Abstract
Purpose
Artificial oocyte activation using calcium ionophores and enhancement of embryonic developmental potential by the granulocyte-macrophage colony-stimulating factor (GM-CSF) have already been reported. In this study, we evaluated the synergistic effect of these two methods on aged human unfertilized oocytes after intracytoplasmic sperm injection (ICSI). Then, we cultured the resulting embryos to the blastocyst stage and screened them for chromosomal abnormalities, to assess the safety of this protocol.
Methods
Aged human oocytes deemed unfertilized after ICSI were activated, either by briefly applying the calcium ionophore A23187 alone (group A) or by briefly applying the ionophore and then supplementing the culture medium with recombinant human GM-CSF (rhGM-CSF) (group B). Next, the development was monitored in a time-lapse incubator system, and ploidy was analyzed by array comparative genomic hybridization (aCGH), after whole embryo biopsy and whole genome amplification. Differences between oocytes and resulting embryos in both groups were evaluated statistically.
Results
Oocytes unfertilized after ICSI can be activated with the calcium ionophore A23187 to show two pronuclei and two polar bodies. Addition of rhGM-CSF in the culture medium of A23187-activated oocytes enhances their cleaving and blastulation potential and results in more euploid blastocysts compared to the culture medium alone.
Conclusions
This study shows that activating post-ICSI aged human unfertilized oocytes with a combination of a calcium ionophore and a cytokine can produce good-morphology euploid blastocysts.
Keywords: Calcium ionophore A23187, GM-CSF, Artificial oocyte activation, aCGH, ICSI, Fertilization failure
Introduction
Fertilization is one of the major events in in vitro fertilization (IVF). In cases of severe male factor infertility, intracytoplasmic sperm injection (ICSI) has revolutionized treatment [1]. Total fertilization failure has been reported to occur in 1–3 % of ICSI cycles [2, 3] with devastating psychological results for the couple [4]. For patients, the psychological burden is equivalent to that experienced after low fertilization post ICSI. In such cases, repeating the ICSI procedure may result in fertilization failure again, despite normal semen parameters and satisfactory ovarian response [3, 5]. The mechanism underlying post-ICSI fertilization failure (PIFF) is linked to failure of oocyte activation after the injection [6]. Sperm-derived phospholipase C-ζ (PLCζ) has been characterized as an important physiological agent of oocyte activation since 2002 [7]. The phosphatidylinositol pathway triggered by PLCζ is responsible for calcium release from the oocyte’s endoplasmic reticulum, resulting in oocyte activation and subsequent fertilization, with characteristic Ca2+ flux oscillations [8]. The role and mechanism of PLCζ and other factors in oocyte activation are still under investigation [9–11].
In PIFF cases, different methods have been applied to achieve artificial oocyte activation (AOA): activation using an electric current [12–14], modifications of ICSI techniques [5, 15], and the use of chemical agents, such as strontium chloride [16], puromycin [17], and Ca2+ ionophores, with calcium ionophore A23187 being the most widely used [8, 18–23].
The use of calcium ionophore A23187 has been found to salvage oocyte activation failure cases, leading to pregnancies in cycles with diminished oocyte reserves [24], although this is still a matter of discussion [25]. Delivery of healthy babies has been reported in severe male factor IVF cycles [18–20, 26–28] and in normozoospermic IVF cycles [21]. Furthermore, A23187 treatment improved embryo development and IVF outcome in couples with embryos showing developmental problems in previous IVF attempts [29].
Granulocyte-macrophage colony-stimulating factor (GM-CSF) has been found to improve embryo developmental potential in IVF. GM-CSF is a hematopoietic cytokine that was initially identified as a derivative of activated T lymphocytes, playing a role in the proliferation and differentiation of myeloid hematopoietic cells [30]. In humans, GM-CSF expression has been identified in different sites of the female reproductive tract, such as endometrial tissue [31], fallopian tubes [32], placenta and trophoblastic cells [33, 34], theca cells around large follicles, ovarian luteal cells [35], and follicular fluid [36].
In human embryos, the GM-CSF receptor is expressed from the fertilized oocyte to the blastocyst stage [37]. In vitro culture of human preimplantation embryos in culture media containing 2 ng/ml of recombinant human GM-CSF (rhGM-CSF) has led to an improved and accelerated embryonic development, an increase of the number of early-cleavage embryos that develop to blastocyst, an increase of blastomere survival, an increase of inner cell mass viability, a decrease in the number of apoptotic nuclei, an increased hatching rate of blastocysts in vitro, and generally, an enhancement of human blastocyst development in vitro [37–40]. Based on the above, GM-CSF has been considered as a cytokine involved in human preimplantation embryo development and has been highlighted as a major maternal determinant of pregnancy outcome [41]. Recent studies confirm that GM-CSF supplementation of culture media enhances the implantation rate and pregnancy rate in cases of repeated IVF failures [42] and this is also seen in a randomized clinical trial [43].
While there are several studies highlighting the value of calcium ionophore A23187 for AOA immediately after or within a short time post ICSI, there are only two studies on its use in salvaging aged human unfertilized oocytes, at either 20 h post ICSI (combination of A23187 and puromycin) [17] or 3 days post ICSI [16]. So far, only one live birth after activation of 1-day-old unfertilized oocytes with calcium ionophore A23187 has been reported [22].
There is recent evidence that AOA does not cause a widespread increase in chromosome segregation errors during meiosis [44], thereby indicating that this method may be safe for clinical use. The same observation applies to GM-CSF supplementation in culture media. There was only one previous study, based on fluorescence in situ hybridization (FISH) analysis of only seven chromosomes [38], indicating that GM-CSF has no effect on the chromosomal constitution of human embryos.
For this study, we applied a modified AOA protocol, in which we combined exposure to calcium ionophore A23187 with supplementation of the culture medium with GM-CSF, to activate human oocytes deemed unfertilized 18 h post ICSI. We compared the results with the standard AOA protocol where calcium ionophore A23187 alone was used. We hypothesized that GM-CSF may exert its effects on activated oocytes and on the resulting embryos. Our primary goal was to evaluate the synergistic effect of both of these stimuli on activated oocytes and subsequent embryonic development; our secondary goal was to examine the safety of this modified protocol on oocytes and developing embryos, by means of array comparative genomic hybridization (aCGH) analysis of the full chromosomal complement of the resulting embryos.
