Abstract
Atypical functional lateralization and specialization for language have been proposed to account for developmental language disorders, yet results from functional neuroimaging studies are sparse and inconsistent. This functional magnetic resonance imaging study compared children with a specific subtype of specific language impairment affecting structural language (n=21), to a matched group of typically-developing children using a panel of four language tasks neither requiring reading nor metalinguistic skills, including two auditory lexico-semantic tasks (category fluency and responsive naming) and two visual phonological tasks based on picture naming. Data processing involved normalizing the data with respect to a matched pairs pediatric template, groups and between-groups analysis, and laterality indexes assessment within regions of interest using single and combined task analysis. Children with specific language impairment exhibited a significant lack of left lateralization in all core language regions (inferior frontal gyrus-opercularis, inferior frontal gyrus-triangularis, supramarginal gyrus, superior temporal gyrus), across single or combined task analysis, but no difference of lateralization for the rest of the brain. Between-group comparisons revealed a left hypoactivation of Wernicke’s area at the posterior superior temporal/supramarginal junction during the responsive naming task, and a right hyperactivation encompassing the anterior insula with adjacent inferior frontal gyrus and the head of the caudate nucleus during the first phonological task. This study thus provides evidence that this specific subtype of specific language impairment is associated with atypical lateralization and functioning of core language areas.
Introduction
Some children fail to develop typical language for no obvious reason. Their impairment typically reflects difficulties with producing and understanding oral language, and cannot be attributed to sensory-motor or intellectual deficits or other developmental impairment, especially autistic spectrum disorders, and is not the consequence of evident brain lesion or socio-affective deprivation (Bishop, 1997; Rapin et al., 2003). It usually leads to literacy difficulties (Bishop and Snowling, 2004) and, in a substantial number of cases, to persistent language difficulties through adolescence (Stothard et al., 1998). This condition is known as developmental dysphasia (Parisse and Maillart, 2009), developmental language disorder (Rapin et al., 2003), or specific language impairment (SLI; Bishop, 1997).
The current optimal diagnostic procedure of SLI involves both psychometric assessment and clinical appraisal and expertise (Rapin et al., 2003; Bishop and Hayiou-Thomas, 2008), since psychometric criteria alone do not provide sufficient sensitivity, specificity, and clinical congruence (Dunn et al., 1996; Bishop, 2004). Psychometric criteria may include a normal nonverbal intelligence quotient and abnormal language scores (Tomblin et al., 1996). Nonword repetition, sentence repetition, and syntactic manipulation are known to be more sensitive and specific than other tests (Conti-Ramsden, 2003; Bishop, 2004).
SLI is known to be heterogeneous, encompassing distinct clinical profiles that may reflect distinct underlying deficits. A convergent trend distinguishes structural impairments affecting the core components of language (especially morphosyntax and phonology), from those affecting language reception, articulation or social use (auditory verbal processing, oro-motor verbal gesture, and pragmatics) (Rapin and Allen, 1983; Conti-Ramsden et al., 1997; Rapin et al., 2003; Bishop, 2004). Structural impairments are referred to as grammatical SLI (van der Lely, 2005), linguistic dysphasia (Parisse and Maillart, 2009), or typical SLI (T-SLI; Bishop, 2004).
The etiology of SLI remains largely unknown. There is growing evidence that SLI has a genetic component and can be inherited, but a complex picture is emerging of interaction between several genes and environmental risk factors (Fisher et al., 2003; Bishop, 2006). This interaction may affect the anatomo-functional development and organization of the brain langage network (Bates, 1997; Bishop, 2000; Rapin et al., 2003; Friederici, 2006a). In addition, given the left lateralization of language in most typically-developing individuals, atypical language lateralization has been hypothesized to explain literacy and language developmental disorders (Annett, 1985; Geshwind and Galaburda, 1985). Moreover, as acquired childhood aphasias show that unilateral lesion of the dominant hemisphere in childhood rarely leads to a persistent deficit (van Hout, 2003), the abnormality of brain development in SLI is suspected to be bilateral (Vargha-Khadem et al., 1998; Rapin et al., 2003).
Although some studies have reported morphometric and functional brain anomalies in SLI, the results remain inconsistent and heterogeneous (Webster and Shevell, 2004; Friederici, 2006a; Herbert and Kenet, 2007).
