Abstract
Background
How the longitudinal asthma control status and other socio-demographic factors influence the changes of health-related quality of life (HRQOL) among asthmatic children, especially from low-income families, has not been fully investigated.
Objectives
This study aimed to describe the trajectories of asthma-specific HRQOL over 15 months, and examine the effect of asthma control status on HRQOL by taking socio-demographic factors into consideration.
Methods
229 dyads of asthmatic children and their parents enrolled in public insurance programs were recruited for assessing asthma control status and HRQOL over 4 time points of assessment. Asthma control status was measured using the Asthma Control and Communication Instrument and asthma-specific HRQOL was assessed using the Patient-Reported Outcomes Measurement Information System’s Pediatric Asthma Impact Scale. Latent growth models (LGMs) were applied to examine the trajectory of HRQOL and the factors contributing to the changes of HRQOL.
Results
Unconditional LGM revealed that HRQOL was improved over time. Conditional LGM suggested that accounting for asthma control and participants’ socio-demographic factors, the variation in the initial level of HRQOL was significant, yet the rate of change was not. Conditional LGM also revealed that poorly-controlled asthma status was associated with poor HRQOL at each time point (p’s<0.05). Lower parental education was associated with lower baseline HRQOL (p<0.05). Hispanic children had a larger increase in HRQOL over time (p<0.01) than non-Hispanic White children.
Conclusions
Vulnerable socio-demographic characteristics and poorly controlled asthma status affect HRQOL in children. This finding encourages interventions to improve asthma control status and HRQOL in minority children.
Keywords: Asthma control, children, health-related quality of life, patient-reported outcomes, latent growth models
INTRODUCTION
Approximately 7 million (9%) American children and adolescents had asthma in 2012 (1). The National Asthma Education and Prevention Program’s Expert Panel Report-3 (NAEPP EPR-3) guideline provides a clear definition for asthma control and underscores the importance of achieving adequate asthma control as the goal of asthma management (2). The Asthma Outcomes Workshop, sponsored by the National Institutes of Health and the Agency for Healthcare Research and Quality, emphasizes the significance of improving asthma control for optimizing health-related quality of life (HRQOL) of asthmatic individuals (3).
The relationship between asthma control and HRQOL is complex. Although numerous studies reported the association between poorly-controlled asthma status and impaired HRQOL, these studies are largely based on cross-sectional designs (4, 5). Sparse longitudinal studies are available to elucidate the progression of asthma in children and adolescents, especially the associations between the changes in asthma control status and the changes in HRQOL.
Several risk factors (e.g., low levels on physical activity (6), self-efficacy (6), and health literacy (7)) might contribute to the changes of asthma control status and HRQOL. The changes in clinical parameters (forced expiratory volume in one second (8)) were also shown a significant association with the variations in HRQOL. In pediatric asthma, child, parental, and family factors including older age (9–11), low-income family (12, 13), ethnic minority group (14, 15), and low maternal educational attainment (12, 16) determined asthma control status and HRQOL.
The present study aimed to examine the change of HRQOL over a 15-month study period, and to identity important factors determining the changes of HRQOL. A group of asthmatic children between 8 and 17.9 years of ages engaged in this study, and HRQOL was measured using the Patient-Reported Outcomes Measurement Information System (PROMIS®) Pediatric Asthma Impact Scale (17). Given that HRQOL assessed by legacy measures (e.g., the Mini-Asthma Quality of Life Questionnaire (7) and the EQ-5D (18)) was improved over time in previous prospective, observational cohorts, we hypothesized that HRQOL in asthmatic children would increase overall during the study period. However, individuals whose asthma status was poorly-controlled would experience impaired HRQOL at individual time points of assessment. Socio-demographic factors including children’s race/ethnicity, comorbidities with other chronic conditions, and parental education were hypothesized to explain the initial status and the rate of changes in HRQOL.
METHODS
Participants and Data Collection
This study used data collected from the PROMIS Pediatric Asthma Study that was primarily designed to test responsiveness and minimally important differences (MIDs) for the PROMIS Pediatric measures. After the University of Florida’s institutional review board approved the protocol, a total of 229 dyads of asthmatic children and their parents were recruited from Florida Medicaid and the State Children’s Health Insurance Program (SCHIP). Inclusion criteria for study participation were: age between 8 and 17.9 years for children and 18 years or older for parents; continuous enrollment (≥ 6 months) in Florida Medicaid and SCHIP; asthma diagnosis (ICD-9-CM: 493.1, 493.2 or other 493.x) listed in Florida Medicaid and SCHIP claims/enrollment files; at least two asthma-related health care visits during the past year; and family accessibility to internet and telephone in the past 6 weeks.
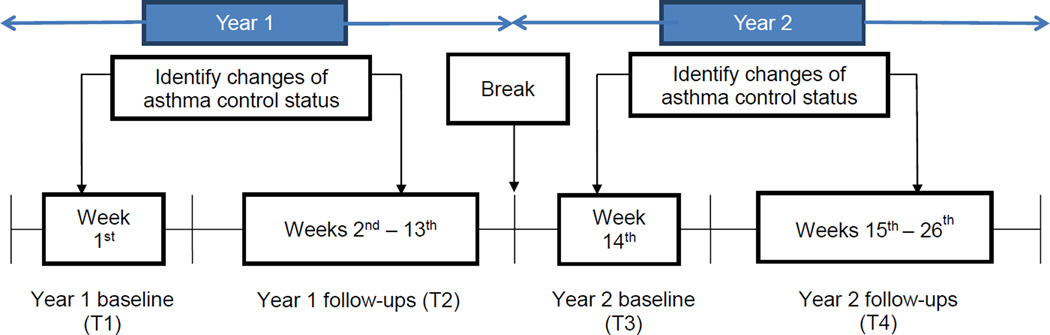
PROMIS Pediatric Asthma Study uses a prospective design (Figure 1) to identify the changes of asthma control status and the corresponding changes of HRQOL in 15 months. Asthma control status was reported weekly (26 weeks in total) by parents through the research website: weeks 1–13 in the first year (09/2010–05/2011) and weeks 14–26 in the second year (09/2011–05/2012). This study only analyzed asthma control status reported at baseline of the first year (T1), the first change of asthma control during weeks 2–13 (T2) as compared to T1, baseline of the second year (T3), and the first change of asthma control during weeks 15–26 (T4) as compared to T3. Pediatric HRQOL was reported through telephone interviews with the participating children at 4 time points of assessment once the first change of asthma control status was occurred: the first year baseline (T1) and follow-up (T2), and the second year baseline (T3) and follow-up (T4).
Figure 1.
Study design to identify the changes of asthma control status
The attrition rate of participation was low, with 7% and 11% at the end of years 1 and 2, respectively. The characteristic significantly different among those retained versus dropped out was the child’s age (p<0.05). Children who were retained at T4 were slightly younger than those who dropped out at T4 (p=0.03).
