2 D). The decreased PRDM16 protein levels in HFD offspring was largely prevented by maternal RES treatment (Fig. 2 E and F). The HFD decreased AMPKα phosphorylation, with no effect on total AMPKα (t‐AMPKα), resulting in a decrease in the p‐AMPKα/t‐AMPKα ratio, which was recovered by RES (Fig. 2 E and F). A similar pattern of changes was observed for Sirt1 (Fig. 2 E and F). Together, these results provide evidence that maternal administration of RES increased the thermogenic activity of BAT in HFD offspring.
Figure 2. Maternal resveratrol supplementation increased BAT metabolic activity in male HFD offspring at weaning.
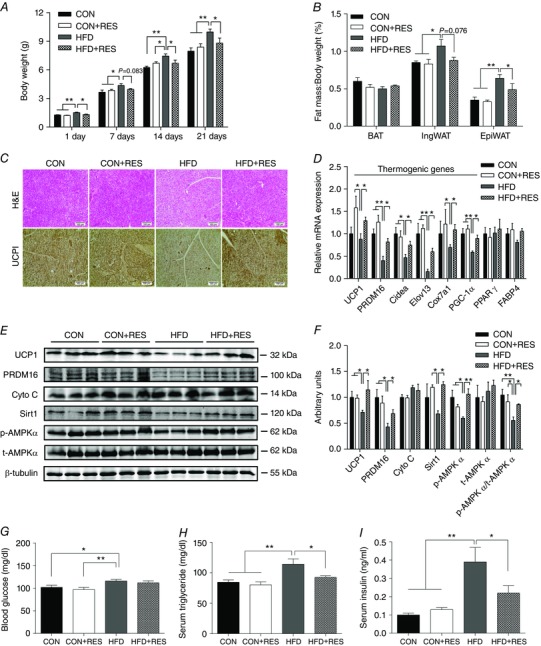
A, body weight (n = 8). B, fat index (percentage of fat pad weight relative to the whole body weight) of BAT, inguinal WAT and epididymal WAT (n = 8). C, representative H&E staining and UCP1 staining for BAT sections (n = 8). D, mRNA expression of thermogenic genes in BAT (n = 6). E and F, immunoblotting analysis for thermogenic genes and AMPKα and Sirt1 (n = 6). G–I, blood glucose (G) and serum triglyceride (H) and insulin concentrations (I,) of the offspring at weaning (n = 8). * P < 0.05 and ** P < 0.01. Data are shown as means + SEM. Abbreviations: AMPKα, AMP‐activated protein kinase; H&E, Haematoxylin and Eosin; Sirt1, Sirtuin 1; UCP1, uncoupling protein 1; for other abbreviations, see legend to Fig. 1. [Color figure can be viewed at wileyonlinelibrary.com]
At weaning, non‐fasting glucose was higher in HFD offspring than that of CON and CON+RES offspring, but maternal RES supplementation did not decrease the glucose concentration in HFD offspring (Fig. 2 G). There were significant increases in serum triglyceride and insulin concentrations in HFD offspring compared with CON and CON+RES offspring, which were reduced by maternal RES treatment, but remained slightly higher than CON (P < 0.05; Fig. 2 H and I).
Resveratrol induces brown fat‐like changes in WAT of HFD offspring at weaning
Besides BAT activity, browning of WAT is another important process in adaptive thermogenesis. We investigated the effects of maternal RES supplementation on thermogenic programming in WAT, both IngWAT and EpiWAT. Maternal RES supplementation noticeably increased the abundance of multilocular adipocytes in IngWAT (Fig. 3 A) and EpiWAT (Fig. 4 A) of HFD offspring, which is a key characteristic of brown fat‐like cells. UCP1 IHC showed enhanced UCP1 levels in IngWAT (Fig. 3 A) and EpiWAT (Fig. 4 A) of HFD+RES offspring compared with HFD offspring. Moreover, maternal RES administration decreased the adipocyte diameter in IngWAT (Fig. 3 A and B) and EpiWAT (Fig. 4 A and B). Consistent with the changes in UCP1 immunostaining, UCP1 mRNA and UCP1 protein expression were reduced in IngWAT (Fig. 3 C, E and F) and EpiWAT (Fig. 4 C, E and F) of HFD mice. This reduction was prevented by RES. Correspondingly, maternal RES supplementation increased the expression of a series of thermogenic genes in IngWAT of HFD offspring, including PRDM16, Cidea, Elovl3 and PGC‐1α (Fig. 3 C). A similar pattern of thermogenic gene expression was noticed in EpiWAT (Fig. 4 C). Moreover, maternal RES treatment also induced the mRNA expression of beige adipocyte‐selective markers in IngWAT (Fig. 3 C) and EpiWAT (Fig. 4 C) of HFD offspring, including cluster of differentiation 137 (CD137), T‐box 1 (Tbx1) and transmembrane protein 26 (Tmem26). In addition, the decreased PRDM16 protein levels in IngWAT (Fig. 3 E and F) and EpiWAT (Fig. 4 E and F) of HFD offspring were increased by maternal RES treatment. Compared with CON and CON+RES groups, HFD decreased the protein levels of cytochrome c (Cyto C), an important component of mitochondrial respiratory chain, in IngWAT (Fig. 3 E and F) and EpiWAT (Fig. 4 E and F). Resveratrol treatment of dams fed HFD increased Cyto C protein levels, accompanied by u‐regulated expression of p‐AMPKα and Sirt1 in IngWAT (Fig. 3 E and F) and EpiWAT (Fig. 4 E and F), indicating that RES activated the AMPK/Sirt1 pathway. We found that maternal RES supplementation had no effect on the expression of PPARγ and FABP4 at both mRNA and protein levels in IngWAT (Fig. 3 D–F) and EpiWAT (Fig. 4 D–F). These results suggested that maternal RES drives browning of WAT during early development.
