Abstract
There is a great need for improved diagnostic and prognostic accuracy of potential cardiac toxicity in drug development. This study reports the evaluation of several commercially available biomarker kits by three institutions (SRI, Eli Lilly and Pfizer) for the discrimination between myocardial degeneration/necrosis and cardiac hypertrophy as well as the assessment of the inter-laboratory and inter-platform variation in results. Serum concentrations of natriuretic peptides (NT-proANP, NT-proBNP), cardiac and skeletal troponins (cTnI, cTnT, sTnI), myosin light chain 3 (Myl3) and fatty acid binding protein 3 (FABP3) were assessed in rats treated with minoxidil and isoproterenol. Minoxidil caused increased heart-to-body weight ratios and prominent elevations in NT-proANP and NT-proBNP concentrations detected at 24 hr postdose without elevation in troponins, Myl3 or FABP3 and with no abnormal histopathological findings. Isoproterenol caused ventricular leukocyte infiltration, myocyte fibrosis and necrosis with increased concentrations of the natriuretic peptides, cardiac troponins and Myl3. These results reinforce the advantages of a multi-marker strategy in elucidating the underlying cause of cardiac insult and detecting myocardial tissue damage at 24 hr post-treatment. The inter-laboratory and inter-platform comparison analyses also showed that the data obtained from different laboratories and platforms are highly correlated and reproducible, making these biomarkers widely applicable in preclinical studies.
Keywords: Rat, Cardiotoxicity, Biomarker, Natriuretic peptides, Cardiac troponins, Hypertrophy, Myocardial necrosis
INTRODUCTION
Drug failures both in clinical development and following post-market approval contribute significantly to the rising cost of drug discovery and development, with amortization of drug failures leading to the cost of development of new drugs being estimated as high as $5 billion (Herper, August 11, 2013). Drug induced cardiac injury is a major cause of drug development failures and 28% of drug withdrawals in the USA have been associated with negative cardiovascular effects (Gwathmey et al., 2009, Feenstra et al., 1999, Ferri et al., 2013). Cardiotoxicity is also an important consideration for drugs with proven effectiveness in high risk diseases such as HIV/AIDS as some patients may be more susceptible to drug-related cardiovascular complications than others (Fantoni et al., 2001, Lambert et al., 2015, Lipshultz et al., 2012, Bozkurt, 2004). There is therefore a significant need for development of improved tools for both preclinical and clinical research that provide more sensitive predictors of potential cardiac effects of candidate drugs.
Serum biomarkers provide a minimally invasive measure for diagnosing cardiac effects of drugs. Several myocardium-specific proteins have been reported as useful biomarkers for congestive heart failure, myocardial infarction and other heart diseases. These biomarkers include cardiac troponins I and T, cardiac natriuretic peptides, creatine kinase isoenzyme MB (CK-MB) and lactate dehydrogenase (LDH) (Selvais et al., 1998, Babuin and Jaffe, 2005, Lott and Stang, 1989, O'Brien, 2008, Lim et al., 2011). Among the biomarkers, cardiac troponins are considered the gold standard for cardiac injury considering their sensitivity and specificity; however, the troponins have a half-life of about two hours, which makes them difficult to use for routine evaluation in toxicology studies (Babuin and Jaffe, 2005, Kehl et al., 2012, Dunn et al., 2011). The early clinical laboratory biomarkers for assessing cardiomyocyte degeneration/necrosis, myosin light chain 3 (Myl3) and fatty acid binding protein 3 (FABP3) are abundant in myocyte cytoplasm and rapidly released with cell injury; however, they are present in both heart and skeletal muscle, thus limiting these biomarkers with regards to tissue-specificity, and are rapidly cleared from the blood stream as well (Tanaka et al., 1991, Ravkilde et al., 1995, Tonomura et al., 2012). One major problem associated with these current biomarkers is that they typically only show significant changes in the first 12–24 hr post-dose, returning to normal levels after 24 hr (Rajappa and Sharma, 2005, Maynard et al., 2000). Therefore, there is an urgent need for cardiac biomarkers that can evaluate drug-induced cardiotoxicity over longer periods of time and be incorporated in routine preclinical toxicology studies.
The natriuretic peptides, atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) are cardiac hormones involved in plasma volume homeostasis, cardiovascular remodeling and counterregulation of the renin–angiotensin–aldosterone system. ANP is produced primarily in the cardiac atria in response to cardiac ischemia, myocardial stretch or cardiac hypertrophy due to pressure or volume overload (Mukoyama et al., 1991, Levin et al., 1998). The messenger RNA transcript for ANP is approximately 1 kb in size and encodes a precursor protein (pro-ANP) of 126 amino acids. BNP was originally identified in extracts of porcine brain but, there is considerably more present in the cardiac ventricles. Human pro-BNP contains 108 amino acids. Amino-terminal fragments derived from the ANP and BNP precursors, N-terminal pro-ANP (NT-proANP) and N-terminal pro-BNP (NT-proBNP) have been identified as predictors of cardiac dysfunction in many clinical settings including congestive heart failure, myocardial infarction or septic shock (Mukoyama et al., 1991, Jarai et al., 2009, Meyer et al., 2007). The natriuretic peptide plasma levels are strong prognostic markers of 30-day-mortality in patients with decompensated heart failure from ischemic and non-ischemic origins (Luers et al., 2013, Scrutinio et al., 2013). In clinical studies of myocardial infarction, plasma BNP has been correlated with the severity of left ventricular dysfunction and remodeling (Morrow and Braunwald, 2003) and also served as a predictor of future heart failure or other cardiovascular events in asymptomatic patients without obvious heart failure (Wang et al., 2004). When evaluated in combination with serum cardiac troponins, NT-proANP and NT-proBNP have been shown to be promising biomarkers bridging preclinical and clinical testing for chronic and/or functional cardiac injury (Walker, 2006, Gaggin and Januzzi, 2013).
Minoxidil (MNX), a direct vasodilator is capable of reducing blood pressure in patients with resistant hypertension; however, its effect can be limited because of its association with idiosyncratic onset of a pericardial effusion, plasma volume expansion and aggravation of myocardial ischemia leading to left ventricular hypertrophy (Sica, 2004, Bryan et al., 1977, Grim et al., 1979, Sanz et al., 1990). MNX is used in many preclinical studies for cardiac toxicity evaluation to induce cardiac hypertrophy by volume overload without primary myocardial injury (Turcani and Jacob, 1998, van der Velden et al., 1999, Engle and Watson, 2016).
Isoproterenol (ISO), a beta-adrenergic agonist used to treat bradycardia or heart block, has been used as a model compound to induce myocardial infarct-like lesions. Various studies have been conducted to evaluate myocyte damage following exposure to this drug (York et al., 2007, Kaufman, 1985, Hasic et al., 2011, Acikel et al., 2005, Brady et al., 2010). We have previously reported on a variety of serum proteins showing significant changes in African green monkeys, Rhesus macaques and Cynomolgus macaques; these proteins can potentially be used for predicting long-term cardiac injury (Liu et al., 2013, Song et al., 2014), but are not currently available in standardized kits.
A wide range of cardiac effects that may eventually lead to the clinical syndrome of heart failure includes myocyte stretch/hypertrophy, myocardial injury, oxidative stress, neurohormonal activation, inflammation, apoptosis, etc. (Gaggin and Januzzi, 2013). Of these, this study focuses on assessing a set of biomarkers that can distinguish cardiac hypertrophy (as evidenced by increased heart weights) from direct myocardial injury (degeneration/necrosis). Utilizing biomarkers that are insult-specific and tissue-specific, the improved diagnostic and prognostic accuracy of potential cardiac toxicity that may have not yet symptomized may become more feasible and reliable in both preclinical and clinical studies. As different laboratories use different platforms, or even subtle differences in application of the same platform, it is also important to assess the reproducibility of these biomarkers across different laboratories using the same platform, as well as across different technology platforms. Recently, a cross-laboratory comparison study presented results demonstrating the ELISA assay detection of serum NT-proANP in rats to be technically adequate with acceptable intra- and inter-assay and inter-laboratory precision and accuracy (Vinken et al., 2016). There have been reports of several successful uses of NT-proANP and NT-proBNP to detect cardiotoxicity in preclinical settings (Dunn et al., 2016, Stokes et al., 2015, Colton et al., 2011, Casartelli et al., 2011).
Here we report results of the evaluation of several commercially available biomarker kits for the discrimination between myocardial degeneration/necrosis and cardiac hypertrophy. In addition, we compare the results from different laboratories, with both the same and different assay technologies, to assess the inter-laboratory and inter-platform variation in results.
MATERIALS AND METHODS
Animal Care
Animal care and housing were in accordance with the National Research Council (NRC) Guide for the Care and Use of Laboratory Animals, 8th edition (2011) and the Animal Welfare Standards incorporated in 9 CFR Part 3, 1991. All use of animals was approved by the Institutional Animal Care and Use Committee and conducted at SRI International (Menlo Park, California) in a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International.