Measures
Parents reported asthma control status for their children using the Asthma Control and Communication Instrument (ACCI) (20) that was developed according to the 2007 NAEPP EPR-3 (2). The ACCI has demonstrated satisfactory psychometric properties including concurrent, discriminant, and clinical known-groups (e.g., forced expiratory volume in 1 second and peak expiratory flow rate) validity (20). This instrument comprises of 11 items covering five domains of asthma outcomes: asthma control (5 items), short-term asthma-related health care (3 items), direction of asthma symptoms (1 item), adherence to daily asthma medication (1 item), and asthma concerns (1 item). The overall asthma control status for individuals is determined by the 5 items measuring asthma control. A child was assigned to adequate control status if all 5 items were endorsed as mild-intermittent status opposed to mild-, moderate-, or severe-persistent status (equivalent to EPR-3 well-controlled); otherwise, a child was assigned to poor control status (equivalent to EPR-3 not well-controlled and very poorly controlled) (2).
Pediatric HRQOL was self-reported by children using the PROMIS Asthma Impact Scale which is one of PROMIS Pediatric measures. PROMIS Pediatric measures were created using qualitative (e.g., focus group and cognitive debriefing (21, 22)) and quantitative methodologies (e.g., item response theory (23)) with good psychometric properties including test-retest, construct, convergent-discriminant, and known-groups validity (17, 24–26). The domain scores of Asthma Impact Scale were calculated through 8 item scores, and converted to a T-score of 50 (a SD of 10) derived from the original calibration sample (27). Higher domain scores indicate worse HRQOL.
This study used the parental report to assess asthma control status because some asthma control items in the ACCI asked for medication use, occurrence of asthma attack, etc., and we believe that parents can provide more accurate information than children. In contrast, pediatric HRQOL was assessed through the child self-report which is in consistent with the common practice of PROMIS Pediatric measures.
Children’s age, gender, race/ethnicity, comorbidities with other chronic conditions, and parental age, marital status, and educational attainment reported by parents at baseline of the first year were included in the analyses. Treatment data were not emphasized because we focused on the common fluctuation of disease activities (e.g., asthma control) that occurred in children to assess the concomitant changes in HRQOL.
Statistical Analyses
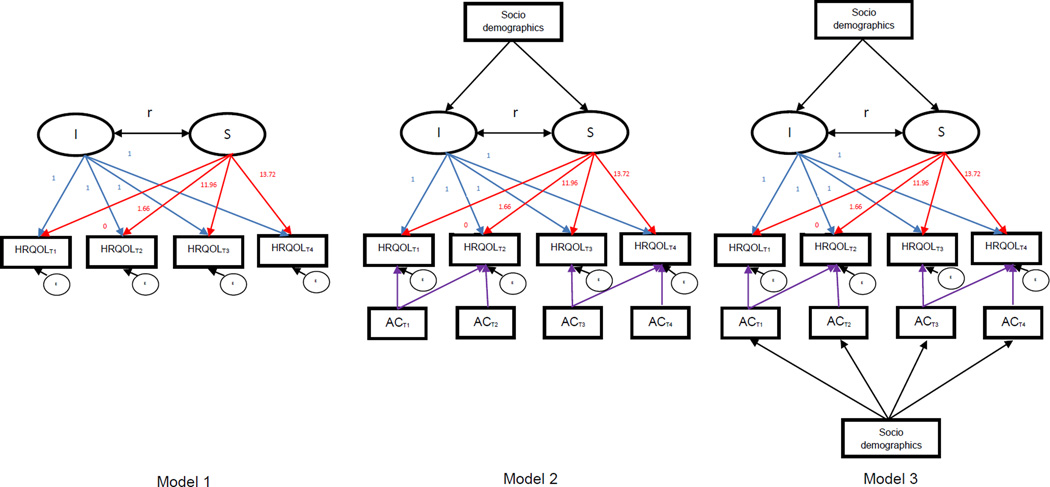
T-tests, ANOVA or Χ2 tests were conducted to examine bivariate associations of asthma control status with socio-demographic characteristics and HRQOL, respectively, by each time point. A linear regression was performed using HRQOL as the dependent variable and asthma control as the main independent variable, controlling for participants’ socio-demographic variables. Two latent growth models (LGMs) were implemented: unconditional LGM (Model 1 in Figure 2) for exploring the changes in HRQOL over time and conditional LGMs (Models 2 and 3 in Figure 2) for quantifying the longitudinal relationship between asthma control and HRQOL by adjusting for socio-demographic factors collected at baseline of the first year. LGM possesses advantages over the traditional models (e.g., generalized estimating equations and repeated-measure analysis of covariance (28)), typically parameterizing the initial status and rate of change in outcomes over times. Because the trend in mean HRQOL scores across time points was linear (Appendix 1), LGMs were performed to examine the factors that contributed to linear changes of HRQOL.
Figure 2.
Unconditional (Model 1) and conditional (Models 2 and 3) latent growth analytic scheme
Specifically, unconditional LGM (Model 1) was performed to describe the trajectories of children’s HRQOL across T1 through T4 by testing the intercept and slope parameters. The intercept parameter represents the initial status of HRQOL, and the slope parameter indicates the rate of HRQOL change over 4 time points. For model identification purpose, the factor loadings for the intercept were fixed to 1, and the factor loadings for the slope were assigned based on the time spacing of T2, T3, and, T4 different from T1, respectively. For all participants, each time point was centered to baseline of year 1 (T1) (29). After the centering, the factor loading of T1 was 0, and the average time spacing was 1.66 months between T2 and T1, 11.96 months between T3 and T1, and 13.72 months between T4 and T1. Because the variability in measurement occasions between participants was not substantial and not expected to affect results (30), we used the average time spacing to define an expected growth trajectory.
Two conditional LGMs (Models 2 and 3) were performed to identify factors contributing to the changes in HRQOL. The first conditional LGM (Model 2) was performed to test the relationships of asthma control status with HRQOL, and the influence of participants’ socio-demographic factors on the growth of HRQOL. Socio-demographic factors were hypothesized to influence the variations in intercept and slope of HRQOL scores. Built on Model 2, the second conditional LGM (Model 3) was performed by additionally accounting for the effect of socio-demographic factors on asthma control status at each time point.
Comparative fit index (CFI) and the root mean square error of approximation (RMSEA) were used to evaluate the adequacy of the model fit for LGMs. CFI ≥ 0.95 and RMSEA ≤ 0.06 were deemed as satisfactory fits (31, 32). LGMs were performed using Mplus 7.3.2 (Muthén & Muthén, Los Angeles, CA), and other analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Participant Characteristics (Table 1)
Table 1.