Figure 3. Maternal resveratrol supplementation induced brown fat‐like changes in IngWAT of HFD offspring at weaning.
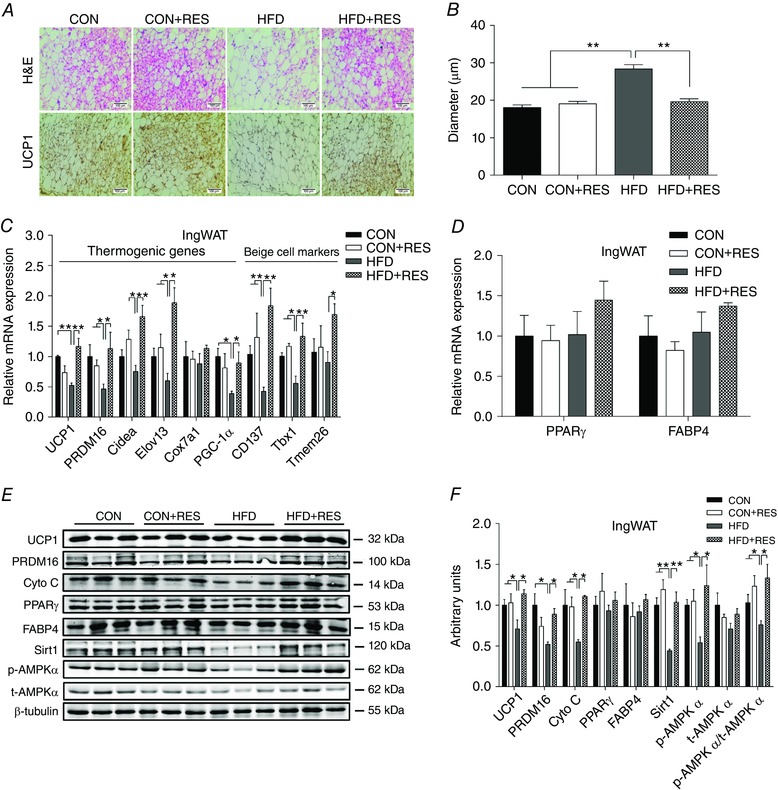
A and B, representative H&E staining and UCP1 staining (A) and average adipocyte diameters (B) in IngWAT (n = 8). C, mRNA expression of thermogenic genes and beige adipocyte‐selective markers in IngWAT (n = 6). D, mRNA expression of PPARγ and FABP4 in IngWAT (n = 6). E and F, immunoblotting analysis of thermogenic genes and AMPKα and Sirt1 in IngWAT (n = 6). * P < 0.05 and ** P < 0.01. Data are shown as means + SEM. Abbreviations: AMPKα, AMP‐activated protein kinase; FABP4, fatty‐acid binding protein 4; H&E, Haematoxylin and Eosin; PPARγ, peroxisomal proliferator‐activated receptor γ; Sirt1, Sirtuin 1; UCP1, uncoupling protein 1; for other abbreviations, see legend to Fig. 1. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 4. Maternal resveratrol supplementation induced brown fat‐like changes in EpiWAT of HFD offspring at weaning.
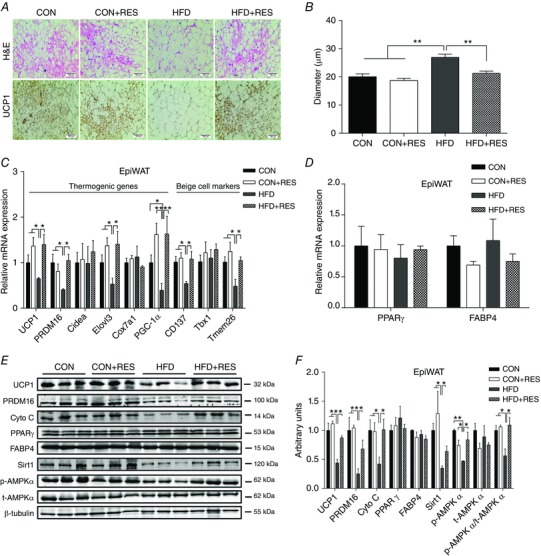
A and B, representative H&E staining and UCP1 staining (A) and average adipocyte diameters (B) in EpiWAT (n = 8). C, mRNA expression of thermogenic genes and beige adipocyte‐selective markers in EpiWAT (n = 6). D, mRNA expression of PPARγ and FABP4 in EpiWAT (n = 6). E and F, immunoblotting analysis of thermogenic genes and AMPKα and Sirt1 in EpiWAT (n = 6). * P < 0.05 and ** P < 0.01. Data are shown as means + SEM. Abbreviations: AMPKα, AMP‐activated protein kinase; FABP4, fatty‐acid binding protein 4; H&E, Haematoxylin and Eosin; PPARγ, peroxisomal proliferator‐activated receptor γ; Sirt1, Sirtuin 1; UCP1, uncoupling protein 1; for other abbreviations, see legend to Fig. 1. [Color figure can be viewed at wileyonlinelibrary.com]
Resveratrol reduces adiposity and improves insulin sensitivity in adult offspring challenged with HFD
To determine whether the increased brown and beige adipogenesis during the early developmental stage could exert long‐time benefits in preventing diet‐induced obesity in adulthood, we examined the body composition and metabolic characteristics of offspring mice at 3 months of age. The HFD offspring showed a marked increase in body weight (Fig. 5 A) and WAT mass (Fig. 5 B) compared with CON and CON+RES. Maternal RES treatment during pregnancy and lactation protected adult offspring against HFD‐induced body weight gain (Fig. 5 A) and fat accumulation (Fig. 5 B). These responses occurred without differences in energy intake (Fig. 5 C). Compared with CON and CON+RES offspring, HFD offspring showed higher serum triglyceride and insulin concentrations, which were attenuated by maternal RES treatment to a level comparable with CON (Fig. 5 D and E). Maternal RES supplementation improved glucose tolerance in HFD offspring (Fig. 5 F and G). These data suggested that maternal RES supplementation prevented diet‐induced obesity and increased insulin sensitivity in adulthood.
