Abstract
Background
Case fatality ratios among children with tuberculosis disease are poorly understood, particularly among HIV-infected cases and those not receiving tuberculosis treatment.
Methods
We carried out a systematic review of the published literature to identify studies of population-representative samples of pediatric (<15 years old) tuberculosis cases. We used random effects meta-analysis to produce pooled estimates of case fatality ratios. We stratified our analyses by whether or not children received tuberculosis treatment, age (0–4 years, 5–14 years), and HIV status.
Findings
We identified 31 papers comprising 35 datasets representing 82,436 children with tuberculosis disease, of whom 9,273 died. Among children with tuberculosis from the pretreatment era, the pooled case fatality ratio was 21.9% (95% confidence interval [CI]: 18.1%, 26.4%). The pooled case fatality ratio was significantly higher among children aged 0–4 years (43.6%; 95% CI: 36.8%, 50.6%) than among children aged 5–14 years (14.9%; 95% CI: 11.5%, 19.1%). In recent studies where the majority of children had tuberculosis treatment, the pooled case fatality ratio was 0.9% (95% CI: 0.5%, 1.6%). USA surveillance data suggest a substantially higher case fatality ratio among HIV-infected children receiving TB treatment, compared with HIV-uninfected children, especially without antiretroviral treatment.
Interpretation
Without adequate treatment, children with tuberculosis disease, especially those under five years of age, are at high risk of death. HIV-infected children have an increased mortality risk, even when receiving tuberculosis treatment.
Funding
US National Institutes of Health, Janssen Global Public Health
Introduction
With recent increased attention to the long-neglected global epidemic of childhood tuberculosis, there is a growing consensus that there are far more children with tuberculosis globally than previously thought, with the majority undiagnosed and untreated.1–3 Current estimates suggest that around 1 million children develop tuberculosis annually,3 of whom only 36% are notified.3 The greatest case detection failure occurs in children under five years old, who likely account for around half of all pediatric cases.2 The under-five mortality associated with these more than 500,000 new cases of tuberculosis disease each year is largely preventable and may contribute more to global under-five mortality than previously thought.
Given that so many children with tuberculosis are never diagnosed, estimating childhood tuberculosis mortality presents unique challenges. Cohort studies from the past three decades include very few children who did not receive tuberculosis treatment. Current mortality estimates (including the World Health Organization [WHO] global estimate of 136,000 annual tuberculosis deaths among children3) often rely on vital registration data,3 but posthumously attributing deaths to undiagnosed tuberculosis disease is hampered by autopsy cost and the unreliability of vital records and verbal autopsy.4 Since tuberculosis symptoms in children are so non-specific, many deaths caused by tuberculosis may be erroneously attributed to more common diseases.5 An alternative source of information on children who did not receive tuberculosis treatment is the scientific literature published before the discovery of antituberculosis chemotherapy.
Quantifying the mortality impact of the global failure to diagnose and treat childhood tuberculosis requires a better understanding of the mortality risks associated with tuberculosis disease in relevant age groups, with and without access to appropriate treatment. Therefore, we conducted a systematic review and meta-analysis to estimate case fatality ratios — i.e. the proportion of children with tuberculosis who die — stratified by treatment, age group, and HIV infection.
Methods
Search strategy for systematic review
We first searched the literature for systematic reviews on tuberculosis mortality or outcomes in children with all forms of tuberculosis disease. We found only systematic reviews of outcomes among children with tuberculous meningitis6 and children with multidrug-resistant tuberculosis (i.e. strains of tuberculosis that are resistant to the drugs isoniazid and rifampicin);7 both reviews examined only cohorts receiving treatment. We then searched the literature for studies reporting deaths among child tuberculosis cases, including all cases classed by the authors as active tuberculosis or tuberculosis disease. We aimed to identify publications in which the child tuberculosis cases were representative of a general population of children with tuberculosis in the study setting; our rationale was that including publications reporting on children who were more or less likely to die than the “average” pediatric tuberculosis case would overestimate or underestimate the mortality risk.
We searched the PubMed and EMBASE electronic databases for reports published before 12 August 2016, that included terms related to tuberculosis (e.g., the MeSH term “tuberculosis” or the text string “tuberculosis”), children (e.g., the MeSH term “child” or the text string “child*”), mortality (e.g., the MeSH term “mortality” or the text string “death”), and population representativeness (e.g., the MeSH term “population” or the text string “surveillance”). See Appendix for a complete list of search terms.
In addition, we reviewed the reference lists of primary studies and review articles for additional references as well as the authors’ personal libraries. We focused this additional search on papers from the pre-treatment era, since untreated children were rare in studies conducted since the discovery of antituberculosis drugs.
Review of studies
After excluding duplicate citations found through the database search, two reviewers (two of HEJ, CMY, CAR, MMM) independently screened each title and abstract and resolved discrepancies by consensus. Cohort studies reporting deaths among children with tuberculosis disease and cross-sectional studies reporting child tuberculosis cases and child tuberculosis deaths in a given year were eligible for inclusion. We excluded cohort studies where ≥10% of children had unknown outcomes. We excluded reviews, editorials, publications restricted to non-representative samples of patients, and publications in a language other than English, Spanish, French, or Portuguese.
We excluded the following as non-representative: case reports/series; publications on specific forms of tuberculosis, other than pulmonary, extrapulmonary, or bacteriologically confirmed; studies restricted to specific types of tuberculosis-associated deaths; and facility-based studies, as these could represent biased samples of the tuberculosis patient population. However, we did not exclude publications that only excluded drug-resistant TB patients or retreatment patients as these are a minority of pediatric tuberculosis cases. Neither did we exclude publications restricted to HIV-infected or HIV-uninfected patients, as we planned to analyze these groups separately. We obtained full texts of citations selected for review, and two reviewers (two of HEJ, CMY, CAR, RRN) independently assessed each publication for inclusion, resolving discrepancies by consensus. We subjected publications found through manual searching of references and in authors’ personal libraries to the same inclusion and exclusion criteria.
We contacted authors for additional information if the report was published after 31 December 2009, and either (a) included children and reported the desired type of mortality data, but did not disaggregate these data by age, or (b) were already included but had information on HIV status that was not disaggregated by age.
Data extraction
The same pairs of reviewers extracted all study data independently, resolving discrepancies by consensus. Data were double-entered into a Microsoft Access database. For each included study, we extracted the number of children (aged under 15 years) with tuberculosis disease, and the number of children with tuberculosis that died. For cohort studies, we extracted tuberculosis-associated deaths if available or all-cause deaths otherwise. For studies where the follow-up period exceeded one year, we attempted to extract data on deaths in the year after diagnosis or treatment. If information was available, we extracted data stratified by HIV status and age subgroup (e.g., 0–4, 5–14). Additional data extracted included location, enrollment year(s), follow-up time (after diagnosis), and whether or not patients received antituberculosis chemotherapy or antiretroviral treatment.