Characteristics of study participants at baseline of the first year (N=229)
| Number of subject (%) or mean (SD) | ||
|---|---|---|
| Child’s age in years | 12.2 (2.6) | |
| Child’s gender, % | ||
| Boy | 135 (58.9 %) | |
| Girl | 94 (41.1%) | |
| Child’s race/ethnicity, % | ||
| White/non-Hispanic | 87 (38.0%) | |
| Black/non-Hispanic | 59 (25.8%) | |
| Hispanic | 63 (27.5%) | |
| Other | 20 (8.7%) | |
| Parental age in years | 40.6 (8.7) | |
| Parental race/ethnicity, % | ||
| White/non-Hispanic | 97 (42.4%) | |
| Black/non-Hispanic | 60 (26.2%) | |
| Hispanic | 59 (25.8%) | |
| Other | 13 (5.7%) | |
| Parental education background, % | ||
| High school or below | 74 (32.7%) | |
| Some college, associate, and college degree |
136 (60.2%) | |
| Advanced degree | 16 (7.1%) | |
| Family income,% | ||
| < $14,999 | 47 (20.5%) | |
| $15,000– $34,999 | 102 (44.5%) | |
| $35,000 –$54,999 | 57 (24.9%) | |
| >55,000 | 23 (10.0%) | |
| Parental marital status,% | ||
| Never married | 40 (17.5%) | |
| Married | 118 (51.5%) | |
| Living with partner in committed relations |
10 (4.4%) | |
| Separated | 9 (3.9%) | |
| Divorced | 45 (19.7%) | |
| Widowed | 7 (3.1%) | |
| Child’s number of chronic conditions | 1.5 (0.8) | |
Abbreviation: SD, standard deviation.
At baseline, the mean age of the children (N=229) was 12.2 years old (SD=2.6); 59% were boys; and 38% were non-Hispanic White. For parents, the mean age was 40.6 years old (SD=8.7); most had an education of some college, associate degree, or college degree (60.2%), and had family income between $15,000 and $35,000 (44.5%).
Bivariate Associations of Asthma Control with HRQOL and Participants’ Characteristics (Table 2)
Table 2.
Bivariate analyses for asthma control associated with socio demographic factors and HRQOLa by individual time points
| T1 | T2 | T3 | T4 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poor asthma control (N=97) |
Adequate asthma control (N=121) |
t-/F- or X2 value |
Poor asthma control (N=77) |
Adequate asthma control (N=114) |
t-/F- or X2 value |
Poor asthma control (N=62) |
Adequate asthma control (N=104) |
t-/F- or X2 value |
Poor asthma control (N=65) |
Adequate asthma control (N=93) |
t-/F- or X2 value |
||
| HRQOL, mean (SD)a | 51.37 (10.19) |
45.18 (9.08) |
4.73 *** |
50.24 (8.64) |
43.95 (10.15) |
4.42 *** |
49.57 (10.62) |
43.23 (8.97) |
4.09 *** |
47.35 (9.13) |
43.37 (10.60) |
2.46 * |
|
| Child’s age in years, mean (SD) |
12.18 (2.44) |
12.26 (2.64) |
−0.23 | 12.38 (2.56) |
12.25 (2.63) |
0.34 | 11.71 (2.39) |
12.11 (2.42) |
−1.03 | 11.69 (2.28) |
12.18 (2.50) |
−1.26 | |
| Child’s gender, % | |||||||||||||
| Boy | 45.67 | 54.33 | 0.17 | 41.82 | 58.18 | 0.24 | 37.23 | 62.77 | 0.01 | 41.11 | 58.89 | 0.00 | |
| Girl | 42.86 | 42.98 | 38.27 | 61.73 | 38.03 | 61.97 | 41.18 | 58.82 | |||||
| Child’s race/ethnicity, % | |||||||||||||
| White/non-Hispanic | 41.67 | 58.33 | 1.67 | 38.03 | 61.97 | 1.29 | 29.82 | 70.18 | 8.48 * |
47.27 | 52.73 | 3.15 | |
| Black/non-Hispanic | 51.79 | 48.21 | 46.94 | 53.06 | 54.35 | 45.65 | 31.91 | 68.09 | |||||
| Hispanic | 41.67 | 58.33 | 37.04 | 62.96 | 35.56 | 64.44 | 46.15 | 53.85 | |||||
| Other | 44.44 | 55.56 | 41.18 | 58.82 | 23.53 | 76.47 | 35.29 | 64.71 | |||||
| Parental age in years, mean (SD) |
40.23 (8.75) |
41.08 (8.82) |
−0.71 | 42.38 (10.36) |
40.61 (7.65) |
1.28 | 39.58 (9.34) |
42.08 (8.44) |
−1.77 | 40.28 (8.61) |
42.32 (9.27) |
−1.40 | |
| Education, % | |||||||||||||
| High school or below | 55.71 | 44.29 | 5.14 * |
39.66 | 60.34 | 0.00 | 43.14 | 56.86 | 1.03 | 39.22 | 60.78 | 0.10 | |
| College degree or above |
39.31 | 60.69 | 40.15 | 59.85 | 34.82 | 65.18 | 41.90 | 58.10 | |||||
| Marital status, % | |||||||||||||
| Married | 48.57 | 51.43 | 1.36 | 39.13 | 60.87 | 0.10 | 44.87 | 55.13 | 3.36 | 40.26 | 59.74 | 0.05 | |
| Others | 40.71 | 59.29 | 41.41 | 58.59 | 31.03 | 68.97 | 41.98 | 58.02 | |||||
| Smoking status,% | |||||||||||||
| Yes | 42.46 | 57.54 | 1.68 | 37.34 | 62.66 | 3.36 | 38.24 | 61.76 | 0.14 | 40.15 | 59.85 | 0.32 | |
| No | 53.85 | 46.15 | 54.55 | 45.45 | 34.48 | 65.52 | 46.15 | 53.85 | |||||
| Number of chronic conditions, mean (SD) |
1.68 (0.80) |
1.37 (0.77) |
2.90 ** |
1.55 (0.85) |
1.48 (0.78) |
0.53 | 1.73 (0.91) |
1.38 (0.70) |
2.58 * |
1.58 (0.86) |
1.47 (0.77) |
0.85 | |
Abbreviations: SD, standard deviation; HRQOL, health-related quality of life.
Higher scores indicate worse HRQOL.
p<0.05;
p<0.01;
p<0.001
Children with poorly-controlled asthma were 44.5% at T1, 40.3% at T2, 37.4% at T3, and 41.1% at T4. The mean HRQOL consistently improved from T1 to T4 with scores 48.1 (SD=10.2) at T1, 46.5 (SD=10.1) at T2, 45.4 (SD=10.1) at T3, and 44.9 (SD=10.2) at T4 (p<0.001; Appendix 1). Children with poorly-controlled asthma status had significantly worse HRQOL than those with adequate control status across 4 time points (p’s<0.05). Parents with educational attainment of high school and below (p<0.05), and children with more chronic conditions (p<0.01) had higher rates of poorly-controlled asthma at T1. Children who were non-Hispanic Black (p<0.05) and had more chronic conditions (p<0.05) were also more likely to have poorly-controlled asthma at T3 than children who were non-Hispanic White and had fewer chronic conditions.
Multivariable Associations of HRQOL with Asthma Control and Participants’ Characteristics (Table 3)
Table 3.
