Abstract
Cannabinoid hyperemesis syndrome (CHS) is a syndrome of cyclic vomiting associated with cannabis use. Our objective is to summarize the available evidence on CHS diagnosis, pathophysiology, and treatment. We performed a systematic review using MEDLINE, Ovid MEDLINE, Embase, Web of Science, and the Cochrane Library from January 2000 through September 24, 2015. Articles eligible for inclusion were evaluated using the Grading and Recommendations Assessment, Development, and Evaluation (GRADE) criteria. Data were abstracted from the articles and case reports and were combined in a cumulative synthesis. The frequency of identified diagnostic characteristics was calculated from the cumulative synthesis and evidence for pathophysiologic hypothesis as well as treatment options were evaluated using the GRADE criteria. The systematic search returned 2178 articles. After duplicates were removed, 1253 abstracts were reviewed and 183 were included. Fourteen diagnostic characteristics were identified, and the frequency of major characteristics was as follows: history of regular cannabis for any duration of time (100%), cyclic nausea and vomiting (100%), resolution of symptoms after stopping cannabis (96.8%), compulsive hot baths with symptom relief (92.3%), male predominance (72.9%), abdominal pain (85.1%), and at least weekly cannabis use (97.4%). The pathophysiology of CHS remains unclear with a dearth of research dedicated to investigating its underlying mechanism. Supportive care with intravenous fluids, dopamine antagonists, topical capsaicin cream, and avoidance of narcotic medications has shown some benefit in the acute setting. Cannabis cessation appears to be the best treatment. CHS is a cyclic vomiting syndrome, preceded by daily to weekly cannabis use, usually accompanied by symptom improvement with hot bathing, and resolution with cessation of cannabis. The pathophysiology underlying CHS is unclear. Cannabis cessation appears to be the best treatment
Keywords: Cannabinoid hyperemesis syndrome, Cyclic vomiting syndrome, Cannabis, Marijuana
Introduction
The United Nations reported that 277 million people worldwide have used cannabis—roughly 4.9% of the total population [1]. In 2014, 22.2 million Americans aged 12 or older (8.4% of the population) were current cannabis users [2]. Cannabis policy in the USA is undergoing a transformation; following the 2016 elections, 26 states and Washington D.C. currently have laws legalizing cannabis in some form, and three other states are soon to adopt legislation. Seven states and Washington D.C. now allow some form of recreational use [3] . Cannabis has been approved for treatment of a range of medical conditions including nausea and vomiting due to chemotherapy, appetite stimulation in HIV/acquired immunodeficiency syndrome (AIDS), chronic pain, spasticity due to multiple sclerosis, depression, and many more [4] depending upon state laws. Adverse effects from cannabis, from both recreational and medical use, have been reported for decades, though reports have increased with increased cannabis access [5]. Among the more common adverse effects is the cannabinoid hyperemesis syndrome (CHS), first reported in 2004 [6]. CHS is a syndrome of cyclic vomiting in the setting of chronic, high-dose cannabis use that is frequently associated with compulsive hot baths/showers, used in attempt to control symptoms.
Patients with CHS present frequently to various health care settings with intractable nausea and vomiting. These patients often undergo expensive medical testing, may require hospital admission for symptom management, and often experience significant delays in diagnosis [7]. CHS is under-recognized due to a combination of factors including the paradoxical use for treatment of nausea and vomiting, the stigma associated with cannabis use, and the illegal status of cannabis in many regions leading to under-reporting of use. The frequency of emergency department visits and high rates of hospital admission for CHS exemplify the difficulty in symptom management. The lack of knowledge and treatment recommendations regarding CHS compounds this issue. Subsequently, CHS is a costly illness to manage. In an observational study of CHS patients followed over 2 years, the median charge for ED visits and hospital admissions was $95,023 (IQR = $62,420–$268,110) [7].
Cannabis has presumably been used by humans for thousands of years, [8] yet CHS is only now being recognized. CHS has been reported numerous times in [9–11] large case series, small case series, and more than 80 individual case reports [6, 8, 11–82]. There are many suggested pathophysiologic mechanisms of CHS, though evidence for each is minimal (see Table 3). It is not clear why cannabis appears to suppress emesis under certain circumstances and induces it in others. It is also unknown why only some individuals develop CHS when the use of cannabis is so widespread.
Table 3.
Proposed pathophysiologic mechanisms for CHS
| Mechanism | GRADE rating |
|---|---|
| The emetogenic and anti-emetic effects of Δ9-THC and its analogs are mediated through CB-1 receptors (CB1r) and thus underlie the syndrome of CHS [106, 135] | Very low |
| Cannabinoids may bind to CB-1 receptors in the gastrointestinal tract and decrease GI motility and gastric emptying, which may override brainstem-mediated antiemetic effects and precipitate hyperemesis [9, 92, 95, 132] | Very low |
| Chronic cannabis use may lead to paradoxical and plastic changes in expression and downstream effects of cannabinoid receptors [133] | Very low |
| Chronic cannabis use leads to desensitization and downregulation of CB1 receptors that ordinarily have peripheral antiemetic effects, causing rebound vomiting and spasmodic pain that abates with abstinence and corresponding recovery of CB-1 receptor activity [98, 136, 185] | Very low |
| In chronic cannabis users, cannabinoid metabolites may accumulate in the brain and fatty tissues inducing a toxic effect [90, 94] | Very low |
| CHS may be caused by a non-THC, cannabinoid-like structure within Cannabis sativa, such as cannabidiol [96, 186] | Very low |
| Patients susceptible to developing CHS may have genetic variation in their metabolic enzymes resulting in toxic levels of cannabinoid metabolites [131] | Very low |
| Δ9-THC may act as a partial agonist on CB1 receptors and thus relatively antagonize the effects of full endogenous agonists on these receptors, thus precipitating sudden withdrawal and hyperemesis in sensitive patients [97, 105] | Very low |
| THC causes dilation of splanchnic vasculature, resulting in CHS. Hot bathing leads to peripheral venodilation and shunts blood away from the splanchnic bed, resulting in symptom improvement [102, 137] | Very low |
Given the relatively new recognition of CHS as a clinical syndrome, diagnosis and treatment practices vary widely. Many authors have proposed diagnostic criteria (see Table 7), but it is unclear if these criteria consistently capture patients with the diagnosis. Additionally, criteria vary significantly, which may contribute to diagnostic uncertainty among providers. Therefore, our objectives are to summarize and evaluate the available scientific evidence on CHS diagnosis, pathophysiology, and treatment utilizing systematic literature review methodology.
Table 7.
Proposed diagnostic frameworks for cannabinoid hyperemesis syndrome
Materials and Methods
Search Strategy
The systematic review was performed utilizing the PRISMA guidelines [83]. Relevant publications were identified by searching the following databases: MEDLINE (via PubMed, Ovid MEDLINE (1946-current), Ovid MEDLINE In-Process & Other Non-Indexed Citations, and Ovid MEDLINE Daily), Embase (via Embase.com), Web of Science, and the Cochrane Library (via Wiley Online Library). Publication date was limited from January 2000 through articles indexed in the databases as of September 24, 2015. English language limits were applied, and human and animal studies were included. Multiple subject headings (including Medical Subject Headings (MeSH) terms in MEDLINE and Emtree terms in Embase) and text words were used to identify each concept (cannabinoids and hyperemesis) and develop the search strategies (see Appendix for a list of all database search strategies). Additional records were identified through the following sources: ClinicalTrials.gov, ProQuest Dissertations & Theses (which includes COS Conference Papers Index; ProQuest Dissertations & Theses: UK & Ireland; ProQuest Dissertations & Theses A&I), and conference proceedings and meeting abstracts available online since 2000 from North American Congress of Clinical Toxicology, American Academy of Clinical Toxicology, American College of Medical Toxicology, and Society for Academic Emergency Medicine. The references of included studies were cross-referenced, and additional studies not identified by the initial search strategy were added. The references of included studies were cross-referenced, and additional studies not identified by the initial search strategy were added.
Study Selection
Two reviewers (CS and AM) independently reviewed all titles generated by the search to identify potentially relevant articles. Duplicates were removed. Articles that were clearly not relevant based on title and abstract were excluded. The full text of all relevant articles were obtained and reviewed. The articles were then segregated into diagnosis, pathophysiology, or treatment categories. The articles were eligible for inclusion under the category of “diagnosis” if they (1) directly studied the criteria used to diagnose CHS from a population or case-based perspective or (2) presented a specific set of diagnostic criteria to define CHS as part of a larger research question or (3) presented a case or cases of CHS and explained the diagnostic criteria used to identify these patients. Articles eligible for inclusion under the category of “pathophysiology” were those that (1) used animal, human, or in vitro models to explore the pathophysiology of CHS or cyclic vomiting or (2) animal or human studies that investigated the role of the endocannabinoid system in vomiting. Articles eligible for inclusion under the category of “treatment” were those that (1) presented results of treatment outcomes using specific interventions, both behavioral and pharmacologic, to manage CHS or (2) used animal studies to evaluate treatments for CHS or cyclic vomiting. When the two reviewers disagreed on article eligibility, a consensus was reached through discussion.
Data Collection
The reviewers assigned each included article to nonexclusive groups of diagnosis, pathophysiology, or treatment. Relevant study characteristics were abstracted including study design and primary findings.
The following data, when available, were extracted from each case report or case series: sample size, gender, age at presentation, age of first cannabis use, age at CHS symptom onset, duration and frequency of cannabis use, presence of cyclic vomiting, presence of abdominal pain, weight loss, presence of compulsive hot baths/showers, response to trial of abstinence, time to improvement of symptoms with abstinence, and amount of weight loss, if present.
Reviewers identified hypotheses to explain the pathophysiology of CHS. For each mechanism proposed, reviewers explored the supportive evidence and the study design.
Similarly, reviewers identified treatment modalities proposed for CHS. For each treatment modality, reviewers explored the evidence and the study design.
Assessment of Study Quality
The strength and quality of each study were evaluated using the GRADE working group metrics [83]. Grading and Recommendations Assessment, Development, and Evaluation (GRADE) defines high-quality studies as randomized trials or double-upgraded observational studies. Moderate-quality studies are defined as downgraded randomized trials or upgraded observational studies. Low-quality studies are defined as double-downgraded randomized trials or observational studies. Very low-quality studies are defined as triple-downgraded randomized trials or downgraded observational studies or case series/case reports.
The quality of evidence for each main outcome of this systematic review was evaluated using the GRADE working group metrics [83]. High-quality outcomes are defined as those for which further research is very unlikely to change our confidence in the estimate of effect. Moderate-quality outcomes are defined as those for which further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low-quality outcomes are those for which further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low-quality outcomes are those for which any estimate of effect is very uncertain.
Data Analysis
Data were extracted from individual case reports and summarized as a single large cohort (individual case report synthesis). Next, after exclusion of duplicate cases, the individual case reports were combined with data from the case series and summarized as a single larger cohort (cumulative synthesis). Median values (plus interquartile range) were calculated for age at diagnosis, cannabis use age of onset, and age at symptom onset. The duration of cannabis use prior to symptom onset was categorized as either less than or equal to 1, 2 to 5, 6 to 10, and greater than 11 years. The amount of cannabis consumed was categorized as either less than daily, daily, weekly, less than weekly, or dose not specified. Cannabis use reported as “heavy” was interpreted to mean “daily.” Cannabis use reported as “frequent” was interpreted to mean “weekly.” The presence of cyclic vomiting, abdominal pain, relief with compulsive hot baths/showers, relief with abstinence, and ongoing symptoms with ongoing cannabis use was determined from each article and summarized as percentages of the total number of patients where the specific symptom category was documented.
The frequency of each individual proposed diagnostic characteristic was calculated by dividing the total number of CHS patients manifesting that characteristic (from the cumulative synthesis) by the total number of patients for which that diagnostic characteristic was reported.