Materials and methods
Study design
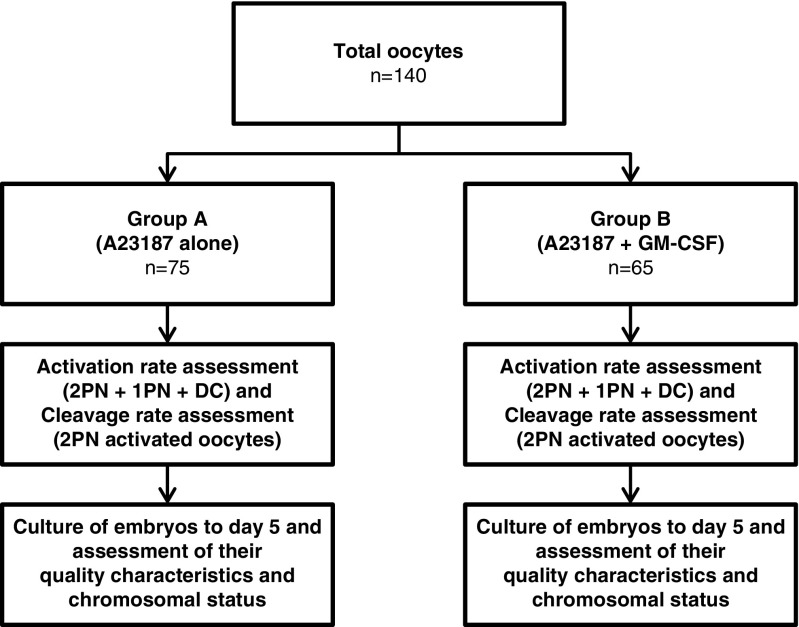
The present study is a prospective randomized trial. We included couples presenting for ICSI treatment in our clinic. Pools of post-ICSI unfertilized oocytes were randomly allocated, by flipping a coin, into two groups: group A (exposure to calcium ionophore A23187 alone, n = 75) and group B (exposure to calcium ionophore A23187 + GM-CSF supplementation, n = 65). We used only unfertilized oocytes showing the first polar body and no apparent pronucleus in the cytoplasm upon fertilization assessment by time-lapse imaging. Target characteristics of oocytes and produced embryos in both groups were statistically analyzed, and the chromosomal constitution of the embryos was evaluated by aCGH analysis. Patient consent and ethical approval were obtained as described below in the “Compliance with ethical standards” section. The experimental workflow is illustrated in Fig. 1.
Fig. 1.
Schematic representation of the experimental workflow (2PN two pronuclei, 1PN one pronucleus, DC direct cleavage)
Patients
All participating patients consented to donate unfertilized oocytes for inclusion in this study. These oocytes were collected from 66 controlled ovarian stimulation cycles. Oocytes in group A derived from 30 couples with an average female age of 35.83 ± 5.10 years (median 37 years) and an average male age of 39.83 ± 6.10 years (median 39.5 years). Oocytes in group B derived from 36 couples with an average female age of 36.61 ± 4.75 years (median 37 years) and an average male age of 40.47 ± 5.45 years (median 40 years). The number of treatment cycles per ICSI indication is shown in Table 1.
Table 1.
Number of treatment cycles per ICSI indication
| ICSI indications | |
|---|---|
| Oligozoospermia | 28 |
| Asthenoteratozoospermia | 34 |
| Obstructive azoospermia | 2 |
| Antisperm antibodies | 1 |
| Unexplained infertility | 1 |
Ovarian stimulation, oocyte collection, and fertilization
Ovarian stimulation was performed with the GnRH antagonist protocol, and oocytes were retrieved by ultrasound-guided transvaginal aspiration 36 h after 5000 IU β-human chorionic gonadotropin (β-hCG) administration.
Standard ICSI procedure was performed [1]. After ICSI, the injected oocytes were cultured in single drops of culture media (Sage 1-Step™, Origio a/s, Måløv, Denmark) under oil (Sage In-Vitro Fertilization, Inc., Trumbull, CT, USA) and observed by time-lapse imaging (EmbryoScope® time-lapse incubator, Vitrolife, Gothenburg, Sweden).
Oocytes having extruded the first polar body (PB), but showing no pronuclei 18 h after ICSI, were deemed unfertilized and would normally be discarded; instead, we used them for our study. In the event of doubt about the appearance of pronuclei in an oocyte during fertilization assessment, the time-lapse video was cross-examined by a second embryologist and, if any doubt persisted, the oocyte was manually checked outside the time-lapse system, under a high-power inverted microscope.
Oocyte activation with calcium ionophore A23187 and culture with rhGM-CSF
Oocyte activation was performed according to the following protocol: unfertilized oocytes in both groups were batch-cultured in 50 μl drops of human tubal fluid medium (Modified HTF, Irvine Scientific, Santa Ana, CA, USA) supplemented with 10 % serum substitute supplement (SSS; Irvine Scientific, Santa Ana, CA, USA), containing 5 μM calcium ionophore A23187 (Sigma, St. Louis, MO, USA; stored at 1 mg/ml in dimethyl sulfoxide (DMSO), at −20 °C). The drops were placed under tissue culture oil (Sage In-Vitro Fertilization, Inc., Trumbull, CT, USA) in the dark and incubated at 37 °C for 10 min. Then, oocytes were thoroughly washed through five drops of fresh HTF medium supplemented with 10 % SSS, previously equilibrated at 37 °C. Oocytes in group A were cultured in single 29.8 μl drops of culture medium (Sage 1-Step™, Origio a/s, Måløv, Denmark), in EmbryoSlide® (Vitrolife, Gothenburg, Sweden) culture dishes, under 1.4 ml of tissue culture oil (Sage In-Vitro Fertilization, Inc., Trumbull, CT, USA). After activation with A23187, oocytes in group B were cultured in 29.8 μl drops of culture medium containing 2 ng/ml of rhGM-CSF (R&D Systems, Minneapolis, MN, USA). The cytokine was stored as a stock solution at 2 μg/ml in Dulbecco’s Phosphate Buffer Saline (Gibco®) containing 0.1 % SSS, at −20 °C. Oocytes in group B were cultured in the same way in the time-lapse system.
The time-lapse images were examined 18 h later for signs of activation. Oocytes in either group, showing one or two pronuclei (PN) with one or two PBs (bipronucleate zygotes may rarely show only one PB due to degeneration or heavy fragmentation of the first PB) or undergoing direct cleavage after treatment with calcium ionophore A23187, were deemed activated; these were further cultured until developmental arrest in the time-lapse incubator, in order to assess embryo quality. Oocyte activation rate, cleavage rate, embryo quality, development to day 5, and blastulation rate were calculated and compared between the two groups. Embryo quality of cleavage-stage embryos was categorized into four grades (A–D) as follows: grade A, no fragmentation present and equally sized blastomeres; grade B, <20 % fragmentation with similarly sized blastomeres; grade C, 20–50 % fragmentation with unequally sized blastomeres; and grade D, >50 % fragmentation with unequally sized blastomeres [17]. Blastocyst-stage embryo quality was assessed and categorized according to a well-defined scoring system [45], assessing the extent of blastocoel expansion, and the inner cell mass and trophectoderm quality. Grade A and B cleavage-stage embryos and grade 4AA, 4AB, 4BA, and 4BB blastocysts were considered high quality embryos.