Multivariate linear regression analyses for HRQOLa associated with asthma control and socio demographic factors by individual time points
| T1 (N=214) | T2 (N=186) | T3 (N=162) | T4 (N=156) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β (SE) | 95% CI | β (SE) | 95% CI | β (SE) | 95% CI | β (SE) | 95% CI | ||
| Asthma control (Ref: Adequate control) |
|||||||||
| Poor control | 5.25*** (1.38) |
[2.53, 7.97] | 6.24*** (1.43) | [3.42, 9.05] | 6.68*** (1.67) |
[3.39, 9.98] | 3.83* (1.65) |
[0.56, 7.09] | |
| Child’s age | −0.05 (0.28) |
[−0.59, 0.50] | 0.40 (0.29) |
[−0.17, 0.97] | 0.40 (0.34) |
[−0.27, 1.07] | 0.41 (0.36) |
[−0.30, 1.13] | |
| Child’s gender (Ref: Girl) |
|||||||||
| Boy | −1.21 (1.35) |
[−3.87, 1.46] | 1.26 (1.42) |
[−1.55, 4.07] | −2.97 (1.55) |
[−6.03, 0.09] | −3.59* (1.63) |
[−6.82, −0.36] | |
| Child’s race/ethnicity (Ref: White/non-Hispanic) |
|||||||||
| Black/non-Hispanic | 1.04 (1.74) |
[−2.38, 4.46] | 0.87 (1.85) |
[−2.78, 4.51] | −2.20 (2.00) |
[−6.16, 1.75] | −0.09 (2.05) |
[−4.14, 3.96] | |
| Hispanic/non-Hispanic | 2.95 (1.67) |
[−0.34, 6.24] | 2.44 (1.77) |
[−1.06, 5.94] | −2.44 (2.00) |
[−6.39, 1.51] | −1.52 (2.15) |
[−5.77, 2.72] | |
| Others | 3.17 (2.58) |
[−1.91, 8.25] | 4.27 (2.57) |
[−0.80, 9.35] | −1.20 (2.76) |
[−6.66, 4.25] | −0.64 (2.87) |
[−6.31, 5.03] | |
| Parental age | −0.02 (0.08) |
[−0.19, 0.14] | −0.08 (0.09) |
[−0.26, 0.10] | −0.04 (0.10) |
[−0.23, 0.16] | −0.12 (0.10) |
[−0.32, 0.08] | |
| Marital status (Ref: Married) |
|||||||||
| Not married | 2.14 (1.39) |
[−0.61, 4.88] | 2.57 (1.46) |
[−0.32, 5.46] | 0.28 (1.61) |
[−2.90, 3.45] | 1.15 (1.68) |
[−2.18, 4.48] | |
| Education (Ref: College or above) |
|||||||||
| High school or below | 3.47* (1.47) |
[0.57, 6.37] | 3.80* (1.56) |
[0.72, 6.88] | 0.90 (1.67) |
[−2.41, 4.21] | 3.12 (1.75) |
[−0.34, 6.58] | |
| Smoking status (Ref: Not smoking) |
|||||||||
| Smoking at home | −1.32 (1.75) |
[−4.77, 2.13] | −2.88 (1.88) |
[−6.59, 0.83] | −2.81 (2.03) |
[−6.82, 1.21] | −1.42 (2.20) |
[−5.77, 2.93] | |
| Number of chronic conditions | 0.54 (0.86) |
[−1.15, 2.23] | 0.82 (0.87) |
[−0.91, 2.55] | −0.38 (0.99) |
[−2.34, 1.59] | −0.02 (1.01) |
[−2.03,1.98] | |
Abbreviations: CI, confidence interval; HRQOL, health-related quality of life.
Higher scores indicate worse HRQOL.
p<0.05;
p<0.01;
p<0.001
Compared with adequately-controlled asthma status, poorly-controlled status was significantly associated with impaired HRQOL with score differences by 5.25 at T1 (p<0.001), 6.24 at T2 (p<0.001), 6.68 at T3 (p<0.001), and 3.83 at T4 (p<0.05), respectively, after adjusting for participants’ characteristics. Parents with educational attainment of high school or below had children with poorer HRQOL at T1 (β=3.47, p<0.05) and T2 (β=3.80, p<0.05) compared with parents with educational attainment of college or an advanced degree. Boys had better HRQOL than girls at T4 (β=−3.59, p<0.05).
Change of HRQOL based on Latent Growth Model (Table 4)
Table 4.
Change of HRQOLa based on unconditional latent growth model (Model 1) and conditional latent growth models with covariate adjustment (Models 2 & 3)
| Model 1 | Model 2 | Model 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intercept (I) |
Slope (S) |
Correlation between I & S |
Intercept (I) |
Slope (S) |
Correlation between I & S |
Intercept (I) |
Slope (S) |
Correlation between I & S |
||
| Mean | 47.80 *** |
−0.19 ** |
−0.41 *** |
41.31 *** |
0.10 | −0.45 *** |
41.68 *** |
0.31 |
−0.56 *** |
|
| Variance | 69.32 *** |
0.29 *** |
57.75 *** |
0.25 *** |
63.97 *** |
0.28 *** |
||||
| Model fit indices | ||||||||||
| X2 (df) | 7.00 (5) | 83.71 (69) | 37.53 (33) | |||||||
| p-value | 0.22 | 0.11 | 0.27 | |||||||
| CFI | 0.99 | 0.95 | 0.98 | |||||||
| RMSEA | 0.04 | 0.03 | 0.03 | |||||||
Abbreviations: CFI, comparative fit index; RMSEA, root mean square error of approximation.
Higher scores indicate worse HRQOL.
p<0.05;
p<0.01;
p<0.001
The unconditional LGM (Model 1) shows an excellent model performance (X2=7.00, df=5, p=0.22, CFI=0.99, RMSEA=0.04). For the HRQOL trajectory, the mean intercept was 47.8 (p<0.001), and the variance of the intercepts was 69.32 (p<0.001), suggesting different HRQOL at the baseline among individuals. The mean slope was −0.19 (p<0.01), suggesting a significant improvement in HRQOL from T1 to T4. The variance of the slopes was 0.29 (p<0.001), indicating the rates of increase in HRQOL among individuals varied significantly. Correlation between the intercept and slope parameters was −0.41 (p<0.001), implying that individuals with higher HRQOL at baseline tended to have slower rates of changes over time.
The conditional LGMs (Models 2 and 3) were performed by adding asthma control as a time-varying predictor and participants’ year 1 baseline socio-demographic characteristics into the unconditional LGM. This model reveals an excellent model performance (X2=83.71, df=69, p=0.11, CFI=0.95, RMSEA=0.03). The mean intercept for Model 2 was 41.31 (p<0.001) and the variance of the intercepts was 57.75 (p<0.001), suggesting that children possessed different levels of HRQOL at the beginning after adjusting for asthma control status and socio-demographic characteristics. Model 3 (Figure 2) revealed an excellent model performance (X2=37.35, df=33, p=0.28; CFI=0.98; RMSEA=0.03).