Results
The systematic search returned 2154 articles. Twenty-four additional articles were identified through the bibliographies of articles returned in the primary search. After removal of duplicates, 1253 abstracts were independently screened by reviewers, of which 170 satisfied criteria for inclusion. The low quality of the articles precluded meta-analysis.
There was a significant overlap in the sorting of articles into the categories of diagnosis, pathophysiology, and treatment. Thus, some articles were classified in more than one category. We identified 116 articles related to diagnosis, 61 to pathophysiology, and 13 to treatment. There were 24 animal studies related to pathophysiology [84–107]. No animal studies were identified that addressed diagnosis or treatment. There were 88 case reports and 8 case series of four or more patients. Of the 88 case reports, 8 were excluded secondary to inability to access the original text, the report being published in a non-English language or if the case did not ultimately yield a clinical diagnosis of CHS as reported by the authors [108–118]. Our search did not yield any randomized trials assessing the diagnosis, pathophysiology, or treatment of CHS. Given that evidence statements are based primarily on case reports and case series, the vast majority of evidence is considered limited. Therefore, limited evidence ratings should not be misconstrued as negative clinical findings but, rather, that there are no higher-level studies to qualify the statement as higher-level evidence. The primary findings for each category are summarized in the following sections.
Diagnosis of CHS
Seven unique diagnostic frameworks were identified [6, 9, 30, 51, 119–121]. There was a significant overlap among major diagnostic characteristics between the authors. The major diagnostic characteristics and frequency of each are as follows: history of regular cannabis use for over 1 year (74.8%), severe nausea and vomiting (100%), vomiting that recurs in a cyclic pattern over months (100%), resolution of symptoms after stopping cannabis (96.8%), compulsive hot baths/showers with symptom relief (92.3%), male predominance (72.9%), abdominal pain (85.1%), at least weekly cannabis use (97.4%), history of daily cannabis use (76.6%), and age less than 50 at time of evaluation (100%) (see Table 2). The following symptoms were inconsistently captured, and thus, their frequency could not be determined: reliable return of symptoms within weeks of resuming use, normal bowel habits, negative medical workup, and weight loss >5 kg (see table 2).
Table 2.
Major commonly cited diagnostic characteristics
| Diagnostic characteristic | Frequency (%) (total n with criterion reported) | GRADE rating |
|---|---|---|
| History of regular cannabis use for years (over 1 year) [6, 16, 30, 51, 119, 120] | 74.8 (179) | Low |
| Severe nausea and vomiting [16, 119, 120] | 100 (211) | Low |
| Vomiting that recurs in a cyclic pattern over months [6, 30, 119, 121] | 100 (211) | Low |
| Resolution of symptoms after stopping cannabis [6, 16, 30, 119–121] | 96.8 (64) | Low |
| Reliable return of symptoms within weeks of resuming use [30] | a | Low |
| Compulsive hot baths with symptom relief [6, 16, 30, 51, 119–121] | 92.3 (170) | Low |
| Male predominance [121] | 72.9 (227) | Low |
| Abdominal pain [30, 119–121] | 85.1 (202) | Low |
| At least weekly cannabis use [16, 120] | 97.4 (197) | Low |
| History of daily cannabis use [121] | 76.6 (197) | Low |
| Age less than 50 at onset of illness [16, 120, 121] | 100 (227) | Low |
| Normal bowel habits [16] | a | Very low |
| Negative medical workup [16, 30, 119, 121] | a | Very low |
| Weight loss >5 kg [16, 120] | a | Very low |
aCriterion inconsistently documented in case reports, thus limiting frequency analysis
Cumulative Synthesis
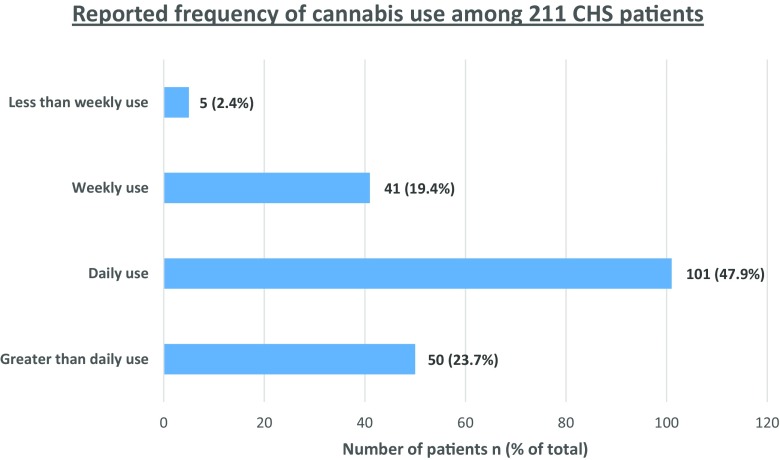
The cumulative synthesis represents data from the individual case report synthesis as well as the case series, excluding duplicate cases (Table 1) [6, 8, 11–82]. In the cumulative synthesis, 211 individual patients are represented. Secondary to heterogeneity across studies and incomplete reporting, not all diagnostic characteristics were reported for each patient. There were 154 males (72.9%) and 57 females (27.1%). The median age at diagnosis was 28 years (IQR = 22–34) (n = 111). The median age of onset of cannabis use was 16 (IQR = 14–18) (n = 84). The median age at symptom onset was 24 (IQR = 19–29) (n = 101). In all cases, the use of cannabis predated the onset of CHS symptoms. The duration of cannabis use prior to onset of symptoms for the 179 patients in which it was captured was as follows: 45 patients (25.1%) used it for ≤1 year, 65 patients (36.3%) used it for 2–5 years, 30 patients (16.8%) used it for 6–10 years, and 39 patients (21.8%) it used for ≥11 years. The frequency of cannabis use was reported in 211 patients as follows: 50 (23.7%) used greater than daily, 101 (47.9%) used daily, 41 (19.4%) used weekly, 5 (2.4%) used less than once per week, and in 14 (6.6%), the quantity was not specified (see Fig. 1). Cyclic nausea and vomiting was reported in 100% of patients. Abdominal pain was reported in 85.1% of cases. Relief of symptoms with compulsive hot baths/showers was reported in 92.3% of cases (n = 170).
Table 1.
Overview of case series, individual case report synthesis, and cumulative synthesis
| Article | Sample (n) | Male n (%) | Female n (%) | Age (years) at diagnosis median (IQR) | Cannabis use age (years) of onset median (IQR) | Age (years) at symptom onset median (IQR) | Duration of cannabis use (years) prior to symptom onset median (IQR) | Cannabis use n (frequency of use) | Cyclic vomiting n (%) | Abdominal pain n (%) | Relief with hot bathing % (n/total queried) | Relief with abstinence or reduction % (n/total queried) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Simonetto* [12] | 98 | 66(67) | 32(33) | Mean = 32.3 (±9.9) | na | Mean = 25.3 ±8.9 (n = 98) | 32 (≤1), 42 (2–5), 11 (6–10), 13 (≥11) (n = 98) | 58 (daily), 35 (weekly), 5 (<weekly) (n = 98) | 98 (100) | 84 (86) | 91 (52/57) | 100 (6/6) |
| Allen [6] | 9 | 6(66) | 3(33) | 29 (21–37) (n = 9) | 17 (15.5–18.5) (n = 9) | 22 (15.8–28.3) (n = 9) | 0 (≤1), 5 (2–5), 1 (6–10), 3 (≥11) (n = 9) | 9 (>daily) (n = 9) | 9 (100) | na | 88 (8/9) | 100 (7/7) |
| Soriano-Co [12] | 8 | 5(63) | 3(37) | 34 (31.9–36.2) (n = 8) | 13 (11.3–14.8) (n = 8) | 30.5 (32.4–32.4) (n = 8) | 0 (≤1), 0 (2–5), 1 (6–10), 7 (≥11) (n = 8) | 8 (>daily) (n = 8) | 8 (100) | 8 (100) | 100 (8/8) | 80 (4/5) |
| Wallace b [10] | 31 | 24(78) | 7(22) | 34 (27.8–40.3) (n = 32) | 16 (14–18) (n = 27) | 24 (18.3–29.8) (n = 27) | 2 (≤1), 9 (2–5), 3 (6–10), 12 (≥11) (n = 26) | 28(>daily), 3 (quantity not specified) (n = 31) | 31 (100) | na | 96 (29/30) | 96 (24/25) |
| Sofka [13] | 4 | 3(75) | 1(25) | 25.5 (22.3–28.6) (n = 4) | 16 (14.5–17.5) (n = 4) | 14 (11.5–16.5) (n = 4) | 0 (≤1), 0 (2–5), 3 (6–10), 1 (≥11) (n = 4) | 4 (>daily) (n = −4) | 4 (100) | 4 (100) | 100 (4/4) | 100 (1/1) |
| Patterson [10] | 4 | 4(100) | 0(0) | 29 (24.6–33.4) (n = 4) | 15.5 (14.9–16.1) (n = 4) | 27.5 (18.5–36.5) (n = 4) | 1 (≤1), 1 (2–5), 0 (6–10), 2 (≥11) (n = 4) | 1 (>daily) 3 (daily) (n = 4) | 4 (100) | 3 (75) | 100 (4/4) | 100 (4/4) |
| Nicolson [15] | 4 | 2(50) | 2(50) | 23 (21.3–24.6) (n = 4) | 16.5 (15.6–17.4) (n = 4) | 20 (17.5–22.5) (n = 4) | 1 (≤1), 1 (2–5), 2 (6–10), 0 (≥11) (n = 4) | 1 (>daily) 3 (daily) (n = 4) | 4 (100) | 3 (75) | 100 (4/4) | 100 (2/2) |
| Masri [14] | 4 | 4(100) | 0(0) | 25 (22.6–27.4) (n = 4) | 16 (14–18) (n = 2) | 22 (19.4–24.6) (n = 4) | 0 (≤1), 0 (2–5), 1 (6–10), 0 (≥11) (n = 1) | 1 (>daily), 1 (daily), 0 (weekly), 2 (quantity not specified) (n = 4) | 4 (100) | 4 (100) | 100 (4/4) | 100 (1/1) |
| Individual case report synthesis | 80 | 64(80) | 16(20) | 28.5 (22.6–34.4) (n = 78) | 16 (12–18) (n = 53) | 24 (19.5–28.5) (n = 68) | 11 (≤1), 16 (2–5), 11 (6–10), 13 (≥11) (n = 51) | 26 (>daily), 36 (daily), 6 (weekly), 12 (quantity not specified) (n = 80) | 80 (100) | 66 (82) | 91 (73/80) | 97 (37/38) |
| Cumulative synthesis | 211 | 154 (72.9) | 57 (27.1) | 28 (12) (n = 111) | 16 (4) (n = 84) | 24 (10) (n = 101) | 45 (≤1) 65 (2–5) 30 (6–10) 39 (≥11) (n = 179) | 50 (>daily), 101 (daily), 41 (weekly), 5 (< weekly), 14 (quantity not specified) (n = 211) | 100 (211) | 172 (85.1) (n = 202) | 92.3 (157/170) | 96.8 (62/64) |
aRaw data not available, statistics represented as reported by study authors
bNot included in cumulative synthesis as raw data not available
Fig. 1.
Reported frequency of cannabis use from cumulative synthesis
The cumulative synthesis identified two potentially novel diagnostic characteristics for CHS. The median age of onset of cannabis use was 16 (IQR = 14–18) (age of onset reported in 84/211 cases), suggesting that many patients who develop CHS begin their cannabis use early in life. Additionally, the median age of symptom onset was found to be 24 (IQR = 19–29) (age of symptom onset reported in 101/211 cases). Underreporting of this clinical characteristic limits its generalizability and should be considered as supporting the diagnosis if present, although should not preclude the diagnosis.