Figure 5. Maternal resveratrol supplementation reduced body weight gain and adiposity and improved insulin sensitivity in 14‐week‐old offspring challenged with HFD.
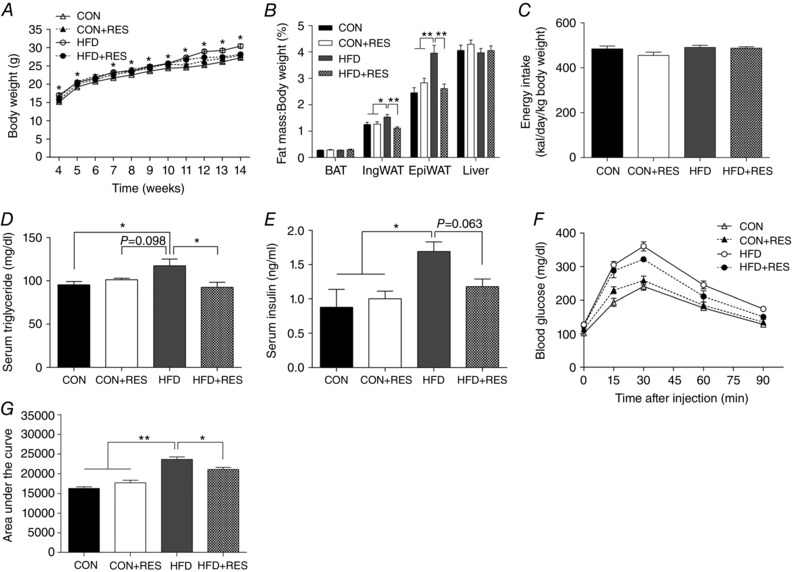
A, offspring body weight (n = 8–10). B, ratio of adipose tissue and liver weights to body weights (n = 8–10). C, offspring energy intake (n = 8–10). D, serum triglyceride concentrations (n = 6). E, serum insulin concentrations (n = 6). F and G, glucose tolerance test and area under the curve (n = 6). * P < 0.05 and ** P < 0.01. Data are expressed as means ± SEM. For abbreviations, see legend to Fig. 1.
Resveratrol increases whole‐body energy expenditure in adult offspring challenged with HFD
To explore why maternal RES feeding protected against diet‐induced obesity in adult offspring, we measured offspring whole‐body energy expenditure. Compared with CON and CON+RES offspring, HFD offspring showed markedly decreased oxygen consumption during light and dark phases, which was prevented by maternal RES treatment (Fig. 6 A and B). There was no difference in CO2 production among groups (Fig. 6 C and D). As a result, the increased RER in HFD offspring tended to be decreased by maternal RES treatment (Fig. 6 E and F), suggesting that a higher ratio of lipids was being oxidized, consistent with the decreased circulating triglyceride concentrations in HFD+RES offspring. Furthermore, in accordance with oxygen consumption, the decreased heat production in HFD offspring was prevented by maternal RES treatment (Fig. 6 G and H). Moreover, HFD+RES offspring had a higher body temperature than HFD offspring during the cold challenge, indicating that maternal RES treatment enhanced the capacity for adaptive thermogenesis (Fig. 6 I). Taken together, these results showed that maternal RES supplementation increased metabolic activity in HFD adult offspring.
Figure 6. Maternal resveratrol supplementation increased energy expenditure in 14‐week‐old offspring under HFD challenge.