Longitudinal Associations between Asthma Control and HRQOL based on Latent Growth Model (Table 5)
Table 5.
The associations of HRQOL change, asthma control, and socio demographic factors based on conditional latent growth models (Models 2 & 3)
| Model 2 | Model 3 | ||||
|---|---|---|---|---|---|
| β | SE | β | SE | ||
| Effects on HRQOLa at T1 | |||||
| Asthma control at T1 (Ref: Adequate control) | |||||
| Poor control | 0.23*** | 0.06 | 0.35*** | 0.08 | |
| Effects on HRQOLa at T2 | |||||
| Asthma control at T1 (Ref: Adequate control) | |||||
| Poor control | −0.01 | 0.06 | 0.16 | 0.10 | |
| Asthma control at T2 (Ref: Adequate control) | |||||
| Poor control | 0.23*** | 0.05 | 0.45*** | 0.08 | |
| Effects on HRQOLa at T3 | |||||
| Asthma control T3 (Ref: Adequate control) | |||||
| Poor control | 0.31*** | 0.06 | 0.41*** | 0.09 | |
| Effects on HRQOLa at T4 | |||||
| Asthma control at T3 (Ref: Adequate control) | |||||
| Poor control | 0.26*** | 0.07 | 0.51*** | 0.11 | |
| Asthma control at T4 (Ref: Adequate control) | |||||
| Poor control | 0.18*** | 0.05 | 0.40*** | 0.08 | |
| Effects on Intercept (I) | |||||
| Child’s age | 0.02 | 0.08 | 0.01 | 0.08 | |
| Child’s gender (Ref: Girl) | |||||
| Boys | −0.05 | 0.16 | −0.06 | 0.16 | |
| Child’s race/ethnicity (Ref: White/non-Hispanic) | |||||
| Black/non-Hispanic | 0.20 | 0.20 | 0.08 | 0.20 | |
| Hispanic/non-Hispanic | 0.36 | 0.19 | 0.33 | 0.20 | |
| Others | 0.49 | 0.29 | 0.40 | 0.25 | |
| Parental age | −0.03 | 0.08 | −0.03 | 0.08 | |
| Marital status (Ref: Married) | |||||
| Not married | 0.26 | 0.16 | 0.23 | 0.16 | |
| Education (Ref: College or above) | |||||
| High school or below | 0.51** | 0.16 | 0.39* | 0.17 | |
| Number of chronic condition | 0.08 | 0.08 | 0.02 | 0.09 | |
| Effects on Slope (S) | |||||
| Child’s age | 0.13 | 0.11 | 0.16 | 0.11 | |
| Child’s gender (Ref: Girl) | |||||
| Boy | −0.43* | 0.19 | −0.37 | 0.20 | |
| Child’s race/ethnicity (Ref: White/non-Hispanic) | |||||
| Black/non-Hispanic | −0.43 | 0.24 | −0.47 | 0.26 | |
| Hispanic/non-Hispanic | −0.64** | 0.24 | −0.75** | 0.26 | |
| Others | −0.60 | 0.34 | −0.42 | 0.36 | |
| Parental age | −0.04 | 0.10 | 0.02 | 0.11 | |
| Marital status (Ref: Married) | |||||
| Not married | −0.31 | 0.20 | −0.26 | 0.21 | |
| Education (Ref: College or above) | |||||
| High school or below | −0.32 | 0.21 | −0.28 | 0.23 | |
| Number of chronic condition | −0.12 | 0.10 | −0.16 | 0.10 | |
| Effects on asthma control at T1 | |||||
| Child’s age | 0.01 | 0.09 | |||
| Child’s gender (Ref: Girl) | |||||
| Boy | 0.11 | 0.17 | |||
| Child’s race/ethnicity (Ref: White/non-Hispanic) | |||||
| Black/non-Hispanic | 0.26 | 0.21 | |||
| Hispanic/non-Hispanic | 0.11 | 0.21 | |||
| Others | 0.14 | 0.32 | |||
| Parental age | −0.06 | 0.09 | |||
| Marital status (Ref: Married) | |||||
| Not married | 0.19 | 0.17 | |||
| Education (Ref: College or above) | |||||
| High school or below | 0.51** | 0.18 | |||
| Number of chronic condition | 0.26** | 0.08 | |||
| Effects on asthma control at T2 | |||||
| Child’s age | 0.04 | 0.09 | |||
| Child’s gender (Ref: Girl) | |||||
| Boy | 0.17 | 0.18 | |||
| Child’s race/ethnicity (Ref: White/non-Hispanic) | |||||
| Black/non-Hispanic | 0.26 | 0.24 | |||
| Hispanic/non-Hispanic | −0.01 | 0.22 | |||
| Others | 0.09 | 0.32 | |||
| Parental age | 0.07 | 0.09 | |||
| Marital status (Ref: Married) | |||||
| Not married | −0.07 | 0.18 | |||
| Education (Ref: College or above) | |||||
| High school or below | −0.05 | 0.19 | |||
| Number of chronic condition | 0.05 | 0.09 | |||
| Effects on asthma control atT3 | |||||
| Child’s age | −0.04 | 0.12 | |||
| Child’s gender (Ref: Girl) | |||||
| Boy | 0.07 | 0.20 | |||
| Child’s race/ethnicity (Ref: White/non-Hispanic) | |||||
| Black/non-Hispanic | 0.56* | 0.24 | |||
| Hispanic/non-Hispanic | 0.23 | 0.25 | |||
| Others | −0.18 | 0.37 | |||
| Parental age | −0.12 | 0.10 | |||
| Marital status (Ref: Married) | |||||
| Not married | 0.21 | 0.20 | |||
| Education (Ref: College or above) | |||||
| High school or below | 0.32 | 0.21 | |||
| Number of chronic condition | 0.26** | 0.10 | |||
| Effects on asthma control at T4 | |||||
| Child’s age | −0.08 | 0.11 | |||
| Child’s gender (Ref: Girl) | |||||
| Boy | −0.04 | 0.20 | |||
| Child’s race/ethnicity (Ref: White/non-Hispanic) | |||||
| Black/non-Hispanic | −0.36 | 0.24 | |||
| Hispanic/non-Hispanic | 0.02 | 0.26 | |||
| Others | −0.33 | 0.37 | |||
| Parental age | −0.10 | 0.11 | |||
| Marital status (Ref: Married) | |||||
| Not married | −0.03 | 0.21 | |||
| Education (Ref: College or above) | |||||
| High school or below | −0.02 | 0.21 | |||
| Number of chronic conditions | 0.07 | 0.09 | |||
Abbreviations: SE, standard error; HRQOL, health-related quality of life.