The synthesis also highlights the difficulty of diagnosis, as the mean delay of symptom onset to diagnosis of CHS was 4.1 years (SD = 4.6).
Our results suggest that the following individual diagnostic characteristics have the highest sensitivity for identifying patients with CHS: at least weekly cannabis use for greater than 1 year, severe nausea and vomiting that recurs in a cyclic pattern over months and that is usually accompanied by abdominal pain, resolution of symptoms after stopping cannabis, and compulsive hot baths/showers with symptom relief (Table 2).
Pathophysiology of CHS
Many hypotheses have been proposed to explain the pathophysiology of CHS (Table 3); however, the GRADE quality of evidence to support any of the proposed mechanisms was very low. Some data suggest that CHS is caused by dysregulation of the endocannabinoid system, a group of endogenous cannabinoid receptors (CB-1 and CB-2) located in the brain, gastrointestinal tract, peripheral nervous system, and immune system of mammals [122]. The endocannabinoid system is thought to play a role in gastrointestinal motility [123, 124] appetite [125], nausea/vomiting [126], inflammation [127], mood [128], sleep [129], pain [130], and more. There was very limited evidence that the emetogenic and anti-emetic effects of Δ9-tetrahydrocannabinol (THC) and its analogs are mediated through CB-1 receptors (CB1r) and thus underlie the syndrome of CHS, though this mechanism is the most parsimonious and is supported by both animal and in vitro studies [88, 106]. We found very limited evidence to support the theory that a genetic variation in metabolic enzymes accounts for the appearance of CHS symptomatology; however, this theory is attractive in that it may explain why some, but not all, chronic cannabis users develop CHS [131]. There is also some evidence to suggest that cannabinoids interact directly with CB-1 receptors in the gastrointestinal tract and alter gastrointestinal motility [16, 92, 95, 132–134]. Animal studies lend some credibility to this theory; however, results are not consistently reproducible in human studies. Additionally, many inferential hypotheses were identified that attribute different aspects of CHS to a myriad of dysregulatory issues at CB-1 receptors throughout the body (brain, gastrointestinal tract, and vasculature) [97, 98, 102, 105, 133, 135–137]. The experimental evidence behind these inferences illustrates the complexity of the pathophysiology of CHS and raises many additional questions. Other hypotheses were identified, but the data to support them were so limited as to not warrant discussion in this review [57, 84–87, 89, 91, 99–105, 107, 122, 126, 138–165].
The pathophysiology of CHS is unclear secondary to a dearth of research dedicated to explicitly investigating its underlying mechanism. Nevertheless, some limited evidence suggests a dynamic interplay between cannabinoid metabolism and complex pharmacodynamics at the CB-1 receptor. Limited evidence also suggests that an individual’s genetics, as well as variability in the cannabinoid components of individual plants [131], may play a role in the manifestation of CHS.
Treatment of CHS
The only definitive treatment identified for CHS was abstinence (low GRADE quality of evidence). Several studies demonstrated this effect. Wallace et al. reported that among 25 patients with CHS who abstained, 24 had complete symptom resolution [10]. Similarly, Allen et al., Simonetto et al., Patterson et al., and Soriano-Co et al. reported symptom resolution in seven out of seven [6], six out of six [9], four out of four [11], and four out of five [12] patients, respectively. The cumulative synthesis (see Table 1) demonstrated that among 64 patients with documented cannabis cessation, 62 (96.8%) had complete resolution of symptoms. The two patients who reported no resolution of symptoms did not have urine testing performed to confirm abstinence [8, 56]. Among 21 patients who did not abstain, all had ongoing symptoms.
There has been limited research into the supportive and symptomatic care of CHS patients. Secondary to intractable vomiting and high-temperature baths/showers, patients may present with moderate to severe dehydration and acute renal failure requiring aggressive fluid resuscitation [20, 30, 38, 166]. In regard to symptomatic care, there is very limited evidence to support the use of dopamine antagonists [40, 167]. For example, Hickey et al. reported complete resolution of emesis 1 h after administration of 5 mg haloperidol to a CHS patient with intractable vomiting [40]. Δ9-THC has been shown to increase dopamine synthesis, turnover, efflux, and dopamine cell firing [103], which may explain clinical improvement with haloperidol treatment. There is also some limited evidence to support the use of capsaicin cream to treat the symptoms of CHS. In a case series of five patients, LaPoint et al. reported complete resolution of nausea and emesis in all patients after application of topical capsaicin cream to the abdomen [168]. Similar positive responses are documented elsewhere [169]. The authors suggest that capsaicin may exert its effects via the TRVP-1 receptor, capsaicin’s only known receptor, which is known to interact with the endocannabinoid system [169]. Some authors recommend the avoidance of narcotic pain medication in the treatment of CHS as opioid analgesic use is associated with bowel dysfunction and could theoretically worsen CHS symptoms and additionally create opioid dependence [170] (see Table 5 for a summary of treatments and associated data to support each modality).
Table 5.
Proposed treatments for CHS
| Proposed treatment | Supportive evidence | Author | Study design | GRADE rating |
|---|---|---|---|---|
| Cessation of cannabis | Cessation of cannabis led to resolution of symptoms in 30 of 31 patients | Wallace [10] | Case series | Very low |
| Among 10 CHS patients with follow-up, 30% did not abstain and continued to have symptoms and 60% stopped using and had complete resolution of symptoms. One patient experienced no symptomatic improvement with self-reported abstinence. | Simonetto [16] | Case series | Very low | |
| Among 10 patients, 7 of 10 abstained and had resolution of symptoms. Three patients did not and illness continued. Three patients rechallenged themselves after a period of abstinence, and all suffered a return to illness | Allen [6] | Case series | Very low | |
| Application of capsaicin cream to the abdomen | Five CHS patients experienced near complete resolution of symptoms after application of topical capsaicin cream to the abdomen | LaPoint [168] | Case series | Very low |
| Case report of dramatic relief of symptoms in CHS patient with topical application of capsaicin | Biary [169] | Case report | Very low | |
| Dopamine antagonists | Δ9-THC increases dopamine synthesis, turnover, and efflux and dopamine cell firing. Δ9-THC withdrawal, induced by abrupt discontinuation of chronic Δ9-THC treatment or administration of rimonabant, results in decreased dopamine efflux and neurotransmission | Schulze [103] | Experimental model | Very low |
| CHS patient received 5 mg haloperidol and experienced complete resolution of symptoms within 1 h | Hickey [40] | Case report | Very low | |
| Avoidance of opiate-based medications | Opioid analgesic use is associated with bowel dysfunction, and GI side effects have been reported in up to 47% of opioid-treated patients | Argoff [188] | Review | Very low |
| Opiates should be used with caution as they have the potential to cause emesis | Galli [170] | Case report | Very low |
Cannabis cessation appears to be the best treatment. Supportive care in the form of intravenous hydration and anti-emetics may be necessary secondary to profound dehydration and acute renal failure. Very limited evidence suggests that dopamine antagonist medications and the application of capsaicin cream to the abdomen may be helpful strategies to manage acute symptoms. Though there is not any direct evidence, avoidance of narcotic pain medication may be useful due to the possibility of worsening symptoms and creating dependence.
Limitations
This study is limited by the heterogeneity of the case series and case reports and the lack of controlled studies examining this syndrome. While case reports and case series demonstrate a consistent syndrome characterized by heavy cannabis use and relief of symptoms with hot showers, there is inherent publication bias; clinicians are more likely to report cases consistent with the initially reported index case. The internal validity of our findings is limited by the possibility of missing articles from our search strategy. We limited the search to English-language articles, so any relevant articles published in foreign languages were not included, and articles not indexed in MEDLINE, Embase, Web of Science, or the Cochrane Library would be missed. Second, due to lack of diagnostic criteria, there was significant heterogeneity in the reporting of patient characteristics for the case series and case reports, resulting in limited data for several of the diagnostic characteristics analyzed. Therefore, the presented diagnostic frequency is reflective only of reported criteria and low frequency may simply be a reflection of failure to report the item. Therefore, the proposed criteria need validation in a prospective cohort. Third, reporting of cannabis use patterns was subjective with variable metrics (cones, bongs, joints, cigarettes, grams/day etc.) resulting in possible lack of accuracy for this statistic. Fourth, there was limited reporting of effective treatment modalities for CHS, and publication bias favors reporting of successful treatments rather than ineffective therapies. For instance, one Colorado cannabis prescriber anecdotally reports two cases in which patients developed symptoms consistent with CHS after the patients switched to a different cannabis product. Their symptoms subsequently resolved when they switched back to their original brand. This information is not published but raises questions regarding the role of an unidentified molecule, whether cannabinoid or non-cannabinoid (e.g., a pesticide) may precipitate of the syndrome. In practice, clinicians may have evolved treatment algorithms for managing CHS patients, but our search did not identify any studies reporting this.
Discussion
There is a large body of literature describing the diagnosis, pathophysiology, and treatment of CHS. However, most of the evidence is considered low quality because it is in the form of case reports and case series. When these reports are combined, a larger and more robust set of diagnostic characteristics, evaluation of pathophysiology, and determination of effective treatments can be generated. Based on our knowledge of 211 unique CHS patients, the following characteristics are associated with the syndrome: (1) severe cyclic vomiting that is usually accompanied with abdominal pain, (2) symptom onset preceded by at least weekly cannabis use, (3) temporary relief of symptoms with compulsive hot baths/showers, (4) resolution of symptoms with cessation of cannabis use, (5) onset of cannabis use in the teenage years, and (6) symptom onset in the third decade of life. While not all these criteria must be met to make the diagnosis, of 133 cases for which raw data were available, 85 met at least four criteria (75.2%) and 104 (92%) met at least three criteria. Secondary to incomplete and inconsistent reporting of the proposed diagnostic characteristics in the case report literature, the aforementioned data may be an underestimate of the actual occurrence of these symptoms in CHS patients.
CHS shares many clinical similarities with cyclic vomiting syndrome (CVS), a functional gastrointestinal disorder. A diagnostic dilemma arises when CVS patients concurrently use cannabis, as it can be difficult to discern if the true underlying disorder is CHS or CVS that is being symptomatically managed with cannabis. Several studies were identified in our search that attempted to isolate and diagnose CHS patients among cohorts of CVS patients and deserve special mention [171–173]. The Rome criteria were developed by an international committee of experts in order to delineate and standardize the diagnosis of functional gastrointestinal disorders [174]. The Rome III diagnostic criteria for CVS are as follows: stereotypical episodes of acute onset of vomiting lasting less than 1 week, three or more discrete episodes in the prior year, and absence of nausea and vomiting between episodes in the absence of organic disease. Kim et al. demonstrated a near doubling of the prevalence of CVS following legalization of cannabis in Colorado (prevalence ratio 1.92, 95% confidence interval [CI] = 1.33 to 2.79), and these patients were more likely to have cannabis use documented (OR = 3.59, 95% CI = 1.44–9.00) [5]. Additionally, five observational studies were identified which reported a significant prevalence of cannabis use and compulsive hot baths/showers among CVS patients (see Table 4) [175–179]. For example, Venkatesan et al. found that among 437 CVS patients, 81% were cannabis users and 67% reported using hot showers for symptom relief [179]. Bathing behavior was associated with cannabis use (OR 2.54, 95% CI = 1.2–4.3, P = 0.0006). Additionally, Fajardo et al. found that among 48 CVS patients, 22% of cases and 12% of controls were active or previous cannabis users (OR = 2.1, 95% CI = 0.7–6.1) [175]. Cannabis use, when present, preceded symptom onset in CVS patients. These studies suggest that there is a cohort of CHS patients who may be misdiagnosed with CVS. In situations where the diagnosis is in question, cannabis use that precedes the onset of the vomiting syndrome as well as compulsive hot baths/showers should alert the clinician to consider recommending a trial of cannabis abstinence.
Table 4.