Meta-analysis
We used meta-analyses to estimate the percentage of children with tuberculosis disease that died from tuberculosis within one year of disease diagnosis (“case fatality ratio” [CFR]). Because tuberculosis treatment has a substantial effect on risk of death,8 we stratified studies into three eras:
“Pre-treatment era” studies were conducted before 1946. We assumed children in these studies were untreated because streptomycin, the first antituberculosis drug, was not yet available.
“Middle-era” studies were conducted from 1946–1980, possibly also including children diagnosed before 1946. We assumed that some children could have received effective treatment, although universal treatment was unlikely.
“Recent-era” studies included children with tuberculosis disease after 1980. Even if not explicitly stated, we assumed the majority of children described in these studies had received tuberculosis treatment, as the Directly Observed Therapy Short-course model of TB control was adopted by many countries beginning in the 1980s.
We excluded from the meta-analysis any data that could have included subjects already contained in another included dataset. Due to substantial variability in study design, we used a random effects meta-analysis to account for this heterogeneity, and the CFR was logit-transformed to account for any violation of the assumption of normality in this variable. We assessed heterogeneity and bias using the I2 statistic and examination of funnel plots. The meta-analyses and resulting figures were produced using R version 3.0.2.9 CFRs in HIV-infected and HIV-uninfected children within individual studies were compared with Fisher’s exact test.
Role of the funding source
The funders had no role in study design, data collection or analysis, writing the report or the decision to publish. The corresponding author had access to all the data in the study and final responsibility for decision to submit the manuscript.
Results
Systematic review
Of the 1,087 unique citations found through the database search, 253 studies were eligible for full-text review, from which 22 studies were included in the analysis (Figure 1). Three of these papers were only included following the receipt of additional information from the authors. We also reviewed 89 papers identified in the reference lists of reviewed papers or obtained from the authors’ personal libraries; of these 89, we included nine.
Figure 1.
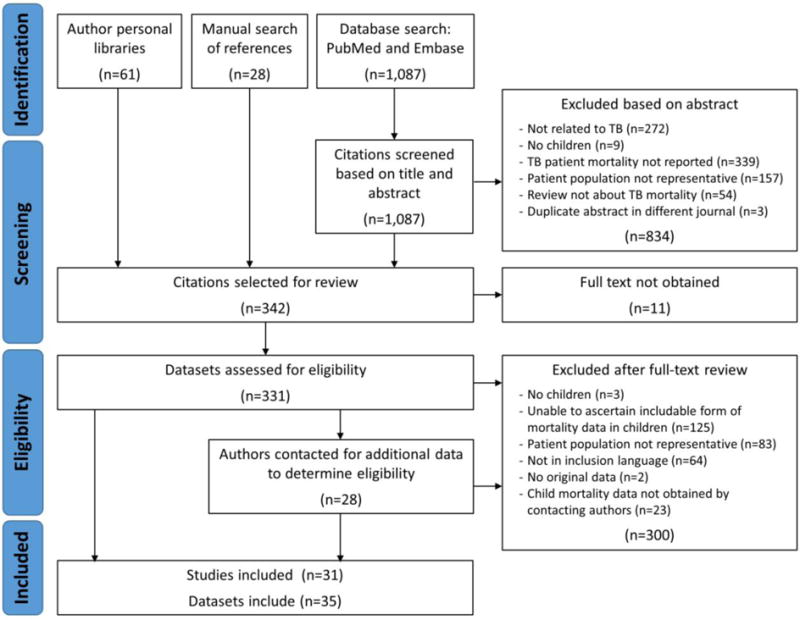
Literature search flow chart
The 31 included papers10–40 comprised 35 datasets (one paper contained five separate datasets19), representing 82,436 children diagnosed with tuberculosis, of whom 9,273 died (Table 1). Six studies were from the pre-treatment era, seven from the middle era, and 18 from the recent era; studies were from multiple geographic regions (Figure 2). Three studies stratified data by HIV status. The majority of studies, including the largest ones, included all forms of tuberculosis (including clinical diagnoses); a few studies included only pulmonary and/or culture-confirmed cases (Table 1).
Table 1.
Characteristics of childhood tuberculosis (TB) studies included in the meta-analysis
| Paper | Location | Years of enrollment/ surveillance |
Age range of children |
TB/patient types with outcome data |
Study design | Type of deaths reported |
Number of children with TB |
Number (%) who died |
|---|---|---|---|---|---|---|---|---|
| Pre-treatment era studies | ||||||||
| Myers23 | USA (Minneapolis) | 1921–41 | 0–14 years | All TB forms | Cohort, variable follow-up (up to 1 year after diagnosis) | TB-related | 246 | 7 (2.8) |
| Lindhart31 | Denmark | 1925–34 | 0–14 years | Pulmonary TB | Cross-sectional | TB-related | 3,152 | 860 (27.3) |
| Hopkins22 | Canada (Montreal) | 1926–38 | 0–1 years | All TB forms | Cohort, variable follow-up (up to 1 year after diagnosis) | TB-related | 17 | 5 (29.4) |
| Drolet19 | UK (England and Wales) | 1933–34 | 0–14 years | All TB forms | Cross-sectional | TB-related | 27,688 | 6,568 (23.7) |
| Drolet19 | USA (New York City) | 1933–34 | 0–14 years | All TB forms | Cross-sectional | TB-related | 1,429 | 544 (38.1) |
| Drolet19 | USA (Chicago) | 1933–34 | 0–14 years | All TB forms | Cross-sectional | TB-related | 1,478 | 276 (18.7) |
| Drolet19 | USA (New York State minus New York City) | 1933–34 | 0–14 years | All TB forms | Cross-sectional | TB-related | 1,561 | 260 (16.7) |
| Drolet19 | USA (New Jersey) | 1933–34 | 0–14 years | All TB forms | Cross-sectional | TB-related | 889 | 213 (24.0) |
| Ferguson & Simes20 | Canada (Saskatchewan) | 1933–45 | 0–4 years | All TB forms | Cohort, variable follow-up | TB-related | 35 | 11 (31.4) |
| Townsend et al.18 | USA (Native American reservations in five states) | 1936–38 | 4–13 years | All TB forms | Cohort, variable follow-up (up to 1 year after diagnosis) | TB-related | 3 | 1 (33.3) |
| Middle-era studies | ||||||||
| Puffer et al.21 | USA (Williamson County, TN) | 1931–51 | 0–14 years | All TB forms | Cohort, variable follow-up | TB-related | 23 | 9 (39.1) |
| Rosenthal et al.17 | USA (Chicago) | 1941–60 | 0–14 years | All TB forms | Cohort, follow-up for 1 year after diagnosis | TB-related | 14 | 3 (21.4) |
| Cammock & Miller30 | UK (Newcastle) | 1945–51 | 0–6 years | All TB forms | Cohort, variable follow-up | TB-related | 258 | 39 (15.1) |