Whole embryo biopsy and preparation for aCGH analysis
All embryos analyzed by aCGH were morula and blastocyst-stage embryos from either group A or B, derived from two pronuclei (2PN) or from direct cleavage, 16–18 h after activation with calcium ionophore A23187.
In order to avoid any residual contamination from cumulus or corona cells, the zona pellucida of all analyzed embryos was removed by micromanipulation, using a non-contact laser system (Saturn, Research Instruments Ltd., Cornwall, UK). Holding the embryo firmly by gentle suction with a holding pipette (Research Instruments Ltd., Cornwall, UK) at 9 o’clock, the zona pellucida was completely cut from 12 to 6 o’clock clockwise using the laser, and the embryo was removed from the remaining zona pellucida (whole embryo biopsy) by gentle suction using a biopsy pipette (The Pipette Company (TPC), Thebarton, South Australia). The procedure was performed in microdrops of buffered culture medium (Modified HTF, Irvine Scientific, Santa Ana, CA, USA) under tissue culture oil (Sage In-Vitro Fertilization, Inc., Trumbull, CT, USA).
The denuded embryos were then transferred in the minimum possible volume of medium (less than 0.5 μl) to 0.2-ml PCR tubes containing 2 μl phosphate-buffered saline (BlueGnome, Cambridge, UK) in a sterile hood. A pulled sterilized micropipette was used for this transfer, and embryos were observed under a stereomicroscope throughout the entire transfer procedure. A different sterile micropipette was used to collect a negative wash control drop.
Whole genome amplification and aCGH analysis
Whole genome amplification (SurePlex, Illumina, Inc.) and aCGH (24sure V3) analysis of morulae and blastocysts was carried out according to the manufacturer’s instructions and as previously described [46]. The slides were scanned using a 10-μm laser scanner (Innopsys 710A; Innopsys S.A., Carbonne, France), and the images analyzed with the BlueFuse Multi software (BlueGnome, Cambridge, UK). The aCGH ratio plots of each embryo were analyzed for gains and losses by at least two independent assessors, to determine the euploid or aneuploid status of each embryo. The chromosomal status of an embryo showing three to four aneuploidies was defined as complex; embryos showing more than four aneuploidies were defined as complex chaotic.
Statistical analysis
The activation rate, cleavage rate, high embryo quality rate, day 5 development rate, and blastulation rate were compared between groups A and B by using Pearson’s chi-square test (two-tailed) or Fisher’s exact test (two-tailed) as appropriate (depending on the expected cell count). All p values in the “Results” section below are chi-square test values (except those marked [F] for Fisher’s exact test). SPSS Statistics (Statistics Package for Social Sciences, version 17) was used for all statistical analyses. A p value of <0.05 was considered statistically significant.
Results
The effect of calcium ionophore A23187 on oocyte activation of unfertilized oocytes after ICSI
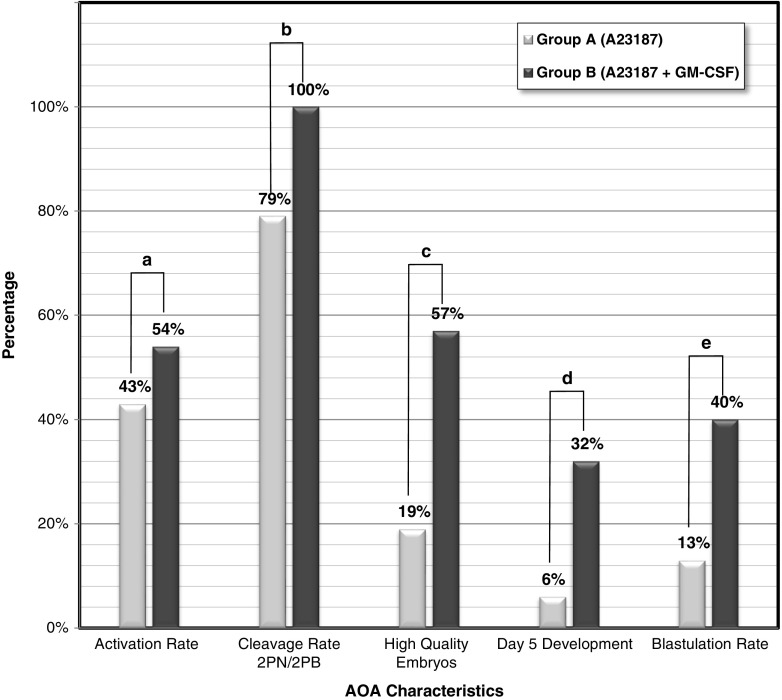
In the present study, a total of 140 oocytes deemed unfertilized after ICSI by monitoring in the time-lapse system were randomly allocated in either group A or B (see Fig. 1). In group A, 75 oocytes were treated with calcium ionophore A23187. In group B, 65 oocytes were initially treated with A23187 as in group A and then cultured in medium supplemented with rhGM-CSF. In group A, 32/75 oocytes were activated (42.66 %), of which 19 showed 2PN/two polar bodies (2PB) (59.37 %), 8 showed 1PN/2PB (25.00 %), and 5 were directly cleaved (15.62 %). In group B, 35/65 oocytes were activated (53.84 %), of which 20 showed 2PN/2PB (one activated oocyte showed two distinct pronuclei and three satellite micronuclei) (57.14 %), 4 showed 1PN/2PB (11.43 %), and 11 were directly cleaved (31.42 %). No differences were observed (see Fig. 2) in the activation rate between the two groups (p = 0.187), nor was any statistically significant difference observed between the 2PN/2PB rate (p = 0.853), the 1PN/2PB rate (p = 0.148), and the directly cleaving embryos (p = 0.130). The cleavage rate of the 2PN/2PB activated oocytes 24–48 h post activation was 15/19 (78.94 %) in group A and 20/20 (100 %) in group B, which was statistically significant (p = 0.047 [F]) in favor of group B (Fig. 2). There was no statistical significance in the cleavage rate of 1PN/2PB oocytes between the two groups (p = 0.333 [F], data not shown).
Fig. 2.
