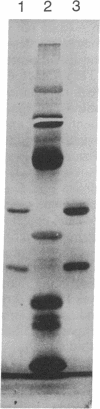
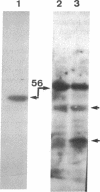
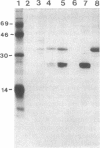
Abstract
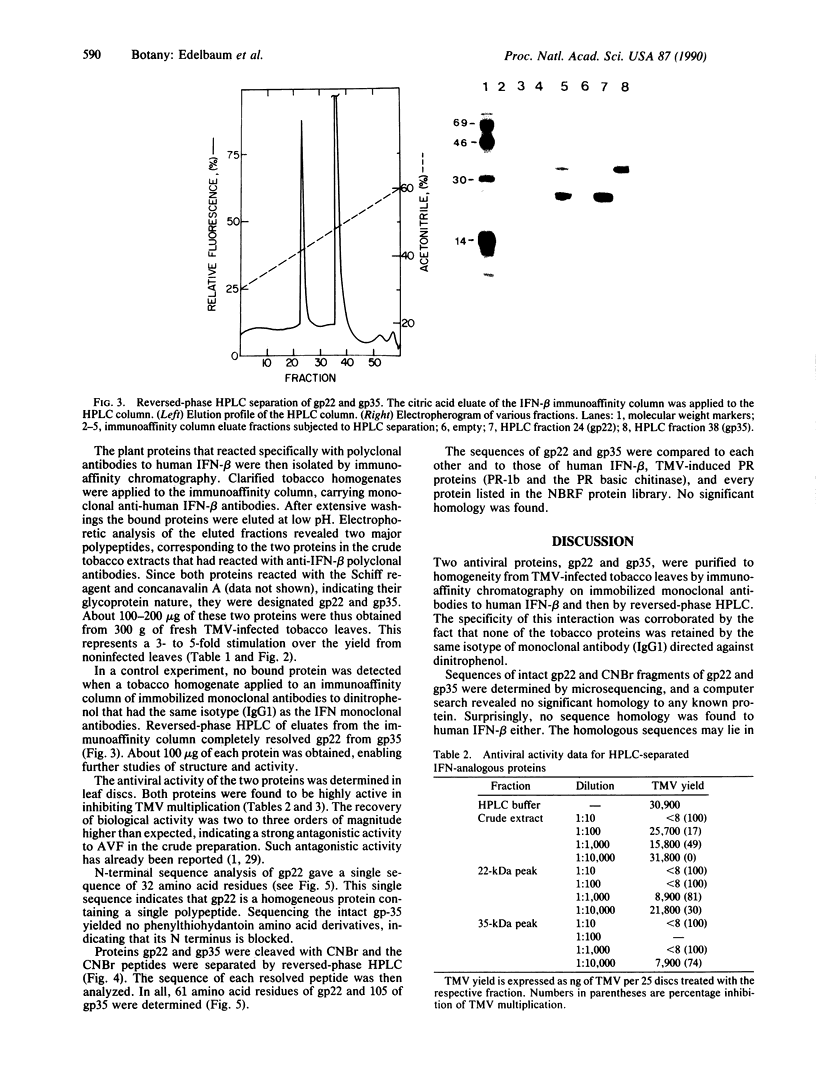
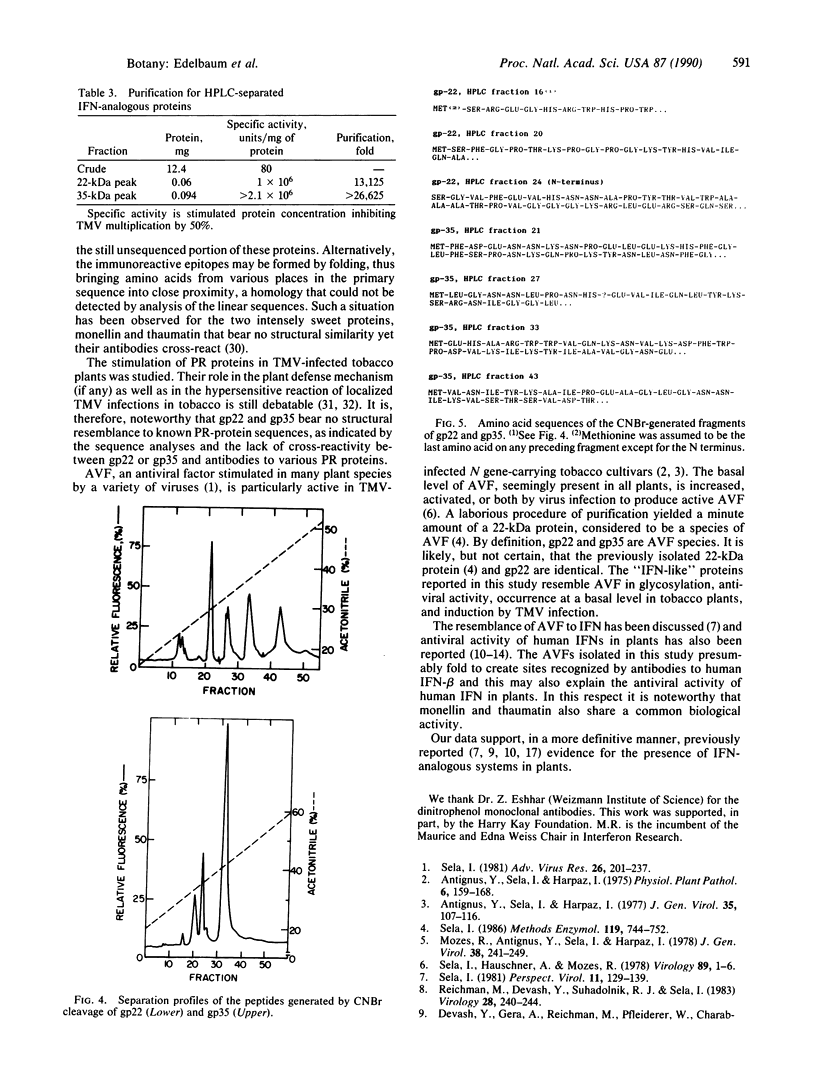
Polyclonal antibodies to human beta-interferon reacted specifically with two plant proteins (gp22 and gp35) by Western blot analysis of crude protein extracts from tobacco leaves infected with tobacco mosaic virus. Immunoaffinity chromatography of these extracts on a column of immobilized monoclonal antibodies to human beta-interferon and then reversed-phase HPLC yielded gp22 and gp35 in a pure state. Both proteins reacted with the Schiff reagent and concanavalin A (indicating their glycoprotein nature) and exhibited antiviral activity (inhibiting tobacco mosaic virus replication in tobacco-leaf discs at concentrations of ng/ml). Each protein was cleaved by cyanogen bromide and the resultant peptides, separated by HPLC, were sequenced as far as the Edman degradation allowed, giving a total of 61 amino acid residues for gp22 and 105 residues for gp35, which represent 30-50% of their expected length. Computer analyses of the sequenced segments revealed no significant homology to human beta-interferon, each other, or any other recorded sequence.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antignus Y., Sela I., Harpaz I. Further studies on the biology of an antiviral factor (AVF) from virus-infected plants and its association with the N-gene of Nicotiana species. J Gen Virol. 1977 Apr;35(1):107–116. doi: 10.1099/0022-1317-35-1-107. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carter W. A., Swartz H., Gillespie D. H. Independent evolution of antiviral and growth-modulating activities of interferon. J Biol Response Mod. 1985 Oct;4(5):447–459. [PubMed] [Google Scholar]