| Gothi et al.32 | India (Bangalore) | 1961 | 5–14 years | Pulmonary TB | Cohort, follow-up for five years after diagnosis | All-cause | 39 | 2 (5.1) |
| Krebs29 | East Germany (Pre-unification) | 1969 | 0–14 years | All TB forms | Cross-sectional | TB-related | 137 | 2 (1.5) |
| Medical Research Council11 | Tanzania (15 districts) | 1969–70 | 0–14 years | All TB forms | Cohort, follow-up for 1 year after diagnosis | All-cause | 433 | 39 (9.0) |
| Medical Research Council10 | Kenya (11 districts) | 1974 | 0–14 years | Pulmonary TB | Cohort, follow-up for 1 year after diagnosis | All-cause | 263 | 47 (17.9) |
| Recent-era studies | ||||||||
| Martineau et al.36 | UK (England and Wales)c | 1988–2001 | 0–14 years | All TB forms | Cross-sectional | TB-related | 6,046 | 22 (0.4) |
| Tocque et al.28 | UK (Liverpool)a | 1989–96 | 1–14 years | All TB forms | Cohort, variable follow-up | All cause | 28 | 0 (0) |
| Bao et al.35 | China (Two districts in Guangzhou) | 1993–2002 | 0–14 years | Pulmonary TB | Cohort followed until treatment outcome | All-cause | 80 | 1 (1.3) |
| Nguyen et al.16 | USA (North Carolina)b | 1993–2003 | 0–14 years | All TB forms | Cohort followed until treatment outcome | All cause | 232 | 2 (0.9) |
| Shah et al.15 | USA | 1993–2011 | 0–14 years | All TB forms | Cohort followed until treatment outcome | All cause | 19,508 | 105 (0.5) |
| Rodwell et al.14 | USA (San Diego County)b | 1994–2005 | 0–14 years | Culture-positive | Cohort followed until treatment outcome | All cause | 54 | 1 (1.9) |
| Abreu et al.13 | Cuba | 1995–2005 | 0–14 years | All TB forms | Cohort followed until treatment outcome | TB-related | 157 | 4 (2.5) |
| Shen et al.34 | China (Shanghai) | 2000–04 | 11–14 years | Culture-positive, pulmonary TB | Cohort followed under treatment outcome | TB-related | 33 | 0 (0) |
| Crofts et al.26 | UK (England and Wales)a | 2001–02 | 0–4 years | All TB forms | Cohort, follow-up for 1 year after diagnosis | TB-related | 856 | 2 (0.2) |
| Abubakar et al.27 | UK (England and Wales) | 2001–05 | 0–15 years | All TB forms | Cohort, follow-up for 1 year after diagnosis | All cause | 1,937 | 13 (0.7) |
| Dangisso et al.37 | Ethiopia (Sidama Zone) | 2003–12 | 0–14 years | All TB forms, new patients | Cohort followed until treatment outcome | All cause | 4,544 | 136 (3.0) |
| Abuaku et al.33 | China (Hunan province) | 2005–06 | 0–14 years | All TB forms | Cohort followed until treatment outcome | All-cause | 371 | 0 (0) |
| Minion et al.12 | Canada | 2005–12 | 0–14 years | Drug-sensitive | Cohort followed until treatment outcome | TB-related | 250 | 4 (1.6) |
| Erkens et al.25 | Netherlands | 2005–12 | 0–14 years | All TB forms | Cohort followed until treatment outcome | TB-related | 395 | 3 (0.8) |
| Gadoev et al.40 | Uzbekistan | 2006–10 | 0–14 years | All TB forms, first-line treatment | Cohort followed until treatment outcome | All cause | 11,519 | 75 (0.7) |
| Korzeniewska-Kosela24 | Poland | 2010 | 0–14 years | All TB forms | Cross-sectional | TB-related | 62 | 0 (0) |
| García-Basteiro et al.39 | Mozambique (Manhiça District) | 2011–12 | 0–14 years | All TB forms | Cohort followed until treatment outcome | All cause | 224 | 24 (10.7) |
| Korzeniewska-Kosela38 | Poland | 2012 | 0–14 years | All TB forms | Cross-sectional | TB-related | 95 | 0 (0) |
These datasets were excluded from analyses related to the 0–14 years age group to avoid double counting; they are subsets of Martineau et al.36 and Abubakar et al.27. However, they were included in analyses related to the 0–4 years and 5–14 years age group because Martineau et al.36 and Abubakar et al.27 did not disaggregate by age in this way.
These datasets were excluded from almost all analyses to avoid double counting; they are subsets of the data from Shah et al.15
Figure 2.
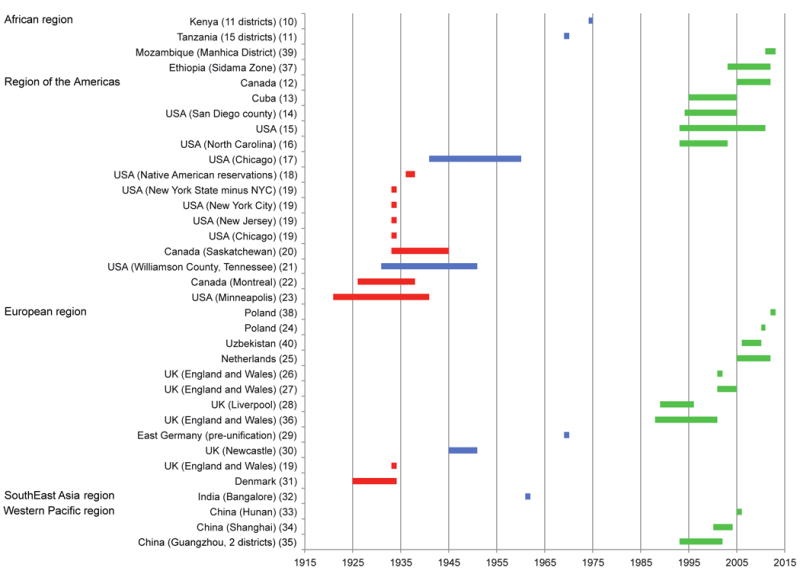
Time periods and geographic regions covered by the included studies. Studies are grouped by location and the time period of enrollment or surveillance is shown by the shaded areas. Red indicates pre-treatment era studies, blue indicates middle-era studies, and green indicates recent-era studies. Numbers in round brackets indicate references in the reference list
Case fatality ratios in the pre-treatment era
Ten datasets reported on children diagnosed with tuberculosis disease during the pre-treatment era, all from North America or Western Europe (Figure 3). We assumed included children were HIV-uninfected. For seven of these datasets, we assumed children were not BCG-vaccinated based on the vaccination policies in their countries during the study period;19,23,31 the other three studies specified which children were not BCG-vaccinated, and we included only unvaccinated children in our meta-analysis. Therefore our meta-analysis estimated the CFR for children who were untreated, not BCG-vaccinated, and not HIV-infected. Within this group, the pooled CFR was 21.9% (95% confidence interval (CI): 18.1%, 26.4%) for children aged 0–14 years (Table 2). The CFR among children aged 0–4 years was 43.6% (95% CI: 36.8%, 50.6%), which was significantly higher than among children aged 5–14 years (14.9%; 95% CI: 11.5%, 19.1%; p<0.001). Heterogeneity among the studies was high (I2>0.94, Table 2). Funnel plots gave no indication of a systematic bias since studies were evenly spread either side of the central dashed line (Appendix Figure A1).