Effect of A23187 (group A) and combination of A23187 and rhGM-CSF (group B) on oocyte activation and subsequent embryonic development (p > 0.05 (a); p < 0.05 (b–e))
The effect of rhGM-CSF on embryo quality and developmental potential of the embryos produced after activation with A23187
After activation with calcium ionophore A23187, the oocytes were further cultured in the time-lapse system, either alone (group A) or in the presence of rhGM-CSF (group B), for 5 days after PN appearance or after direct cleavage. In group A, 6/32 (18.75 %) activated oocytes resulted in high quality embryos, whereas in group B, 20/35 (57.14 %) activated oocytes resulted in high quality embryos; this difference was found to be highly significant (p = 0.001, see Fig. 2). In group A, 5/6 (83.33 %) of the top quality embryos derived from the 2PN/2PB activated group, while the sixth high quality embryo derived from a directly cleaving activated oocyte (16.66 %). In group B, 11/20 (55.00 %) top quality embryos derived from 2PN/2PB embryos, 8/20 (40.00 %) derived from directly cleaving embryos, and 1/20 (5.00 %) from 1PN/2PB embryos. In group A, 4/32 embryos (2 blastocysts and 2 morulae; 12.5 %) bypassed the day 3 developmental arrest stage and reached the day 5 stage, while in group B, 14/35 embryos (11 blastocysts and 3 morulae; 40.00 %) reached the day 5 stage. This difference was also found to be statistically significant (p = 0.011, see Fig. 2). In group A, 2/32 (6.25 %) embryos reached the blastocyst stage on day 5, while in group B, 11/35 (31.42 %) embryos reached the blastocyst stage 5 days after activation; this was found to be highly significant (p = 0.009, see Fig. 2).
Array CGH chromosomal analysis of day 5 embryos in the two groups
In group A, 4 embryos (2 blastocysts and 2 morulae) that reached the day 5 stage were analyzed by aCGH to determine their chromosomal status; in group B, 14 embryos (11 blastocysts and 3 morulae) were also analyzed.
As summarized in Table 2, the analysis of day 5 embryos in group A showed that all four embryos were chromosomally abnormal. Two of the 2PN day 5 embryos in group A showed a double aneuploidy, while the third had complex chromosomal abnormalities (chaotic embryo). The directly cleaving embryo in group A also showed a single aneuploidy (trisomy 22).
Table 2.
Characteristics and aCGH results of day 5 embryos created in groups A and B
| Group | Embryo no. | Activation status | Embryo quality | Sex | aCGH outcome |
|---|---|---|---|---|---|
| A | 1 | 2PN | Blastocyst, 4CC | Turner (XO) | Mon 7, Mon X |
| A | 2 | 2PN | Morula | Male | Mon 6, Tri 15 |
| A | 3 | 2PN | Morula | Klinefelter (XXY) | Complex Chaotic |
| A | 4 | DC | Blastocyst, 4BA | Female | Tri 22 |
| B | 1 | 2PN | Blastocyst, 4AB | Female | 46,XX |
| B | 2 | 2PNa | Blastocyst, 4AA | Male | 46,XY |
| B | 3 | 2PN | Blastocyst, 4AA | Male | 46,XY |
| B | 4 | 2PN | Blastocyst, 4BB | Female | 46,XX |
| B | 5 | 2PN | Blastocyst, 4BB | Male | 46,XY |
| B | 6 | 2PN | Blastocyst, 3CC | Triple X (XXX) | Complex |
| B | 7 | 2PN | Morula | Male | Complex chaotic |
| B | 8 | 2PN | Morula | Female | Mon 6, Mon 22 |
| B | 9 | DC | Blastocyst, 4BB | Turner (XO) | Mon 8, Mon 10 |
| B | 10 | DC | Morula | Female | Complex chaotic |
| B | 11 | DC | Blastocyst, 4BA | Female | Mon 18, Mon 19 |
| B | 12 | DC | Blastocyst, 3CB | Male | Mon 19, Tri 20 |
| B | 13 | DC | Blastocyst, 5AA | Female | Mon 19 |
| B | 14 | DC | Blastocyst, 5AA | Female | Tri 20 |
DC direct cleavage, 2PN two pronuclei, Mon monosomy, Tri trisomy
aThis embryo (B2) showed two distinct pronuclei and three satellite micronuclei
In group B, five of the 2PN-stage embryos were found to be chromosomally normal for all 23 pairs of chromosomes (autosomes and sex chromosomes). All five embryos produced good quality blastocysts on day 5. The rest of the 2PN-stage and directly cleaving embryos were found to be chromosomally abnormal, with six embryos showing single aneuploidies, three embryos showing complex chromosomal abnormalities (two of them being chaotic with more than four chromosomes abnormal), one embryo showing complex abnormalities, and two embryos being chaotic. No chromosomally normal embryo that derived from directly cleaving embryos was identified in either group.
As regards to the 2PN-stage embryos, it is obvious that while none of the 2PN-stage embryos that reached the day 5 stage were chromosomally normal (0/3–0 %) in group A, 5/8 (62.5 %) of the 2PN-stage embryos that reached the day 5 stage in group B were chromosomally normal.
Of the analyzed embryos in group A, one was confirmed as male and one as female and two showed a sex chromosome abnormality; in group B, five embryos were male, seven were female, and two showed a sex chromosome abnormality. Three of the male embryos in group B were among those with a normal chromosomal status.
Discussion
An unexpectedly low fertilization rate, or complete PIFF, is perhaps the worst case scenario for couples undergoing assisted reproductive treatment. An effective treatment for cases of previous partial or complete PIFF with the use of calcium ionophore A23187 for artificial oocyte activation (AOA) has already been established [20]. This technique has been reported to result in improved fertilization rates [18, 47], embryonic developmental potential [8], pregnancy rates [48, 49], and live birth rates [20, 21]. However, the safety of the AOA technique has been questioned [50, 51].
The present study is the first to examine the effect of AOA using the calcium ionophore A23187 in combination with rhGM-CSF supplementation on 18-h aged human unfertilized oocytes after ICSI. Our primary endpoint was to apply a modified AOA protocol on aged human unfertilized oocytes 18 h after ICSI, using calcium ionophore A23187 and then culturing the embryos produced with rhGM-CSF. The goal was to compare this protocol to standard AOA, in which A23187 alone is applied to activate the oocytes artificially, and then embryos are cultured in a standard medium.
We cultured the oocytes with the calcium ionophore A23187 under oil at 37 °C, as per standard IVF laboratory practice. This may introduce a limitation, since the ionophore is soluble in oil and could therefore partly leak out of the medium; however, given the short incubation time (10 min), this depletion seems unlikely.
The comparison of the two study groups showed no difference in the oocyte activation rate. We report a significant difference in favor of group B in the cleavage rate of 2PN/2PB activated oocytes, in high quality embryo formation rate, in the potential of embryos produced to develop to the day 5 stage, and in blastulation rate. These results suggest that rhGM-CSF may significantly improve the developmental potential not only of embryos derived from conventional ICSI but also of embryos produced after AOA with calcium ionophore A23187 of 18-h aged unfertilized oocytes after ICSI. Nevertheless, the activation rate for both groups is lower than the one reported in a previous study [17], and this could be explained by the higher mean age of our patients.