Volumetric studies have reported abnormal volume and/or asymmetries of the perisylvian fronto-temporo-parietal region (Plante et al., 1991), inferior frontal region (Jernigan et al., 1991) and posterior perisylvian region or planum temporale (PT) (Jernigan et al., 1991; Leonard et al., 2002). However, other studies have reported normal (Gauger et al., 1997; Preis et al., 1998) and even exaggerated (De Fossé et al., 2004; Herbert et al., 2005) asymmetry of the PT. Furthermore, voxel-based morphometry (VBM) studies have reported no differences of grey matter for core language areas (Jäncke et al., 2007) and grey matter increase in the right posterior superior temporal gyrus (pSTG; Soriano-Mas et al., 2009).
Studies using single photon emission computed tomography (SPECT) have also detected perisylvian functional anomalies, including hypoactivation of the anterior perisylvian region at rest (Lou et al., 1984) and the left inferior parietal region during phoneme discrimination (Tzourio et al., 1994), as well as Broca’s area during a dichotic verbal task (Chiron et al., 1999). However, Ors et al. (2005) reported hypoactivation at rest affecting the right parietal region and a right hyperactivation with lower left asymmetry involving the temporal lobes.
To our knowledge, three studies have investigated SLI using functional magnetic resonance imaging (fMRI). In a study of 8 adolescents with SLI, Ellis-Weismer et al. (2005) reported hypoactivation in the left parietal region and precentral gyrus during sentence comprehension, as well as in the left “insular portion of the inferior frontal gyrus (IFG)” during final word recognition, without any difference of lateralization. Dibbets et al. (2006), in a study of 4 adolescents with SLI, found hyperactivations in frontal, temporal and angular regions during a non-verbal executive paradigm. Finally, based on listening to speech sounds in 5 members of the same family, Hugdhal et al. (2004) reported a leftward but smaller and weaker temporal activation than in controls.
Thus, the results from neuroimaging studies of SLI remain inconsistent, which may be partly due to the heterogeneity of SLI (Rapin et al., 2003; Friederici, 2006a; Herbert and Kenet, 2007; Whitehouse and Bishop, 2008). In addition, although fMRI is promising for investigating developmental language disorders (Friederici, 2006a; Gaillard et al., 2006), there are as yet very few fMRI studies.
One issue of fMRI language mapping is its sensitivity and specificity, i.e. the ability to draw a comprehensive and selective picture of the essential language network (Medina et al., 2007; Tie et al., 2008). Since any single language task is unlikely to engage all aspects of language, and be limited to language processing alone, one strategy is to use several tasks targeting distinct aspects of language (Deblaere et al., 2002; Roux et al., 2003; Gaillard et al., 2004). This makes it possible to focus separately on parts of the network that are distinctly recruited by the tasks, while combined tasks analysis provides a more robust laterality assessment (Ramsey et al., 2001; Rutten et al., 2002).
FMRI in children requires special precautions due to the risk of movement, attentional constraints, task design, and preparation of the child (O’Shaughnessy et al., 2008; Leach and Holland, 2010). The procedure may be even more problematic with young and language-disordered children, who may not be able to do classical tasks involving reading or verbs-to-words and words-to-letter generation, which have been used with typically-developing children. Similar difficulty arises with metalinguistic tasks, which, in addition, require explicit forced-choice analysis and are likely to involve undesired executive effects (Blank et al., 2002; Crinion et al., 2003).
Following fMRI studies that developed task panels for language mapping in children (Gaillard et al., 2004; Wilke et al., 2006; Holland et al., 2007), we developed and tested with typically-developing children a four-task panel that was specifically designed to be feasible for use with young and language-disordered children (de Guibert et al., 2010). The whole procedure avoids reading, metalinguistic or complex executive requirements. It makes use of auditory and visual stimuli, and involves language comprehension and production.
In the present fMRI study, we use this task panel to compare a group of children with typical SLI (T-SLI) to a matched group of typically-developing children. All T-SLI children were referred to a hospital specialized centre and diagnosed on both psychometric and clinical grounds. Data processing includes single task, group and between-group analysis, as well as laterality indexes assessment and comparison within regions of interest using single tasks and combined tasks analysis.
Subjects and methods
Participants
The study was approved by the regional ethics committee of the University Hospital. Parents and children were informed about the experiment; parents signed the informed consent and children gave their verbal assent.