Figure 3.
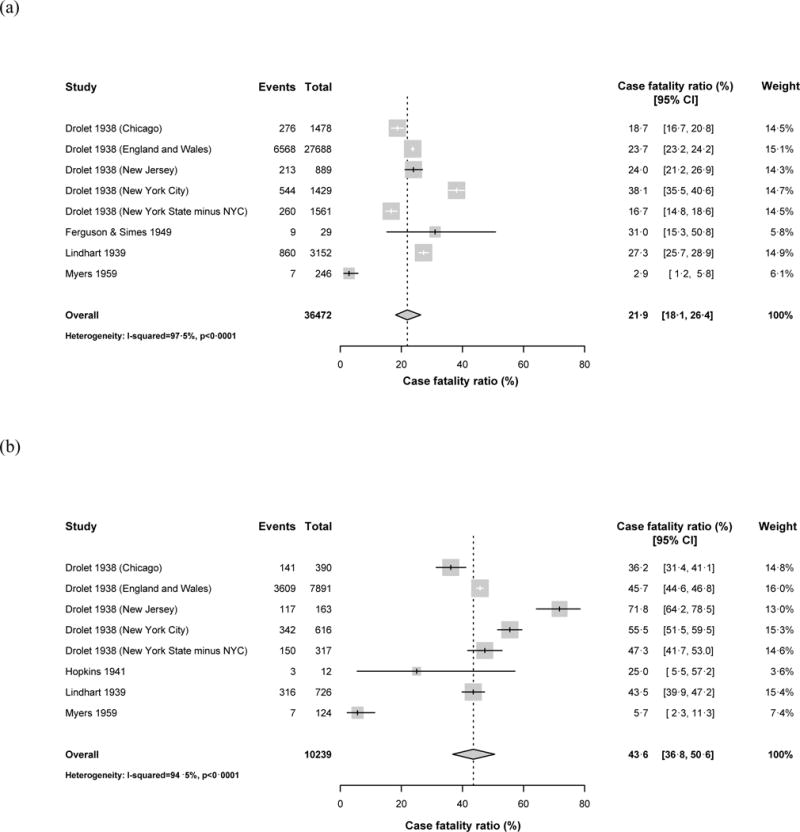
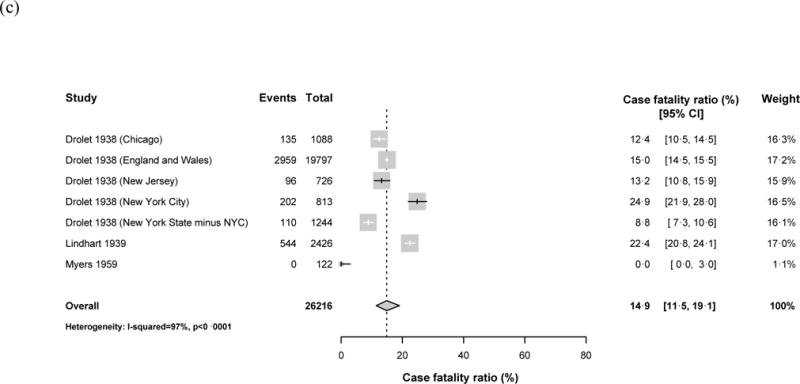
Forest plots for the case fatality ratios from studies in the pre-treatment era. Results are shown for (a) children aged 0–14 years, (b) children aged <5 years old, (c) children aged 5–14 years old. Horizontal lines represent the confidence intervals around the point estimates for each study and the grey shaded areas are proportional to the weight given to each study.
Table 2.
Pooled estimates of case fatality ratios.
| Era and age group | Datasets | Children | Deaths | Pooled estimate (95% CI) | I-squared |
|---|---|---|---|---|---|
| Pre-treatment era studies | |||||
| 0–14 years | 8 | 36,472 | 8,737 | 21.9% (18.1%, 26.4%) | 0.975 |
| 0–4 years1 | 8 | 10,239 | 4,685 | 43.6% (36.8%, 50.6%) | 0.945 |
| 5–14 years | 7 | 26,216 | 4,046 | 14.9% (115%, 19.1%) | 0.970 |
| Middle-era studies | |||||
| 0–14 years | 5 | 870 | 100 | 13.6% (6.7%, 25.4%) | 0.883 |
| 0–4 years2 | 4 | 651 | 102 | 16.5% (11.6%, 23.0%) | 0.671 |
| 5–14 years | 4 | 356 | 28 | 8.3% (5.6%, 12.3%) | 0.104 |
| Recent-era studies | |||||
| 0–14 years5 | 12 | 44,514 | 362 | 0.9% (0.5%, 1.6%) | 0.955 |
| 0–4 years3 | 6 | 12,741 | 118 | 1.9% (0.5%, 7.1%) | 0.967 |
| 5–14 years4 | 7 | 12,873 | 132 | 0.8% (0.3%, 2.1%) | 0.925 |
Includes one study of 0–1 years;
Includes one study of 0–6 years;
Includes one study of 1–4 years;
Includes one study of 11–14 years;
Includes one study of 0–15 years
Case fatality ratios in the middle era
Seven middle-era studies were conducted in Tanzania, Kenya, the USA, the UK, India, and East Germany (pre-unification). Because two of the larger studies from this era were from East Africa after the point at which HIV is hypothesized to have crossed into the human population,41 we did not assume that all middle-era children were HIV-uninfected, although the HIV prevalence among children was likely low. Therefore, our meta-analysis reflects mortality risk in a population of treated and untreated children, and possibly both HIV-infected and uninfected individuals. We estimated CFRs of 13.6% (95% CI: 6.7%, 25.4%) for children aged 0–14 years, 16.5% (95% CI: 11.6%, 23.0%) for children aged 0–4 years, and 8.3% (95% CI: 5.6%, 12.3%) for children aged 5–14 years (p-value for the difference between 0–4 years and 5–14 years = 0.009) (Appendix Figure A2). Heterogeneity was variable (Table 2) but funnel plots gave no indication of a systematic bias (Appendix Figure A3).