Study Design
Three groups of Sprague Dawley rats, each consisting of 10 males and 10 females, were administered 1% methylcellulose in sterile water (Vehicle Control, oral daily doses for 7 days), MNX at 30 mg/kg/day (oral daily doses for 7 days) or ISO at 5 mg/kg (subcutaneous single dose on Day 1), respectively (Table 1). Oral dose administration was by gavage. Blood samples were drawn prestudy and on Days 2 (Main group, 5 animals/sex/group) and 8 (Recovery group, 5 animals/sex/group) for determination of cardiac toxicity biomarker concentrations.
Table 1.
Study design
| Group | Treatment | No. of Doses (Route) |
Dose Level (mg/kg/day) |
Dose Conc. (mg/ml) |
Volume (ml/kg) |
Total No. of Animals |
No. of Animals | |
|---|---|---|---|---|---|---|---|---|
| Main, Day 2 |
Recovery, Day 8 |
|||||||
| 1 | Vehicle Control | 7 (po) | 0 | 0 | 10 | 10M/10F | 5M/5F | 5M/5F |
| 2 | Minoxidil | 7 (po) | 30 | 3 | 10 | 10M/10F | 5M/5F | 5M/5F |
| 3 | Isoproterenol | 1 (sc) | 5 | 1 | 5 | 10M/10F | 5M/5F | 5M/5F |
| 30M/30F | 15M/15F | 15M/15F | ||||||
Serum was prepared from the blood samples by centrifugation within 30 minutes of collection then flash frozen in liquid nitrogen and stored at ≤ −60°C until analysis. The samples were analyzed by the participating laboratories (Table 2) using commercially available assays described under Biomarker Analysis.
Table 2.
Assay platforms used by each laboratory for cardiac toxicity biomarker evaluation
| Cardiac Biomarkers |
Participating Laboratories | ||
|---|---|---|---|
| SRI | Lilly | Pfizer | |
| NT-proANP | MSD | MSD Alpco | |
| NT-proBNP | MSD | MSD | |
| Myl3 | MSD | MPI | |
| FABP3 | MSD | ||
| cTnI | MSD | Siemens | |
| cTnT | MSD | ||
| sTnI | MSD | ||
Biomarker Analysis
NT-proANP, NT-proBNP, MIP-1(MSD)
Cardiac biomarkers were evaluated using electrochemiluminescent assays, including the Rat Muscle Injury Panel 1 Assay (MIP-1: Myl3, FABP3, cTnI, cTnT, sTnI) kit K15181C, Rat NT-proANP kit K153MBD, and Rat NT-proBNP kit K153JKD from Meso Scale Discovery (MSD; Gaithersburg, MD). Serum samples were diluted 1:4 and 25 μL of the diluted sample (for MIP-1) or 50 μL of diluted sample (for Rat NT-proANP and Rat NT-proBNP) were dispensed and tested in duplicate according to the manufacturer’s protocol. After the completion of the procedure based on MSD assay protocol, the plates were analyzed using a MSD Sector Imager, Model 2400. The average of the two values was used to report the final results.
NT-proANP (Alpco)
N-terminal proANP was also measured by ELISA (Cat# 04-BI-20892, Alpco Diagnostics, Salem, NH) (Vinken et al., 2016). 10μL of serum per sample were dispensed and tested in duplicate according to the manufacturer’s protocol. Plates were analyzed on a Molecular Devices (Sunnyvale, CA) Spectramax Plus 384 Microplate Reader using Softmax Pro data acquisition and analysis software. Results were reported as the average of duplicate values.
Myl3 (MPI)
Myl3 was quantitated in rat serum on a custom assay by MPI Research (Mattawan, MI). Samples (5μL) were analyzed in duplicate on Gyrolab™ Bioaffy 1000 CDs (Gyros, Uppsala, Sweden) using the Gyrolab xP workstation with a biotinylated (EZ Link™ Micro Sulfo-NHS-LC biotinylation kit, Thermo Scientific) capture antibody (BiosPacific, Emeryville, CA) and a fluorescently labeled (Alexa Fluor®647 labeling kit, Invitrogen) detection antibody (Abcam, Cambridge, UK). Prior to analysis, samples were diluted 1:4 in Rexxip A buffer (Gyros). Concentrations were interpolated from a 7-point standard curve using a recombinant; his-tagged rat specific myl3 expressed in-house (Eli Lilly and Company, Indianapolis, IN) as a calibrator and fit with a 5-parameter logistic curve. Capture antibody was diluted in PBS + 0.01% Tween 20, detection antibody was diluted in Rexxip F buffer (Gyros), and standards were diluted in Rexxip A. Prior to sample analysis, the performance of the assay was characterized using positive controls (assay calibrator spiked into rat serum) at six different concentrations (approximately mid-way between each standard concentration) ran on 3 different CDs on 3 different days to assess precision and accuracy. Myl3 was quantifiable from 0.05 ng/mL to 200 ng/mL with <12.5 %CV and −5 to −12% relative error.
cTnI (Siemens)
The Siemens Advia Centaur cTnI-Ultra assay (Tarrytown, NY) was used to measure cardiac troponin I. It is a three-site sandwich immunoassay using direct chemiluminometric technology that was optimized to detect human cardiac troponin I but has been shown to cross-react in multiple species including rats (Apple et al., 2008). Serum samples were tested for cTnI on the Siemens Advia Centaur according to the manufacturer’s protocol.
Organ Weights and Histopathology
Hearts were collected from all animals at necropsy and weighed. Left and right ventricular free walls were dissected from hearts and retained in 10% neutral buffered formalin. Sections of the tissues were embedded in paraffin, cut approximately 5 μm thick, stained with hematoxylin and eosin and evaluated by a board-certified veterinary pathologist. Each lesion was listed and coded by the most specific topographic and morphologic diagnoses, severity, and distribution, using International Harmonization of Nomenclature and Diagnostic Criteria for Lesions (INHAND) as a guide. A four-step grading system (minimal, mild, moderate, and marked) was used to define gradable lesions for comparison between treated and control groups.
Statistical Analysis
Data were analyzed using two-way analysis of variance (ANOVA) followed by Dunnett’s test for post hoc multiple comparison. Results were expressed as mean, standard deviations (SD), percentage of coefficient of variation (%CV) and correlation coefficient (r). The intra-assay %CV was defined as the ratio of the standard deviation and the mean of the measurements within each run. The inter-platform and inter-laboratory %CV was defined as the ratio of the standard deviation and the mean of the group mean values from each platform or laboratory, respectively. For correlation analysis, the two-tail Pearson test was used. Statistical analysis was performed using GraphPad Prism, version 6 for Windows (GraphPad software, La Jolla, CA).