Cyclic vomiting syndrome and cannabinoid hyperemesis syndrome diagnostic dilemmas
| Author | Study design | Primary findings | GRADE rating |
|---|---|---|---|
| Kim [5] | Observational, retrospective | Following legalization of cannabis in Colorado, the prevalence of cyclic vomiting nearly doubled (prevalence ratio 1.92, 95% confidence interval [CI] = 1.33 to 2.79) and patients were more likely to have cannabis use documented (OR = 3.59, 95% CI = 1.44–9.00) | Low |
| Fajardo [175] | Observational, retrospective, case-control | Among 48 CVS patients, 22% of cases and 12% of controls were active or previous cannabis users (OR = 2.1, 95% CI = 0.7–6.1). Cannabis use, when present, preceded symptom onset in CVS patients. | Very low |
| Hejazi [187] | Observational, case-control | Among 132 CVS patients, 53% of patients deemed “non-responders” to standard therapy were chronic cannabis users | Very low |
| Namin [177] | Observational, cross-sectional survey | Of 31 patients diagnosed with cyclic vomiting syndrome, 72% reported hot showers as a self-therapy. Forty-two percent used cannabis daily to weekly and 2 experienced resolution of symptoms with cessation. | Very low |
| Oruganti [178] | Observational, cross-sectional survey | Of 20 patients diagnosed with CVS, 12 used cannabis chronically and all took hot baths/showers to alleviate symptoms. | Very low |
| Venkates [179] | Observational, cross-sectional survey | Among 437 CVS patients, 81% were cannabis users and 67% reported using hot showers for symptom relief. Bathing behavior was associated with cannabis use (OR 2.54, 95% confidence interval [CI] = 1.2–4.3, P = 0.0006) | Low |
CHS shares many clinical features with other CVSs. We identified four case reports of patients initially diagnosed as hyperemesis gravidarium but were ultimately diagnosed as CHS, with a history of chronic cannabis use, compulsive hot baths/showers, and a history of cyclic vomiting predating the pregnancy [18, 64, 180, 181]. These studies exemplify the diagnostic difficulty in the identification of CHS and suggest that CHS may be more common than is reported.
The pathophysiologic processes underlying CHS are unclear at this time. Many hypotheses exist, yet there is very limited evidence to support any one unifying mechanism. The best evidence suggests a dynamic interplay between cannabinoid metabolism and complex pharmacodynamics at the CB-1 receptor. In addition, our study identified three unique cases of CHS caused by synthetic cannabinoids [23, 41, 182]. These agents are potent agonists of the cannabinoid CB1 receptors, similar to THC, suggesting that agonism at the CB1 receptor may be responsible for CHS. Supportive care with IV fluids and anti-emetics is the mainstay of treatment in the acute phase of illness. There is very limited evidence to suggest that agents such as dopamine antagonists and capsaicin cream and avoidance of opiate pain medications may be of benefit. While many providers utilize these agents in practice and find them effective, prospective case-control studies are needed before recommendations can be made based upon effectiveness. The authors of this study acknowledge the potential difficulties of such studies, owing to the fact that CHS is a heterogeneous clinical entity, and thus, selecting a comparable group of patients may prove difficult. Additionally, agents such as topical capsaicin cream are not commonly stocked in hospitals and emergency departments but could be made available in the outpatient setting. There is moderate evidence to support that the definitive treatment for CHS is cannabis cessation. In this study, 96.8% of patients who ceased cannabis use experienced complete resolution of symptoms.
This systematic review is the first and most comprehensive characterization of the CHS literature. We believe that these evidence-based recommendations will improve diagnosis, highlight the limitations of pathophysiology understanding, and provide guidelines for treatment of this difficult condition. Our proposed diagnostic characteristics are listed in Table 6 with corresponding GRADE quality recommendations.
Table 6.
Diagnostic characteristics for cannabinoid hyperemesis syndrome
| Diagnostic characteristic | GRADE rating |
|---|---|
| Severe cyclic vomiting usually accompanied by abdominal pain | Low |
| Symptom onset preceded by at least weekly cannabis use | Low |
| Temporary relief of symptoms with hot bathing | Low |
| Resolution of symptoms with cannabis cessation | Low |
| Supportive features: male gender, cannabis use onset in teenage years, symptom onset in third decade of life | Low |
A careful social history is needed in all cyclic vomiting patients. Cannabis has various therapeutic properties including anti-emesis, appetite stimulation, and analgesia [4]. These properties have led to cannabis use in patients with cachexia associated with AIDS, chemotherapy-induced nausea and vomiting, peripheral neuropathy, chronic pain, and CVSs [4]. Cyclic vomiting patients and those using cannabis for its myriad of health benefits may perceive that cannabis is helping their condition instead of exacerbating it. This systematic review suggests that there may be a demographic of cyclic vomiting patients who have been mislabeled. This may also be true for hyperemesis gravidarum and gastroparesis patients. Simonetto et al. reported that among 61 CHS patients who underwent gastric emptying studies, 18 (30%) demonstrated delayed emptying [9]. Since cannabis use has been shown to delay gastric emptying [92, 132], this finding should not preclude the diagnosis of CHS. When diagnosis is in doubt, a trial of cannabis cessation may support the diagnosis [183].
It is difficult to quantify the precise amount of cannabis consumed by patients who manifest CHS. Reporting is often subjective and qualitative, and there is no metric for how much physiologically active compounds are contained in one joint, cone, bong, etc. In addition, higher THC content through selective breeding of plants and more selective use of female buds that contain more concentrated THC levels may cause CHS to appear in patients who report lower amounts of cannabis use [170].
Initial reports describe an average of 7.1 emergency department visits, 3.1 hospitalizations, and 5.0 clinic visits prior to diagnosis [12], but as the syndrome is recognized more and cannabis availability increases, more rapid diagnosis is likely. Exclusion of a major medical etiology is mandatory prior to the consideration of CHS. Other medical conditions that may present similarly to CHS include, but are not limited to, bowel perforation, cholangitis, pancreatitis, and ruptured aortic aneurysm [184]. Martinez et al. suggest that a minimum workup should include basic laboratories, abdominal ultrasound (US) and/or computerized tomography (CT) scan, and esophagogastroduodenoscopy (EGD) with initial admission. However, the literature suggests that patients often undergo multiple CT scans, EGDs, and even exploratory surgeries prior to diagnosis. In one multicenter cohort study, the mean numbers of abdominal CT studies and abdominal/pelvic US and abdominal radiographs were 5.3 ± 4.1, 3.8 ± 3.6, and 5.5 ± 6, respectively [7]. For one participating ED with seven patients enrolled in the study, the median charge for ED visits and hospital admissions over the course of a patient’s illness was $95,023 (range $62,420 to 268,110).
We would suggest a pragmatic approach to diagnosis that starts with a complete history of cannabis use including age of initial onset, amount used per week, and behavioral factors associated with symptom relief (such as hot showers). Following this, underlying medical etiology of the symptoms should be ruled out using laboratory evaluation and imaging. However, this workup should only be performed initially and repeated only if there is a change in the patient’s clinical status that suggests a new emergent condition. Greater education is needed among clinicians in order to limit repeated “exclusionary” workups and iatrogenesis. In addition, as the trend toward cannabis legalization spreads, states need to create public health messages to warn cannabis users of the possibility of developing this syndrome.
Cessation of cannabis appears to be the best treatment for CHS. Our review revealed that CHS patients may often have poor follow-up. Follow-up was documented in only 85 of 211 patients (40.2%). Multidisciplinary care may be necessary for management and diagnosis. Patients may deny cannabis use as a cause of their symptoms and fail to follow-up or seek medical care at other facilities resulting in repeated testing and resource utilization [8]. Therefore, substance abuse experts should be involved when the diagnosis is made.
Further targeted basic science research is needed to elucidate the pathophysiology underlying CHS. Strong prospective epidemiologic studies are needed to determine the prevalence of the disorder and the rate of disease development among cannabis users. In addition, randomized studies are needed to establish effective treatments to terminate acute exacerbations. As demonstrated by Kim et al., with liberalization of cannabis laws, growing prevalence of CHS is likely. In addition, results from the 2014 National Survey on Drug Use and Health (NSDUH) indicate that 7.4% of adolescents aged 12–17 were current users [2]. Our results show the average age of cannabis use onset among CHS patients to be 16 (IQR = 14–18). Given a large, young aged demographic of chronic cannabis users, a prospective, case-control study would increase our understanding as to who is at risk for the development of CHS.
Conclusion
CHS is characterized by severe cyclic vomiting, usually accompanied by abdominal pain associated with early age of cannabis use with symptoms that most commonly develop in the third decade of life. Symptom onset is preceded by daily to weekly cannabis use. Patients usually report temporary relief of symptoms with compulsive hot baths/showers and experience resolution of symptoms with cessation of cannabis use. The pathophysiology underlying CHS is unclear. Cannabis cessation appears to be the best treatment.
Acknowledgements
We acknowledge the Colorado Department of Public Health and Environment Retail Marijuana Public Health Advisory Committee.
Appendix: Search strategies
Cochrane Library (via Wiley Online Library)
MeSH descriptor: [Cannabinoids] explode all trees
MeSH descriptor: [Cannabis] explode all trees
MeSH descriptor: [Marijuana Abuse] explode all trees
MeSH descriptor: [Marijuana Smoking] explode all trees
MeSH descriptor: [Medical Marijuana] explode all trees
cannab*:ti,ab,kw (Word variations have been searched)
marijuana:ti,ab,kw (Word variations have been searched)
phytocannab*:ti,ab,kw (Word variations have been searched)
tetrahydrocannab*:ti,ab,kw (Word variations have been searched)
THC:ti,ab,kw (Word variations have been searched)
#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10
MeSH descriptor: [Vomiting] explode all trees
emes*:ti,ab,kw (Word variations have been searched)
hypereme*:ti,ab,kw (Word variations have been searched)
vomit*:ti,ab,kw (Word variations have been searched)
#12 or #13 or #14 or #15
#11 and #16
Results: 34
Embase (via Embase.com)
‘cannabinoid’/exp
‘cannabis’/exp
‘cannabis addiction’/exp
‘cannabis use’/exp
‘medical cannabis’/exp
cannab*:ab,ti
marijuana:ab,ti
phytocannab*:ab,ti
tetrahydrocannab*:ab,ti
thc:ab,ti
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
‘vomiting’/exp
emes*:ab,ti
hypereme*:ab,ti
vomit*:ab,ti
#12 OR #13 OR #14 OR #15
#11 AND #16
#11 AND #16 AND [english]/lim AND [2000-2015]/py AND [embase]/lim
Results: 1099
Ovid Medline (Ovid MEDLINE (1946-current), Ovid MEDLINE In-Process & Other Non-Indexed Citations, and Ovid MEDLINE Daily)
exp Cannabinoids/
exp Cannabis/
exp Marijuana Abuse/
exp Marijuana Smoking/
exp Medical Marijuana/
cannab$.mp.
marijuana.mp.
phytocannab$.mp.
tetrahydrocannab$.mp.