Case fatality ratios in the recent era
Eighteen recent-era studies were from Canada, China, Cuba, Ethiopia, Mozambique, the Netherlands, Poland, the USA, the UK, and Uzbekistan. In all 11 studies that discussed treatment, more than 96% of children initiated treatment; the remaining studies were carried out in settings where policies and resources suggest that the vast majority of children described received tuberculosis treatment. All the countries represented by these publications had adult HIV prevalences <2% in 2015 except for Mozambique, which had a 10.5% adult HIV prevalence42. We therefore excluded the Mozambique study39 from the meta-analysis because the HIV prevalence among child TB patients was likely substantially higher than in the other studies. Also, not all studies were incorporated into each meta-analysis due to overlapping populations (Table 1). Among children 0–14 years old in low-HIV-prevalence countries, we estimated a CFR of 0.9% (95% CI: 0.5%, 1.6%). The CFR was higher for children 0–4 years old (1.9%; 95% CI: 0.5%, 7.1%) than children 5–14 years old (0.8%; 95% CI: 0.3%, 2.1%), but this difference was not statistically significant (p=0.29) (Appendix Figure A4). Despite significant heterogeneity (Table 2), funnel plots provided no evidence of systematic bias (Appendix Figure A5).
Only three studies provided HIV status information.12,15,25 In the USA surveillance dataset, which provided the vast majority of the HIV-stratified data, CFRs were higher for HIV-infected children than HIV-uninfected children (Table 3). We were unable to extract data about HIV-infected children’s immune status or whether they were receiving antiretroviral therapy, but stratifying the USA surveillance data into time periods before and after the widespread availability of highly active antiretroviral therapy suggests a protective effect15 (Table 3). Of HIV-infected children receiving tuberculosis treatment before widespread access to antiretroviral therapy, 14.3% (95% CI: 7.4%, 24.1%) died. However, even among children who received tuberculosis treatment and had access to antiretroviral therapy, the CFR was significantly higher (3.4%, 95% CI: 0.7%, 9.6%) than that of HIV-uninfected children treated for tuberculosis (0.4%, 95% CI: 0.3%, 0.7%) in the same time period (p<0.001). In the cohort from Mozambique, the only study identified in our review from a high-HIV-prevalence country, 10.7% of children died; although we do not know the HIV prevalence among this pediatric subgroup, the overall HIV prevalence among TB patients in the cohort was 72%.
Table 3.
Raw data and pooled estimates of case fatality ratios (CFR) from studies stratifying by HIV-status. Includes children aged 0–14 years with known HIV test result.
| Study | Time period | HIV-uninfected children | HIV-uninfected deaths | HIV-uninfected CFR % (95% CI) | HIV-infected children | HIV-infected deaths | HIV-infected CFR % (95% CI) | P-value for difference between HIV-infected CFR and HIV-uninfected CFR2 |
|---|---|---|---|---|---|---|---|---|
| Minion et al. (Canada) | 2005–2012 | 71 | 2 | 2.8% (0.3%, 9.8%) | 3 | 0 | 0.0% (0.0%, 70.8%) | <0.99 |
| Erkens et al. (Netherlands) | 2005–2012 | 74 | 0 | 0.0% (0.0%, 4.9%) | 3 | 0 | 0.0% (0.0%, 70.8%) | <0.99 |
| Shah et al.1 (USA) | 1993–1996 | 679 | 8 | 1.2% (0.5%, 2.3%) | 77 | 11 | 14.3% (7.4%, 24.1%) | 0.0001 |
| 1997–2011 | 3831 | 17 | 0.4% (0.3%, 0.7%) | 88 | 3 | 3.4% (0.7%, 9.6%) | 0.0095 |
Data are presented separately for 1993–1996 and 1997–2011 because highly active antiretroviral therapy including protease inhibitors for treatment of HIV became the standard of care in the United States in 199764. Therefore, children during the earlier period were unlikely to be receiving antiretroviral therapy, while those in the later period would have had access to antiretroviral therapy.
P-values calculated using Fisher’s exact test
Discussion
This is the first comprehensive analysis of CFRs among children with tuberculosis. We found that children receiving adequate treatment had a low mortality risk. In contrast, children managed before the widespread availability of antituberculosis chemotherapy and BCG vaccination had far worse outcomes, with around one in five dying; more than 40% in those under 5 years of age. CFRs among younger children were consistently higher than among older children. Finally, CFRs among children with HIV infection were markedly higher than among children without HIV infection, even when antituberculosis treatment was delivered. The difference was greatly reduced but persisted after the availability of antiretroviral therapy.
The striking difference in CFRs between younger (0–4 years) and older (5–14 years) children is consistent with the natural history of tuberculosis in children.43,44 Given the difficulty in obtaining a diagnosis of tuberculosis in young children,45 our finding that nearly half of these younger children died before the availability of antituberculosis treatment and BCG vaccination suggests a worrisome unrecognized global burden of childhood mortality due to undiagnosed tuberculosis. Our estimated CFR for young children in the pre-treatment era (44%) is virtually identical to the 43% tuberculosis CFR among untreated, HIV-uninfected persons of all ages estimated by the WHO.3
Risk of death among HIV co-infected children appears higher than among HIV-uninfected children, but our results are insufficient to produce reliable CFR estimates for HIV co-infected children given the small number of studies that met our selection criteria. However, hospital-based studies from sub-Saharan Africa have reported CFRs of 14%–41% in HIV-infected children receiving tuberculosis treatment.46–49 Therefore, the mortality risks of 14% and 3% from the USA prior to and after the availability of combination antiretroviral therapy respectively, likely represent the “best case scenario”; CFRs in low-resource settings where treatment access and supportive care are greatly limited are likely to be higher. The WHO estimates a CFR of 78% among HIV-infected persons of all ages in the absence of both tuberculosis treatment and antiretroviral therapy.3 The TB/HIV combination is likely to be even more lethal in young children who have high HIV viral loads following vertical HIV infection, and who often experience rapid tuberculosis disease progression, even in the absence of immune compromise. Our results highlight the importance of HIV testing in children with tuberculosis exposure or infection, to assist active HIV case-finding and improve access to both early antiretroviral treatment initiation and tuberculosis preventive therapy.50
Our study was subject to limitations in the data we were able to collect. First, we did not have data on the form or severity of tuberculosis disease in child patients, or (for the majority of studies) on BCG vaccination status; we were therefore unable to separate the effect of these factors from those of treatment availability and HIV infection. For example, HIV infection increases the risk of disseminated disease,51 and both tuberculous meningitis and miliary tuberculosis are associated with high mortality risks,6 while BCG vaccination reduces risk of both disseminated disease and death.52,53 Furthermore, tuberculosis patients with limited access to health care are more likely to be diagnosed at an advanced stage of disease with associated high mortality risk, but we were unable to control for this. Data limitations also prevented more informative age stratification, in particular to assess CFRs in children less than 2 years of age who are most vulnerable according to studies of the natural history of tuberculosis in children.44 Only one study (pre-treatment era) reported narrower age bands,31 confirming a higher risk in very young children (CFR=70% in infants under one and 36% in children aged 1–4).