Children with T-SLI were native French speakers recruited among children referred to the Centre for Language and Learning Disorders (University Hospital). They were diagnosed on psychometric and clinical grounds by the interdisciplinary team, after neuropediatric, neuroradiological, neuropsychological and language examinations. Out of the twenty-five children initially recruited, four were finally excluded, because of associated attention-deficit hyperactivity disorder (n=1), non-verbal index within the deficit range (i.e. <70; n=1), and fear (n=1) or teeth braces (n=1) preventing the MRI session. This resulted in a group of 21 T-SLI children aged from 7 to 18 years (mean age=11.4±3.3), with 9 boys (mean age=11.4±3.7) and 12 girls (mean age=11.4±3.1). Three children were left-handed (14.3%), as assessed using the Edinburgh inventory (Oldfield, 1971), which is within the estimation of 8–15% left-handers for the general population (Hardyck and Petrinovitch, 1977). None exhibited any neurological anomalies or auditory deficit, or was affected by communication, behavioural or attentional disorders. The visual inspection of anatomical 3D T1 and FLAIR images by an experienced neuroradiologist showed no significant abnormalities.
A matched group of typically-developing children was recruited excluding non-French native language speakers, previous or current neurological, developmental or psychiatric illness, as well as learning disability or abnormal academic performance. They did not undergo psychometric testing. This resulted in a group of 18 children aged from 8.7 to 17.7 years (mean age=12.7±3), with 9 boys (mean age=12.3±3.2) and 9 girls (mean age=13±3). Two children (11.1%) were left-handed.
The T-SLI and control groups were similar for sex and handedness, and no significant between-group difference was found for age (p= 0.22).
Neuropsychological and language assessment
Neuropsychological assessment includes achievement of the full Wechsler Intelligence Scale for Children–Fourth version (WISC-IV; Wechsler, 2005). This version provides a Verbal Comprehension Index (VCI) and a Perceptual Reasoning Index (PRI) replacing the previous verbal and nonverbal intelligence quotients. Two subjects older than 16 years performed the full Wechsler Adult Intelligence Scale–Third version (WAIS-III; Wechsler, 2000).
T-SLI children as a group showed a discrepancy between low verbal (VCI) and higher nonverbal (PRI) indexes (Table 1). Individually, nonverbal scores were all above the intellectual deficit range (i.e. PRI≥70).
Table 1.
Groups characteristics
| Control group | |
| N | 18 |
| Age (years) | 12.7±3 (8.7––17.7) |
| Gender (male:female) | 9:9 |
| Handedness (left:right) | 2:16 |
|
| |
| T-SLI Group | |
| N | 21 |
| Age (years) | 11.4±3.3 (7––18) |
| Gender (male:female) | 9:12 |
| Handedness (left:right) | 3:18 |
| Intellectual indexes (WISC-IV) | |
| Verbal index (VCI) | 77.0±15.7 (45––112) |
| Non-verbal index (PRI) | 90.0±13.1 (73––121) |
| Language z-scores (L2MA)* | |
| Phonology (complex unfamiliar word repetition) | −2.4±1.7 (−6.9––0.4) |
| Vocabulary (picture naming) | −0.3±1.0 (−2.6––1.3) |
| Morphosyntactic integration (sentence completion) | −1.2±1.5 (−5.0––1.3) |
| Complex instructions comprehension | −0.2±1.1 (−2.2––1.7) |
| Morphosyntax-Comprehension (sentence-picture match) | −0.5±1.0 (−2.5––1.1) |
| Sentence repetition | −1.4±0.8 (−2.5––0.8) |
Ranges are reported in brackets. Bold font indicates language scores <1 SD below the normal mean.
Including scores of the four youngest children who performed equivalent subtests from the Nouvelles Epreuves pour l’Evaluation du Langage (N-EEL; see text).
L2MA= battery Langage oral, Langage écrit, Mémoire, Attention; WISC-IV= Wechsler Intelligence Scale for Children-Fourth version; VCI and PRI= Verbal Comprehension and Perceptual Reasoning Indexes.
The language assessment included subtests from the Langage oral, Langage écrit, Mémoire, Attention battery (L2MA; Chevrie-Muller et al., 1997). Since the L2MA is standardized from 8 years 7 months, four children below this age performed analogous subtests from the Nouvelles Epreuves pour l’Examen du Langage, standardized for younger children (N-EEL; Chevrie-Muller and Plaza, 2001). All children performed the 6 following subtests: phonology (repetition of complex unfamiliar words); vocabulary (picture naming); morphosyntactic integration and comprehension (sentence completion; sentence-picture matching); comprehension of complex instructions; sentence repetition.