THC.mp.
or/1-10
exp Vomiting/
emes$.mp.
hypereme$.mp.
vomit$.mp.
or/12-15
11 and 16
limit 17 to (english language and yr=“2000 - 2015”)
Results: 341
PubMed
“Cannabinoids”[Mesh]
“Cannabis”[Mesh]
“Marijuana Abuse”[Mesh]
“Marijuana Smoking”[Mesh]
“Medical Marijuana”[Mesh]
cannab*
marijuana
phytocannab*
tetrahydrocannab*
THC
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
“Vomiting”[Mesh]
emes*
hypereme*
vomit*
#12 OR #13 OR #14 OR #15
#11 AND #16
(#11 AND #16) AND “english”[Language] AND (“2000”[Date - Publication]: “2015”[Date - Publication])
Results: 357
Web of Science
TS=cannab*
TS=marijuana
TS=phytocannab*
TS=tetrahydrocannab*
TS=THC
#1 OR #2 OR #3 OR #4 OR #5
TS=emes*
TS=hypereme*
TS=vomit*
#7 OR #8 OR #9
(#6 AND #10) AND LANGUAGE: (English) Timespan=2000-2015
Results: 323
Compliance with Ethical Standards
Funding and Financial Disclosures
Dr. Sorensen has no conflicts of interest to declare. Kristen DeSanto has no conflicts of interest to declare. Dr. Borgelt receives support from the Colorado Department of Public Health and Environment and the University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences. Dr. Phillips receives support from NIH on Drug Abuse R15 DA041656 and R01 DA034957, and Dr. Monte receives support from NIH 1 K23 GM110516 and NIH CTSI UL1 TR001082.
References
- 1.United Nations Office on Drugs and Crime. World Drug Report [Internet]. 2014 [cited 26 November 2016] Available at: http://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf.
- 2.Results from the 2014 National Survey on Drug Use and Health: summary of national findings [Internet]. 2014 [cited 27 November 2016] Available at: http://www.samhsa.gov/data/sites/default/files/NSDUH-FRR1-2014/NSDUH-FRR1-2014.pdf.
- 3.[No author listed] State marijuana laws in 2016. Governing the states and localities [Internet]. 2016 [cited 26 November 2016] Available at: http://www.governing.com/gov-data/state-marijuana-laws-map-medical-recreational.html.
- 4.Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456–2473. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- 5.Kim HS, Anderson JD, Saghafi O, Heard KJ, Monte AA. Cyclic vomiting presentations following marijuana liberalization in Colorado. Acad Emerg Med. 2015;22(6):694–699. doi: 10.1111/acem.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen JH, de Moore GM, Heddle R, Twartz JC. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut. 2004;53(11):1566–1570. doi: 10.1136/gut.2003.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perrotta G, Miller J, Stevens T, Chauhan A, Musunuru H, Salciccioli J, et al. Cannabinoid hyperemesis: relevance to emergency medicine. Acad Emerg Med. 2012;19:S286–S2S7. [Google Scholar]
- 8.Cox B, Chhabra A, Adler M, Simmons J, Randlett D. Cannabinoid hyperemesis syndrome: case report of a paradoxical reaction with heavy marijuana use. Case Rep Med. 2012;2012:757696. doi: 10.1155/2012/757696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonetto DA, Oxentenko AS, Herman ML, Szostek JH. Cannabinoid hyperemesis: a case series of 98 patients. Mayo Clin Proc. 2012;87(2):114–119. doi: 10.1016/j.mayocp.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace EA, Andrews SE, Garmany CL, Jelley MJ. Cannabinoid hyperemesis syndrome: literature review and proposed diagnosis and treatment algorithm. South Med J. 2011;104(9):659–664. doi: 10.1097/SMJ.0b013e3182297d57. [DOI] [PubMed] [Google Scholar]
- 11.Patterson DA, Smith E, Monahan M, Medvecz A, Hagerty B, Krijger L, et al. Cannabinoid hyperemesis and compulsive bathing: a case series and paradoxical pathophysiological explanation. J Am Board Fam Med. 2010;23(6):790–793. doi: 10.3122/jabfm.2010.06.100117. [DOI] [PubMed] [Google Scholar]
- 12.Soriano-Co M, Batke M, Cappell MS. The cannabis hyperemesis syndrome characterized by persistent nausea and vomiting, abdominal pain, and compulsive bathing associated with chronic marijuana use: a report of eight cases in the United States. Dig Dis Sci. 2010;55(11):3113–3119. doi: 10.1007/s10620-010-1131-7. [DOI] [PubMed] [Google Scholar]
- 13.Sofka S, Lerfald N. Cannabinoid hyperemesis syndrome: a case series. W V Med J. 2013;109(3):20–23. [PubMed] [Google Scholar]
- 14.Masri KR, Moussa R, Licke H, El Haddad B. Chronic cannabis use with hyperemesis, epigastric pain and conditioned showering behavior. Journal of Gastroenterology and Hepatology Research. 2012;1(6):107–110. [Google Scholar]
- 15.Nicolson SE, Denysenko L, Mulcare JL, Vito JP, Chabon B. Cannabinoid hyperemesis syndrome: a case series and review of previous reports. Psychosomatics. 2012;53(3):212–219. doi: 10.1016/j.psym.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Simonetto DA, Oxentenko AS, Herman ML, Szostek JH. Cannabinoid and hyperemesis. Mayo Clin Proc. 2012;87(5):503. doi: 10.1016/j.mayocp.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Achanta L, Kelkhoff AJ. Cannabinoid hyperemesis—is it more common than we think? J Ark Med Soc. 2013;109(8):158. [PubMed] [Google Scholar]
- 18.Alaniz VI, Liss J, Metz TD, Stickrath E. Cannabinoid hyperemesis syndrome: a cause of refractory nausea and vomiting in pregnancy. Obstet Gynecol. 2015;125(6):1484–1486. doi: 10.1097/AOG.0000000000000595. [DOI] [PubMed] [Google Scholar]
- 19.Bagdure S, Smalligan RD, Sharifi H, Khandheria B. Waning effect of compulsive bathing in cannabinoid hyperemesis. Am J Addict. 2012;21(2):184–185. doi: 10.1111/j.1521-0391.2011.00209.x. [DOI] [PubMed] [Google Scholar]
- 20.Baron M, Haymann JP, Wolfromm A, Rondeau E, Mesnard L. The smoker and the nephrologist cannabinoid hyperemesis syndrome. Kidney Int. 2011;79(12):1385–1386. doi: 10.1038/ki.2011.98. [DOI] [PubMed] [Google Scholar]
- 21.Basaviah P, Liao C, Ramsey M. Hot water: cannabinoid hyperemesis. J Gen Intern Med. 2010;25:521. [Google Scholar]
- 22.Beech RA, Sterrett DR, Babiuk J, Fung H. Cannabinoid hyperemesis syndrome: a case report and literature review. J Oral Maxillofac Surg. 2015. [DOI] [PubMed]
- 23.Bick BL, Szostek JH, Mangan TF. Synthetic cannabinoid leading to cannabinoid hyperemesis syndrome. Mayo Clin Proc. 2014;89(8):1168–1169. doi: 10.1016/j.mayocp.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Bourke MG, McCormack O. Response to “desperate for a hot shower”. Ir Med J. 2014;107(8):258–259. [PubMed] [Google Scholar]
- 25.Braver O, Leibman Y. Cannabinoid hyperemesis syndrome: descriptive overview of an under-recognized diagnosis. Isr Med Assoc J. 2015;17(5):324–325. [PubMed] [Google Scholar]
- 26.Brenna O, Aasarod K, Gustafsson BI. A man in his 30s with recurrent vomiting and abdominal pain relieved by hot showers. Tidsskr Nor Laegeforen. 2011;131(21):2134–2136. doi: 10.4045/tidsskr.10.0959. [DOI] [PubMed] [Google Scholar]
- 27.Cha JM, Kozarek RA, Lin OS. Case of cannabinoid hyperemesis syndrome with long-term follow-up. World J Clin Cases. 2014;2(12):930–933. doi: 10.12998/wjcc.v2.i12.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, McCarron RM. Cannabinoid hyperemesis syndrome: a result of chronic, heavy cannabis use. Current Psychiatry. 2013;12(10):48–54. [Google Scholar]
- 29.Chepyala P, Olden KW. Cyclic vomiting and compulsive bathing with chronic cannabis abuse. Clin Gastroenterol Hepatol. 2008;6(6):710–712. doi: 10.1016/j.cgh.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 30.Cheung E, Ng C, Foote J. A hot mess: a case of hyperemesis. Can Fam Physician. 2014;60(7):633–637. [PMC free article] [PubMed] [Google Scholar]
- 31.Desjardins N, Jamoulle O, Taddeo D, Stheneur C. Cannabinoid hyperemesis syndrome in a 17-year-old adolescent. J Adolesc Health. 2015. [DOI] [PubMed]
- 32.Donnino MW, Cocchi MN, Miller J, Fisher J. Cannabinoid hyperemesis: a case series. J Emerg Med. 2011;40(4):e63–e66. doi: 10.1016/j.jemermed.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 33.Enuh HA, Chin J, Nfonoyim J. Cannabinoid hyperemesis syndrome with extreme hydrophilia. Int J Gen Med. 2013;6:685–687. doi: 10.2147/IJGM.S49701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Figueroa-Rivera IM, Estremera-Marcial R, Sierra-Mercado M, Gutierrez-Nunez J, Toro DH. Cannabinoid hyperemesis syndrome: a paradoxical cannabis effect. Case Rep Gastrointest Med. 2015;2015:405238. doi: 10.1155/2015/405238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleig S, Brunkhorst R. Hyperemesis and a high water bill. Z Gastroenterol. 2011;49(11):1479–1481. doi: 10.1055/s-0029-1246107. [DOI] [PubMed] [Google Scholar]
- 36.Gessford AK, John M, Nicholson B, Trout R. Marijuana induced hyperemesis: a case report. W V Med J. 2012;108(6):20–22. [PubMed] [Google Scholar]
- 37.Gupta N, Ojo O, Muruthettuwegama K. Cannabinoid hyper-emesis syndrome: an enigma. Indian J Psychol Med. 2013;35(4):405–406. doi: 10.4103/0253-7176.122241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Habboushe J, Sedor J. Cannabinoid hyperemesis acute renal failure: a common sequela of cannabinoid hyperemesis syndrome. Am J Emerg Med. 2014;32(6):690.e1–690.e2. doi: 10.1016/j.ajem.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 39.Heise L. Cannabinoid hyperemesis syndrome. Adv Emerg Nurs J. 2015;37(2):95–101. doi: 10.1097/TME.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 40.Hickey JL, Witsil JC, Mycyk MB. Haloperidol for treatment of cannabinoid hyperemesis syndrome. Am J Emerg Med. 2013;31(6):1003.e5–1003.e6. doi: 10.1016/j.ajem.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 41.Hopkins CY, Gilchrist BLA. Case of cannabinoid hyperemesis syndrome caused by synthetic cannabinoids. J Emerg Med. 2013;45(4):544–546. doi: 10.1016/j.jemermed.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 42.Shaq S, Ismail S, Ghaus S, Roop EZ, Rostami K. Cannabinoid hyperemesis should be recognised as an effect of chronic cannabis abuse. Gastroenterol Hepatol Bed Bench. 2014;7(3):173–176. [PMC free article] [PubMed] [Google Scholar]
- 43.Khayambashi S. Unusual cause of nausea, vomiting and abdominal pain. J Gen Intern Med. 2012;27:S497–S4S8. [Google Scholar]
- 44.Krishnan SK, Khaira H, Ganipisetti VM. Cannabinoid hyperemesis syndrome: truly an oxymoron. J Gen Intern Med. 2014;29:S328. doi: 10.1007/s11606-013-2637-4. [DOI] [Google Scholar]