The present study is also the first one where the full chromosomal complement of human embryos derived from AOA of aged oocytes is analyzed by array comparative genomic hybridization (aCGH). A secondary endpoint of our study was indeed to examine the safety of the modified protocol. In the majority of AOA studies, the calcium ionophore A23187 is applied a short time after ICSI [18, 20, 21, 29], in cases of previously known partial or complete PIFF. Failed oocyte activation after ICSI can be foreseen to a certain extent, by assessing the relative expression of PLCζ protein [52]. However, complete or near-complete fertilization failure cannot be predicted for all fresh cycles in the assisted reproduction clinic and the cause of PIFF may be only examined a posteriori [53]. This limits the use of calcium ionophore A23187 in AOA only to known cases with previous fertilization problems. The ideal strategy would be to develop a protocol that would allow AOA and salvage of unfertilized oocytes 18 h after ICSI, should partial or complete fertilization failure occur. However, this may only have a clinical value if the procedure is safe. Indeed, most of the studies on AOA safety have been conducted in cases where the AOA stimulus was applied during or immediately after ICSI [54, 55]. Furthermore, if the procedure proves to be safe by appropriate studies, it may lead to yet another general source of human embryonic stem cell lines [56] for therapeutic purposes.
We found that only embryos derived from group B were euploid, while no day 5 embryos were chromosomally normal in group A. The presence of euploid embryos in group B only should be considered as a random finding, due to the low number of group A embryos reaching the desired developmental stage on day 5, in order to be analyzed by aCGH. On the other hand, rhGM-CSF may have exerted a direct positive effect. It is well known that mitotic chromosomal errors are a common aneuploidy source in all developmental stages of human preimplantation embryos [57–59]. Cytokinesis errors are a common cause for mitotic aneuploidies [60–64]. Failed or asymmetric cytokinesis results in the formation of binucleated cells, tetraploidy or spindle pole abnormalities, and chromosomal chaos [65]. The supplementation of the culture medium with rhGM-CSF, a cytokine that promotes proper cell division and cytokinesis in general, may have contributed in limiting or eliminating such errors, therefore yielding a higher rate of euploid embryos in group B. This GM-CSF effect on alleviating chromosomal errors needs to be examined further.
We were able to accurately identify the artificially activated oocytes that produced two pronuclei, and to distinguish them from the directly cleaving ones, by observing the culture in the time-lapse system. Among the 2PN/2PB euploid embryos in group B, we identified embryo B2 (see Table 2), which showed two distinct pronuclei and three satellite micronuclei after activation. It is known that aCGH technology cannot detect polyploidy, resulting in an estimated 0.2 % missed abnormalities [66]. Despite the theoretical chance of embryo B2 being uniformly polyploid due to the presence of the micronuclei, the distinctive male sex of the embryo leads us to consider that it is euploid.
In addition, only the day 5 embryos in group B that developed from 2PN/2PB artificially activated oocytes were found to be chromosomally normal by aCGH analysis, while no embryos developed from oocytes activated by direct cleavage were euploid, despite their good morphology on day 5 (Table 2). Some of the directly cleaving embryos in group B may have resulted from the cytokinetic effect of GM-CSF itself. In a non-AOA study [67], direct cleavage has been correlated to aneuploidy and our study confirms this finding as well.
Among the day 5 embryos analyzed in both groups, we identified six male embryos (three among the euploid embryos in group B; see Table 2) and one carrying the Y chromosome (XXY). The identification of such embryos bearing a Y chromosome is important, since it is indicative of sperm participation in the formation of pronuclei in activated unfertilized oocytes after ICSI, as has been previously reported [17]. Our results indicate that AOA in combination with rhGM-CSF supplementation can lead to the safe activation of unfertilized oocytes and to the development of chromosomally healthy blastocysts on day 5.
In conclusion, we applied and compared two AOA protocols on 18-h aged human unfertilized oocytes after ICSI: a traditional protocol, in which oocytes were briefly treated with calcium ionophore A23187 alone, and a modified protocol, in which oocytes were cultured with rhGM-CSF after activation with A23187. By using time-lapse and aCGH technologies, we demonstrated that activating 18-h aged unfertilized oocytes by the combination of A23187 and rhGM-CSF can lead to the development of 2PN/2PB embryos showing a high cleavage rate and having the potential to develop into euploid, good-morphology blastocysts on day 5. If our findings are confirmed by further and larger-scale studies, blastocysts derived from aged unfertilized oocytes salvaged with the technique described above could be used clinically for embryo transfer, in cases of unexpected partial or complete post-ICSI fertilization failure.
Acknowledgments
The authors wish to thank Mr. Christos Karamalegos for the help with statistical analysis and Ms. Maria Papadopoulou for collecting the patient consent forms.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
The study was approved by the institutional research ethics committee, and then it was submitted to and obtained authorization by the Hellenic National Authority of Medically Assisted Reproduction (authorization ref. 134/15), as required by national legislation for research use of human embryos not destined to embryo transfer.
Informed consent
All patients who donated oocytes and embryos to be included in the study signed consent forms, after receiving exhaustive information on the purpose of the research carried out, on the procedures to be used, on the possibility to withdraw their consent at any time up to the first actual use of each oocyte or embryo in an experiment, and on the legal and ethical obligation of the authors to destroy all embryos after completion of the experiments, without attempting to transfer them in vivo.
Footnotes
Capsule The exposure of human unfertilized oocytes 18 h after ICSI to a combination of calcium ionophore A23187 and the GM-CSF cytokine can safely salvage the oocytes, resulting in the development of good-morphology, euploid blastocysts, with potential clinical use.