T-SLI children as a group showed scores lower than 1 SD below the normal mean for phonology (repetition of complex unfamiliar words), sentence repetition, and morpho-syntactic integration (sentence completion) (Table 1). Individually, all children performed less than 1.5 SD below the normal mean for at least one of these subtests. Thus, all children demonstrated impairments of the phonological or morpho-syntactic components of language, or both, which is characteristic of T-SLI. No child was diagnosed with developmental verbal dyspraxia, verbal auditory agnosia, or pragmatic language impairment.
fMRI protocol and task panel
The protocol has been previously described (de Guibert et al., 2010) and is summarized here. The session includes four language tasks separately implemented with the same parameters, aiming to minimize attentional complications: a simple block design involves alternating a rest condition with a task, starting with rest. Each paradigm comprises three 27 s blocks of each condition and has a total duration of 2 min 48 s. The session has a duration of 30–35 min, including the anatomical acquisition and the four tasks. All subjects perform the tasks in the same order, as during the preparation step, to avoid the mixing of auditory and visual tasks. During the rest condition, children are asked “not to work”, to listen to the noise of the scanner, while fixing their attention on a red cross displayed on the screen.
The panel of tasks does not involve reading, metalinguistic (i.e. explicit analysis of language) or high-executive skills, and targets anterior and posterior core language areas. It uses auditory and visual stimuli delivery, solicits language comprehension and production, and involves lexico-semantic and phonologic processing.
Two auditory lexico-semantic tasks were chosen from the literature because of their distinct and selective activations of either the left IFG (word generation from categories, Gaillard et al., 2003; hereafter category task) or the left STG (auditory responsive naming, Balsamo et al., 2002; hereafter definition task).
In the category task adapted from Gaillard et al. (2003), children hear category names (e.g. animals, colours) and have to silently generate examples of these categories. A category name is delivered every 9 s, with three categories per block and nine categories for the whole task.
In the definition task adapted from Balsamo et al. (2002), children hear definitions of concepts (e.g. “a big animal with a trunk”) and have to find and silently name the corresponding word (e.g. elephant). Definitions are delivered every 9 s, with three definitions per block and nine definitions for the whole task.
Two new visual phonological tasks are based on picture naming and require the child to silently name three objects repetitively (i.e. triplets) one-by-one. Within the triplets, the names are semantically unrelated, but exhibit a minimal phonological change, either, for the first task, a phonological minimal difference (hereafter phon-diff task), or, for the second task, a change in segmentation (hereafter phon-seg task). These tasks are adapted from so-called minimal pairs in linguistics and from procedures used in the assessment and remediation of phonological disorders. The repetitive evocation of just three familiar but phonologically close words attenuates the lexico-semantic requirements, while stressing the phonological constraints.
In both tasks, line-drawings of objects from each triplet are displayed every 1.4 s, successively and randomly (without any picture being delivered twice in succession), so that the child could not predict the upcoming picture. Three distinct triplets (one per block) are used for each task.
In the phon-diff task, children name objects such as poule, boule and moule (/pul/–/bul/–/mul/; [hen, ball, tin]). In the triplets, a difference of distinctive feature occurs in the first phoneme, e.g. the voicing feature of /p/ and /b/ (voiceless vs. voiced) for poule and moule. Concretely, children may successively name, for example: “poule, moule, boule, moule, poule…” in a first block, then: “banc, dent, gant, dent, banc…” (/bã/, /dã/, /gã/…; [bench, tooth, glove]) in a second block, and so on.
In the phon-seg task, children name objects such as “car, car-te and car-t-on” (/kar/–/kart/–/kartõ/; [car, card, cardboard box]). In the triplets, there is a change of segmentation, with phoneme addition (car and then carte or carton) or subtraction (carton and then carte or car). Concretely, children may successively name, for example: “car, carte, carton, carte, car…” in a first block, then: “croix, roi, oie, roi, croix…” (/krwa/, /rwa/, /wa/…; [cross, king, goose]) in a second block, and so on.
For all tasks, children were prepared extensively prior to entering the scanner, with each task being thoroughly explained and practiced several times, using original task material and both aloud and silently, to check for comprehension and achievement by the child.
Data acquisition
Acquisitions were performed on a 3 T whole-body scanner (Achieva, Philips Medical Systems, Best, The Netherlands) using a 8-channel head coil. Anatomical 3D T1-weighted images were acquired with a Fast Field Echo sequence. The acquisition parameters were as follows: TE/TR/Flip angle: 4.6 ms/9.9 ms/8°; acquired matrix size: 256x256 mm; field of view (FOV): 256 mm; voxel size: 1x1x1 mm; volume: 160 sagittal 1 mm thickness slices; acquisition time: 3 min 56 s. Functional images were acquired using a single-shot T2* weighted gradient-echo echo planar imaging sequence. Twenty-four 4 mm slices were acquired with the following parameters: TE/TR/Flip angle: 35 ms/3000 ms/90°; acquired matrix size: 80x80; reconstructed matrix size: 128x128; FOV: 230x230; acquired voxel size: 2.9x2.9x4 mm; reconstructed voxel size: 1.8x1.8x4 mm. Slices were positioned parallel to the anterior commissure-posterior commissure line, with no gap, and were interleaved from bottom to top. Each functional run consisted of 56 series of image acquisitions for the 24 slices covering the entire brain volume separated by a 3000 ms delay, with a total acquisition time of 2 min 48 s. Children were positioned supine in the system. The subject’s head motion was minimized using straps and foam padding.