Higher scores indicate worse HRQOL.
p<0.05;
p<0.01;
p<0.001
Model 2 shows that poor asthma control status was a significant predictor of impaired HRQOL across different time points, with significant β coefficients ranging from 0.18 to 0.31 (p’s<0.001), except for the coefficient of asthma control status at T1 related to HRQOL at T2 (p>0.05). Parents with education of high school or below had children with worse initial HRQOL at baseline of year 1 (β=0.51, p<0.01) than those with a college education or above. For the rate of HRQOL change, since higher scores represent worse HRQOL, negative coefficients on the slopes indicate improving HRQOL. Therefore, boys and Hispanic children reported increasing HRQOL over time compared with girls (β=−0.43, p<0.05) and non-Hispanic White (β=−0.64, p<0.01), respectively.
Model 3 shows that the effects of poor asthma control on HRQOL impairment across 4 time points were all statistically significant (βs ranged from 0.35 to 0.51; p’s<0.001), except for the effects of asthma control at T1 on HRQOL at T2 (β=0.16, p>0.05). Parents with an education of high school or below reported worse initial HRQOL at baseline of year 1 than those with a college education or above (β=0.39, p<0.05). Hispanic children had increasing HRQOL over time compared with non-Hispanic White (β=−0.75, p<0.01).
DISCUSSION
This study examined the associations between asthma control status and asthma-specific HRQOL in children from low-income families using longitudinal data and LGM methodology. Instead of evaluating treatment effects on asthma outcomes, this study quantified the natural changes of HRQOL among children who received usual care during the changes of asthma control. Because asthma control status was reported prior to HRQOL, our results revealed a temporal relationship between the changes of asthma control status and the consequent variations in HRQOL. This study is among the first attempts to investigate the longitudinal relationship of asthma control status with HRQOL in children with significant baseline covariates.
We found an improvement in HRQOL during the study period; however, the magnitude of improvement decreased when asthma control status and participants’ characteristics were taken into consideration. Parental education was related to variations in the initial HRQOL and children’s race/ethnicity was associated with different rates of change in HRQOL. Although the change in HRQOL can be explained by the change of asthma control status and participants’ baseline characteristics, the improvement in HRQOL might also be attributed by regression to the mean in which children tended to report higher HRQOL over time due to their familiarity with the survey (19); or the Hawthorne effect. Our recent publication further reveals that response shift pheromone (i.e., changing the internal standard to interpret the meaning of PROMIS items when study subjects growth within a 1.5-year period) was minimal (33). Thus, we believe that the child’s age is less likely to influence the change of HRQOL during the study period. Nevertheless, our finding is consistent with the previous non-interventional study showing improved HRQOL over the observational period (7).
The significant beta coefficients for the effect of asthma control on HRQOL ranged from 0.18 to 0.51, suggesting that 1 unit change in asthma control was associated with 0.18 to 0.51 unit change in HRQOL on a 1SD metric. Our previous PROMIS study found that MIDs determined by children, caregivers, and pediatricians for the PROMIS Pediatric measures ranged from 0.2 to 0.3 on 1SD metric (34). Therefore, HRQOL changes related to asthma control changes are clinically meaningful.
Our findings have important clinical implications for managing outcomes of asthmatic children. Given that the changes in pediatric HRQOL are significantly driven by asthma control status, it is crucial to design innovative approaches to assess children’s asthma control and provide individualized therapy to maintain asthma in well-controlled status. Implementing effective and efficient monitoring systems in both clinical and community settings are the key to assist asthmatic children, their parents, and clinicians to monitor asthma symptoms, to identify poorly-controlled asthma status, and to adjust medication. Rapid learning approaches, as an example, have been successfully used in people with chronic conditions (e.g., chronic pulmonary diseases (35) and cancer survivors (36)) to assist clinicians to receive decision supports, to provide timely treatment regimens, and to improve long-term HRQOL through greater confidence in self-care. Future studies may apply the rapid learning approach to design asthma control monitoring systems for asthmatic children to improve HRQOL.
We found that parental education was related to variations in the initial HRQOL and children’s race/ethnicity was associated with the change of HRQOL. This is because individuals of different cultures, education, ethnicities possess varied beliefs and perceptions toward managing asthma disease (37–39). Our finding echoes the broader literature showing that cultural background, language proficiency, health literacy, and health beliefs are inter-related and collectively influence healthcare utilization and HRQOL in asthmatic populations (40, 41). Specifically, Hispanics with low health literacy are likely to have insufficient health knowledge about the causes of asthma and inappropriate use of asthma medication, leading to poor disease progression (37). Interestingly, our study found that Hispanic children reported a negative rate of change in HRQOL over time compared with non-Hispanic White children after taking asthma control status into consideration (Model 2: β= −0.64, p<0.01; Model 3: β= −0.75, p<0.01). The negative rate of changes represents the increase of HRQOL during the observational period and implies that appropriate interventions targeted at asthma control status for Hispanic children may lead to more promising results than non-Hispanic White children. In this regard, clinical practice should implement literacy- and culture-tailored approaches to monitor and improve outcomes for children with asthma; for example, using community health workers received specific training to manage individuals who have literacy/education and culture/ethnic-sensitive demand (42, 43).
This study has several limitations. First, asthmatic children were selected from economically disadvantaged families. Therefore, the findings may not be generalizable to the general population. Second, we collected asthma control status through parental reports but HRQOL through the child’s self-reports. As described in the Methods section, a parent-report was used because several items in the ACCI assess medication use and parents often have better knowledge of the types and dosages of asthma medicine than do children. Evidence shows that both parents and children are likely to over-report adherence to medication compared with the use of objective assessments (e.g., electronic canister measures) (44). However, medication use reported by parents and children was not significantly different (44). Because differences in asthma control status between parent proxy-reports and child self-reports are under studied, further research should compare validity of asthma control data collected from proxy-reports and self-reports for increasing the precision of asthma control measures. For HRQOL, evidence suggests that differences in pediatric HRQOL rated by parents and children are smaller in physical domains than in psychosocial domains (45–47). We have previously emphasized the importance of collecting dyadic ratings of pediatric HRQOL for clinical decision-making, especially among children with severe chronic conditions (47). However, it is not feasible to collect a dyadic report of pediatric HRQOL in this asthma study because data collection processes at each time point is very time consuming (approximately 30 to 35 minutes to complete by the parent and child, respectively). We believe that the use of advanced technology (e.g., computerized adaptive tests; CATs) to decrease response burden can facilitate the collection of dyadic data for pediatric HRQOL assessment. Third, we examined the longitudinal associations of asthma control changes with HRQOL changes after controlling for socio-demographic factors. However, the longitudinal associations may be confounded by coping skills and environmental stressors (e.g., air pollution) which were not included in this study. Last, we did not collect information of written action plans or other standards of care from individual participants which may confound the association of asthma control status with HRQOL. Our sample was recruited from Florida Medicaid and the Children’s Health Insurance Program itself, and the children enrolled attend multiple practices across the state of Florida. All participants are able to access primary care physicians or pediatricians (i.e., usual source of care) which may and should offer routine asthma care; therefore, we believe the variation of asthma care in our study sample is small. Additionally, a previous study found that if the action plan is offered, physicians discuss the action plans with parents and children who enroll in public insurance programs more often than those in other insurance programs (48). Unfortunately, we did not collect data of written action plans or standards of care from participants, and the extent to which these treatment factors impact the association of asthma control with HRQOL is warranted in further studies.