References
- 1.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–8. doi: 10.1016/0140-6736(92)92425-F. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Nagy Z, Joris H, Tournaye H, Smitz J, Camus M, et al. Analysis of 76 total fertilization failure cycles out of 2732 intracytoplasmic sperm injection cycles. Hum Reprod. 1995;10:2630–6. doi: 10.1093/oxfordjournals.humrep.a135758. [DOI] [PubMed] [Google Scholar]
- 3.Moomjy M, Sills ES, Rosenwaks Z, Palermo GD. Implications of complete fertilization failure after intracytoplasmic sperm injection for subsequent fertilization and reproductive outcome. Hum Reprod. 1998;13:2212–6. doi: 10.1093/humrep/13.8.2212. [DOI] [PubMed] [Google Scholar]
- 4.de Klerk C, Macklon NS, Heijnen EM, Eijkemans MJ, Fauser BC, Passchier J, et al. The psychological impact of IVF failure after two or more cycles of IVF with a mild versus standard treatment strategy. Hum Reprod. 2007;22:2554–8. doi: 10.1093/humrep/dem171. [DOI] [PubMed] [Google Scholar]
- 5.Tesarik J, Rienzi L, Ubaldi F, Mendoza C, Greco E. Use of a modified intracytoplasmic sperm injection technique to overcome sperm-borne and oocyte-borne oocyte activation failures. Fertil Steril. 2002;78:619–24. doi: 10.1016/S0015-0282(02)03291-0. [DOI] [PubMed] [Google Scholar]
- 6.Rawe VY, Kopelman S, Nodar FN, Olmedo SB, Chillik CF. Pronuclear abnormalities and cytoskeletal organization during assisted fertilization in a patient with multifollicular ovarian response. J Assist Reprod Genet. 2002;19:152–7. doi: 10.1023/A:1014745006944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, et al. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129:3533–44. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- 8.Ebner T, Oppelt P, Wober M, Staples P, Mayer RB, Sonnleitner U, et al. Treatment with Ca2+ ionophore improves embryo development and outcome in cases with previous developmental problems: a prospective multicenter study. Hum Reprod. 2015;30:97–102. doi: 10.1093/humrep/deu285. [DOI] [PubMed] [Google Scholar]
- 9.Nomikos M. Novel signalling mechanism and clinical applications of sperm-specific PLCζ. Biochem Soc Trans. 2015;43:371–6. doi: 10.1042/BST20140291. [DOI] [PubMed] [Google Scholar]
- 10.Kashir J, Nomikos M, Swann K, Lai FA. PLCζ or PAWP: revisiting the putative mammalian sperm factor that triggers egg activation and embryogenesis. Mol Hum Reprod. 2015;21:383–8. doi: 10.1093/molehr/gav009. [DOI] [PubMed] [Google Scholar]
- 11.Nikiforaki D, Vanden Meerschaut F, de Roo C, Lu Y, Ferrer-Buitrago M, de Sutter P, et al. Effect of two assisted oocyte activation protocols used to overcome fertilization failure on the activation potential and calcium releasing pattern. Fertil Steril. 2016;105:798–806. doi: 10.1016/j.fertnstert.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Baltaci V, Ayvaz OU, Unsal E, Aktas Y, Baltaci A, Turhan F, et al. The effectiveness of intracytoplasmic sperm injection combined with piezoelectric stimulation in infertile couples with total fertilization failure. Fertil Steril. 2010;94:900–4. doi: 10.1016/j.fertnstert.2009.03.107. [DOI] [PubMed] [Google Scholar]
- 13.Mansour R, Fahmy I, Tawab NA, Kamal A, El-Demery Y, Aboulghar M, et al. Electrical activation of oocytes after intracytoplasmic sperm injection: a controlled randomized study. Fertil Steril. 2009;91:133–9. doi: 10.1016/j.fertnstert.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Yanagida K, Katayose H, Yazawa H, Kimura Y, Sato A, Yanagimachi H, et al. Successful fertilization and pregnancy following ICSI and electrical oocyte activation. Hum Reprod. 1999;14:1307–11. doi: 10.1093/humrep/14.5.1307. [DOI] [PubMed] [Google Scholar]
- 15.Ebner T, Moser M, Sommergruber M, Jesacher K, Tews G. Complete oocyte activation failure after ICSI can be overcome by a modified injection technique. Hum Reprod. 2004;19:1837–41. doi: 10.1093/humrep/deh325. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Han XJ, Liu MH, Wang SY, Jia CW, Yu L, et al. Three-day-old human unfertilized oocytes after in vitro fertilization/intracytoplasmic sperm injection can be activated by calcium ionophore a23187 or strontium chloride and develop to blastocysts. Cell Reprogram. 2014;16:276–80. doi: 10.1089/cell.2013.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Q, Zhao Y, Gao X, Li Y, Ma S, Mullen S, et al. Combination of calcium ionophore A23187 with puromycin salvages human unfertilized oocytes after ICSI. Eur J Obstet Gynecol Reprod Biol. 2006;126:72–6. doi: 10.1016/j.ejogrb.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 18.Ebner T, Koster M, Shebl O, Moser M, Van der Ven H, Tews G, et al. Application of a ready-to-use calcium ionophore increases rates of fertilization and pregnancy in severe male factor infertility. Fertil Steril. 2012;98:1432–7. doi: 10.1016/j.fertnstert.2012.07.1134. [DOI] [PubMed] [Google Scholar]
- 19.Ebner T, Maurer M, Oppelt P, Mayer RB, Duba HC, Costamoling W, et al. Healthy twin live-birth after ionophore treatment in a case of theophylline-resistant Kartagener syndrome. J Assist Reprod Genet. 2015;32:873–7. doi: 10.1007/s10815-015-0486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebner T, Montag M, Oocyte Activation Study Group. Montag M, Van der Ven K, Van der Ven H, et al. Live birth after artificial oocyte activation using a ready-to-use ionophore: a prospective multicentre study. Reprod Biomed Online. 2015;30:359–65. doi: 10.1016/j.rbmo.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Eldar-Geva T, Brooks B, Margalioth EJ, Zylber-Haran E, Gal M, Silber SJ. Successful pregnancy and delivery after calcium ionophore oocyte activation in a normozoospermic patient with previous repeated failed fertilization after intracytoplasmic sperm injection. Fertil Steril. 2003;79s3:1656–8. doi: 10.1016/S0015-0282(03)00369-8. [DOI] [PubMed] [Google Scholar]
- 22.Lu Q, Chen X, Li Y, Zhang XH, Liang R, Zhao YP, et al. A live birth of activated one-day-old unfertilized oocyte for a patient who experienced repeatedly near-total fertilization failure after intracytoplasmic sperm injection. Chin Med J. 2012;125:546–8. [PubMed] [Google Scholar]