Visual stimuli were delivered through a screen placed within the head-coil (IFIS-SA fMRI system, Invivo, Orlando, FL) just in front of the face, and synchronised with the scanner. If necessary, the children wore corrective glasses compatible with the high-magnetic-field environment. Auditory verbal stimuli were delivered by an experienced member of the staff using the machine microphone, via specially converted high-fidelity stereo headphones.
Data processing
MRI data were pre-processed and analysed using the General Linear Model (Friston et al., 1995) with SPM5 (Statistical Parametric Mapping; Wellcome Department of Imaging Neuroscience, University College, London; www.fil.ion.ucl.ac.uk). The first two volumes of fMRI data were discarded to allow for signal stabilization. Slice timing and motion correction were applied to the remaining 54 volumes. Data were excluded if associated with excessive motion (more than 3 mm of translation in any direction or 3° of rotation throughout the session). To prevent bias caused by the normalization of pediatric data on adult templates (Wilke et al., 2003a), we used the match pair option of the Template-O-Matic toolbox (Wilke et al., 2008) to generate a customized pediatric template based on the age and sex of our 39 subjects. Structural MRI were segmented using unified segmentation (Ashburner and Friston, 2005), and then normalized. Functional MRI data were registered on structural images, normalized and then smoothed using an isotropic 8-mm Full Width at Half Maximum (FWHM) 3D Gaussian kernel.
Statistical activation maps were obtained using a mixed effects analysis. At the subject level, a high-pass filter was applied to fMRI data to remove slow signal drifts due to undesired effects. The hemodynamic response was modelled by the Informed Basis Set (Friston et al., 1998) to account for possible delay and dispersion of the response from the canonical hemodynamic response function. For each task, group activations were identified by contrasting out the effect of temporal and dispersion derivative, focusing on the canonical variable, at a threshold of p<0.05 Family-Wise-Error corrected for multiple comparisons (FWE-corr.) at the cluster level with a cluster-defining threshold of p<0.001. Between-group comparisons were considered significant at the cluster level at a threshold of p<0.05 FWE-corr., with a cluster-defining threshold of p<0.005.
For regional analyses, ROIs covering brain areas involved in language were selected from the literature: the IFG-opercularis, IFG-triangularis, STG, SMG, and insula. For laterality assessment, the following extended ROIs were also computed: “frontal language” (IFG-opercularis, IFG-triangularis, insula), “temporo-parietal language”“ (STG, SMG), “language” (i.e. combining the two previous regions) and “non-language” (i.e. all brain ROIs except the “language ROI”). Left and right ROIs as delineated in the Automated Anatomical Labeling atlas (AAL; Tzourio-Mazoyer et al., 2002) were adapted to our customized pediatric template using an approach by Wilke et al. (2003b). To match our template, we performed a non-linear deformation of the structural image on which the AAL regions were delineated. The deformation parameters were then applied to the ROIs.
Laterality indexes (LIs) were estimated using the LI toolbox (Wilke and Lidzba, 2007). For each subject, the average t-value within each ROI was measured and voxels smaller than this threshold were discarded. The laterality index was then calculated with the remaining voxels as follows:
where ΣActivationL and ΣActivationR denote the sum of the remaining voxels in the left and right parts of the ROI, respectively.
LI was calculated for each single task and also using combined task analysis (Ramsey et al., 2001), the latter being known to yield more robust LI when dealing with a panel of tasks (Rutten et al., 2002). Boxplots based on these values were created for each region and each task. T-tests were performed on each ROI to determine significant group lateralization (i.e. left or right if LI significantly greater or smaller than zero, respectively; otherwise bilateral). Correlation with age was assessed using covariance analysis including the factors group, age and interaction. When the effect of interaction and age were non-significant, and based on current hypotheses about SLI, a one-tailed two-sample t-test was performed to highlight laterality differences in language ROIs, while a two-sided two-sample t-test was performed in non-language ROIs.
Results
The results are reported successively for each single task, focusing on language areas, including group analysis (Fig. 1), between-group comparisons (Fig. 2), as well as LI measurements and comparison (Fig. 3). Finally, LIs comparison using the combined task analysis is reported (Fig. 4).