RESULTS
Serum NT-proANP and NT-proBNP
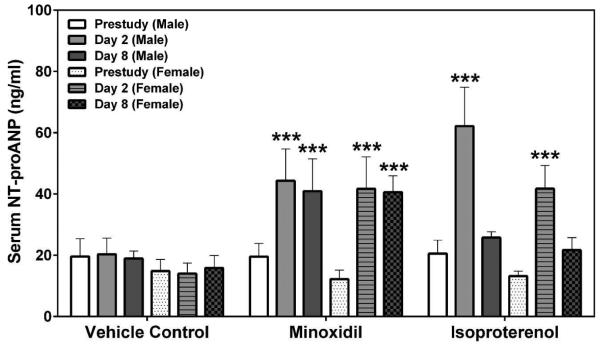
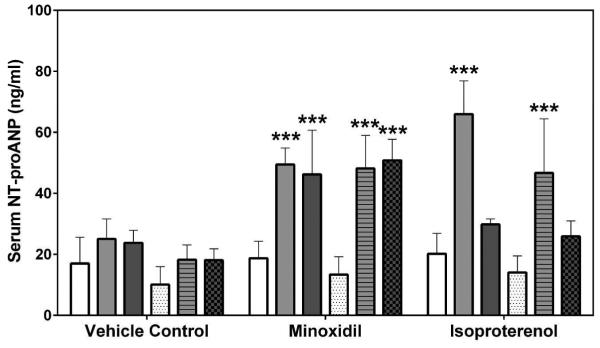
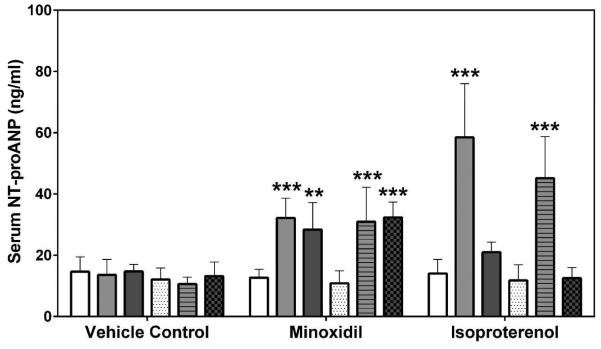
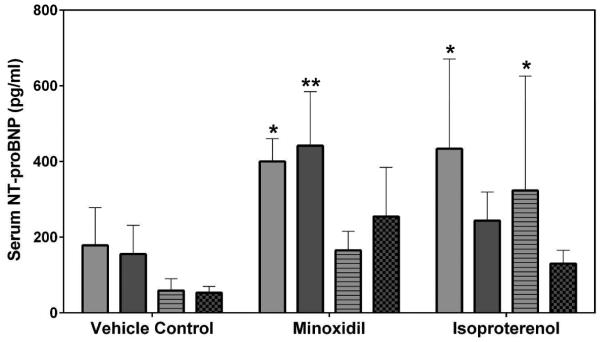
Two laboratories, SRI International (SRI) and Eli Lilly and Company (Lilly), analyzed the natriuretic peptides, NT-proANP and NT-proBNP, using assays from MSD (SRI/Lilly) or Alpco (Lilly). The results from all analyses showed that the group mean NT-proANP levels at 30 mg/kg/day MNX for 7-daily doses were increased on Days 2 and 8 in both male and female rats (a 2.0- to 3.0-fold increase over the concurrent vehicle control mean value [p<0.001; p<0.01 on Day 8 males from Alpco assay], Figure 1). After a single dose of 5 mg/kg ISO, the group mean NT-proANP level was elevated on Day 2 in both male and female rats (a 2.6- to 4.3-fold increase over the concurrent vehicle control mean value [p<0.001], Figure 1). The intra-assay %CVs of NT-proANP ranged between 7.5~29.9% from SRI-MSD analysis, 6.1~58.2% from Lilly-MSD analysis and 15.4~43.68% from Lilly-Alpco analysis (Supplemental Table 1). Statistically significant increases in group mean NT-proBNP levels at 30 mg/kg/day MNX for 7-daily doses on Days 2 and 8 in male rats were shown in results from both laboratories (a 2.4- to 2.7-fold increase over the concurrent vehicle control mean value [p<0.01], Figure 2A; a 2.2- to 2.8-fold increase over the concurrent vehicle control mean value [p<0.05 on Day 2; p<0.01 on Day 8], Figure 2B). At a single dose of 5 mg/kg ISO, the group mean NT-proBNP level was higher on Day 2 in both male and female rats (a 2.4-fold increase over the concurrent vehicle control mean value for males [p<0.01 from SRI; p<0.05 from Lilly]; a 5- to 5.5-fold increase over the concurrent vehicle control mean value for females [p<0.05], Figure 2) and on Day 8 in male rats (from SRI analysis only; a 2.4-fold increase over the concurrent vehicle control mean value [p<0.01], Figure 2A). The intra-assay %CVs of NT-proBNP ranged between 11.6~102.1% from SRI analysis and 15~93.5% from Lilly analysis (Supplemental Table 2).
Figure 1.
Serum NT-proANP levels at prestudy, Days 2 and 8 in rats treated with vehicle control, 7-daily oral doses of MNX at 30 mg/kg/day or a single subcutaneous dose of ISO at 5 mg/kg, analyzed by (A) MSD kit at SRI, and (B) MSD or Alpco kits at Lilly (B, MSD; C, Alpco). Values are means, SD as error bars; n=5. **Significantly different from the concurrent vehicle control, p-value<0.01; ***p-value<0.001.
Figure 2.
Serum NT-proBNP levels on Days 2 and 8 in rats treated with vehicle control, 7-daily oral doses of MNX at 30 mg/kg/day or a single subcutaneous dose of ISO at 5 mg/kg, analyzed by MSD kits at (A) SRI and (B) Lilly. Values are means, SD as error bars; n=5. *Significantly different from the concurrent vehicle control, p-value<0.05; **p-value<0.01.
Serum Myl3 and FABP3
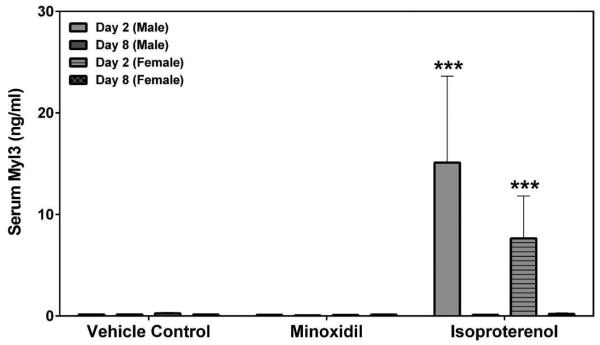
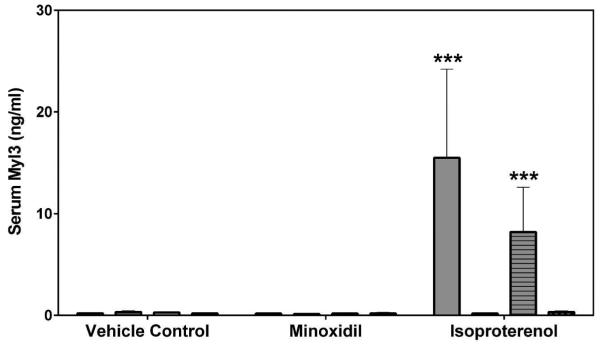
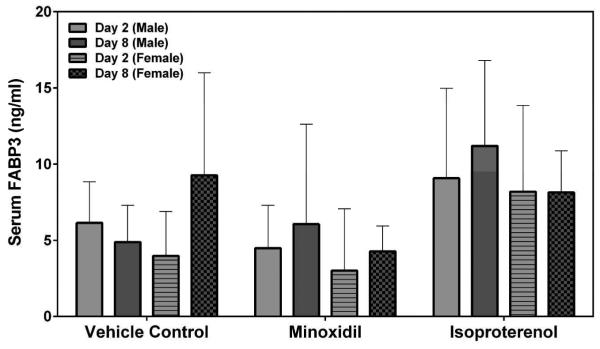
Myl3 was analyzed by SRI and Lilly using assays from MSD or MPI, respectively. FABP3 was analyzed by SRI using an assay from MSD. The results from both laboratories showed that the group mean Myl3 levels after a single dose of 5 mg/kg ISO were increased on Day 2 in both male and female rats (a 73- to 108-fold increase over the concurrent vehicle control mean value for males [p<0.001]; a 30-fold increase over the concurrent vehicle control mean value for females [p<0.001], Figure 3). The intra-assay %CVs of Myl3 ranged between 25.3~56.4% from SRI analysis and 22.3~56.3% from Lilly analysis (Supplemental Table 3). No statistically significant differences were noted in the group mean FABP3 levels by MNX or ISO treatment in this study (Figure 4). The intra-assay %CVs of FABP3 ranged between 33.6~134.6% (Supplemental Table 4).
Figure 3.
Serum Myl3 levels on Days 2 and 8 in rats treated with vehicle control, 7-daily oral doses of MNX at 30 mg/kg/day or a single subcutaneous dose of ISO at 5 mg/kg, analyzed by (A) MSD kit at SRI and (B) MPI kit at Lilly. Values are means, SD as error bars; n=5. ***Significantly different from the concurrent vehicle control, p-value<0.001.
Figure 4.
Serum FABP3 levels on Days 2 and 8 in rats treated with vehicle control, 7-daily oral doses of MNX at 30 mg/kg/day or a single subcutaneous dose of ISO at 5 mg/kg, analyzed by MSD kit SRI. Values are means, SD as error bars; n=5.