Another limitation is the generalizability of our results. The recent-era datasets identified may not be representative of settings where the majority of tuberculosis cases occur, as a third of them were from high-resource settings with low tuberculosis incidence. CFRs in high-tuberculosis-burden settings are likely to be higher than our estimates due to delayed diagnosis and comorbidities such as malnutrition.55 In addition, while the TB/HIV CFRs from the USA may not be representative of settings where the majority of TB/HIV cases occur, the observed trend of higher CFRs among HIV-infected children than HIV-uninfected children is consistent with the findings of the aforementioned facility-based studies from sub-Saharan Africa.47–49
Pre-treatment era studies were conducted in North America and Europe, which may limit the generalizability of these studies to untreated populations today, given geographic and temporal differences in socioeconomic conditions, nutrition, burden of disease, and other factors. However, many of the societal conditions that characterized the pre-treatment era cohorts — high-density households, malnutrition, lack of access to medical care, poor air quality, and high background tuberculosis incidence rates — share commonalities with high-tuberculosis-burden settings today.56 Nevertheless, socioeconomic improvements and increases in health system capacity could have contributed to the decrease in CFRs observed between the pre-treatment and recent eras; thus further studies such as tuberculosis prevalence surveys and population-based autopsy studies are required to estimate the true burden of mortality from undiagnosed tuberculosis. Additional limitations to the pre-treatment studies were the preponderance of cross-sectional rather than cohort data and a lack of uniformity in tuberculosis diagnostic criteria and follow-up periods among the cohort studies.
Several sources of error in reported CFRs could also have affected our estimates. Given the number of children likely diagnosed clinically and the difficulties in posthumously ascertaining tuberculosis as a cause of death, both the numerators and denominators of reported CFRs could have been affected by misclassification error. We also conservatively assumed children with unknown outcomes (e.g., “lost to follow-up”) to have survived, which would underestimate CFRs. In addition, many cases in the included studies were studied and cared for by childhood tuberculosis experts, and therefore the resulting mortality might be lower than in many settings where fewer specialists are available.
Finally, it is possible that some useful papers could have been excluded due to our selection of search terms (Appendix) and our inclusion of reports in only four languages. These limitations are difficult to avoid in systematic reviews, in which the potential for increased yield from a wider search must be weighed against the increased feasibility of a tighter search.
While more work is needed to quantify the burden of tuberculosis-associated mortality among children, immediate steps can be taken to mitigate it. These include targeted active case-finding to diagnose children early, promoting clinical diagnoses and empiric treatment when required, and providing preventive therapy to young children with recent tuberculosis exposure or tuberculosis infection.50 Active case-finding strategies that could improve early detection of childhood tuberculosis include contact investigation, and integrating tuberculosis screening into other child health services such as HIV, nutrition, and Integrated Management of Childhood Illness screening programs.57 Preventive therapy is widely recognized as crucial to reduce unnecessary tuberculosis deaths in young children58 and is now increasingly an area of focus in global policy discourse,58,59 but it is poorly implemented in tuberculosis-endemic areas. Preventive therapy could also limit the probability of chronic tuberculosis suffered by some childhood survivors of tuberculosis (estimated to occur in 8.7% of child tuberculosis survivors60), as well as future re-activation disease, phenomena outside the scope of our current review.
Additionally, we urgently need diagnostics that are accurate and feasible in young children, since traditional sputum smear microscopy and culture methods have poor sensitivity in children.61,62 Sputum-based diagnostic methods are inadequate in young children, who cannot expectorate. Development must thus be accelerated for non-sputum-based diagnostics, such as technologies that analyze peripheral blood, stool, urine, or volatile organic compounds in breath.63
In sum, this systematic review and meta-analysis suggests that the risk of death in children with tuberculosis disease is particularly high for children also infected with HIV and children who do not receive tuberculosis treatment, demonstrating the urgent need to extend tuberculosis treatment to young children in tuberculosis endemic areas. Our findings point to a large and invisible burden of preventable child deaths related to tuberculosis, particularly in areas with uncontrolled tuberculosis transmission where children have poor access to appropriate care. In the absence of sensitive diagnostic tools, the most important strategies that programs can deploy are empiric treatment of young children with clinical suspicion of tuberculosis disease and extensive use of preventive therapy in vulnerable children in close contact with an infectious tuberculosis case.
Supplementary Material
Acknowledgments
This work was supported by the U.S. National Institutes of Health (US NIH K01AI102944 award to HEJ) and by a research grant to Harvard Medical School from Janssen Global Public Health (MCB, CMY, CAR). The content is solely the responsibility of the authors and does not 17 necessarily represent the views of the U.S. National Institute of Allergy and Infectious Diseases or the U.S. National Institutes of Health.
The authors would like to thank J. Sean Cavanaugh, C.C. Leung, Jason E. Stout, and Connie Erkens for providing additional data. The authors would like to thank the Public Health Agency of Canada for providing updated data from the Canadian Tuberculosis Reporting System. The authors would also like to thank C. Robert Horsburgh, Jr. and Michael LaValley for helpful comments on the analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
HEJ, CMY, and MCB designed the study. HEJ, CMY, CAR, and MCB designed the literature search. CMY and CAR supervised the literature search and review process. PD and BM identified additional reports from their personal libraries. HEJ, CMY, CAR, RRN, and MMM reviewed citations and reports, and extracted data. HEJ and CAR contacted authors for additional data. HEJ analyzed the data. HEJ, CMY, PD, BM, and MCB interpreted the results. HEJ and CMY prepared the tables and figures. HEJ and CMY wrote the first draft of the paper. All authors revised it critically for important intellectual content. HEJ and CMY wrote the final version of the paper. All authors have approved this version for publication.
Declaration of interests
HEJ received funding from the U.S. National Institutes of Health (Award number: K01AI102944). MCB, CMY, and CAR received funding from Janssen Global Public Health.