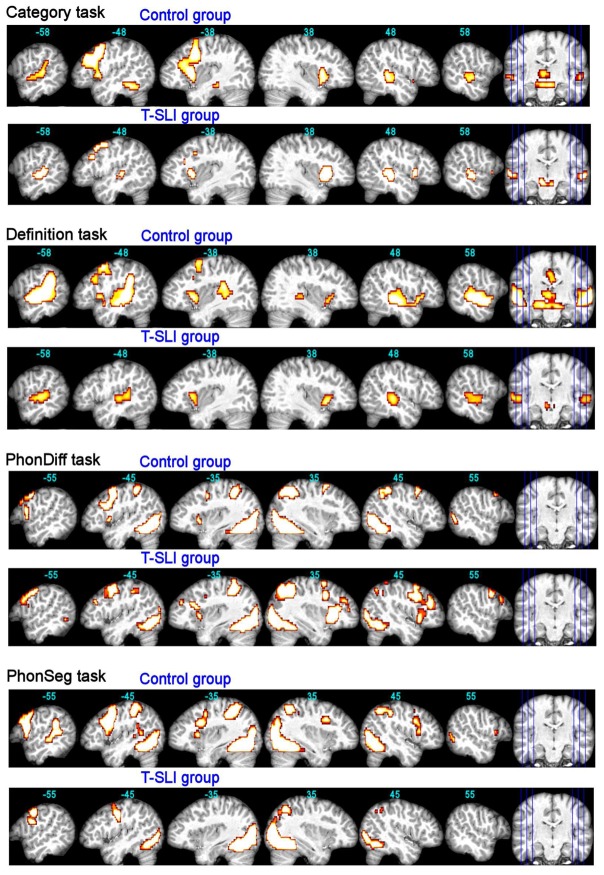
Fig. 1.
fMRI control and T-SLI group effects for each language task (p<0.05 FWE-corr.). The functional maps are superimposed onto an individual brain normalized with respect to our customized pediatric template, with x-coordinates in Montreal Neurological Institute space. Left slices are left hemisphere.
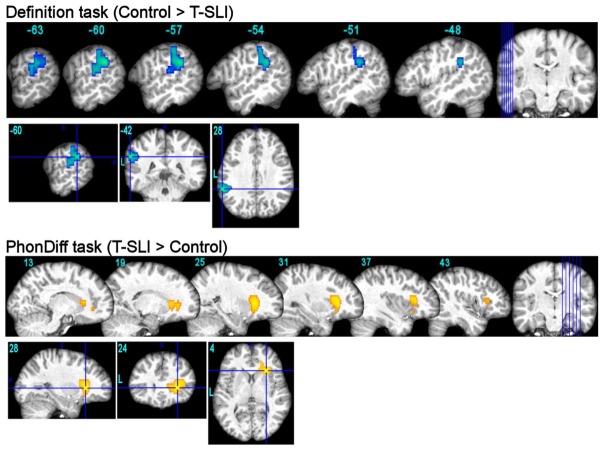
Fig. 2.
fMRI between-group comparisons for definition and phon-diff tasks (p<0.05 FWE-corr.). Blue and yellow colours indicate, respectively, the hypo- and hyper-activations for the T-SLI group compared with the control group. The three-dimensional view focuses on the peak contrasts. Functional maps are superimposed on an individual brain normalized with respect to our customized pediatric template. Coordinates are in Montreal Neurological Institute space.
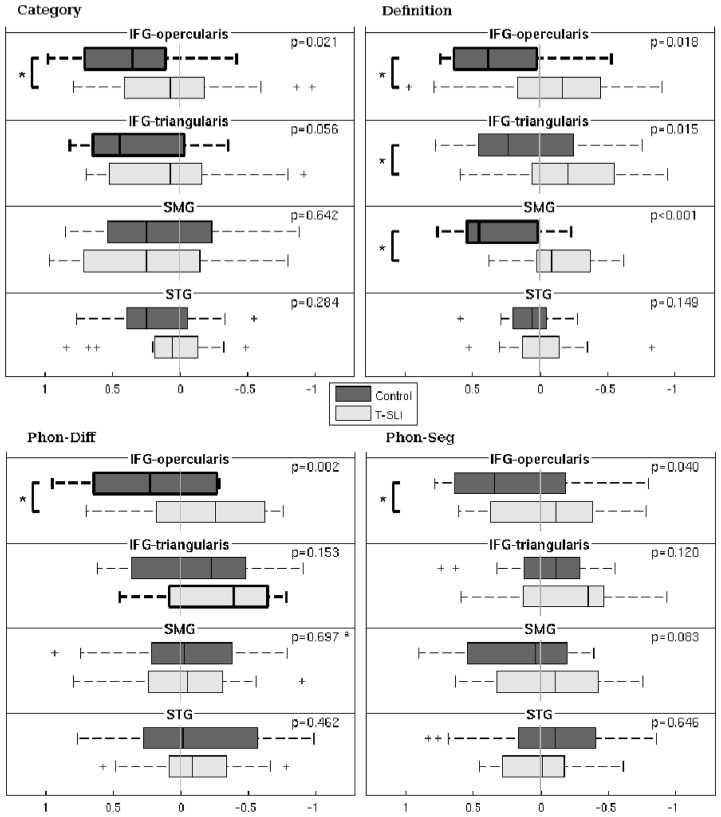
Fig. 3.