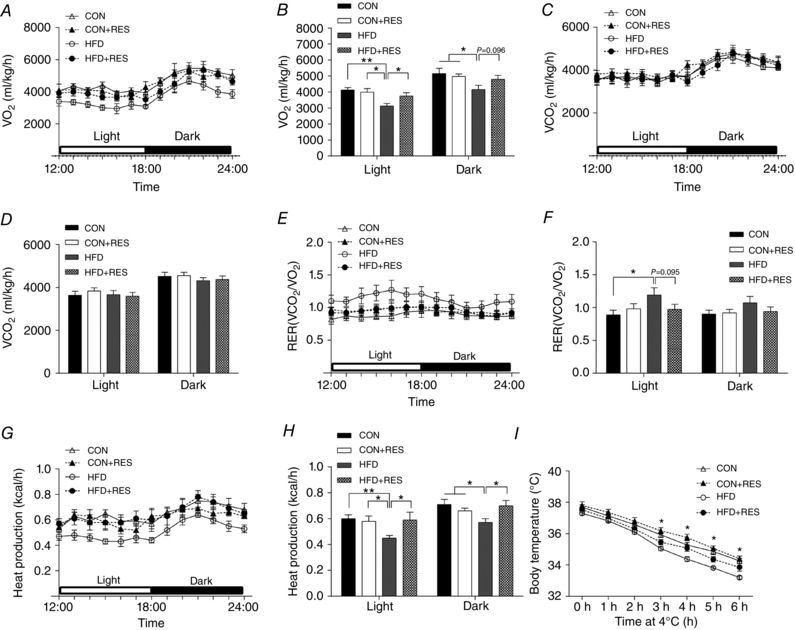
A and B, during a 6 h light–6 h dark cycle measured in a metabolic cage (A) and the average values (B). C and D, during a 6 h light–6 h dark cycle (C) and the average values (D). E and F, the values of RER (ratio of to ) were calculated from metabolic chamber data during a 6 h light–6 h dark cycle. G and H, heat production during a 6 h light–6 h dark cycle (G) and the average values (H). I, acute cold tolerance test. * P < 0.05 and ** P < 0.01. Data are expressed as means ± SEM (n = 6). Abbreviations: RER, respiratory exchange rate; , carbon dioxide production; , oxygen consumption rate; for other abbreviations, see legend to Fig. 1.
Resveratrol promotes BAT function and IngWAT browning in adult offspring challenged with HFD
To explain the enhanced energy expenditure in HFD+RES offspring, we examined whether BAT function and WAT thermogenic programme are elevated in these mice. Haematoxylin and Eosin staining results revealed that the average adipocyte diameters in WAT of HFD+RES offspring were smaller than those of HFD offspring (Fig. 7 A). Large adipocytes were most abundant in WAT of HFD offspring, whereas maternal RES treatment attenuated this HFD‐induced effect (Fig. 7 B and C). In addition, histological analysis also indicated that HFD+RES offspring displayed fewer lipid deposits and greater UCP1 expression in BAT compared with HFD offspring (Fig. 7 A). Similar to their BAT, IngWAT of HFD+RES offspring mice also displayed more beige adipocytes and higher UCP1‐positive areas than those of HFD offspring mice (Fig. 7 A and D). Consistent with these findings, maternal RES supplementation increased UCP1 protein expression in BAT and IngWAT of adult HFD offspring (Fig. 7 E and F). However, the morphological changes (Fig. 7 A and D) and expressional alteration of UCP1 (Fig. 7 G) were not observed in EpiWAT of HFD+RES offspring. These data suggested that maternal RES supplementation increased BAT function and induced beige adipocyte development in IngWAT of adult offspring.
Figure 7. Maternal resveratrol supplementation enhanced BAT function and IngWAT thermogenic programme in 14‐week‐old offspring challenged with HFD.
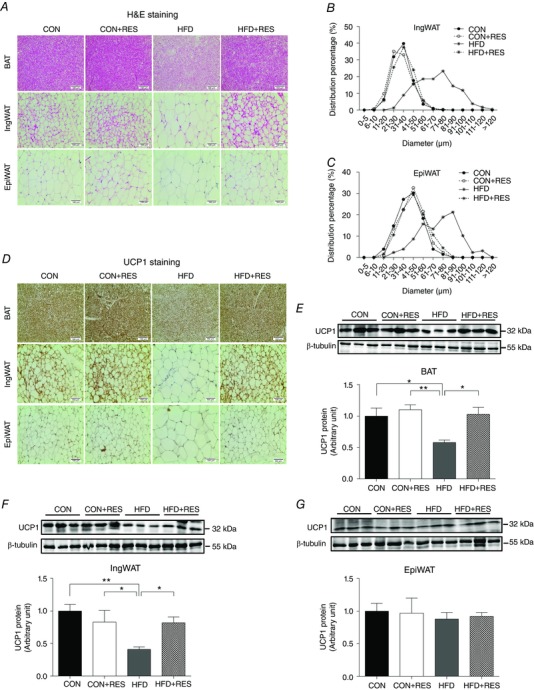
A, representative H&E staining in sections of BAT and WAT (n = 8). B and C, percentage distribution of adipocyte diameters of IngWAT (B) and EpiWAT (C) (n = 8). D, representative UCP1 staining in sections of BAT and WAT (n = 8). E–G, immunoblotting analysis for UCP1 in BAT (E), IngWAT (F) and EpiWAT (G) (n = 6). * P < 0.05 and ** P < 0.01. Data are shown as means + SEM. Abbreviations: H&E, Haematoxylin and Eosin; UCP1, uncoupling protein 1; for other abbreviations, see legend to Fig. 1. [Color figure can be viewed at wileyonlinelibrary.com]
Discussion
It has been well established that maternal obesity and high energy intake impair fetal development, with subsequent long‐term negative effects on offspring health. Several studies have shown that maternal polyphenol supplementation during the fetal and lactation stages improves offspring development, reducing body fat accumulation and metabolic disorders, such as hypertension, insulin resistance and inflammation, in adult life (Dolinoy et al. 2006; Resende et al. 2013; Wu et al. 2014). However, the mechanistic links remain to be established.