In conclusion, while HRQOL changed over time in publicly insured children, these changes were explained by asthma control status and vulnerable socio-demographic factors. It is important to design appropriate interventions to improve asthma control status that could lead to optimal HRQOL for minority children and potentially result in a reduction of health disparities.
Supplementary Material
Acknowledgments
Funding sources:
National Institutes of Health U01 AR052181 and American Lebanese Syrian Associated Charities
Footnotes
CONFLICTS OF INTEREST
No conflicts of interest to all co-authors.
REFERENCES
- 1.Bloom B, Jones LI, Freeman G. Summary health statistics for U.S. children: National health interview survey, 2012. Vital Health Stat 10. 2013;258:1–81. [PubMed] [Google Scholar]
- 2.National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 3.Busse WW, Morgan WJ, Taggart V, Togias A. Asthma outcomes workshop: Overview. J Allergy Clin Immunol. 2012;129:S1–S8. doi: 10.1016/j.jaci.2011.12.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seid M, Limbers CA, Driscoll KA, Opipari-Arrigan LA, Gelhard LR, Varni JW. Reliability, validity, and responsiveness of the pediatric quality of life inventory (PedsQL) generic core scales and asthma symptoms scale in vulnerable children with asthma. J Asthma. 2010;47(2):170–177. doi: 10.3109/02770900903533966. [DOI] [PubMed] [Google Scholar]
- 5.Gandhi PK, Kenzik KM, Thompson LA, DeWalt DA, Revicki DA, Shenkman EA, et al. Exploring factors influencing asthma control and asthma-specific health-related quality of life among children. Respir Res. 2013;14:26,9921-14-26. doi: 10.1186/1465-9921-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eilayyan O, Gogovor A, Mayo N, Ernst P, Ahmed S. Predictors of perceived asthma control among patients managed in primary care clinics. Qual Life Res. 2015;24:55–65. doi: 10.1007/s11136-014-0700-1. [DOI] [PubMed] [Google Scholar]
- 7.Apter AJ, Wan F, Reisine S, Bender B, Rand C, Bogen DK, et al. The association of health literacy with adherence and outcomes in moderate-severe asthma. J Allergy Clin Immunol. 2013;132(2):321–327. doi: 10.1016/j.jaci.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hesselink AE, van der Windt DA, Penninx BW, Wijnhoven HA, Twisk JW, Bouter LM, et al. What predicts change in pulmonary function and quality of life in asthma or COPD? J Asthma. 2006;43(7):513–519. doi: 10.1080/02770900600856954. [DOI] [PubMed] [Google Scholar]
- 9.Mosnaim G, Li H, Martin M, Richardson D, Belice PJ, Avery E, et al. Factors associated with levels of adherence to inhaled corticosteroids in minority adolescents with asthma. Ann Allergy Asthma Immunol. 2014;112(2):116–120. doi: 10.1016/j.anai.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahut B, Trinquart L, Delclaux C. Influence of age on the risk of severe exacerbation and asthma control in childhood. J Asthma. 2011;48(1):65–68. doi: 10.3109/02770903.2010.529225. [DOI] [PubMed] [Google Scholar]
- 11.Koster ES, Raaijmakers JA, Vijverberg SJ, Koenderman L, Postma DS, Koppelman GH, et al. Limited agreement between current and long-term asthma control in children: The PACMAN cohort study. Pediatr Allergy Immunol. 2011;22(8):776–783. doi: 10.1111/j.1399-3038.2011.01188.x. [DOI] [PubMed] [Google Scholar]
- 12.Mansour ME, Kotagal U, Rose B, Ho M, Brewer D, Roy-Chaudhury A, et al. Health-related quality of life in urban elementary schoolchildren. Pediatric. 2003;111:1372–1381. doi: 10.1542/peds.111.6.1372. [DOI] [PubMed] [Google Scholar]
- 13.Patel MR, Brown RW, Clark NM. Perceived parent financial burden and asthma outcomes in low-income, urban children. J Urban Health. 2013;90(2):329–342. doi: 10.1007/s11524-012-9774-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasbinder E, Dahhan N, Wolf B, Zoer J, Blankman E, Bosman D, et al. The association of ethnicity with electronically measured adherence to inhaled corticosteroids in children. Eur J Clin Pharmacol. 2013;69(3):683–690. doi: 10.1007/s00228-012-1380-9. [DOI] [PubMed] [Google Scholar]
- 15.Ortega H, Miller DP, Li H. Characterization of asthma exacerbations in primary care using cluster analysis. J Asthma. 2012;49(2):158–169. doi: 10.3109/02770903.2011.649872. [DOI] [PubMed] [Google Scholar]
- 16.Deger L, Plante C, Goudreau S, Smargiassi A, Perron S, Thivierge RL, et al. Home environmental factors associated with poor asthma control in montreal children: A population-based study. J Asthma. 2010;47(5):513–520. doi: 10.3109/02770901003615778. [DOI] [PubMed] [Google Scholar]
- 17.Yeatts KB, Stucky B, Thissen D, Irwin D, Varni JW, DeWitt EM, et al. Construction of the pediatric asthma impact scale (PAIS) for the patient-reported outcomes measurement information system (PROMIS) J Asthma. 2010;47(3):295–302. doi: 10.3109/02770900903426997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terzano C, Cremonesi G, Girbino G, Ingrassia E, Marsico S, Nicolini G, et al. 1-year prospective real life monitoring of asthma control and quality of life in Italy. Respir Res. 2012;13:112,9921-13-112. doi: 10.1186/1465-9921-13-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howell CR, Thompson L, Gross H, Reeve BB, DeWalt D, Huang I. Responsiveness to change in PROMIS® measures among children with asthma: A report from the PROMIS pediatric asthma study. Value Health. 2016;19:192–201. doi: 10.1016/j.jval.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patino CM, Okelo SO, Rand CS, Riekert KA, Krishnan JA, Thompson K, et al. The asthma control and communication instrument: A clinical tool developed for ethnically diverse populations. J Allergy Clin Immunol. 2008;122(5):936, 943.e6. doi: 10.1016/j.jaci.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh TR, Irwin DE, Meier A, Varni JW, DeWalt DA. The use of focus groups in the development of the PROMIS pediatrics item bank. Qual Life Res. 2008;17(5):725–735. doi: 10.1007/s11136-008-9338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irwin DE, Varni JW, Yeatts K, DeWalt DA. Cognitive interviewing methodology in the development of a pediatric item bank: A patient reported outcomes measurement information system (PROMIS) study. Health Qual Life Outcomes. 