Laterality indexes (LIs), significant group lateralizations, and between-group comparisons within the ROIs for each single language task. The box plots depict group lateralization for each ROI, with positive and negative LIs reflecting left and right, respectively, and with significant left or right lateralizations (p<0.05) outlined by bold lines. The p-values of between-group comparisons are indicated, with significant between-group differences outlined by a square bracket with a star.
ap-value of group factor from analysis of covariance with factors age and group.
Oper=opercularis; Tri=triangularis; Sup=superior.
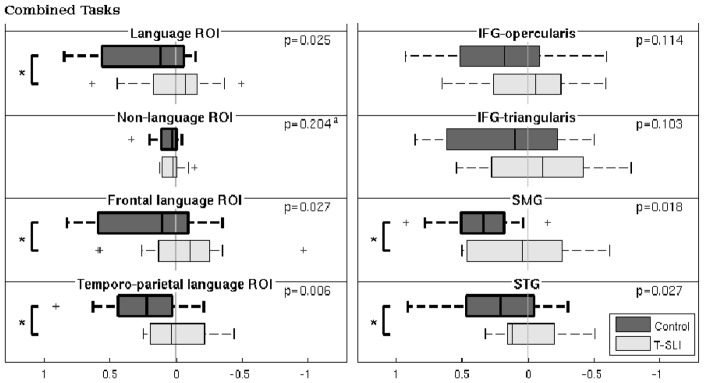
Fig. 4.
Lateralization indexes (LIs), significant group lateralizations, and between-group comparisons using combined tasks analysis (CTA), within single (right panel) and extended (left panel) ROIs. The box plots depict group lateralization for each ROI, with positive and negative LIs reflecting left and right, respectively, and with significant left or right lateralizations (p<0.05) outlined by bold lines. The p-values of between-group comparisons are indicated, with significant between-group differences outlined by a square bracket with a star.
ap-value from two-sided two sample t-test.
IFG=inferior frontal gyrus; STG=superior temporal gyrus; SMG=supramarginal gyrus.
“Temporo-parietal language ROI”=STG and SMG; “Frontal language ROI”=IFG (opercularis and triangularis) and insula; “Language ROI”=“frontal language” and “temporo-parietal language” ROIs; “Non-language ROI”=whole brain (i.e. all AAL ROIs) except “language ROI”.
Auditory language tasks
Category task
The control group shows left-only activations in the dorsal IFG as well as in the pSTG with, as expected, a predominance of the former. A left-dominant activation is situated in the anterior insula and extends into the ventral IFG. According to the LIs, the left lateralization is significant in the IFG-opercularis and -triangularis.
By contrast, the T-SLI group shows small activations in the left dorsal IFG, and no activation in the most posterior STG. The activation of the anterior insula is right-dominant and extends into the ventral IFG on the right. According to the LIs, no ROI is significantly lateralized.
The between-group analysis does not reveal any significant between-group differences. However, the LI comparison reveals a significant lack of left lateralization of the IFG-opercularis in the T-SLI group (Fig. 3).
Definition task
The control group shows left-only activations in the pSTG/adjacent SMG, and in the dorsal IFG, with an expected predominance of the former. A left-dominant activation occurs in the anterior insula and extends into the adjacent ventral IFG. According to the LIs, the SMG and IFG-opercularis are significantly left-lateralized.
By contrast, the T-SLI group shows no activation in the pSTG/adjacent SMG or in the dorsal IFG. A bilateral activation is centred on the anterior insula and extends, superiorly on the right, into the ventral IFG. According to the LIs, no ROI is significantly lateralized, although the IFG and the SMG tend towards the right.