- 45.Acopetti CL, Packer CD. Cannabinoid hyperemesis syndrome: a case report and review of pathophysiology. Clin Med Res. 2014;12(1–2):65–67. doi: 10.3121/cmr.2013.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luther V, Yap L. A hot bath to calm what ails you: the cannabis hyperemesis syndrome. Acute Med. 2012;11(1):23–24. [PubMed] [Google Scholar]
- 47.Mahmad AI, Jehangir W, Littlefield JM, John S, Yousif A. Cannabis hyperemesis syndrome: a case report review of treatment. Toxicology Reports. 2015;2:889–890. doi: 10.1016/j.toxrep.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mattens V, Aerts M, Mana F, Urbain D. Daily cannabis use and the digestive tract: an underrecognized relationship. Acta Gastroenterol Belg. 2010;73(3):403–405. [PubMed] [Google Scholar]
- 49.Miller JB, Walsh M, Patel PA, Rogan M, Arnold C, Maloney M, et al. Pediatric cannabinoid hyperemesis: two cases. Pediatr Emerg Care. 2010;26(12):919–920. doi: 10.1097/PEC.0b013e3181fe9189. [DOI] [PubMed] [Google Scholar]
- 50.Mohammed F, Panchoo K, Bartholemew M, Maharaj D. Compulsive showering and marijuana use—the cannabis hyperemesis syndrome. Am J Case Rep. 2013;14:326–328. doi: 10.12659/AJCR.884001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morris R, Fisher M. Cannabinoid hyperemesis syndrome: a specific cause of cyclical vomiting. Int J Adolesc Med Health. 2014;26(1):153–156. doi: 10.1515/ijamh-2012-0113. [DOI] [PubMed] [Google Scholar]
- 52.Nogi M, Fergusson D, Chiaco JM. Mid-ventricular variant takotsubo cardiomyopathy associated with cannabinoid hyperemesis syndrome: a case report. Hawaii J Med Public Health. 2014;73(4):115–118. [PMC free article] [PubMed] [Google Scholar]
- 53.Nour SA, Nour HA, Byrd R, Mehta J, Roy T. Bath time: an unusual etiology for hypovolemic shock in a young patient. Crit Care Med. 2012;40(12):289. [Google Scholar]
- 54.Oruganti VV, Ward LD. Mid-Atlantic regional resident award winner: reverse munchies: a case of cannabinoid hyperemesis. J Gen Intern Med. 2009;24:378–379. [Google Scholar]
- 55.Pandey TS, Salim T. Clinical vingnettes “I am always in the hot shower.” cannabinoid hyperemesis syndrome—a case report. J Gen Intern Med. 2014;29:S262. doi: 10.1007/s11606-013-2580-4. [DOI] [Google Scholar]
- 56.Parikh M, Gould M. Cyclical vomiting syndrome: is pot really at the bottom of the pot? Am J Gastroenterol. 2010;105:S363. doi: 10.1038/ajg.2009.580. [DOI] [Google Scholar]
- 57.Price SL, Fisher C, Kumar R, Hilgerson A. Cannabinoid hyperemesis syndrome as the underlying cause of intractable nausea and vomiting. J Am Osteopath Assoc. 2011;111(3):166–169. doi: 10.7556/jaoa.2011.111.3.166. [DOI] [PubMed] [Google Scholar]
- 58.Raja MW, Patel D, Chemitiganti R, Burks JK. Cannabinoid hyperemesis syndrome: a consideration in patients with refractory emesis. J Investig Med. 2012;60(1):313–314. [Google Scholar]
- 59.Rashid S, Dahl K, Moise D, Subramani K, Rizvon K, Mustacchia P. Cannabinoid hyperemesis syndrome—an obscure clinical diagnosis. Am J Gastroenterol. 2009;104:S366. doi: 10.1038/ajg.2009.492_14. [DOI] [Google Scholar]
- 60.Robinson TL, Cheng FK, Domingo CA, Kim CH, Ally MT, Itzkowitz SL. Spicing up the differential for cyclical vomiting. Am J Gastroenterol. 2013;108(8):1371. doi: 10.1038/ajg.2013.170. [DOI] [PubMed] [Google Scholar]
- 61.Roche E, Foster PN. Cannabinoid hyperemesis: not just a problem in Adelaide Hills. Gut. 2005;54(5):731. doi: 10.1136/gut.2004.057471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roca-Pallin JM, Lopez-Pelayo H, Sugranyes G, Balcells-Olivero MM. Cannabinoid hyperemesis syndrome. CNS Neurosci Ther. 2013;19(12):994–995. doi: 10.1111/cns.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sannarangappa V, Tan C. Cannabinoid hyperemesis. Intern Med J. 2009;39(11):777–778. doi: 10.1111/j.1445-5994.2009.02047.x. [DOI] [PubMed] [Google Scholar]
- 64.Schmid SM, Lapaire O, Huang DJ, Jurgens FE, Guth U. Cannabinoid hyperemesis syndrome: an underreported entity causing nausea and vomiting of pregnancy. Arch Gynecol Obstet. 2011;284(5):1095–1097. doi: 10.1007/s00404-010-1811-8. [DOI] [PubMed] [Google Scholar]
- 65.Sharma AN, Hoffman RJ. Cyclical hyperemesis associated with frequent marijuana use: a case report. Clin Toxicol. 2008;46(5):394. [Google Scholar]
- 66.Wild K, Wilson H. Cannabinoid hyperemesis. BMJ Case Rep. 2010. [DOI] [PMC free article] [PubMed]
- 67.Williamson JE, July M, Gonzalez LM, Amin HH, Chaudhari S. Cannabinoid hyperemesis syndrome: cyclical vomiting behind the cloud of smoke. Am J Med. 2014;127(4):e1–e2. doi: 10.1016/j.amjmed.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 68.Woods JA, Wright NJ, Gee J, Scobey MW. Cannabinoid hyperemesis syndrome: an emerging drug-induced disease. Am J Ther. 2014. [DOI] [PubMed]
- 69.Boeckxstaens GE. Cannabinoid hyperemesis with the unusual symptom of compulsive bathing. Ned Tijdschr Geneeskd. 2005;149(26):1468–1471. [PubMed] [Google Scholar]
- 70.Alfonso Moreno V, Ojesa F, Moreno-Osset E. Cannabinoid hyperemesis. Gastroenterol Hepatol. 2006;29(7):434–435. doi: 10.1157/13091459. [DOI] [PubMed] [Google Scholar]
- 71.Wallace D, Martin AL, Park B. Cannabinoid hyperemesis: marijuana puts patients in hot water. Australas Psychiatry. 2007;15(2):156–158. doi: 10.1080/10398560701196778. [DOI] [PubMed] [Google Scholar]
- 72.Chang YH, Windish DM. Cannabinoid hyperemesis relieved by compulsive bathing. Mayo Clin Proc. 2009;84(1):76–78. doi: 10.4065/84.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ochoa-Mangado E, Jimenez Gimenez M, Salvador Vadillo E, Madoz-Gurpide A. Cyclical hyperemesis secondary to cannabis abuse. Gastroenterol Hepatol. 2009;32(6):406–409. doi: 10.1016/j.gastrohep.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 74.Watts M. Cannabinoid hyperemesis presenting to a New Zealand hospital. N Z Med J. 2009;122(1290):116–118. [PubMed] [Google Scholar]
- 75.Budhraja V. Confirming the diagnosis of cannabinoid hyperemesis. Mayo Clin Proc. 2009;84(5):483. doi: 10.1016/S0025-6196(11)60569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carhill A, Wiese J. Your stomach on drugs: cyclic vomiting in association with chronic cannabis abuse. J Gen Intern Med. 2007;22:255. [Google Scholar]
- 77.Cheng FK, Robinson T, Domingo C, Ally M, Kim CH, Itzkowitz S. Spicing up the differential for cyclic vomiting: a case of synthetic-cannabinoid induced hyperemesis syndrome. Am J Gastroenterol. 2012;107:S268–S2S9. [Google Scholar]
- 78.Estremera R, Figueroa I, Sierra M, Toro DA. paradoxical cannabis effect. Am J Gastroenterol. 2014;109:S54. [Google Scholar]
- 79.Muschart X, Flament J. A non-classical cannabinoid syndrome. Acta Clin Belg. 2015;70(4):299–300. doi: 10.1179/2295333714Y.0000000116. [DOI] [PubMed] [Google Scholar]
- 80.Ramos S, Rodrigues R, Almeida N, Sa JM, Fonseca L. Cannabinoid hyperemesis syndrome. Psychother Psychosom. 2013;82:90. [Google Scholar]
- 81.Sadiq M. Cannabis hyperemesis syndrome. Journal of Addiction Medicine. 2013;7(4):E3. [Google Scholar]
- 82.Shah S, Gilbert C, Toth J, Reed M. Cannabinoid hyperemesis syndrome causing pneumomediastinum and pneumorachis. Chest. 2014;146(4).
- 83.Atkins D. Grading quality of evidence and strength of recommendations. Brit Med Jou. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abalo R, Cabezos PA, Vera G, Lopez-Perez AE, Martin MI. Cannabinoids may worsen gastric dysmotility induced by chronic cisplatin in the rat. Neurogastroenterol Motil. 2013;25(5):373–382. doi: 10.1111/nmo.12073. [DOI] [PubMed] [Google Scholar]
- 85.Barann M, Molderings G, Bruss M, Bonisch H, Urban BW, Gothert M. Direct inhibition by cannabinoids of human 5-HT3A receptors: probable involvement of an allosteric modulatory site. Br J Pharmacol. 2002;137(5):589–596. doi: 10.1038/sj.bjp.0704829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beaumont H, Jensen J, Carlsson A, Ruth M, Lehmann A, Boeckxstaens G. Effect of delta9-tetrahydrocannabinol, a cannabinoid receptor agonist, on the triggering of transient lower oesophageal sphincter relaxations in dogs and humans. Br J Pharmacol. 2009;156(1):153–162. doi: 10.1111/j.1476-5381.2008.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bornheim LM, Kim KY, Li J, Perotti BY, Benet LZ. Effect of cannabidiol pretreatment on the kinetics of tetrahydrocannabinol metabolites in mouse brain. Drug Metab Dispos. 1995;23(8):825–831. [PubMed] [Google Scholar]
- 88.Darmani NA. Delta-9-tetrahydrocannabinol differentially suppresses cisplatin-induced emesis and indices of motor function via cannabinoid CB(1) receptors in the least shrew. Pharmacol Biochem Behav. 2001;69(1–2):239–249. doi: 10.1016/S0091-3057(01)00531-7. [DOI] [PubMed] [Google Scholar]
- 89.Darmani NA. The potent emetogenic effects of the endocannabinoid, 2-AG (2-arachidonoylglycerol) are blocked by delta(9)-tetrahydrocannabinol and other cannabinoids. J Pharmacol Exp Ther. 2002;300(1):34–42. doi: 10.1124/jpet.300.1.34. [DOI] [PubMed] [Google Scholar]
- 90.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 91.Fennessy MR, Taylor DA. The effect of delta9-tetrahydrocannabinol on body temperature and brain amine concentrations in the rat at different ambient temperatures. Br J Pharmacol. 1977;60(1):65–71. doi: 10.1111/j.1476-5381.1977.tb16748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Izzo AA, Mascolo N, Pinto L, Capasso R, Capasso F. The role of cannabinoid receptors in intestinal motility, defaecation and diarrhoea in rats. Eur J Pharmacol. 1999;384(1):37–42. doi: 10.1016/S0014-2999(99)00673-1. [DOI] [PubMed] [Google Scholar]
- 93.Izzo AA, Mascolo N, Capasso R, Germano MP, De Pasquale R, Capasso F. Inhibitory effect of cannabinoid agonists on gastric emptying in the rat. Naunyn Schmiedeberg’s Arch Pharmacol. 1999;360(2):221–223. doi: 10.1007/s002109900054. [DOI] [PubMed] [Google Scholar]
- 94.Kreuz DS, Axelrod J. Delta-9-tetrahydrocannabinol: localization in body fat. Science. 1973;179(4071):391–393. doi: 10.1126/science.179.4071.391. [DOI] [PubMed] [Google Scholar]