- 23.Vanden Meerschaut F, Nikiforaki D, De Gheselle S, Dullaerts V, Van den Abbeel E, Gerris J, et al. Assisted oocyte activation is not beneficial for all patients with a suspected oocyte-related activation deficiency. Hum Reprod. 2012;27:1977–84. doi: 10.1093/humrep/des097. [DOI] [PubMed] [Google Scholar]
- 24.Check JH, Summers-Chase D, Cohen R, Brasile D. Artificial oocyte activation with calcium ionophore allowed fertilization and pregnancy in a couple with long-term unexplained infertility where the female partner had diminished EGG reserve and failure to fertilize oocytes despite intracytoplasmic sperm injection. Clin Exp Obstet Gynecol. 2010;37:263–5. [PubMed] [Google Scholar]
- 25.Caglar Aytac P, Kilicdag EB, Haydardedeoglu B, Simsek E, Cok T, Parlakgumus HA. Can calcium ionophore “use” in patients with diminished ovarian reserve increase fertilization and pregnancy rates? A randomized, controlled study. Fertil Steril. 2015;104:1168–74. doi: 10.1016/j.fertnstert.2015.07.1163. [DOI] [PubMed] [Google Scholar]
- 26.Borges E, Jr, de Almeida Ferreira Braga DP, de Sousa Bonetti TC, Iaconelli A, Jr, Franco JG., Jr Artificial oocyte activation with calcium ionophore A23187 in intracytoplasmic sperm injection cycles using surgically retrieved spermatozoa. Fertil Steril. 2009;92:131–6. doi: 10.1016/j.fertnstert.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 27.Kang HJ, Lee SH, Park YS, Lim CK, Ko DS, Yang KM, et al. Artificial oocyte activation in intracytoplasmic sperm injection cycles using testicular sperm in human in vitro fertilization. Clin Exp Reprod Med. 2015;42:45–50. doi: 10.5653/cerm.2015.42.2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nasr-Esfahani MH, Razavi S, Javdan Z, Tavalaee M. Artificial oocyte activation in severe teratozoospermia undergoing intracytoplasmic sperm injection. Fertil Steril. 2008;90:2231–7. doi: 10.1016/j.fertnstert.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 29.Darwish E, Magdi Y. A preliminary report of successful cleavage after calcium ionophore activation at ICSI in cases with previous arrest at the pronuclear stage. Reprod Biomed Online. 2015;31:799–804. doi: 10.1016/j.rbmo.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Ruef C, Coleman DL. Granulocyte-macrophage colony-stimulating factor: pleiotropic cytokine with potential clinical usefulness. Rev Infect Dis. 1990;12:41–62. doi: 10.1093/clinids/12.1.41. [DOI] [PubMed] [Google Scholar]
- 31.Giacomini G, Tabibzadeh SS, Satyaswaroop PG, Bonsi L, Vitale L, Bagnara GP, et al. Epithelial cells are the major source of biologically active granulocyte macrophage colony-stimulating factor in human endometrium. Hum Reprod. 1995;10:3259–63. doi: 10.1093/oxfordjournals.humrep.a135899. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y, Chegini N. The expression of granulocyte macrophage-colony stimulating factor (GM-CSF) and receptors in human endometrium. Am J Reprod Immunol. 1999;42:303–11. doi: 10.1111/j.1600-0897.1999.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 33.Berkowitz RS, Faris HM, Hill JA, Anderson DJ. Localization of leukocytes and cytokines in chorionic villi of normal placentas and complete hydatidiform moles. Gynecol Oncol. 1990;37:396–400. doi: 10.1016/0090-8258(90)90375-U. [DOI] [PubMed] [Google Scholar]
- 34.Uzumaki H, Okabe T, Sasaki N, Hagiwara K, Takaku F, Tobita M, et al. Identification and characterization of receptors for granulocyte colony-stimulating factor on human placenta and trophoblastic cells. Proc Natl Acad Sci U S A. 1989;86:9323–6. doi: 10.1073/pnas.86.23.9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Y, Rong H, Chegini N. Expression and selective cellular localization of granulocyte-macrophage colony-stimulating factor (GM-CSF) and GM-CSF alpha and beta receptor messenger ribonucleic acid and protein in human ovarian tissue. Biol Reprod. 1995;53:923–30. doi: 10.1095/biolreprod53.4.923. [DOI] [PubMed] [Google Scholar]
- 36.Jasper MJ, Brannstrom M, Olofsson JI, Petrucco OM, Mason H, Robertson SA, et al. Granulocyte-macrophage colony-stimulating factor: presence in human follicular fluid, protein secretion and mRNA expression by ovarian cells. Mol Hum Reprod. 1996;2:555–62. doi: 10.1093/molehr/2.8.555. [DOI] [PubMed] [Google Scholar]
- 37.Sjoblom C, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor (GM-CSF) acts independently of the beta common subunit of the GM-CSF receptor to prevent inner cell mass apoptosis in human embryos. Biol Reprod. 2002;67:1817–23. doi: 10.1095/biolreprod.101.001503. [DOI] [PubMed] [Google Scholar]
- 38.Agerholm I, Loft A, Hald F, Lemmen JG, Munding B, Sorensen PD, et al. Culture of human oocytes with granulocyte-macrophage colony-stimulating factor has no effect on embryonic chromosomal constitution. Reprod Biomed Online. 2010;20:477–84. doi: 10.1016/j.rbmo.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 39.Sjoblom C, Roberts CT, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor alleviates adverse consequences of embryo culture on fetal growth trajectory and placental morphogenesis. Endocrinology. 2005;146:2142–53. doi: 10.1210/en.2004-1260. [DOI] [PubMed] [Google Scholar]
- 40.Sjoblom C, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor promotes human blastocyst development in vitro. Hum Reprod. 1999;14:3069–76. doi: 10.1093/humrep/14.12.3069. [DOI] [PubMed] [Google Scholar]
- 41.Robertson SA. GM-CSF regulation of embryo development and pregnancy. Cytokine Growth Factor Rev. 2007;18:287–98. doi: 10.1016/j.cytogfr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Tevkin S, Lokshin V, Shishimorova M, Polumiskov V. The frequency of clinical pregnancy and implantation rate after cultivation of embryos in a medium with granulocyte macrophage colony-stimulating factor (GM-CSF) in patients with preceding failed attempts of ART. Gynecol Endocrinol. 2014;30s1:9–12. doi: 10.3109/09513590.2014.945767. [DOI] [PubMed] [Google Scholar]