- Chernajovsky Y., Mory Y., Chen L., Marks Z., Novick D., Rubinstein M., Revel M. Efficient constitutive production of human fibroblast interferon by hamster cells transformed with the IFN-beta 1 gene fused to an SV40 early promoter. DNA. 1984 Aug;3(4):297–308. doi: 10.1089/dna.1.1984.3.297. [DOI] [PubMed] [Google Scholar]
- Clark M. F., Adams A. N. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J Gen Virol. 1977 Mar;34(3):475–483. doi: 10.1099/0022-1317-34-3-475. [DOI] [PubMed] [Google Scholar]
- Devash Y., Biggs S., Sela I. Multiplication of tobacco mosaic virus in tobacco leaf disks is inhibited by (2'-5) oligoadenylate. Science. 1982 Jun 25;216(4553):1415–1416. doi: 10.1126/science.6178155. [DOI] [PubMed] [Google Scholar]
- Devash Y., Gera A., Willis D. H., Reichman M., Pfleiderer W., Charubala R., Sela I., Suhadolnik R. J. 5'-dephosphorylated 2',5'-adenylate trimer and its analogs. Inhibition of tobacco mosaic virus replication in tobacco mosaic virus-infected leaf discs, protoplasts, and intact tobacco plants. J Biol Chem. 1984 Mar 25;259(6):3482–3486. [PubMed] [Google Scholar]
- Gander J. E. Gel protein stains: glycoproteins. Methods Enzymol. 1984;104:447–451. doi: 10.1016/s0076-6879(84)04112-4. [DOI] [PubMed] [Google Scholar]
- Kijimoto-Ochiai S., Katagiri Y. U., Ochiai H. Analysis of N-linked oligosaccharide chains of glycoproteins on nitrocellulose sheets using lectin-peroxidase reagents. Anal Biochem. 1985 May 15;147(1):222–229. doi: 10.1016/0003-2697(85)90031-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Loesch-Fries L. S., Halk E. L., Nelson S. E., Krahn K. J. Human leukocyte interferon does not inhibit alfalfa mosaic virus in protoplasts or tobacco tissue. Virology. 1985 Jun;143(2):626–629. doi: 10.1016/0042-6822(85)90402-7. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Van Keuren M. L. Gel protein stains: silver stain. Methods Enzymol. 1984;104:441–447. doi: 10.1016/s0076-6879(84)04111-2. [DOI] [PubMed] [Google Scholar]