Serum Troponins
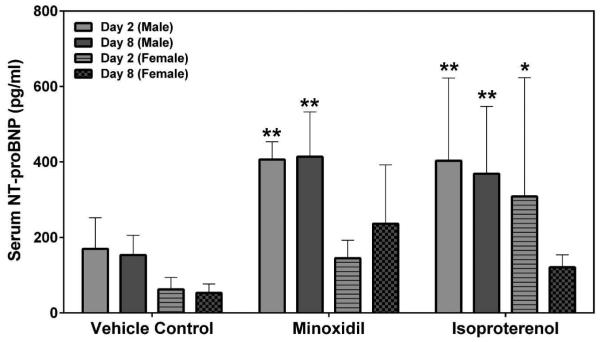
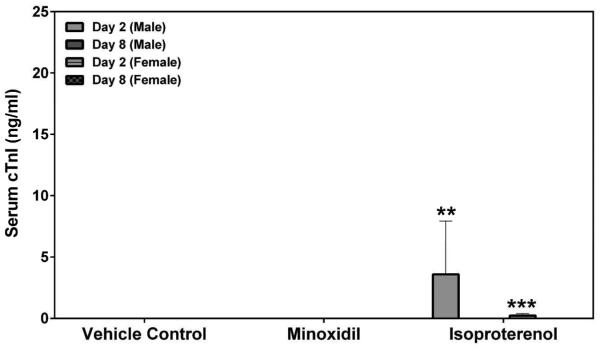
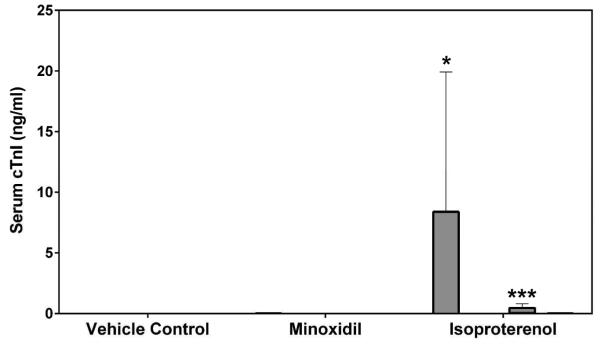
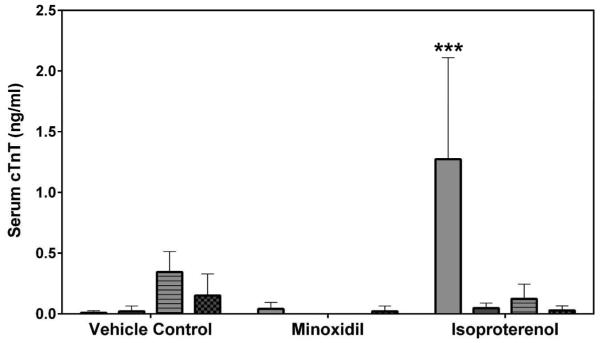
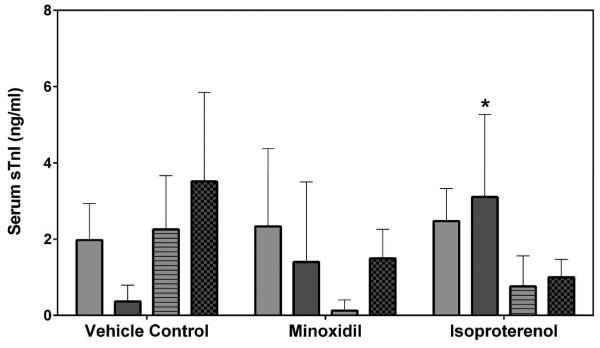
Serum cardiac troponin I (cTnI), cardiac troponin T (cTnT) and skeletal troponin I (sTnI) were analyzed by SRI and Pfizer (cTnI only) using assays from MSD or Siemens, respectively. Statistically significant increases in cTnI levels after a single dose of 5 mg/kg ISO on Day 2 were observed in both male and female rats by both laboratories (a >449-fold increase over the concurrent vehicle control mean value for males [p<0.01 from SRI; p<0.05 from Pfizer]; a >56-fold increase over the concurrent vehicle control mean value for females [p<0.001], Figures 5A and 5B). The intra-assay %CVs of cTnI ranged between 76.7~223.6% from SRI analysis and 81.8~223.6% from Pfizer analysis (Supplemental Table 5). The group mean cTnT level after a single dose of 5 mg/kg ISO was increased on Day 2 in male rats (a 159-fold increase over the concurrent vehicle control mean value [p<0.001], Figure 5C). The intra-assay %CVs of cTnT ranged between 49~223.6% (Supplemental Table 6). The group mean sTnI level after a single dose of 5 mg/kg ISO was increased on Day 8 in male rats (a 8-fold increase over the concurrent vehicle control mean value [p<0.05], Figure 5D). The intra-assay %CVs of sTnI ranged between 34.4~223.6% (Supplemental Table 7).
Figure 5.
Serum cardiac troponin I (cTnI), cardiac troponin T (cTnT) and skeletal troponin I (sTnI) levels on Days 2 and 8 in rats treated with vehicle control, 7-daily oral doses of MNX at 30 mg/kg/day or a single subcutaneous dose of ISO at 5 mg, analyzed by (A), (C), (D) MSD kit at SRI and (B) Siemens kit at Pfizer. Values are means, SD as error bars; n=5. *Significantly different from the concurrent vehicle control, p-value<0.05; **p-value<0.01; ***p-value<0.001.
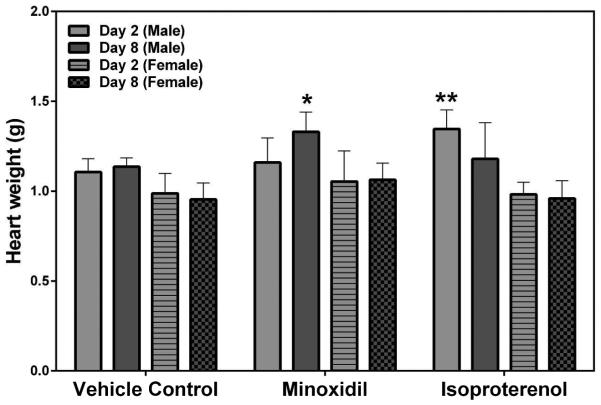
Heart Weight and Histopathology
Statistically significant increases in absolute heart weights at 30 mg/kg/day MNX for 7-daily doses on Day 8 (a 1.2-fold increase over the concurrent vehicle control mean value [p<0.05]), and after a single dose of 5 mg/kg ISO on Day 2 (a 1.2-fold increase over the concurrent vehicle control mean value [p<0.01]) were observed in male rats (Figure 6). Histopathology findings included ventricle leukocyte infiltration, ventricle fibrosis and necrosis in the ISO-treated animals but not in any of the MNX-treated animals (Figure 7). Cardiac hypertrophy was diagnosed based on the increase in both absolute and relative heart weight, however, hypertrophy was not detectable from histopathologic examination in this study as histopathological changes of hypertrophy are difficult to detect unless the impact is severe. Females also showed better overall recovery from cardiac muscle injury from ISO compared with males on Day 8 (Table 3).
Figure 6.
Heart weights at necropsy on Days 2 and 8 in rats treated with vehicle control, 7-daily oral doses of MNX at 30 mg/kg/day or a single subcutaneous dose of ISO at 5 mg/kg. Values are means, SD as error bars; n=5. *Significantly different from the concurrent vehicle control, p-value<0.05; **p-value<0.01.
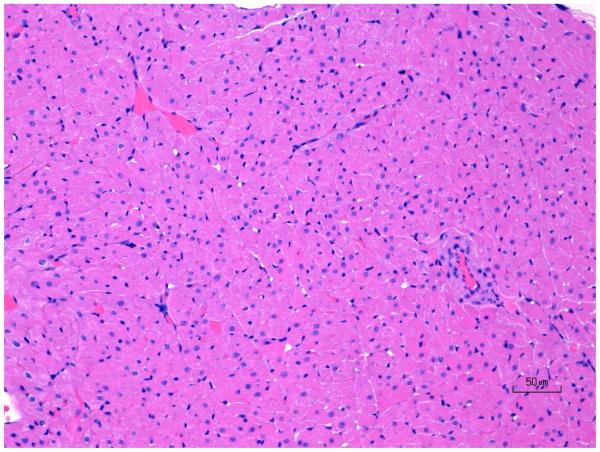
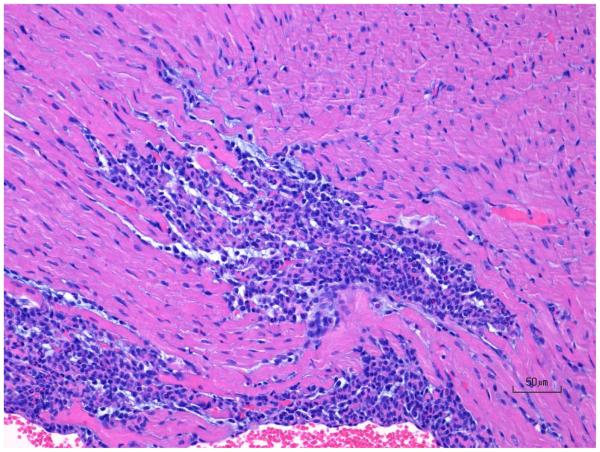
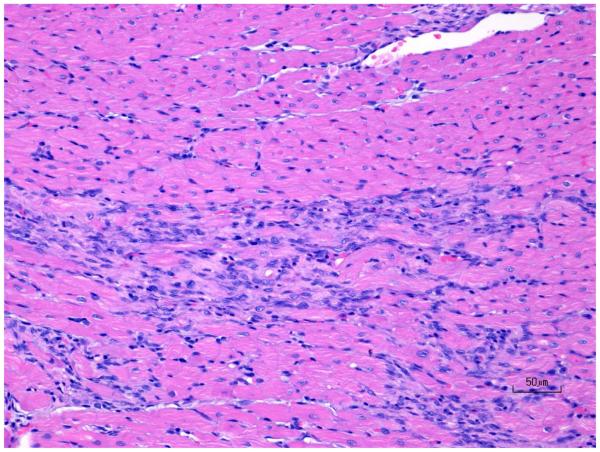
Figure 7.