In the present study, we used the diet‐induced obesity model to test the effect of RES on offspring adipose development. The body weight of HFD neonates was higher than that of CON neonates at postnatal day 1, similar to macrosomia of the babies of obese women, and maternal resveratrol treatment reduced the body weight gain of HFD neonates. The biological effects of RES on fetal development should be attributable to both maternal and direct fetal effects. Resveratrol can cross the placenta and affect the fetus directly (Bourque et al. 2012). In addition, RES also alters maternal metabolism to change the intrauterine and early postnatal nutritional environments, which in turn affects the early programming of the offspring (Sun et al. 2012). At the end of lactation, RES supplementation did not significantly alter blood glucose concentrations in HFD dams and their weaned offspring; findings which are similar to previous reports demonstrating that maternal polyphenol administration had no significant effect on blood glucose in HFD‐fed dams in gestation or lactation (Roberts et al. 2014; Wu et al. 2014). Moreover, the HFD dams had higher body weight, WAT mass and circulating concentrations of triglycerides and insulin compared with CON dams, which was recovered by maternal RES treatment, suggesting a positive effect of RES on maternal metabolic homeostasis.
Two forms of adipose tissue exist, the white and brown adipose tissues. While WAT is responsible for obesity and metabolic dysfunction, BAT burns fatty acids and glucose to generate heat (Virtanen et al. 2009). Brown adipose tissue is capable of producing 300 times more heat than other tissues per unit mass (Symonds, 2013). Thus, the recent discovery of BAT in adult humans provides a promising therapeutic target against obesity and diabetes (Kozak et al. 2010; Peirce et al. 2014). In addition, beige adipocytes have recently been identified in WAT (Harms & Seale, 2013). Importantly, beige adipocytes are inducible, a process termed ‘browning of WAT’ (Wu et al. 2012). In adult animals, RES could reduce body weight gain and adiposity via inhibition of white adipogenesis (Lagouge et al. 2006; Kim et al. 2011) and induction of browning of WAT (Wang et al. 2015). To date, however, no study has assessed the effect of maternal RES administration on adipocyte browning and thermogenesis in offspring born to maternal HFD mothers. In the present study, we found that maternal HFD intake during pregnancy and lactation impaired offspring BAT and beige adipocyte development at weaning and had lasting effects on offspring metabolic health. Importantly, RES treatment of HFD dams not only enhanced BAT activity and beige adipocyte formation and the associated thermogenic programme in offspring, but also had persistent beneficial effects in preventing HFD‐induced obesity and metabolic disorders in adulthood. To our knowledge, this is the first report showing promotion of BAT/beige adipogenesis and thermogenic function attributable to RES supplementation of HFD mothers.
The development and thermogenic function of brown/beige adipocytes are regulated by a complex network of hormones and signalling pathways. PRDM16 is a crucial transcription factor that drives brown adipogenic gene expression and thermogenesis (Hondares et al. 2011). In our previous studies, we found that RES activates AMPK and Sirt1 (Wang et al. 2015), and we also found that AMPK promotes PRDM16 expression (Yang et al. 2016). Consistent with these findings, in the present study, maternal RES treatment increased the expression of PRDM16 and other thermogenic genes in HFD+RES offspring. These findings demonstrated that maternal RES supplementation promotes thermogenic activity of BAT and beige adipocytes in offspring at weaning. As a consequence, the increase in body weight gain and WAT mass of HFD offspring was prevented by RES supplementation. AMPK and Sirt1, two important energy sensors, act together with PGC‐1α to regulate energy homeostasis in response to environmental and nutritional stimuli (Cantó & Auwerx, 2009). We found that maternal RES supplementation increased phosphorylated AMPKα levels and Sirt1 protein contents in BAT and WAT of HFD offspring, consistent with previous reports showing that RES‐induced biological effects are mediated by the AMPK/Sirt1 pathway (Kitada et al. 2011; Price et al. 2012), and also in adult mice, where grape seed procyanidins (Crescenti et al. 2015) and azuki (Mukai et al. 2013) activate the AMPK/Sirt1 pathway.
The development of BAT mainly occurs during the late fetal stage, and its thermogenic function is crucial for an adequate neonatal response to prevent hypothermia. During fetal development, myogenic factor (MYF)5+ cells in the future BAT depots start to express Prdm16, which initiates brown adipogenic gene cascades in myogenic precursor cells (Seale et al. 2008). In addition, based on lineage tracing, a portion of brown adipocytes in BAT are developed from non‐myogenic PDGFRα+ progenitor cells (Tran et al. 2012; Peirce et al. 2014), whereas most of the beige adipocytes in WAT are derived from PDGFRα+ progenitor cells (McDonald et al. 2015; Tharp et al. 2015; Townsend & Tseng, 2015). The density of progenitor cells varies among different fat depots. The subcutaneous fat has a high density of progenitor cells and high expansion capacity, whereas that of EpiWAT is limited (Liang et al. 2016). The low density of progenitor cells in EpiWAT limits de novo formation of beige adipocytes, which might explain the lack of changes in EpiWAT beige adipogenesis of offspring born to HFD+RES mothers, because new beige adipocytes are primarily derived from progenitor cells (Wang et al. 2013; Lee et al. 2015).