- Novick D., Eshhar Z., Gigi O., Marks Z., Revel M., Rubinstein M. Affinity chromatography of human fibroblast interferon (IFN-beta 1) by monoclonal antibody columns. J Gen Virol. 1983 Apr;64(Pt 4):905–910. doi: 10.1099/0022-1317-64-4-905. [DOI] [PubMed] [Google Scholar]
- Ogarkov V. I., Kaplan I. B., Tal'ianskii M. E., Atabekov I. G. Podavlenie reproduktsii virusov kartofelia pod vliianiem interferona cheloveka. Dokl Akad Nauk SSSR. 1984;276(3):743–745. [PubMed] [Google Scholar]
- Ogata C., Hatada M., Tomlinson G., Shin W. C., Kim S. H. Crystal structure of the intensely sweet protein monellin. Nature. 1987 Aug 20;328(6132):739–742. doi: 10.1038/328739a0. [DOI] [PubMed] [Google Scholar]
- Orchansky P., Rubinstein M., Sela I. Human interferons protect plants from virus infection. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2278–2280. doi: 10.1073/pnas.79.7.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Reichman M., Gera A., Sela I. Antiviral activity of natural and recombinant human leukocyte interferons in tobacco protoplasts. Virology. 1985 Jan 15;140(1):173–178. doi: 10.1016/0042-6822(85)90456-8. [DOI] [PubMed] [Google Scholar]
- Sela I. Assay of effect of human interferons on tobacco protoplasts. Methods Enzymol. 1986;119:744–752. doi: 10.1016/0076-6879(86)19100-2. [DOI] [PubMed] [Google Scholar]
- Sela I., Hauschner A., Mozes R. The mechanism of stimulation of the antiviral factor (AVF) in Nicotiana leaves. The involvement of phosphorylation and the role of the N-gene. Virology. 1978 Aug;89(1):1–6. doi: 10.1016/0042-6822(78)90034-x. [DOI] [PubMed] [Google Scholar]
- Sela I. Plant-virus interactions related to resistance and localization of viral infections. Adv Virus Res. 1981;26:201–237. doi: 10.1016/s0065-3527(08)60424-8. [DOI] [PubMed] [Google Scholar]
- Stein S., Moschera J. High-performance liquid chromatography and picomole-level detection of peptides and proteins. Methods Enzymol. 1981;79(Pt B):7–16. doi: 10.1016/s0076-6879(81)79006-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]