H&E images of rat ventricular myocardium after treatment with ISO in rats showing (A) normal, (B) progression of moderate ventricle leukocyte infiltration and necrosis, and (C) progression of mild ventricle multifocal fibrosis. Original objective 20X.
Table 3.
Summary histopathology findings
| Group | Treatment | Histopathology Findings | |||
|---|---|---|---|---|---|
| Main, Day 2 | Recovery, Day 8 | ||||
| Male | Female | Male | Female | ||
| 1 | Vehicle Control |
- | Ventricle Leukocyte Infiltration (1/5) |
- | - |
| 2 | Minoxidil | - | - | - | - |
| 3 | Isoproterenol | Ventricle Leukocyte Infiltration (5/5), Ventricle Necrosis (5/5) |
Ventricle Leukocyte Infiltration (5/5), Ventricle Necrosis (5/5) |
Ventricle Leukocyte Infiltration (4/5), Ventricle Necrosis (4/5) |
Ventricle Leukocyte Infiltration (2/5), Ventricle Fibrosis (2/5) |
Inter-laboratory and Inter-platform Statistical Analysis
Inter-laboratory and inter-platform correlation and precision analyses of each data point on serum NT-proANP, NT-proBNP, Myl3 and cTnI levels were performed. Correlation analysis results are presented in Supplemental Table 8, and CV% and mean values are presented in Supplemental Table 9. Both NT-proANP and NT-proBNP data sets from two laboratories were well-correlated (r=0.9460 for NT-proANP; r=0.9125 for NT-proBNP). The precision analysis showed highly reproducible NT-proANP levels with %CVs of <20% from all groups except female prestudy vehicle samples (26.9%). NT-proBNP data sets also showed strong precision and reproducibility with %CVs of <10% from all groups except male Day 8 ISO samples (28.8%). Two sets of NT-proANP levels each from MSD and Alpco assays, both analyzed by Lilly, were well-correlated (r=0.8777) with %CVs ranging between 2.3~49.2%. Myl3 and cTnI also showed high correlation between the data sets from two different assays (MSD vs. MPI for Myl3, r=0.9982; MSD vs. Siemens for cTnI, r=0.9970). The precision of data was poor at low levels of Myl3 (<1 ng/ml) with %CVs ranging up to 50.1%; however, analysis showed great reproducibility of data at higher concentration of Myl3 in male and female ISO groups on Day 2 with %CVs of less than 5%. The %CVs of cTnI ranged above 50% at concentrations higher than 0.3 ng/ml, and above 100% at lower levels of cTnI.
DISCUSSION
Based on the results of this study using commercially available kits, a multi-marker strategy is beneficial in distinguishing the underlying cause of cardiac insult with or without apparent tissue damage (e.g. hypertrophy vs. myocyte necrosis). Also the changes in the levels of these cardiac injury markers were detectable over longer periods of time at 24 hr (Day 2) or 168 hr (Day 8; e.g. NT-proANP) post-treatment providing a wider time frame to identify the cardiac insult as well as the sex differences in the elevation of these markers. The addition of the natriuretic peptides to the cardiotoxicity biomarker panel along with the direct myocyte injury biomarkers in animal models would enable the researchers to stratify the risk of potential cardiac myopathy caused by various mechanisms of action at early stages of drug development.
While assessing serum biomarkers is a promising strategy for early preclinical detection of potential drug-induced cardiac injury, some of these serum markers have limitations with poor cardiac specificity, sensitivity and short half-life as well as inconsistency across species and low relevance to clinical outcome. Reverse-translational application of established clinical biomarkers, such as cardiac troponins and natriuretic peptides to preclinical models increases clinical relevance and a multi-marker approach offers the advantage of a more comprehensive assessment of risk (Pinsky and Zhu, 2011, Taqui and Daniels, 2013). Prominent elevation of NT-proANP and NT-proBNP concentrations detected at 24 hr postdose following single and multiple doses of MNX, a vasodilator that causes pericardial effusion in man and left ventricular hypertrophy (Moravec et al., 1994, Reichgott, 1981) suggest that these biomarkers can be used to detect the presence of or possibly predict the development of myocardial hypertrophy in the absence of direct cardiomyocyte injury. Similar results, where early changes in NT-proANP after only two doses reflect the eventual development of cardiac hypertrophy after 14 or 28 days in rats treated with a PPAR agonist have also been reported (Engle et al., 2010).
NT-proANP and NT-proBNP concentrations were also elevated at 24 hr after a single ISO administration that caused direct myocardial injury as indicated by ventricular leukocyte infiltration, myocyte fibrosis and necrosis in histopathologic examination. During the course of cardiac injury by ISO, the natriuretic hormones respond as protective hormones that counteract the physiologic abnormalities of heart injury and dysfunction as also demonstrated in several clinical studies (Maisel et al., 2002, Richards et al., 2003, de Lemos et al., 2001, Eggers et al., 2009). The clinical biomarker Myl3 was elevated in response to the direct myocardial injury with a single ISO treatment at 24 hr postdose, but showed no change with MNX-mediated hypertrophy. While FABP3 was shown to be a good early marker (i.e. 2-3 hr post-insult) for detecting acute/active cardiomyocyte injury in rat models (Tonomura et al., 2012, Clements et al., 2010), no changes in protein levels of FABP3 were detected in this study where the serum samples were collected at a later time point (24 hr) after ISO treatment, likely due to its rapid clearance in circulation. The concentrations of the most widely established biomarkers for myocardial injury, cTnI and cTnT, were also elevated only in rats treated with ISO at 24 hr postdose showing their specificity for myocardial degeneration and necrosis. Although increased cardiac troponin concentrations classically indicate cardiomyocyte necrosis, their elevation does not offer further information about the preceding events and also has poor sensitivity in the absence of infarction in people (Balk et al., 2001). The sex differences in some of the biomarkers were also presented with higher serum biomarker levels in male rats induced by the treatments (e.g. NT-proBNP, Myl3, cTnT, cTnI). The males also showed poorer recovery from ISO treatment compared with females on Day 8 on histopathologic evaluation. The intra-assay variation with wide ranges of %CVs were presented in some of the groups or timepoints from NT-proBNP, FABP3 and serum troponin assays presumably due to relatively low basal or response levels to the treatments.
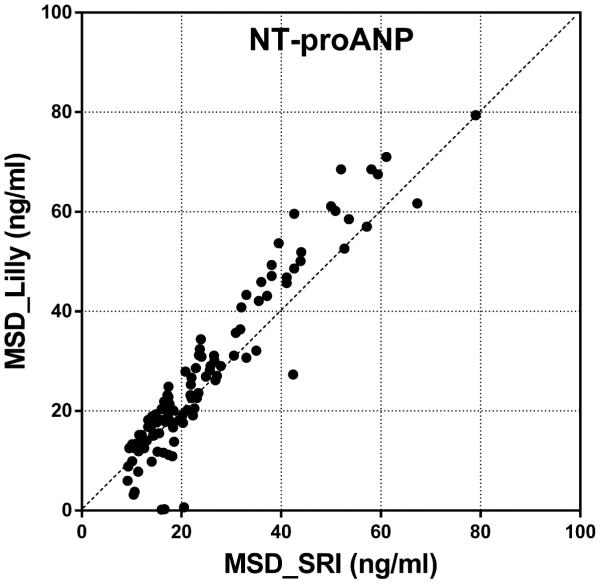
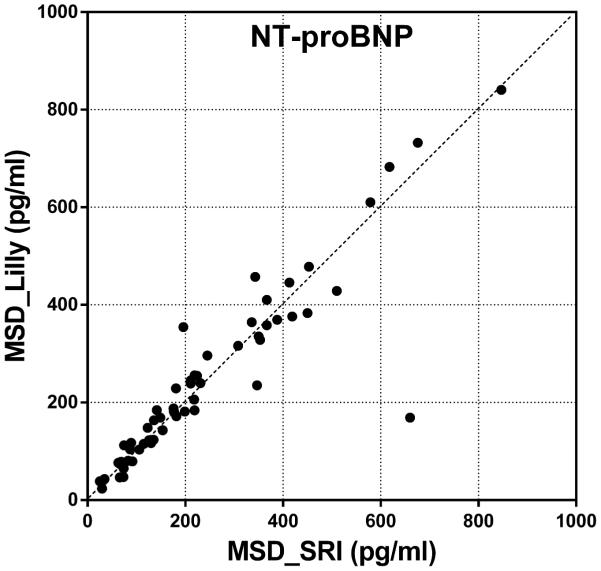
Inter-laboratory correlation and precision analyses were performed for each data point on serum NT-proANP and NT-proBNP levels assessed by SRI and Lilly. Pearson correlation coefficient (r), which quantifies the direction and magnitude of correlation between two data sets, shows both NT-proANP and NT-proBNP data sets from two laboratories were well-correlated and followed close trend lines (Figure 8). The precision of the data (%CV) presents the reproducibility of the biomarker levels from each laboratory and the results showed precise and reproducible NT-proANP levels with %CVs lower than 20%. NT-proBNP data sets also showed strong precision and reproducibility with %CVs lower than 10%. This inter-laboratory comparison analysis shows that the biomarker data obtained using the MSD kits at two participating laboratories are highly reproducible, thus enabling these assays to be used across different institutions to obtain comparable results in preclinical studies.