2009;7:3. doi: 10.1186/1477-7525-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irwin DE, Stucky B, Langer MM, Thissen D, Dewitt EM, Lai JS, et al. An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Qual Life Res. 2010;19(4):595–607. doi: 10.1007/s11136-010-9619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varni JW, Magnus B, Stucky BD, Liu Y, Quinn H, Thissen D, et al. Psychometric properties of the PROMIS (R) pediatric scales: Precision, stability, and comparison of different scoring and administration options. Qual Life Res. 2014;23(4):1233–1243. doi: 10.1007/s11136-013-0544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeWitt EM, Stucky BD, Thissen D, Irwin DE, Langer M, Varni JW, et al. Construction of the eight-item patient-reported outcomes measurement information system pediatric physical function scales: Built using item response theory. J Clin Epidemiol. 2011;64(7):794–804. doi: 10.1016/j.jclinepi.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai JS, Stucky BD, Thissen D, Varni JW, DeWitt EM, Irwin DE, et al. Development and psychometric properties of the PROMIS(R) pediatric fatigue item banks. Qual Life Res. 2013;22(9):2417–2427. doi: 10.1007/s11136-013-0357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irwin DE, Stucky BD, Thissen D, Dewitt EM, Lai JS, Yeatts K, et al. Sampling plan and patient characteristics of the PROMIS pediatrics large-scale survey. Qual Life Res. 2010;19(4):585–594. doi: 10.1007/s11136-010-9618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curran PJ, Obeidat K, Losardo D. Twelve frequently asked questions about growth curve modeling. J Cogn Dev. 2010;11(2):121–136. doi: 10.1080/15248371003699969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blozis SA, Cho YI. Coding and centering of time in latent curve models in the presence of interindividual time heterogeneity. Structural Equation Modeling. 2008;15(3):413–433. [Google Scholar]
- 30.Aydin B, Leite WL, Algina J. The consequences of ignoring variability in measurement occasions within data collection waves in latent growth models. Multivar behav res. 2014;49(2):149–160. doi: 10.1080/00273171.2014.887901. [DOI] [PubMed] [Google Scholar]
- 31.Yu C. Evaluating cutoff criteria of model fit indices for latent variable models with binary and continuous outcomes. Los Angeles: Diss. University of California; 2002. [Google Scholar]
- 32.Wu W, West SG, Taylor AB. Evaluating model fit for growth curve models: Integration of fit indices from SEM and MLM frameworks. Psychol Methods. 2009;14(3):183–201. doi: 10.1037/a0015858. [DOI] [PubMed] [Google Scholar]
- 33.Gandhi PK, Schwartz CE, Reeve BB, DeWalt DA, Gross HE, Huang IC. An item-level response shift study on the change of health state with the rating of asthma-specific quality of life: A report from the PROMIS(R) pediatric asthma study. Qual Life Res. 2016;25(6):1349–1359. doi: 10.1007/s11136-016-1290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thissen D, Liu Y, Magnus B, Quinn H, Gipson DS, Dampier C, et al. Estimating minimally important difference (MID) in PROMIS pediatric measures using the scale-judgment method. Qual Life Res. 2016;25:13–23. doi: 10.1007/s11136-015-1058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamal AH, Bull J, Stinson C, Blue D, Smith R, Hooper R, et al. Collecting data on quality is feasible in community-based palliative care. J Pain Symptom Manage. 2011;42(5):663–667. doi: 10.1016/j.jpainsymman.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Abernethy AP, Herndon JE, 2nd, Coan A, Staley T, Wheeler JL, Rowe K, et al. Phase 2 pilot study of pathfinders: A psychosocial intervention for cancer patients. Support Care Cancer. 2010;18(7):893–898. doi: 10.1007/s00520-010-0823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koinis-Mitchell D, McQuaid EL, Friedman D, Colon A, Soto J, Rivera DV, et al. Latino caregivers' beliefs about asthma: Causes, symptoms, and practices. J Asthma. 2008;45(3):205–210. doi: 10.1080/02770900801890422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu AC, Smith L, Bokhour B, Hohman KH, Lieu TA. Racial/ethnic variation in parent perceptions of asthma. Ambul Pediatr. 2008;8(2):89–97. doi: 10.1016/j.ambp.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson LD, Jr, Calmes DP, Bazargan M. The impact of literacy enhancement on asthma-related outcomes among underserved children. J Natl Med Assoc. 2008;100(8):892–896. doi: 10.1016/s0027-9684(15)31401-2. [DOI] [PubMed] [Google Scholar]
- 40.Wisnivesky JP, Kattan M, Evans D, Leventhal H, Musumeci-Szabo TJ, McGinn T, et al. Assessing the relationship between language proficiency and asthma morbidity among inner-city asthmatics. Med Care. 2009;47(2):243–249. doi: 10.1097/MLR.0b013e3181847606. [DOI] [PubMed] [Google Scholar]
- 41.Poureslami IM, Rootman I, Balka E, Devarakonda R, Hatch J, Fitzgerald JM. A systematic review of asthma and health literacy: A cultural-ethnic perspective in Canada. Med Gen Med. 2007;9(3):40. [PMC free article] [PubMed] [Google Scholar]
- 42.Fox P, Porter PG, Lob SH, Boer JH, Rocha DA, Adelson JW. Improving asthma-related health outcomes among low-income, multiethnic, school-aged children: Results of a demonstration project that combined continuous quality improvement and community health worker strategies. Pediatrics. 2007;120(4):e902–e911. doi: 10.1542/peds.2006-1805. [DOI] [PubMed] [Google Scholar]
- 43.Li P, Guttmann A. Recent innovations to improve asthma outcomes in vulnerable children. Curr Opin Pediatr. 2009;21(6):783–788. doi: 10.1097/MOP.0b013e328332537d. [DOI] [PubMed] [Google Scholar]
- 44.Bender BG, Bartlett SJ, Rand CS, Turner C, Wamboldt FS, Zhang L. Impact of interview mode on accuracy of child and parent report of adherence with asthma-controller medication. Pediatrics. 2007;120(3):e471–e477. doi: 10.1542/peds.2006-3457. [DOI] [PubMed] [Google Scholar]
- 45.Varni JW, Burwinkle TM, Lane MM. Health-related quality of life measurement in pediatric clinical practice: An appraisal and precept for future research and application. Health Qual Life Outcomes. 2005;3:34. doi: 10.1186/1477-7525-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Theunissen NC, Vogels TG, Koopman HM, Verrips GH, Zwinderman KA, Verloove-Vanhorick SP, et al. The proxy problem: Child report versus parent report in health-related quality of life research. Qual Life Res. 1998;7(5):387–397. doi: 10.1023/a:1008801802877. [DOI] [PubMed] [Google Scholar]
- 47.Huang IC, Shenkman EA, Leite W, Knapp CA, Thompson LA, Revicki DA. Agreement was not found in adolescents' quality of life rated by parents and adolescents. J Clin Epidemiol. 2009;62(3):337–346. doi: 10.1016/j.jclinepi.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gillette C, Carpenter DM, Ayala GX, Williams DM, Davis S, Tudor G, et al. How often do providers discuss asthma action plans with children? Analysis of transcripts of medical visits. Clin Pediatr (Phila) 2013;52(12):1161–1167. doi: 10.1177/0009922813506256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.