- 43.Ziebe S, Loft A, Povlsen BB, Erb K, Agerholm I, Aasted M, et al. A randomized clinical trial to evaluate the effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) in embryo culture medium for in vitro fertilization. Fertil Steril. 2013;99:1600–9. doi: 10.1016/j.fertnstert.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 44.Capalbo A, Ottolini CS, Griffin DK, Ubaldi FM, Handyside AH, Rienzi L. Artificial oocyte activation with calcium ionophore does not cause a widespread increase in chromosome segregation errors in the second meiotic division of the oocyte. Fertil Steril. 2016;105:807–14. doi: 10.1016/j.fertnstert.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 45.Gardner DK, Schoolcraft WB. In-vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Towards reproductive certainty: infertility and genetics beyond 1999. Carnforth: Parthenon; 1999. pp. 378–88. [Google Scholar]
- 46.Christopikou D, Tsorva E, Economou K, Shelley P, Davies S, Mastrominas M, et al. Polar body analysis by array comparative genomic hybridization accurately predicts aneuploidies of maternal meiotic origin in cleavage stage embryos of women of advanced maternal age. Hum Reprod. 2013;28:1426–34. doi: 10.1093/humrep/det053. [DOI] [PubMed] [Google Scholar]
- 47.Tesarik J, Sousa M. More than 90% fertilization rates after intracytoplasmic sperm injection and artificial induction of oocyte activation with calcium ionophore. Fertil Steril. 1995;63:343–9. doi: 10.1016/S0015-0282(16)57366-X. [DOI] [PubMed] [Google Scholar]
- 48.Chi HJ, Koo JJ, Song SJ, Lee JY, Chang SS. Successful fertilization and pregnancy after intracytoplasmic sperm injection and oocyte activation with calcium ionophore in a normozoospermic patient with extremely low fertilization rates in intracytoplasmic sperm injection cycles. Fertil Steril. 2004;82:475–7. doi: 10.1016/j.fertnstert.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 49.Rybouchkin AV, Van der Straeten F, Quatacker J, De Sutter P, Dhont M. Fertilization and pregnancy after assisted oocyte activation and intracytoplasmic sperm injection in a case of round-headed sperm associated with deficient oocyte activation capacity. Fertil Steril. 1997;68:1144–7. doi: 10.1016/S0015-0282(97)00378-6. [DOI] [PubMed] [Google Scholar]
- 50.van Blerkom J, Cohen J, Johnson M. A plea for caution and more research in the ‘experimental’ use of ionophores in ICSI. Reprod Biomed Online. 2015;30:323–4. doi: 10.1016/j.rbmo.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Thompson P. HFEA response to ‘A plea for caution and more research in the “experimental” use of ionophores in ICSI’. Reprod Biomed Online. 2015;31:829–30. doi: 10.1016/j.rbmo.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 52.Aghajanpour S, Ghaedi K, Salamian A, Deemeh MR, Tavalaee M, Moshtaghian J, et al. Quantitative expression of phospholipase C zeta, as an index to assess fertilization potential of a semen sample. Hum Reprod. 2011;26:2950–6. doi: 10.1093/humrep/der285. [DOI] [PubMed] [Google Scholar]
- 53.Heindryckx B, Van der Elst J, De Sutter P, Dhont M. Treatment option for sperm- or oocyte-related fertilization failure: assisted oocyte activation following diagnostic heterologous ICSI. Hum Reprod. 2005;20:2237–41. doi: 10.1093/humrep/dei029. [DOI] [PubMed] [Google Scholar]
- 54.Vanden Meerschaut F, D’Haeseleer E, Gysels H, Thienpont Y, Dewitte G, Heindryckx B, et al. Neonatal and neurodevelopmental outcome of children aged 3–10 years born following assisted oocyte activation. Reprod Biomed Online. 2014;28:54–63. doi: 10.1016/j.rbmo.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 55.Miller N, Biron-Shental T, Sukenik-Halevy R, Klement AH, Sharony R, Berkovitz A. Oocyte activation by calcium ionophore and congenital birth defects: a retrospective cohort study. Fertil Steril. 2016;106:590–6. [DOI] [PubMed]
- 56.Trounson AO. The derivation and potential use of human embryonic stem cells. Reprod Fertil Dev. 2001;13:523–32. doi: 10.1071/RD01101. [DOI] [PubMed] [Google Scholar]
- 57.Katz-Jaffe MG, Trounson AO, Cram DS. Mitotic errors in chromosome 21 of human preimplantation embryos are associated with non-viability. Mol Hum Reprod. 2004;10:143–7. doi: 10.1093/molehr/gah017. [DOI] [PubMed] [Google Scholar]
- 58.Katz-Jaffe MG, Trounson AO, Cram DS. Chromosome 21 mosaic human preimplantation embryos predominantly arise from diploid conceptions. Fertil Steril. 2005;84:634–43. doi: 10.1016/j.fertnstert.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 59.Mantikou E, Wong KM, Repping S, Mastenbroek S. Molecular origin of mitotic aneuploidies in preimplantation embryos. Biochim Biophys Acta. 2012;1822:1921–30. [DOI] [PubMed]
- 60.Hardy K, Winston RM, Handyside AH. Binucleate blastomeres in preimplantation human embryos in vitro: failure of cytokinesis during early cleavage. J Reprod Fertil. 1993;98:549–58. doi: 10.1530/jrf.0.0980549. [DOI] [PubMed] [Google Scholar]
- 61.Harrison RH, Kuo HC, Scriven PN, Handyside AH, Ogilvie CM. Lack of cell cycle checkpoints in human cleavage stage embryos revealed by a clonal pattern of chromosomal mosaicism analysed by sequential multicolour FISH. Zygote. 2000;8:217–24. doi: 10.1017/S0967199400001015. [DOI] [PubMed] [Google Scholar]
- 62.Ruangvutilert P, Delhanty JD, Serhal P, Simopoulou M, Rodeck CH, Harper JC. FISH analysis on day 5 post-insemination of human arrested and blastocyst stage embryos. Prenat Diagn. 2000;20:552–60. doi: 10.1002/1097-0223(200007)20:7<552::AID-PD871>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 63.Bielanska M, Tan SL, Ao A. High rate of mixoploidy among human blastocysts cultured in vitro. Fertil Steril. 2002;78:1248–53. doi: 10.1016/S0015-0282(02)04393-5. [DOI] [PubMed] [Google Scholar]
- 64.Clouston HJ, Herbert M, Fenwick J, Murdoch AP, Wolstenholme J. Cytogenetic analysis of human blastocysts. Prenat Diagn. 2002;22:1143–52. doi: 10.1002/pd.502. [DOI] [PubMed] [Google Scholar]
- 65.Chatzimeletiou K, Morrison EE, Prapas N, Prapas Y, Handyside AH. Spindle abnormalities in normally developing and arrested human preimplantation embryos in vitro identified by confocal laser scanning microscopy. Hum Reprod. 2005;20:672–82. doi: 10.1093/humrep/deh652. [DOI] [PubMed] [Google Scholar]
- 66.Lutz EE. Preimplantation genetic diagnosis (PGD) according to medical ethics and medical law. J Turk Ger Gynecol Assoc. 2012;13:50–5. doi: 10.5152/jtgga.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rubio I, Kuhlmann R, Agerholm I, Kirk J, Herrero J, Escribá MJ, et al. Limited implantation success of direct-cleaved human zygotes: a time-lapse study. Fertil Steril. 2012;98:1458–63. doi: 10.1016/j.fertnstert.2012.07.1135. [DOI] [PubMed] [Google Scholar]