In conclusion, we have demonstrated that RES supplementation of mice fed a HFD during pregnancy and lactation promotes a thermogenic programme in BAT and WAT, and induces beige adipocyte development of WAT in their male offspring. Furthermore, the enhanced BAT function and browning of WAT increased energy expenditure, protecting offspring against HFD‐induced obesity and insulin resistance in adulthood. Thus, we propose that maternal intervention with RES throughout pregnancy and lactation has promise as an intervention in the setting of maternal obesity by inducing persistent beneficial programming effects on the BAT/beige adipocyte function and thermogenesis in male offspring, with lasting effects on offspring metabolic health.
Additional information
Competing interests
The authors declare no conflict of interest.
Author contributions
T.Z. and M.D. conceived the project, designed the experiments and wrote the manuscript. T.Z., D.C., Q.Y. and B.W. researched data. T.Z., Q.Y. and B.W. performed the experiments. D.C., M.‐J.Z. and P.W.N. contributed to the discussion and reviewed and edited the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by grants from the National Institutes of Health (R01‐HD067449 and R21‐AG049976) and a grant from the National Processed Raspberry Council to M.D., the National Basic Research Program of China (2012CB124701) to D.C. and a scholarship from the China Scholarship Council to T.Z.
T. Zou and D. Chen contributed equally to this work.
Linked articles This article is highlighted by a Perspective by Aiken & Ozanne. To read this Perspective, visit http://dx.doi.org/10.1113/JP273821.
This is an Editor's Choice article from the 1 March 2017 issue.
References
- Bambini‐Junior V, Zanatta G, Nunes GDF, de Melo GM, Michels M, Fontes‐Dutra M, Freire VN, Riesgo R & Gottfried C (2014). Resveratrol prevents social deficits in animal model of autism induced by valproic acid. Neurosci Lett 583, 176–181. [DOI] [PubMed] [Google Scholar]
- Blackmore HL, Niu Y, Fernandez‐Twinn DS, Tarry‐Adkins JL, Giussani DA & Ozanne SE (2014). Maternal diet‐induced obesity programs cardiovascular dysfunction in adult male mouse offspring independent of current body weight. Endocrinology 155, 3970–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekelheide K, Blumberg B, Chapin RE, Cote I, Graziano JH, Janesick A, Lane R, Lillycrop K, Myatt L, Thayer KA, Waalkes MP & Rogers JM (2012). Predicting later‐life outcomes of early‐life exposures. Environ Health Perspect 120, 1353–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonet ML, Oliver P & Palou A (2013). Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim Biophys Acta 1831, 969–985. [DOI] [PubMed] [Google Scholar]
- Borengasser SJ, Zhong Y, Kang P, Lindsey F, Ronis MJ, Badger TM, Gomez‐Acevedo H & Shankar K (2013). Maternal obesity enhances white adipose tissue differentiation and alters genome‐scale DNA methylation in male rat offspring. Endocrinology 154, 4113–4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque S, Dolinsky V, Dyck J & Davidge S (2012). Maternal resveratrol treatment during pregnancy improves adverse fetal outcomes in a rat model of severe hypoxia. Placenta 33, 449–452. [DOI] [PubMed] [Google Scholar]
- Boyle KE, Patinkin ZW, Shapiro AL, Baker PR, Dabelea D & Friedman JE (2016). Mesenchymal stem cells from infants born to obese mothers exhibit greater potential for adipogenesis: the healthy start BabyBUMP project. Diabetes 65, 647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce KD, Cagampang FR, Argenton M, Zhang J, Ethirajan PL, Burdge GC, Bateman AC, Clough GF, Poston L & Hanson MA (2009). Maternal high‐fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology 50, 1796–1808. [DOI] [PubMed] [Google Scholar]
- Cannon B & Nedergaard J (2004). Brown adipose tissue: function and physiological significance. Physiol Rev 84, 277–359. [DOI] [PubMed] [Google Scholar]
- Cantó C & Auwerx J (2009). PGC‐1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Current Opin Lipidol 20, 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Care AS, Sung MM, Panahi S, Gragasin FS, Dyck JR, Davidge ST & Bourque SL (2016). Perinatal resveratrol supplementation to spontaneously hypertensive rat dams mitigates the development of hypertension in adult offspring. Hypertension 67, 1038–1044. [DOI] [PubMed] [Google Scholar]
- Crescenti A, del Bas JM, Arola‐Arnal A, Oms‐Oliu G, Arola L & Caimari A (2015). Grape seed procyanidins administered at physiological doses to rats during pregnancy and lactation promote lipid oxidation and up‐regulate AMPK in the muscle of male offspring in adulthood. J Nutr Biochem 26, 912–920. [DOI] [PubMed] [Google Scholar]
- Dearden L & Ozanne SE (2015). Early life origins of metabolic disease: developmental programming of hypothalamic pathways controlling energy homeostasis. Front Neuroendocrinol 39, 3–16. [DOI] [PubMed] [Google Scholar]
- Del Bas J, Crescenti A, Arola‐Arnal A, Oms‐Oliu G, Arola L & Caimari A (2015). Grape seed procyanidin supplementation to rats fed a high‐fat diet during pregnancy and lactation increases the body fat content and modulates the inflammatory response and the adipose tissue metabolism of the male offspring in youth. Int J Obes (Lond) 39, 7–15. [DOI] [PubMed] [Google Scholar]