References
- 1.Jenkins HE, Tolman AW, Yuen CM, et al. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. Lancet. 2014;383(9928):1572–9. doi: 10.1016/S0140-6736(14)60195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. The Lancet Global health. 2014;2(8):e453–9. doi: 10.1016/S2214-109X(14)70245-1. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Global Tuberculosis Report 2015. Geneva: 2015. [Google Scholar]
- 4.Setel PW, Whiting DR, Hemed Y, et al. Validity of verbal autopsy procedures for determining cause of death in Tanzania. Trop Med Int Health. 2006;11(5):681–96. doi: 10.1111/j.1365-3156.2006.01603.x. [DOI] [PubMed] [Google Scholar]
- 5.Graham SM, Sismanidis C, Menzies HJ, Marais BJ, Detjen AK, Black RE. Importance of tuberculosis control to address child survival. Lancet. 2014;383(9928):1605–7. doi: 10.1016/S0140-6736(14)60420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang SS, Khan FA, Milstein MB, et al. Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(10):947–57. doi: 10.1016/S1473-3099(14)70852-7. [DOI] [PubMed] [Google Scholar]
- 7.Ettehad D, Schaaf HS, Seddon JA, Cooke GS, Ford N. Treatment outcomes for children with multidrug-resistant tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(6):449–56. doi: 10.1016/S1473-3099(12)70033-6. [DOI] [PubMed] [Google Scholar]
- 8.Donald PR, Ahmed A, Burman WJ, et al. Requirements for the clinical evaluation of new anti-tuberculosis agents in children. Int J Tuberc Lung Dis. 2013;17(6):794–9. doi: 10.5588/ijtld.12.0567. [DOI] [PubMed] [Google Scholar]
- 9.R: A language and environment for statistical computing., Version 3.0.2. R Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2013. http://www.R-project.org/ [Google Scholar]
- 10.The East African and British Medical Research Council. Tuberculosis in Kenya: follow-up of the second (1974) national sampling survey and a comparison with the follow-up data from the first (1964) national sampling survey. An East African and British Medical Research Council co-operative investigation. Tubercle. 1979;60(3):125–49. doi: 10.1016/0041-3879(79)90014-x. [DOI] [PubMed] [Google Scholar]
- 11.Tuberculosis in Tanzania: a follow-up of a national sampling survey of drug resistance and other factors. Tubercle. 1977;58(2):55–78. doi: 10.1016/0041-3879(77)90032-0. [DOI] [PubMed] [Google Scholar]
- 12.Minion J, Gallant V, Wolfe J, Jamieson F, Long R. Multidrug and extensively drug-resistant tuberculosis in Canada 1997–2008: demographic and disease characteristics. PLoS One. 2013;8(1):e53466. doi: 10.1371/journal.pone.0053466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abreu G, Gonzalez JA, Gonzalez E, et al. Cuba’s strategy for childhood tuberculosis control, 1995–2005. MEDICC review. 2011;13(3):29–34. doi: 10.37757/MR2011V13.N3.7. [DOI] [PubMed] [Google Scholar]
- 14.Rodwell TC, Moore M, Moser KS, Brodine SK, Strathdee SA. Tuberculosis from Mycobacterium bovis in binational communities, United States. Emerg Infect Dis. 2008;14(6):909–16. doi: 10.3201/eid1406.071485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah NS, Cavanaugh JS, Pratt R, et al. Epidemiology of smear-negative pulmonary tuberculosis in the United States, 1993–2008. Int J Tuberc Lung Dis. 2012;16(9):1234–40. doi: 10.5588/ijtld.11.0794. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen LT, Hamilton CD, Xia Q, Stout JE. Mortality before or during treatment among tuberculosis patients in North Carolina, 1993–2003. Int J Tuberc Lung Dis. 2011;15(2):257–62. i. [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenthal SR, Loewinsohn E, Graham ML, Liveright D, Thorne MG, Johnson V. BCG vaccination in tuberculous households. Am Rev Respir Dis. 1961;84:690–704. doi: 10.1164/arrd.1961.84.5P1.690. [DOI] [PubMed] [Google Scholar]
- 18.Townsend JG, Aronson JD, Saylor R, Parr I. Tuberculosis control among the North American Indians. American Review of Tuberculosis. 1942;45:41–52. [Google Scholar]
- 19.Drolet G. Present trend of case fatality rates in tuberculosis. American Review of Tuberculosis. 1938;37:125–51. [Google Scholar]
- 20.Ferguson RG, Simes AB. BCG vaccination of Indian infants in Saskatchewan. Tubercle. 1949;30(1):5–11. doi: 10.1016/s0041-3879(49)80055-9. [DOI] [PubMed] [Google Scholar]
- 21.Puffer RR, Zeidberg LD, Dillon A, Gass RS, Hutcheson RH. Tuberculosis attack and death rates of household associates; the influence of age, sex, race, and relationship. Am Rev Tuberc. 1952;65(2):111–27. doi: 10.1164/art.1952.65.2.111. [DOI] [PubMed] [Google Scholar]
- 22.Hopkins JW. BCG vaccination in Montreal: statistical analysis of the results of research by Dr. J.A. Baudouin on BCG vaccination in Montreal. American Review of Tuberculosis. 1941;43:581–99. [Google Scholar]
- 23.Myers JA. The natural history of tuberculosis in the human body. I. The demonstrable primary pulmonary infiltrate. Am Rev Tuberc. 1959;79(1):19–30. doi: 10.1164/artpd.1959.79.1.19. [DOI] [PubMed] [Google Scholar]
- 24.Korzeniewska-Kosela M. Tuberculosis in Poland in 2011. Przeglad epidemiologiczny. 2013;67(2):277–81. 375–8. [PubMed] [Google Scholar]
- 25.Erkens CG, de Vries G, Keizer ST, Slump E, van den Hof S. The epidemiology of childhood tuberculosis in the Netherlands: still room for prevention. BMC Infect Dis. 2014;14:295. doi: 10.1186/1471-2334-14-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crofts JP, Pebody R, Grant A, Watson JM, Abubakar I. Estimating tuberculosis case mortality in England and Wales, 2001–2002. Int J Tuberc Lung Dis. 2008;12(3):308–13. [PubMed] [Google Scholar]
- 27.Abubakar I, Laundy MT, French CE, Shingadia D. Epidemiology and treatment outcome of childhood tuberculosis in England and Wales: 1999–2006. Archives of disease in childhood. 2008;93(12):1017–21. doi: 10.1136/adc.2008.139543. [DOI] [PubMed] [Google Scholar]
- 28.Tocque K, Convrey RP, Bellis MA, Beeching NJ, Davies PD. Elevated mortality following diagnosis with a treatable disease: tuberculosis. Int J Tuberc Lung Dis. 2005;9(7):797–802. [PubMed] [Google Scholar]
- 29.Krebs A. The fight against tuberculosis in the German Democratic Republic, with special reference to prophylaxis and mass miniature radiography. Scandinavian journal of respiratory diseases Supplementum. 1972;80:51–60. [PubMed] [Google Scholar]
- 30.Cammock RM, Miller FJ. Tuberculosis in young children. Lancet. 1953;1(6752):158–60. doi: 10.1016/s0140-6736(53)90779-x. [DOI] [PubMed] [Google Scholar]
- 31.Lindhart M. The Statistics of Pulmonary Tuberculosis in Denmark 1925–1934: A Statistical Investigation on the Occurrence of Pulmonary Tuberculosis in the Period 1925–1934, Worked out on the Basis of the Danish National Health Service File of Notified Cases and of Deaths. Acta tuberculosea Scandinavica. 1939:179. Supplementum III. [Google Scholar]
- 32.Gothi GD, Nair SS, Pyarelal Some epidemiological aspects of tuberculos disease and infection in pediatric age group in a rural community. Indian pediatrics. 1971;8(6):186–94. [PubMed] [Google Scholar]