Figure 8.
Inter-laboratory comparison of (A) serum NT-proANP levels and (B) Serum NT-proBNP levels in rats treated with vehicle control, 7-daily oral doses of MNX at 30 mg/kg/day or a single subcutaneous dose of ISO at 5 mg/kg, analyzed by SRI (X-axis) and Lilly (Y-axis) using assays from MSD.
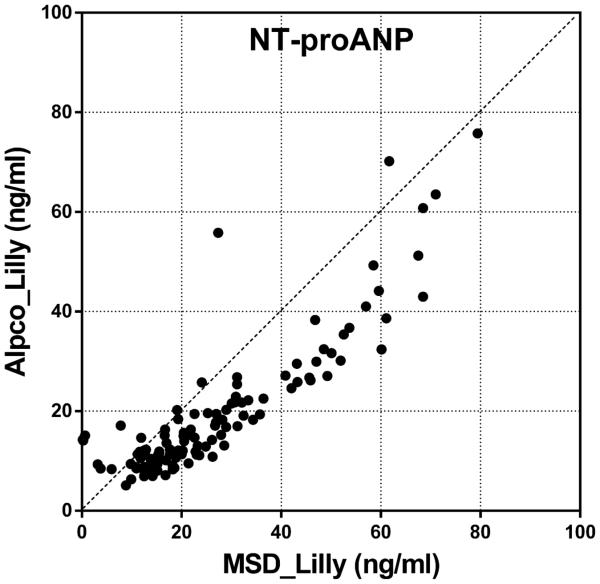
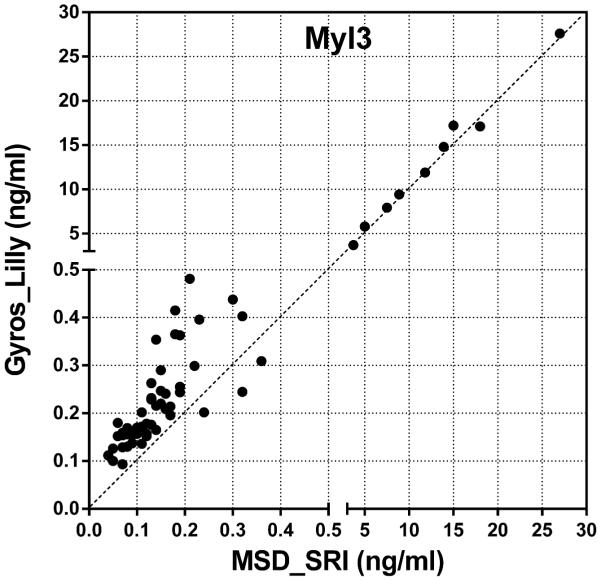
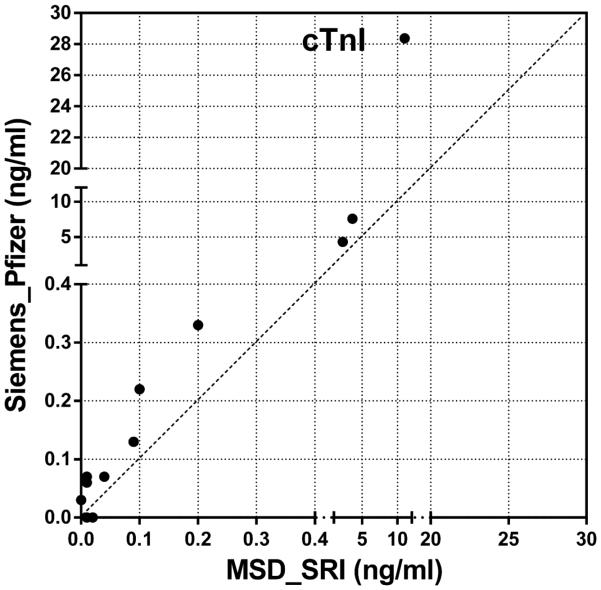
Inter-platform comparison was performed on NT-proANP, Myl3 and cTnI levels analyzed using two different assay kits as shown in Table 2. NT-proANP levels from MSD and Alpco assays, both analyzed by Lilly, were well-correlated (r=0.8777) with moderate precision and reproducibility (Figure 9A). Myl3 and cTnI also showed high correlation between two data sets from different assays (Figures 9B and C). The precision analysis showed great reproducibility of Myl3 data only in male and female ISO groups which had elevated Myl3 concentrations. Although the variability between biomarker levels obtained from different assays are considered to be inevitable and exaggerated with wide ranges of %CVs when the basal and/or expression levels of analytes are relatively low at the time of sample collection, the high correlation between each data set gives confidence in utilizing these biomarkers in preclinical studies using commercially available assay kits to interpret the evidence of cardiac toxicity across different studies and platforms. Although the high %CVs at baseline concentrations of the analytes suggests that subtle changes may not be detected, the advantages of using these kits are still creditable as the meaningful myocardial injury would induce the detectable changes in the levels of biomarkers within a reasonable time frame (~24 hr) of sample collection serving as preliminary diagnosis and/or supplemental toxicity data along with the traditional cardiac toxicity evaluation. In cases where quantitative differences were significant (e.g., The Pfizer Siemens cTnI assay was clearly more sensitive than the corresponding MSD assay), the qualitative differences were not, and all assays correctly identified the cardiac toxicity induced by the drugs at the doses used in this study.
Figure 9.
Inter-platform comparison of (A) serum NT-proANP, (B) serum Myl3 levels and (C) serum cTnI levels in rats treated with vehicle control, 7-daily oral doses of MNX at 30 mg/kg/day or a single subcutaneous dose of ISO at 5 mg/kg, analyzed by SRI (B and C, X-axis, MSD assay), Lilly (A, MSD and Alpco assays; B, Y-axis, MPI assay) or Pfizer (C, Y-axis, Siemens assay).
In summary, we have evaluated several commercially available kits for the assessment of biomarkers of direct cardiac injury as well as cardiac hypertrophy, assessing both different assay platforms as well as inter-laboratory reproducibility of results using the same platform. All cardiac markers performed as expected, and demonstrated excellent differentiation between cardiac hypertrophy (based on increased heart weights) and cardiac injury. Inter-laboratory reproducibility of the MSD kits was excellent, and inter-platform reproducibility, while not as tight as results from the MSD platform in different labs, showed very good comparability. These results indicate that the use of these commercially available biomarker kits for assessment of cardiac toxicity could provide robust and reproducible data to identify drug-induced cardiac injury and provide improved prognostic indication of cardiac liabilities of candidate drugs.
Supplementary Material
ACKNOWLEDGEMENTS
This project has been funded in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract Nos. HHSN272200700043C and HHSN272201400006. The authors gratefully acknowledge Dr. Hao Zhang, NIAID, for his support and insightful suggestions. Diana Vuong, Lynn Chellarajen, Amanda Fields and Lauren Haberland provided expert technical assistance in the conduct of the animal studies. This material is based upon work supported by Critical Path Institute’s (C-Path) Predictive Safety Testing Consortium (PSTC). The authors would like to acknowledge and thank the members of PSTC for their scientific, financial, and in-kind contributions that supported these research activities, as well as the input from FDA and EMA scientists who serve as advisors.