- 95.Krowicki ZK, Moerschbaecher JM, Winsauer PJ, Digavalli SV, et al. Delta9-tetrahydrocannabinol inhibits gastric motility in the rat through cannabinoid CB1 receptors. Eur J Pharmacol. 1999;371(2–3):187–196. doi: 10.1016/S0014-2999(99)00165-X. [DOI] [PubMed] [Google Scholar]
- 96.Kwiatkowska M, Parker LA, Burton P, Mechoulam R. A comparative analysis of the potential of cannabinoids and ondansetron to suppress cisplatin-induced emesis in the Suncus murinus (house musk shrew) Psychopharmacology. 2004;174(2):254–259. doi: 10.1007/s00213-003-1739-9. [DOI] [PubMed] [Google Scholar]
- 97.Lichtman AH, Wiley JL, LaVecchia KL, Neviaser ST, et al. Effects of SR 141716A after acute or chronic cannabinoid administration in dogs. Eur J Pharmacol. 1998;357(2–3):139–148. doi: 10.1016/S0014-2999(98)00558-5. [DOI] [PubMed] [Google Scholar]
- 98.Lundberg DJ, Daniel AR, Thayer SA. Delta(9)-tetrahydrocannabinol-induced desensitization of cannabinoid-mediated inhibition of synaptic transmission between hippocampal neurons in culture. Neuropharmacology. 2005;49(8):1170–1177. doi: 10.1016/j.neuropharm.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 99.Moldrich G, Wenger T. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides. 2000;21(11):1735–1742. doi: 10.1016/S0196-9781(00)00324-7. [DOI] [PubMed] [Google Scholar]
- 100.Rawls SM, Cabassa J, Geller EB, Adler MW. CB1 receptors in the preoptic anterior hypothalamus regulate WIN 55212-2 [(4,5-dihydro-2-methyl-4(4-morpholinylmethyl)-1-(1-naphthalenyl-carbonyl)-6H-pyrr olo[3,2,1ij]quinolin-6-one]-induced hypothermia. J Pharmacol Exp Ther. 2002;301(3):963–8. [DOI] [PubMed]
- 101.Rock EM, Goodwin JM, Limebeer CL, Breuer A, Pertwee RG, Mechoulam R, et al. Interaction between non-psychotropic cannabinoids in marihuana: effect of cannabigerol (CBG) on the anti-nausea or anti-emetic effects of cannabidiol (CBD) in rats and shrews. Psychopharmacology. 2011;215(3):505–512. doi: 10.1007/s00213-010-2157-4. [DOI] [PubMed] [Google Scholar]
- 102.Ros J, Claria J, To-Figueras J, Planaguma A, et al. Endogenous cannabinoids: a new system involved in the homeostasis of arterial pressure in experimental cirrhosis in the rat. Gastroenterology. 2002;122(1):85–93. doi: 10.1053/gast.2002.30305. [DOI] [PubMed] [Google Scholar]
- 103.Schulze DR, Carroll FI, McMahon LR. Interactions between dopamine transporter and cannabinoid receptor ligands in rhesus monkeys. Psychopharmacology. 2012;222(3):425–438. doi: 10.1007/s00213-012-2661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smirnov MS, Kiyatkin EA. Behavioral and temperature effects of delta 9-tetrahydrocannabinol in human-relevant doses in rats. Brain Res. 2008;1228:145–160. doi: 10.1016/j.brainres.2008.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sugiura T, Kishimoto S, Oka S, Gokoh M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog Lipid Res. 2006;45(5):405–446. doi: 10.1016/j.plipres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 106.Van Sickle MD, Oland LD, Mackie K, Davison JS, Sharkey KA. Distribution of the cannabinoid 1 receptor in the brainstem and gut: a novel neuroregulatory system in emesis. Gastroenterology. 2001;120(5):A197. doi: 10.1016/S0016-5085(08)80976-6. [DOI] [Google Scholar]
- 107.Varvel SA, Bridgen DT, Tao Q, Thomas BF, Martin BR, Lichtman AH. Delta9-tetrahydrocannbinol accounts for the antinociceptive, hypothermic, and cataleptic effects of marijuana in mice. J Pharmacol Exp Ther. 2005;314(1):329–337. doi: 10.1124/jpet.104.080739. [DOI] [PubMed] [Google Scholar]
- 108.Fabries P, Ribaud N, Puidupin A, Coton T. Cannabinoid hyperemesis syndrome. Presse Medicale. 2013;42(11):1531–1533. doi: 10.1016/j.lpm.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 109.Felton D, Zitomersky N, Manzi S, Lightdale JR. 13-year-old girl with recurrent, episodic, persistent vomiting: out of the pot and into the fire. Pediatrics. 2015;135(4):e1060–e1063. doi: 10.1542/peds.2014-2116. [DOI] [PubMed] [Google Scholar]
- 110.Gremida A, Gammack J. Marijuana-induced cyclic vomiting: what clinicians need to know. Am J Gastroenterol. 2014;109:S544. [Google Scholar]
- 111.Harris E, McDonagh M, Kennedy N. Cannabis and hyperemesis. Ir J Psychol Med. 2010;27(1):47–48. doi: 10.1017/S0790966700000938. [DOI] [PubMed] [Google Scholar]
- 112.Kraemer RR, La Hoz RM, Willig JH. Some like it hot: erythema ab igne due to cannabinoid hyperemesis. J Gen Intern Med. 2013;28(11):1522. doi: 10.1007/s11606-013-2446-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lemaire N, Douillart C, Deheul S, Bordet R, Gautier S. Cannabis-induced hyperemesis: unusual symptoms associated with chronic cannabis abuse. Fundamental & Clinical Pharmacology. 2010;24:96–97. [Google Scholar]
- 114.Louie RK, Lee JC. Psychiatric interventions for cannabinoid-induced hyperemesis syndrome in a diabetic patient. Am J Addict. 2015;24(1):59–60. [Google Scholar]
- 115.Qipo A, DeLorme J, Anis K, Acharya A, Ansari N. Cannabinoid hyperemesis syndrome (CHS) versus uremia in a patient with end stage renal disease. Am J Kidney Dis. 2014;63(5):A92. [Google Scholar]
- 116.Shinha T, Agarwal R, Lazarides A, Levey R. Cannabinoid-induced gastroparesis. Am J Gastroenterol. 2011;106:S189–SS90. [Google Scholar]
- 117.Velasco A, Pentecost P. An unexpected etiology of cyclical vomiting. J Hosp Med. 2012;7:S281. [Google Scholar]
- 118.Vujasinović M, Ivartnik M, Tretjak M. Cannabinoid hyperemesis syndrome—case report. Zdravniski Vestnik. 2012;81(2):159–162. [Google Scholar]
- 119.Sontineni SP, Chaudhary S, Sontineni V, Lanspa SJ. Cannabinoid hyperemesis syndrome: clinical diagnosis of an underrecognised manifestation of chronic cannabis abuse. World J Gastroenterol. 2009;15(10):1264–1266. doi: 10.3748/wjg.15.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun S, Zimmermann AE. Cannabinoid hyperemesis syndrome. Hosp Pharm. 2013;48(8):650–655. doi: 10.1310/hpj4808-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pattathan MB, Hejazi RA, McCallum RW. Association of marijuana use and cyclic vomiting syndrome. Pharmaceuticals (Basel) 2012;5(7):719–726. doi: 10.3390/ph5070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Darmani NA. Cannabinoid-induced hyperemesis: a conundrum—from clinical recognition to basic science mechanisms. Pharmaceuticals. 2010;3(7):2163–2177. doi: 10.3390/ph3072163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hasenoehrl C, Taschler U, Storr M, Schicho R. The gastrointestinal tract—a central organ of cannabinoid signaling in health and disease. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2016. [DOI] [PMC free article] [PubMed]
- 124.Lee Y, Jo J, Chung HY, Pothoulakis C, Im E. Endocannabinoids in the gastrointestinal tract. American journal of physiology Gastrointestinal and liver physiology. 2016;311(4):G655–GG66. doi: 10.1152/ajpgi.00294.2015. [DOI] [PubMed] [Google Scholar]
- 125.Watkins BA, Kim J. The endocannabinoid system: directing eating behavior and macronutrient metabolism. Front Psychol. 2014;5:1506. doi: 10.3389/fpsyg.2014.01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sharkey KA, Darmani NA, Parker LA. Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur J Pharmacol. 2014;722:134–146. doi: 10.1016/j.ejphar.2013.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McCoy KL. Interaction between cannabinoid system and toll-like receptors controls inflammation. Mediat Inflamm. 2016;2016:5831315. doi: 10.1155/2016/5831315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rubino T, Zamberletti E, Parolaro D. Endocannabinoids and mental disorders. Handb Exp Pharmacol. 2015;231:261–283. doi: 10.1007/978-3-319-20825-1_9. [DOI] [PubMed] [Google Scholar]
- 129.Prospero-Garcia O, Amancio-Belmont O, Becerril Melendez AL, Ruiz-Contreras AE, Mendez-Diaz M. Endocannabinoids and sleep. Neuroscience and biobehavioral reviews. 2016. [DOI] [PubMed]
- 130.Malek N, Starowicz K. Dual-acting compounds targeting endocannabinoid and endovanilloid systems—a novel treatment option for chronic pain management. Front Pharmacol. 2016;7:257. doi: 10.3389/fphar.2016.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Del Mar Ramirez Fernandez M, De Boeck G, Wood M, Lopez-Rivadulla M, Samyn N. Simultaneous analysis of THC and its metabolites in blood using liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;875(2):465–470. doi: 10.1016/j.jchromb.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 132.McCallum R, Soykan I. Delta-9-tetrahydrocannabinol delays the gastric emptying of solid food in humans: a double-blind, randomized study. Aliment Pharmaco Ther. 1999;13(1):77–80. doi: 10.1046/j.1365-2036.1999.00441.x. [DOI] [PubMed] [Google Scholar]
- 133.Abalo R, Vera G, Lopez-Perez AE, Martinez-Villaluenga M, Martin-Fontelles MI. The gastrointestinal pharmacology of cannabinoids: focus on motility. Pharmacology. 2012;90(1–2):1–10. doi: 10.1159/000339072. [DOI] [PubMed] [Google Scholar]
- 134.Al-Mahdawi R, McMann LJ, Alwardi T, Naseer M, Sabbagh H, Kulairi Z. Marijuana induced biliary dyskinesia. J Gen Intern Med. 2015;30:S325–S3S6. [Google Scholar]
- 135.Crowley TJ, MacDonald MJ. Cannabis dependence, withdrawal, and reinforcing effects among adolescents with conduct symptoms and substance use disorders. Drug Alcohol Depend. 1998;50(1):27–37. doi: 10.1016/S0376-8716(98)00003-9. [DOI] [PubMed] [Google Scholar]
- 136.Sim LJ, Hampson RE, Deadwyler SA, Childers SR. Effects of chronic treatment with delta9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci. 1996;16(24):8057–8066. doi: 10.1523/JNEUROSCI.16-24-08057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Parfieniuk A, Flisiak R. Role of cannabinoids in chronic liver diseases. World J Gastroenterol. 2008;14(40):6109–6114. doi: 10.3748/wjg.14.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ashton CH. Adverse effects of cannabis and cannabinoids. Br J Anaesth. 1999;83(4):637–649. doi: 10.1093/bja/83.4.637. [DOI] [PubMed] [Google Scholar]
- 139.Aviello G, Romano B, Izzo AA. Cannabinoids and gastrointestinal motility: animal and human studies. Eur Rev Med Pharmacol Sci. 2008;12(Suppl 1):81–93. [PubMed] [Google Scholar]
- 140.Bashashati M, McCallum RW. Neurochemical mechanisms and pharmacologic strategies in managing nausea and vomiting related to cyclic vomiting syndrome and other gastrointestinal disorders. Eur J Pharmacol. 2014;722(1):79–94. doi: 10.1016/j.ejphar.2013.09.075. [DOI] [PubMed] [Google Scholar]