- 33.Abuaku B, Tan H, Li X, Chen M, Huang X. Treatment default and death among tuberculosis patients in Hunan, China. Scandinavian journal of infectious diseases. 2010;42(4):281–7. doi: 10.3109/00365540903493723. [DOI] [PubMed] [Google Scholar]
- 34.Shen X, Deriemer K, Yuan Z, et al. Deaths among tuberculosis cases in Shanghai, China: who is at risk? BMC Infect Dis. 2009;9:95. doi: 10.1186/1471-2334-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bao QS, Du YH, Lu CY. Treatment outcome of new pulmonary tuberculosis in Guangzhou, China 1993–2002: a register-based cohort study. BMC Public Health. 2007;7:344. doi: 10.1186/1471-2458-7-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martineau AR, Lowey H, Tocque K, Davies PD. Decreasing tuberculosis case fatality in England and Wales, 1988–2001. Int J Tuberc Lung Dis. 2004;8(6):737–42. [PubMed] [Google Scholar]
- 37.Dangisso MH, Datiko DG, Lindtjorn B. Low case notification rates of childhood tuberculosis in southern Ethiopia. BMC pediatrics. 2015;15:142. doi: 10.1186/s12887-015-0461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korzeniewska-Kosela M. Tuberculosis in Poland in 2013. Przeglad epidemiologiczny. 2015;69(2):277–82. 389–93. [PubMed] [Google Scholar]
- 39.Garcia-Basteiro AL, Respeito D, Augusto OJ, et al. Poor tuberculosis treatment outcomes in Southern Mozambique (2011–2012) BMC Infect Dis. 2016;16:214. doi: 10.1186/s12879-016-1534-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gadoev J, Asadov D, Tillashaykhov M, et al. Factors Associated with Unfavorable Treatment Outcomes in New and Previously Treated TB Patients in Uzbekistan: A Five Year Countrywide Study. PLoS One. 2015;10(6):e0128907. doi: 10.1371/journal.pone.0128907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Worobey M, Gemmel M, Teuwen DE, et al. Direct evidence of extensive diversity of HIV-1 in Kinshasa by 1960. Nature. 2008;455(7213):661–4. doi: 10.1038/nature07390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.UNAIDS. AIDSinfo. http://aidsinfo.unaids.org (accessed 26 September 2016.
- 43.Marais BJ, Gie RP, Schaaf HS, et al. The clinical epidemiology of childhood pulmonary tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;8(3):278–85. [PubMed] [Google Scholar]
- 44.Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;8(4):392–402. [PubMed] [Google Scholar]
- 45.Connell TG, Zar HJ, Nicol MP. Advances in the diagnosis of pulmonary tuberculosis in HIV-infected and HIV-uninfected children. J Infect Dis. 2011;204(Suppl 4):S1151–8. doi: 10.1093/infdis/jir413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiseman CA, Schaaf HS, Cotton MF, et al. Bacteriologically confirmed tuberculosis in HIV-infected infants: disease spectrum and survival. Int J Tuberc Lung Dis. 2011;15(6):770–5. doi: 10.5588/ijtld.10.0501. [DOI] [PubMed] [Google Scholar]
- 47.Palme IB, Gudetta B, Bruchfeld J, Muhe L, Giesecke J. Impact of human immunodeficiency virus 1 infection on clinical presentation, treatment outcome and survival in a cohort of Ethiopian children with tuberculosis. Pediatr Infect Dis J. 2002;21(11):1053–61. doi: 10.1097/00006454-200211000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Soeters M, de Vries AM, Kimpen JL, Donald PR, Schaaf HS. Clinical features and outcome in children admitted to a TB hospital in the Western Cape–the influence of HIV infection and drug resistance. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2005;95(8):602–6. [PubMed] [Google Scholar]
- 49.Mukadi YD, Wiktor SZ, Coulibaly IM, et al. Impact of HIV infection on the development, clinical presentation, and outcome of tuberculosis among children in Abidjan, Cote d’Ivoire. AIDS. 1997;11(9):1151–8. doi: 10.1097/00002030-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 50.Marais BJ, Ayles H, Graham SM, Godfrey-Faussett P. Screening and preventive therapy for tuberculosis. Clinics in chest medicine. 2009;30(4):827–46. x. doi: 10.1016/j.ccm.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 51.Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS. 2015;29(15):1987–2002. doi: 10.1097/QAD.0000000000000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367(9517):1173–80. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 53.Marais BJ, Seddon JA, Detjen AK, et al. Interrupted BCG vaccination is a major threat to global child health. The Lancet Respiratory medicine. 2016 doi: 10.1016/S2213-2600(16)00099-0. [DOI] [PubMed] [Google Scholar]
- 54.Sandgren A, Hollo V, Quinten C, Manissero D. Childhood tuberculosis in the European Union/European Economic Area 2000 to 2009. Euro Surveill. 2011;16(12) [PubMed] [Google Scholar]
- 55.Hesseling AC, Westra AE, Werschkull H, et al. Outcome of HIV infected children with culture confirmed tuberculosis. Archives of disease in childhood. 2005;90(11):1171–4. doi: 10.1136/adc.2004.070466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brewer IW. City Life in Relation to Tuberculosis. A Plea for Better Surroundings for Factories and Better Homes for the Working Classes. American journal of public health. 1913;3(9):903–14. doi: 10.2105/ajph.3.9.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuen CM, Amanullah F, Dharmadhikari A, et al. Turning off the tap: stopping tuberculosis transmission through active case-finding and prompt effective treatment. Lancet. 2015 doi: 10.1016/S0140-6736(15)00322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rangaka MX, Cavalcante SC, Marais BJ, et al. Controlling the seedbeds of tuberculosis: diagnosis and treatment of tuberculosis infection. Lancet. 2015 doi: 10.1016/S0140-6736(15)00323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.World Health Organization. The WHO End TB Strategy. 2016 http://www.who.int/tb/post2015_strategy/en/
- 60.Lincoln EW, Sewell EM. Tuberculosis in Children. New York: McGraw-Hill; 1963. [Google Scholar]
- 61.Oberhelman RA, Soto-Castellares G, Gilman RH, et al. Diagnostic approaches for paediatric tuberculosis by use of different specimen types, culture methods, and PCR: a prospective case-control study. Lancet Infect Dis. 2010;10(9):612–20. doi: 10.1016/S1473-3099(10)70141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marais BJ, Hesseling AC, Gie RP, Schaaf HS, Enarson DA, Beyers N. The bacteriologic yield in children with intrathoracic tuberculosis. Clin Infect Dis. 2006;42(8):e69–71. doi: 10.1086/502652. [DOI] [PubMed] [Google Scholar]
- 63.Chiang SS, Swanson DS, Starke JR. New Diagnostics for Childhood Tuberculosis. Infectious disease clinics of North America. 2015;29(3):477–502. doi: 10.1016/j.idc.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 64.AIDS.gov. 30 years of AIDS/HIV timeline. 2016 https://http://www.aids.gov/pdf/aidsgov-timeline.pdf (accessed 6 April 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