Abbreviations
- ISO
Isoproterenol
- MNX
Minoxidil
- NT-proANP
N-terminal pro-atrial natriuretic peptide
- NT-proBNP
N-terminal pro-brain natriuretic peptide
- Myl3
Myosin light chain 3
- FABP3
Fatty acid binding protein 3
- cTnI
Cardiac troponin I
- cTnT
Cardiac troponin T
- sTnI
Skeletal troponin I
REFERENCES
- Acikel M, Buyukokuroglu ME, Erdogan F, Aksoy H, Bozkurt E, Senocak H. Protective effects of dantrolene against myocardial injury induced by isoproterenol in rats: biochemical and histological findings. International journal of cardiology. 2005;98:389–394. doi: 10.1016/j.ijcard.2003.10.054. [DOI] [PubMed] [Google Scholar]
- Apple FS, Murakami MM, Ler R, Walker D, York M. Analytical characteristics of commercial cardiac troponin I and T immunoassays in serum from rats, dogs, and monkeys with induced acute myocardial injury. Clinical chemistry. 2008;54:1982–1989. doi: 10.1373/clinchem.2007.097568. [DOI] [PubMed] [Google Scholar]
- Babuin L, Jaffe AS. Troponin: the biomarker of choice for the detection of cardiac injury. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2005;173:1191–1202. doi: 10.1503/cmaj.050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk EM, Ioannidis JP, Salem D, Chew PW, Lau J. Accuracy of biomarkers to diagnose acute cardiac ischemia in the emergency department: a meta-analysis. Annals of emergency medicine. 2001;37:478–494. doi: 10.1067/mem.2001.114905. [DOI] [PubMed] [Google Scholar]
- Bozkurt B. Cardiovascular toxicity with highly active antiretroviral therapy: review of clinical studies. Cardiovascular toxicology. 2004;4:243–260. doi: 10.1385/ct:4:3:243. [DOI] [PubMed] [Google Scholar]
- Brady S, York M, Scudamore C, Williams T, Griffiths W, Turton J. Cardiac troponin I in isoproterenol-induced cardiac injury in the Hanover Wistar rat: studies on low dose levels and routes of administration. Toxicologic pathology. 2010;38:287–291. doi: 10.1177/0192623309357948. [DOI] [PubMed] [Google Scholar]
- Bryan RK, Hoobler SW, Rosenzweig J, Weller JM, Purdy JM. Effect of minoxidil on blood pressure and hemodynamics in severe hypertension. The American journal of cardiology. 1977;39:796–801. doi: 10.1016/s0002-9149(77)80029-5. [DOI] [PubMed] [Google Scholar]
- Casartelli A, Lanzoni A, Comelli R, Crivellente F, Defazio R, Dorigatti R, Fasdelli N, Faustinelli I, Pagliarusco S, Tontodonati M, Cristofori P. A novel and integrated approach for the identification and characterization of drug-induced cardiac toxicity in the dog. Toxicologic pathology. 2011;39:361–371. doi: 10.1177/0192623310390704. [DOI] [PubMed] [Google Scholar]
- Clements P, Brady S, York M, Berridge B, Mikaelian I, Nicklaus R, Gandhi M, Roman I, Stamp C, Davies D, McGill P, Williams T, Pettit S, Walker D, Turton J. Time course characterization of serum cardiac troponins, heart fatty acid-binding protein, and morphologic findings with isoproterenol-induced myocardial injury in the rat. Toxicologic pathology. 2010;38:703–714. doi: 10.1177/0192623310374969. [DOI] [PubMed] [Google Scholar]
- Colton HM, Stokes AH, Yoon LW, Quaile MP, Novak PJ, Falls JG, Kimbrough CL, Cariello NF, Jordan HL, Berridge BR. An initial characterization of N-terminal-proatrial natriuretic peptide in serum of Sprague Dawley rats. Toxicological sciences : an official journal of the Society of Toxicology. 2011;120:262–268. doi: 10.1093/toxsci/kfr003. [DOI] [PubMed] [Google Scholar]
- de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH, Hall C, Cannon CP, Braunwald E. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. The New England journal of medicine. 2001;345:1014–1021. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- Dunn ME, Coluccio D, Hirkaler G, Mikaelian I, Nicklaus R, Lipshultz SE, Doessegger L, Reddy M, Singer T, Geng W. The complete pharmacokinetic profile of serum cardiac troponin I in the rat and the dog. Toxicological sciences : an official journal of the Society of Toxicology. 2011;123:368–373. doi: 10.1093/toxsci/kfr190. [DOI] [PubMed] [Google Scholar]
- Dunn ME, Manfredi TG, Agostinucci K, Engle SK, Powe J, King NM, Rodriguez LA, Gropp KE, Gallacher M, Vetter FJ, More V, Shimpi P, Serra D, Colton HM. Serum Natriuretic Peptides as Differential Biomarkers Allowing for the Distinction between Physiologic and Pathologic Left Ventricular Hypertrophy. Toxicologic pathology. 2016 doi: 10.1177/0192623316634231. [DOI] [PubMed] [Google Scholar]
- Eggers KM, Lagerqvist B, Venge P, Wallentin L, Lindahl B. Prognostic value of biomarkers during and after non-ST-segment elevation acute coronary syndrome. Journal of the American College of Cardiology. 2009;54:357–364. doi: 10.1016/j.jacc.2009.03.056. [DOI] [PubMed] [Google Scholar]
- Engle SK, Solter PF, Credille KM, Bull CM, Adams S, Berna MJ, Schultze AE, Rothstein EC, Cockman MD, Pritt ML, Liu H, Lu Y, Chiang AY, Watson DE. Detection of left ventricular hypertrophy in rats administered a peroxisome proliferator-activated receptor alpha/gamma dual agonist using natriuretic peptides and imaging. Toxicological sciences : an official journal of the Society of Toxicology. 2010;114:183–192. doi: 10.1093/toxsci/kfp311. [DOI] [PubMed] [Google Scholar]
- Engle SK, Watson DE. Natriuretic Peptides as Cardiovascular Safety Biomarkers in Rats: Comparison With Blood Pressure, Heart Rate, and Heart Weight. Toxicological sciences : an official journal of the Society of Toxicology. 2016;149:458–472. doi: 10.1093/toxsci/kfv240. [DOI] [PubMed] [Google Scholar]
- Fantoni M, Autore C, Del Borgo C. Drugs and cardiotoxicity in HIV and AIDS. Annals of the New York Academy of Sciences. 2001;946:179–199. doi: 10.1111/j.1749-6632.2001.tb03912.x. [DOI] [PubMed] [Google Scholar]
- Feenstra J, Grobbee DE, Remme WJ, Stricker BH. Drug-induced heart failure. Journal of the American College of Cardiology. 1999;33:1152–1162. doi: 10.1016/s0735-1097(99)00006-6. [DOI] [PubMed] [Google Scholar]
- Ferri N, Siegl P, Corsini A, Herrmann J, Lerman A, Benghozi R. Drug attrition during pre-clinical and clinical development: understanding and managing drug-induced cardiotoxicity. Pharmacology & therapeutics. 2013;138:470–484. doi: 10.1016/j.pharmthera.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Gaggin HK, Januzzi JL., Jr. Biomarkers and diagnostics in heart failure. Biochimica et biophysica acta. 2013;1832:2442–2450. doi: 10.1016/j.bbadis.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Grim CE, Luft FC, Grim CM, Klotman PE, Van Huysse JW, Weinberger MH. Rapid blood pressure control with minoxidil: acute and chronic effects on blood pressure, sodium excretion, and the renin-aldosterone system. Archives of internal medicine. 1979;139:529–533. doi: 10.1001/archinte.139.5.529. [DOI] [PubMed] [Google Scholar]
- Gwathmey JK, Tsaioun K, Hajjar RJ. Cardionomics: a new integrative approach for screening cardiotoxicity of drug candidates. Expert opinion on drug metabolism & toxicology. 2009;5:647–660. doi: 10.1517/17425250902932915. [DOI] [PubMed] [Google Scholar]
- Hasic S, Jadric R, Kiseljakovic E, Valjevac A, Mornjakovic Z, Winterhalter-Jadric M. Time-dependent responses of rat troponin I and cardiac injury following isoproterenol administration. Medicinski glasnik : official publication of the Medical Association of Zenica-Doboj Canton, Bosnia and Herzegovina. 2011;8:140–145. [PubMed] [Google Scholar]
- Herper M. The cost of creating a new drug now $5 billion, pushing big pharma to change. Forbes. 2013 Aug 11; [Google Scholar]
- Jarai R, Fellner B, Haoula D, Jordanova N, Heinz G, Karth GD, Huber K, Geppert A. Early assessment of outcome in cardiogenic shock: relevance of plasma N-terminal pro-B-type natriuretic peptide and interleukin-6 levels. Critical care medicine. 2009;37:1837–1844. doi: 10.1097/CCM.0b013e31819fe896. [DOI] [PubMed] [Google Scholar]
- Kaufman PL. Isoproterenol dose-outflow facility response relationships in the vervet monkey. Current eye research. 1985;4:877–883. doi: 10.3109/02713688509095255. [DOI] [PubMed] [Google Scholar]
- Kehl DW, Iqbal N, Fard A, Kipper BA, De La Parra Landa A, Maisel AS. Biomarkers in acute myocardial injury. Translational research : the journal of laboratory and clinical medicine. 2012;159:252–264. doi: 10.1016/j.trsl.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Lambert CT, Sandesara PB, Hirsh B, Shaw LJ, Lewis W, Quyyumi AA, Schinazi RF, Post WS, Sperling L. HIV, highly active antiretroviral therapy and the heart: a cellular to epidemiological review. HIV medicine. 2015 doi: 10.1111/hiv.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER, Gardner DG, Samson WK. Natriuretic peptides. The New England journal of medicine. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- Lim CC, van Gaal WJ, Testa L, Cuculi F, Arnold JR, Karamitsos T, Francis JM, Petersen SE, Digby JE, Westaby S, Antoniades C, Kharbanda RK, Burrell LM, Neubauer S, Banning AP. With the "universal definition," measurement of creatine kinase-myocardial band rather than troponin allows more accurate diagnosis of periprocedural necrosis and infarction after coronary intervention. Journal of the American College of Cardiology. 2011;57:653–661. doi: 10.1016/j.jacc.2010.07.058. [DOI] [PubMed] [Google Scholar]