- Dolinoy DC, Weidman JR, Waterland RA & Jirtle RL (2006). Maternal genistein alters coat colour and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect 114, 567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forhead AJ, Jellyman JK, De Blasio MJ, Johnson E, Giussani DA, Broughton Pipkin F & Fowden AL (2015). Maternal dexamethasone treatment alters tissue and circulating components of the renin‐angiotensin system in the pregnant ewe and fetus. Endocrinology 156, 3038–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco J, Fernandes T, Rocha C, Calviño C, Pazos‐Moura C, Lisboa P, Moura E & Trevenzoli I (2012). Maternal high‐fat diet induces obesity and adrenal and thyroid dysfunction in male rat offspring at weaning. J Physiol 590, 5503–5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Zhu M, Zhang S, Foretz M, Viollet B & Du M (2015). Obesity impairs skeletal muscle regeneration via inhibition of AMP‐activated protein kinase. Diabetes 65, 188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giralt M & Villarroya F (2013). White, brown, beige/brite: different adipose cells for different functions? Endocrinology 154, 2992–3000. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Niu Y, Herrera EA, Richter HG, Camm EJ, Thakor AS, Kane AD, Hansell JA, Brain KL & Skeffington KL (2014). Heart disease link to fetal hypoxia and oxidative stress. Adv Exp Med Biol 814, 77–87. [DOI] [PubMed] [Google Scholar]
- Harms M & Seale P (2013). Brown and beige fat: development, function and therapeutic potential. Nat Med 19, 1252–1263. [DOI] [PubMed] [Google Scholar]
- Hondares E, Rosell M, Díaz‐Delfín J, Olmos Y, Monsalve M, Iglesias R, Villarroya F & Giralt M (2011). Peroxisome proliferator‐activated receptor α (PPARα) induces PPARγ coactivator 1α (PGC‐1α) gene expression and contributes to thermogenic activation of brown fat involvement of PRDM16. J Biol Chem 286, 43112–43122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon BT, Jeong EA, Shin HJ, Lee Y, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS & Roh GS (2012). Resveratrol attenuates obesity‐associated peripheral and central inflammation and improves memory deficit in mice fed a high‐fat diet. Diabetes 61, 1444–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Jin Y, Choi Y & Park T (2011). Resveratrol exerts anti‐obesity effects via mechanisms involving down‐regulation of adipogenic and inflammatory processes in mice. Biochem Pharmacol 81, 1343–1351. [DOI] [PubMed] [Google Scholar]
- Kitada M, Kume S, Imaizumi N & Koya D (2011). Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn‐SOD dysfunction in AMPK/SIRT1‐independent pathway. Diabetes 60, 634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak LP, Koza RA & Anunciado‐Koza R (2010). Brown fat thermogenesis and body weight regulation in mice: relevance to humans. Int J Obes 34, S23–S27. [DOI] [PubMed] [Google Scholar]
- Kumar A, Singh CK, LaVoie HA, DiPette DJ & Singh US (2011). Resveratrol restores Nrf2 level and prevents ethanol‐induced toxic effects in the cerebellum of a rodent model of fetal alcohol spectrum disorders. Mol Pharmacol 80, 446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart‐Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P & Elliott P (2006). Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC‐1α. Cell 127, 1109–1122. [DOI] [PubMed] [Google Scholar]
- Lasa A, Schweiger M, Kotzbeck P, Churruca I, Simón E, Zechner R & del Puy Portillo M (2012). Resveratrol regulates lipolysis via adipose triglyceride lipase. J Nutr Biochem 23, 379–384. [DOI] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Konkar AA & Granneman JG (2015). Cellular origins of cold‐induced brown adipocytes in adult mice. FASEB J 29, 286–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Bouzar C, Cottet‐Rousselle C, Zagotta I, Lamarche F, Wabitsch M, Tokarska‐Schlattner M, Fischer‐Posovszky P, Schlattner U & Rousseau D (2016). Resveratrol inhibits lipogenesis of 3T3‐L1 and SGBS cells by inhibition of insulin signalling and mitochondrial mass increase. BBA‐Bioenergetics 1857, 643–652. [DOI] [PubMed] [Google Scholar]
- Liang X, Yang Q, Fu X, Rogers CJ, Wang B, Pan H, Zhu MJ, Nathanielsz PW & Du M (2016). Maternal obesity epigenetically alters visceral fat progenitor cell properties in male offspring mice. J Physiol 594, 4453–4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ & Schmittgen TD (2001). Analysis of relative gene expression data using real‐time quantitative PCR and the method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- McDonald ME, Li C, Bian H, Smith BD, Layne MD & Farmer SR (2015). Myocardin‐related transcription factor A regulates conversion of progenitors to beige adipocytes. Cell 160, 105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai Y, Sun Y & Sato S (2013). Azuki bean polyphenols intake during lactation upregulate AMPK in male rat offspring exposed to fetal malnutrition. Nutrition 29, 291–297. [DOI] [PubMed] [Google Scholar]
- Nathanielsz PW, Ford SP, Long NM, Vega CC, Reyes‐Castro LA & Zambrano E (2013). Interventions to prevent adverse fetal programming due to maternal obesity during pregnancy. Nutr Rev 71, S78–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce V, Carobbio S & Vidal‐Puig A (2014). The different shades of fat. Nature 510, 76–83. [DOI] [PubMed] [Google Scholar]