- 141.Bergman J, Delatte MS, Paronis CA, Vemuri K, Thakur GA, Makriyannis A. Some effects of CB1 antagonists with inverse agonist and neutral biochemical properties. Physiol Behav. 2008;93(4–5):666–670. doi: 10.1016/j.physbeh.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Coutts AA. Cannabinoid receptor activation and the endocannabinoid system in the gastrointestinal tract. Curr Neuropharmacol. 2004;2(1):91–102. doi: 10.2174/1570159043476963. [DOI] [Google Scholar]
- 143.Coutts AA, Izzo AA. The gastrointestinal pharmacology of cannabinoids: an update. Curr Opin Pharmacol. 2004;4(6):572–579. doi: 10.1016/j.coph.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 144.Darmani NA, Sim-Selley LJ, Martin BR, Janoyan JJ, Crim JL, Parekh B, et al. Antiemetic and motor-depressive actions of CP55,940: cannabinoid CB1 receptor characterization, distribution, and G-protein activation. Eur J Pharmacol. 2003;459(1):83–95. doi: 10.1016/S0014-2999(02)02815-7. [DOI] [PubMed] [Google Scholar]
- 145.Darmani NA. Methods evaluating cannabinoid and endocannabinoid effects on gastrointestinal functions. Methods Mol Med. 2006;123:169–189. doi: 10.1385/1-59259-999-0:169. [DOI] [PubMed] [Google Scholar]
- 146.Fioramonti J, Bueno L. Role of cannabinoid receptors in the control of gastrointestinal motility and perception. Expert Review of Gastroenterology and Hepatology. 2008;2(3):385–397. doi: 10.1586/17474124.2.3.385. [DOI] [PubMed] [Google Scholar]
- 147.Inui A. Emesis, appetite, and endocannabinoids. Gastroenterology. 2002;123(2):655–656. doi: 10.1053/gast.2002.35227. [DOI] [PubMed] [Google Scholar]
- 148.Izzo AA, Mascolo N, Capasso F. Cannabis and cannabinoid receptors—Third Monothematic Meeting of the Italian Society of Pharmacology, Naples, May 2000: Guest editorial. Pharm Pharmacol Commun. 2000;6(6):229. doi: 10.1211/146080800128736033. [DOI] [Google Scholar]
- 149.Izzo AA, Coutts AA. Cannabinoids and the digestive tract. Handb Exp Pharmacol. 2005(168):573–98. [DOI] [PubMed]
- 150.Izzo AA, Camilleri M. Emerging role of cannabinoids in gastrointestinal and liver diseases: basic and clinical aspects. Gut. 2008;57(8):1140–1155. doi: 10.1136/gut.2008.148791. [DOI] [PubMed] [Google Scholar]
- 151.Lin XH, Wang YQ, Wang HC, Ren XQ, Li YY. Role of endogenous cannabinoid system in the gut. Sheng Li Xue Bao. 2013;65(4):451–460. [PubMed] [Google Scholar]
- 152.ML L, Agito MD. Cannabinoid hyperemesis syndrome: marijuana is both antiemetic and proemetic. Cleve Clin J Med. 2015;82(7):429–434. doi: 10.3949/ccjm.82a.14023. [DOI] [PubMed] [Google Scholar]
- 153.Malik Z, Baik D, Schey R. The role of cannabinoids in regulation of nausea and vomiting, and visceral pain. Curr Gastroenterol Rep. 2015;17(2):429. doi: 10.1007/s11894-015-0429-1. [DOI] [PubMed] [Google Scholar]
- 154.Martin BR, Wiley JL. Mechanism of action of cannabinoids: how it may lead to treatment of cachexia, emesis, and pain. J Support Oncol. 2004;2(4):305–314. [PubMed] [Google Scholar]
- 155.Massa F, Storr M, Lutz B. The endocannabinoid system in the physiology and pathophysiology of the gastrointestinal tract. J Mol Med. 2005;83(12):944–954. doi: 10.1007/s00109-005-0698-5. [DOI] [PubMed] [Google Scholar]
- 156.Parker LA, Rock EM, Limebeer CL. Regulation of nausea and vomiting by cannabinoids. Br J Pharmacol. 2011;163(7):1411–1422. doi: 10.1111/j.1476-5381.2010.01176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sanger GJ. Endocannabinoids and the gastrointestinal tract: what are the key questions? Br J Pharmacol. 2007;152(5):663–670. doi: 10.1038/sj.bjp.0707422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sim-Selley LJ. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol. 2003;15(2):91–119. doi: 10.1615/CritRevNeurobiol.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- 159.Tache Y. Cyclic vomiting syndrome: the corticotropin-releasing-factor hypothesis. Dig Dis Sci. 1999;44(8 Suppl):79s–86s. [PubMed] [Google Scholar]
- 160.Traver F, Edo S, Haro G. Cyclic hyperemesis secondary to chronic consumption of cannabis: a reconceptualization of psychogenic vomiting. Addictive Disorders and their Treatment. 2009;8(4):175–184. doi: 10.1097/ADT.0b013e3181a6f8aa. [DOI] [Google Scholar]
- 161.Haiderali A, Menditto L, Good M, Teitelbaum A, Wegner J. Impact on daily functioning and indirect/direct costs associated with chemotherapy-induced nausea and vomiting (CINV) in a US population. Support Care Cancer. 2011;19(6):843–851. doi: 10.1007/s00520-010-0915-9. [DOI] [PubMed] [Google Scholar]
- 162.de Moore GM. Value of the single case report. Marijuana, vomiting and compulsive bathing: a new clinical syndrome? Aust N Z J Psychiatry. 2005;39(11–12):1046. doi: 10.1111/j.1440-1614.2005.01724_1.x. [DOI] [PubMed] [Google Scholar]
- 163.Janczyk P, Donaldson CW, Gwaltney S. Two hundred and thirteen cases of marijuana toxicoses in dogs. Vet Hum Toxicol. 2004;46(1):19–21. [PubMed] [Google Scholar]
- 164.Praharaj SK, Sarkhel S. Rimonabant-induced persistent vomiting. German Journal of Psychiatry. 2013;16(1):49–50. [Google Scholar]
- 165.Storr MA, Sharkey KA. The endocannabinoid system and gut-brain signalling. Curr Opin Pharmacol. 2007;7(6):575–582. doi: 10.1016/j.coph.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 166.Caplow J, Aizenberg DJ. New on the differential for kidney failure: cannabinoid hyperemesis syndrome. J Gen Intern Med. 2015;30:S431. [Google Scholar]
- 167.Witsil JC, Hickey JL, Mycyk MB. Haloperidol successfully treats cannabinoid hyperemesis syndrome. Clin Toxicol. 2013;51(7):591. [Google Scholar]
- 168.Lapoint J. Case series of patients treated for cannabinoid hyperemesis syndrome with capsaicin cream. Clin Toxicol. 2014;52(7):707. [Google Scholar]
- 169.Biary R, Oh A, Lapoint J, Nelson LS, Hoffman RS, Howland MA. Topical capsaicin cream used as a therapy for cannabinoid hyperemesis syndrome. Clin Toxicol. 2014;52(7):787. [Google Scholar]
- 170.Galli JA, Sawaya RA, Friedenberg FK. Cannabinoid hyperemesis syndrome. Curr Drug Abuse Rev. 2011;4(4):241–249. doi: 10.2174/1874473711104040241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Abell TL, Adams KA, Boles RG, Bousvaros A, Chong SKF, Fleisher DR, et al. Cyclic vomiting syndrome in adults. Neurogastroenterol Motil. 2008;20(4):269–284. doi: 10.1111/j.1365-2982.2008.01113.x. [DOI] [PubMed] [Google Scholar]
- 172.Aljomah G, Hutchings R. Cyclic vomiting syndrome (CVS): a management challenge across the ages. Am J Gastroenterol 2011;106:S401.
- 173.Choung RS, Locke GR 3rd, Lee RM, Schleck CD, Zinsmeister AR, Talley NJ. Cyclic vomiting syndrome and functional vomiting in adults: association with cannabinoid use in males. Neurogastroenterol Motil 2012;24(1):20–26, e1. [DOI] [PMC free article] [PubMed]
- 174.Guidelines--Rome III. Diagnostic criteria for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15(3):307–312. [PubMed] [Google Scholar]
- 175.Fajardo NR, Cremonini F, Talley NJ. Cyclic vomiting syndrome and chronic cannabis use. Am J Gastroenterol. 2005;100(9):S343. [Google Scholar]
- 176.Hejazi RA, McCallum RW. Review article: cyclic vomiting syndrome in adults—rediscovering and redefining an old entity. Aliment Pharmacol Ther. 2011;34(3):263–273. doi: 10.1111/j.1365-2036.2011.04721.x. [DOI] [PubMed] [Google Scholar]
- 177.Namin F, Patel J, Lin Z, Sarosiek I, Foran P, Esmaeili P, et al. Clinical, psychiatric and manometric profile of cyclic vomiting syndrome in adults and response to tricyclic therapy. Neurogastroenterol Motil. 2007;19(3):196–202. doi: 10.1111/j.1365-2982.2006.00867.x. [DOI] [PubMed] [Google Scholar]
- 178.Oruganti V, Sachdeva P, Fisher RS, Parkman HP. Cyclic vomiting syndrome in adults: relationship to cannabis use, migraine headaches, and intervening symptoms. Gastroenterology. 2010;138(5):S380. [Google Scholar]
- 179.Venkatesan T, Samuel EA, Kumar N, Sengupta J, Ali M, Faddis M, et al. The endocannabinoid system (ECS) and the hypothalamic-pituitary-adrenal (HPA) axis in adults with cyclic vomiting syndrome (CVS) Gastroenterology. 2013;144(5):S924. doi: 10.1016/S0016-5085(13)63441-1. [DOI] [Google Scholar]
- 180.Manning L, Eckford S. Cannabinoid hyperemesis syndrome in pregnancy. Bjog-an International J Obstet Gynaecol. 2012;119:27. [Google Scholar]
- 181.Andrews KH, Bracero LA. Cannabinoid hyperemesis syndrome during pregnancy: a case report. J Reprod Med. 2015;60(9–10):430–432. [PubMed] [Google Scholar]
- 182.Ukaigwe A, Karmacharya P, Donato AA. Gut gone to pot: a case of cannabinoid hyperemesis syndrome due to K2, a synthetic cannabinoid. Case Rep Emerg Med. 2014;2014:1670–1698. doi: 10.1155/2014/167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hejazi RA, Lavenbarg TH, Foran P, McCallum RW. Who are the nonresponders to standard treatment with tricyclic antidepressant agents for cyclic vomiting syndrome in adults? Aliment Pharmacol Ther. 2010;31(2):295–301. doi: 10.1111/j.1365-2036.2009.04165.x. [DOI] [PubMed] [Google Scholar]
- 184.Martinez AMC, Singh E. Marijuana: anti-emetic or pro-emetic? Am J Gastroenterol. 2012;107:S281. doi: 10.1038/ajg.2011.472. [DOI] [Google Scholar]
- 185.Darmani NA. The cannabinoid CB1 receptor antagonist SR 141716A reverses the antiemetic and motor depressant actions of WIN 55, 212-2. Eur J Pharmacol. 2001;430(1):49–58. doi: 10.1016/S0014-2999(01)01355-3. [DOI] [PubMed] [Google Scholar]
- 186.Turner CE, Elsohly MA, Boeren EG. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J Nat Prod. 1980;43(2):169–234. doi: 10.1021/np50008a001. [DOI] [PubMed] [Google Scholar]
- 187.Hejazi R, Lavenbarg TH, Foran P, McCallum RW. Who are the non-responders to standard therapy for cyclic vomiting syndrome in adults? A large single center experience. Neurogastroenterol Motil. 2009;21:65. [Google Scholar]
- 188.Argoff CE, Brennan MJ, Camilleri M, Davies A, Fudin J, et al. Consensus recommendations on initiating prescription therapies for opioid-induced constipation. Pain Med. 2015;16(12):2324–2337. doi: 10.1111/pme.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]