- Lipshultz SE, Mas CM, Henkel JM, Franco VI, Fisher SD, Miller TL. HAART to heart: highly active antiretroviral therapy and the risk of cardiovascular disease in HIV-infected or exposed children and adults. Expert review of anti-infective therapy. 2012;10:661–674. doi: 10.1586/eri.12.53. [DOI] [PubMed] [Google Scholar]
- Liu Y, Parman T, Schneider B, Song B, Galande AK, Anderson D, Mirsalis J. Serum biomarkers reveal long-term cardiac injury in isoproterenol-treated African green monkeys. Journal of proteome research. 2013;12:1830–1837. doi: 10.1021/pr3011809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott JA, Stang JM. Differential diagnosis of patients with abnormal serum creatine kinase isoenzymes. Clinics in laboratory medicine. 1989;9:627–642. [PubMed] [Google Scholar]
- Luers C, Sutcliffe A, Binder L, Irle S, Pieske B. NT-proANP and NT-proBNP as prognostic markers in patients with acute decompensated heart failure of different etiologies. Clinical biochemistry. 2013;46:1013–1019. doi: 10.1016/j.clinbiochem.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. The New England journal of medicine. 2002;347:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- Maynard SJ, Menown IB, Adgey AA. Troponin T or troponin I as cardiac markers in ischaemic heart disease. Heart. 2000;83:371–373. doi: 10.1136/heart.83.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B, Huelsmann M, Wexberg P, Delle Karth G, Berger R, Moertl D, Szekeres T, Pacher R, Heinz G. N-terminal pro-B-type natriuretic peptide is an independent predictor of outcome in an unselected cohort of critically ill patients. Critical care medicine. 2007;35:2268–2273. doi: 10.1097/01.CCM.0000284509.23439.5B. [DOI] [PubMed] [Google Scholar]
- Moravec CS, Ruhe T, Cifani JR, Milovanovic M, Khairallah PA. Structural and functional consequences of minoxidil-induced cardiac hypertrophy. The Journal of pharmacology and experimental therapeutics. 1994;269:290–296. [PubMed] [Google Scholar]
- Morrow DA, Braunwald E. Future of biomarkers in acute coronary syndromes: moving toward a multimarker strategy. Circulation. 2003;108:250–252. doi: 10.1161/01.CIR.0000078080.37974.D2. [DOI] [PubMed] [Google Scholar]
- Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, Shirakami G, Jougasaki M, Obata K, Yasue H, et al. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. The Journal of clinical investigation. 1991;87:1402–1412. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien PJ. Cardiac troponin is the most effective translational safety biomarker for myocardial injury in cardiotoxicity. Toxicology. 2008;245:206–218. doi: 10.1016/j.tox.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Pinsky PF, Zhu CS. Building multi-marker algorithms for disease prediction-the role of correlations among markers. Biomarker insights. 2011;6:83–93. doi: 10.4137/BMI.S7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajappa M, Sharma A. Biomarkers of cardiac injury: an update. Angiology. 2005;56:677–691. doi: 10.1177/000331970505600605. [DOI] [PubMed] [Google Scholar]
- Ravkilde J, Nissen H, Horder M, Thygesen K. Independent prognostic value of serum creatine kinase isoenzyme MB mass, cardiac troponin T and myosin light chain levels in suspected acute myocardial infarction. Analysis of 28 months of follow-up in 196 patients. Journal of the American College of Cardiology. 1995;25:574–581. doi: 10.1016/0735-1097(94)00430-X. [DOI] [PubMed] [Google Scholar]
- Reichgott MJ. Minoxidil and pericardial effusion: an idiosyncratic reaction. Clinical pharmacology and therapeutics. 1981;30:64–70. doi: 10.1038/clpt.1981.128. [DOI] [PubMed] [Google Scholar]
- Richards AM, Nicholls MG, Espiner EA, Lainchbury JG, Troughton RW, Elliott J, Frampton C, Turner J, Crozier IG, Yandle TG. B-type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation. 2003;107:2786–2792. doi: 10.1161/01.CIR.0000070953.76250.B9. [DOI] [PubMed] [Google Scholar]
- Sanz E, Lopez Novoa JM, Linares M, Digiuni E, Caramelo CA. Intravascular and interstitial fluid dynamics in rats treated with minoxidil. Journal of cardiovascular pharmacology. 1990;15:485–492. doi: 10.1097/00005344-199003000-00020. [DOI] [PubMed] [Google Scholar]
- Scrutinio D, Ammirati E, Guida P, Passantino A, Raimondo R, Guida V, Sarzi Braga S, Pedretti RF, Lagioia R, Frigerio M, Catanzaro R, Oliva F. Clinical utility of N-terminal pro-B-type natriuretic peptide for risk stratification of patients with acute decompensated heart failure. Derivation and validation of the ADHF/NT-proBNP risk score. International journal of cardiology. 2013;168:2120–2126. doi: 10.1016/j.ijcard.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Selvais PL, Donckier JE, Robert A, Laloux O, van Linden F, Ahn S, Ketelslegers JM, Rousseau MF. Cardiac natriuretic peptides for diagnosis and risk stratification in heart failure: influences of left ventricular dysfunction and coronary artery disease on cardiac hormonal activation. European journal of clinical investigation. 1998;28:636–642. doi: 10.1046/j.1365-2362.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- Sica DA. Minoxidil: an underused vasodilator for resistant or severe hypertension. J Clin Hypertens (Greenwich) 2004;6:283–287. doi: 10.1111/j.1524-6175.2004.03585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Liu Y, Parman T, Liu S, Miller JK, Liu X, Tanga MJ, Mirsalis J. Quantitative proteomics for cardiac biomarker discovery using isoproterenol-treated nonhuman primates. Journal of proteome research. 2014;13:5909–5917. doi: 10.1021/pr500835w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes AH, Falls JG, Yoon L, Cariello N, Faiola B, Colton HM, Jordan HL, Berridge BR. Integrated approach to early detection of cardiovascular toxicity induced by a ghrelin receptor agonist. International journal of toxicology. 2015;34:151–161. doi: 10.1177/1091581815573029. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Hirota Y, Sohmiya K, Nishimura S, Kawamura K. Serum and urinary human heart fatty acid-binding protein in acute myocardial infarction. Clinical biochemistry. 1991;24:195–201. doi: 10.1016/0009-9120(91)90571-u. [DOI] [PubMed] [Google Scholar]
- Taqui S, Daniels LB. Putting it into perspective: multimarker panels for cardiovascular disease risk assessment. Biomarkers in medicine. 2013;7:317–327. doi: 10.2217/bmm.13.15. [DOI] [PubMed] [Google Scholar]
- Tonomura Y, Matsushima S, Kashiwagi E, Fujisawa K, Takagi S, Nishimura Y, Fukushima R, Torii M, Matsubara M. Biomarker panel of cardiac and skeletal muscle troponins, fatty acid binding protein 3 and myosin light chain 3 for the accurate diagnosis of cardiotoxicity and musculoskeletal toxicity in rats. Toxicology. 2012;302:179–189. doi: 10.1016/j.tox.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Turcani M, Jacob R. Minoxidil accelerates heart failure development in rats with ascending aortic constriction. Canadian journal of physiology and pharmacology. 1998;76:613–620. doi: 10.1139/cjpp-76-6-613. [DOI] [PubMed] [Google Scholar]
- van der Velden J, Borgdorff P, Stienen GJ. Minoxidil-induced cardiac hypertrophy in guinea pigs. Cellular and molecular life sciences : CMLS. 1999;55:788–798. doi: 10.1007/s000180050332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinken P, Reagan WJ, Rodriguez LA, Buck WR, Lai-Zhang J, Goeminne N, Barbacci G, Liu R, King NM, Engle SK, Colton H. Cross-laboratory analytical validation of the cardiac biomarker NT-proANP in rat. Journal of pharmacological and toxicological methods. 2016;77:58–65. doi: 10.1016/j.vascn.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Walker DB. Serum chemical biomarkers of cardiac injury for nonclinical safety testing. Toxicologic pathology. 2006;34:94–104. doi: 10.1080/01926230500519816. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. The New England journal of medicine. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- York M, Scudamore C, Brady S, Chen C, Wilson S, Curtis M, Evans G, Griffiths W, Whayman M, Williams T, Turton J. Characterization of troponin responses in isoproterenol-induced cardiac injury in the Hanover Wistar rat. Toxicologic pathology. 2007;35:606–617. doi: 10.1080/01926230701389316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.