- Poston L (2012). Maternal obesity, gestational weight gain and diet as determinants of offspring long term health. Bes Pract Res Clin Endocrinol Metab 26, 627–639. [DOI] [PubMed] [Google Scholar]
- Price NL, Gomes AP, Ling AJ, Duarte FV, Martin‐Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G & Teodoro JS (2012). SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15, 675–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayalam S, Yang JY, Ambati S, Della‐Fera MA & Baile CA (2008). Resveratrol induces apoptosis and inhibits adipogenesis in 3T3‐L1 adipocytes. Phytother Res 22, 1367–1371. [DOI] [PubMed] [Google Scholar]
- Resende AC, Emiliano AF, Cordeiro VS, Graziele F, de Cavalho LC, de Oliveira PRB, Neto ML, Costa CA, Boaventura GT & de Moura RS (2013). Grape skin extract protects against programmed changes in the adult rat offspring caused by maternal high‐fat diet during lactation. J Nutr Biochem 24, 2119–2126. [DOI] [PubMed] [Google Scholar]
- Roberts VH, Pound LD, Thorn SR, Gillingham MB, Thornburg KL, Friedman JE, Frias AE & Grove KL (2014). Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. FASEB J 28, 2466–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson A‐M, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L & Taylor PD (2008). Diet‐induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 51, 383–392. [DOI] [PubMed] [Google Scholar]
- Santangelo C, R Varì, Scazzocchio B, Filesi C & Masella R (2014). Management of reproduction and pregnancy complications in maternal obesity: which role for dietary polyphenols? Biofactors 40, 79–102. [DOI] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument‐Bromage H, Tempst P, Rudnicki MA, Beier DR & Spiegelman BM (2008). PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Purcell RH, Terrillion CE, Yan J, Moran TH & Tamashiro KL (2012). Maternal high‐fat diet during gestation or suckling differentially affects offspring leptin sensitivity and obesity. Diabetes 61, 2833–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds ME (2013). Brown adipose tissue growth and development. Scientifica (Cairo) 2013, 305763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P, Samuelsson AM & Poston L (2014). Maternal obesity and the developmental programming of hypertension: a role for leptin. Acta Physiol (Oxf) 210, 508–523. [DOI] [PubMed] [Google Scholar]
- Tharp KM, Jha AK, Kraiczy J, Yesian A, Karateev G, Sinisi R, Dubikovskaya EA, Healy KE & Stahl A (2015). Matrix‐assisted transplantation of functional beige adipose tissue. Diabetes 64, 3713–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D & Kersten S (2011). Calorie restriction‐like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 14, 612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend K & Tseng Y (2015). Of mice and men: novel insights regarding constitutive and recruitable brown adipocytes. Int J Obesity Suppl 5, S15–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka Y, Wada E & Wada K (2009). Diet‐induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. FASEB J 23, 1920–1934. [DOI] [PubMed] [Google Scholar]
- Tran KV, Gealekman O, Frontini A, Zingaretti MC, Morroni M, Giordano A, Smorlesi A, Perugini J, De Matteis R, Sbarbati A, Corvera S & Cinti S (2012). The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab 15, 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um J‐H, Park S‐J, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B & Chung JH (2010). AMP‐activated protein kinase‐deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 59, 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega CC, Reyes‐Castro LA, Rodríguez‐González GL, Bautista CJ, Vázquez‐Martínez M, Larrea F, Chamorro‐Cevallos GA, Nathanielsz PW & Zambrano E (2016). Resveratrol partially prevents oxidative stress and metabolic dysfunction in pregnant rats fed a low protein diet and their offspring. J Physiol 594, 1483–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto N‐J & Enerbäck S (2009). Functional brown adipose tissue in healthy adults. New Engl J Med 360, 1518–1525. [DOI] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK & Scherer PE (2013). Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 19, 1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Liang X, Yang Q, Fu X, Rogers C, Zhu M, Rodgers B, Jiang Q, Dodson M & Du M (2015). Resveratrol induces brown‐like adipocyte formation in white fat through activation of AMP‐activated protein kinase (AMPK) α1. Int J Obesity 39, 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang A‐H, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerbäck S, Schrauwen P & Spiegelman BM (2012). Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Zhao J, Xu H, Lyv Y, Feng X, Fang Y & Xu Y (2014). Maternal quercetin administration during gestation and lactation decrease endoplasmic reticulum stress and related inflammation in the adult offspring of obese female rats. Eur J Nutr 53, 1669–1683. [DOI] [PubMed] [Google Scholar]
- Yang Q, Liang X, Sun X, Zhang L, Fu X, Rogers CJ, Berim A, Zhang S, Wang S, Wang B, Foretz M, Viollet B, Gang DR, Rodgers BD, Zhu MJ & M Du (2016). AMPK/α‐ketoglutarate axis dynamically mediates DNA demethylation in the Prdm16 promoter and brown adipogenesis. Cell Metab 24, 542–554. [DOI] [PMC free article] [PubMed] [Google Scholar]