The between-group analysis highlights a left hypoactivation centred on the pSTG/SMG junction in the T-SLI group (Fig. 2; k=304; T=4.42; p=0.03). According to the LI comparison, there is a significant lack of left lateralization of the SMG, IFG-opercularis and IFG-triangularis in the T-SLI group (Fig. 3).
Visual language tasks
Phon-diff task
The control group shows left-only activations in the ventral and dorsal IFG and in the anterior insula, without activation in the STG. According to the LIs, the IFG-opercularis is significantly left-lateralized.
By contrast, the T-SLI group shows a right-only activation in the IFG and a right-dominant activation in the anterior insula. According to the LIs, the IFG-triangularis is significantly right-lateralized.
Between-group analysis highlights a right hyperactivation centred on the anterior insula, extending into the adjacent ventral IFG (opercularis and triangularis) and into the head of the caudate nucleus in the T-SLI group (Fig. 2; k=362; T=4.34; p=0.02). According to the LIs comparison, there is a significant lack of left lateralization of the IFG-opercularis in the T-SLI group (Fig. 3). A positive effect of age on LI is found in the SMG (p=0.024), but no group difference is detected in this region.
Phon-seg task
The control group shows a left-dominant activation in the ventral and dorsal IFG-opercularis, as well as left-only activations in the pSTG/adjacent SMG and in the anterior insula. According to the LIs, no region is significantly lateralized.
By contrast, the T-SLI group shows no activation in the IFG, the STG, the SMG or the insula. According to the LIs, no ROI is significantly lateralized.
The between-group analysis does not reveal any significant between-group differences. However, the LI comparison highlights a significant lack of left lateralization of the IFG-opercularis in the T-SLI group (Fig. 3).
LI assessment and comparison using combined task analysis (CTA)
According to the assessment of LIs using CTA, the control group exhibits a left lateralization of the SMG and STG. This is not observed with the T-SLI group, where no ROI is lateralized. According to the between-group comparison, the lack of left lateralization in the T-SLI group is significant for the SMG and the STG (Fig. 4).
Subsequently, CTA was carried out using extended ROIs: “frontal language”, “temporo-parietal language, “language” (i.e. combining the two latter) and “non-language ROI” (i.e. all AAL ROIs except the latter). In the control group, LIs from the CTA show a left lateralization in all these extended ROIs. This is not the case for the T-SLI group, where no lateralization appears, despite a right trend for the “frontal language” and “language” ROIs.
The between-group comparison of the LIs highlights a significant lack of left lateralization in all extended language ROIs (i.e. “temporo-parietal language”, “frontal language” and “language”), while, inversely, there is no significant difference for the “non-language” ROI (Fig. 4).
In summary, our main results highlight a left hypoactivation centred on the pSTG/SMG junction (definition task), a right hyperactivation of the anterior insula including the adjacent IFG and extending into the head of caudate (phon-diff task), and a lack of left lateralization of core language areas in the T-SLI group. The lack of left lateralization is found for the IFG-opercularis (all tasks), the IFG-triangularis (definition task), the SMG (definition task and combined tasks), the STG (combined tasks), and in all extended language ROIs when using combined tasks. On the contrary, there is no difference of lateralization for the rest of the brain when the language regions are excluded.
Discussion
Although functional neuroimaging may have an expanding role in the investigation of development language disorders, fMRI studies of SLI are sparse and available results from functional studies remain inconsistent, in parallel with heterogeneous morphometric findings. Based on a comparison with typically-developing children, we studied a group of 21 children with typical SLI, a main form of SLI affecting structural aspects of language, which was diagnosed on psychometric and clinical grounds. To apply an appropriate procedure and to improve the mapping by using several tasks, we set up a panel of tasks without reading, metalinguistic or high attentional requirements. Three main interesting results arise from our study: i) the lack of left lateralization of core language areas; ii) the left hypoactivation centred on the pSTG/SMG junction (definition task); iii) the right hyperactivation of the anterior insula including the adjacent IFG and extending into the head of caudate (phon-diff task).
Lack of left lateralization of core language areas
The study reveals a lack of left functional lateralization for all single language ROIs across single or combined tasks, i.e. in the IFG-opercularis (all tasks), the IFG-triangularis (definition task), the SMG (definition and combined tasks), and the STG (combined tasks). In addition, when using combined tasks analysis, this lack also applies to larger frontal and temporo-parietal language ROIs, while, interestingly, no significant difference appears for the whole brain when language ROIs are excluded. Thus, our study provides evidence that T-SLI is associated with atypical lateralization of language function in core language areas.
No anomaly of lateralization was found or reported in previous fMRI studies of SLI (Hugdhal et al., 2004; Ellis Weismer et al., 2005; Dibbets et al., 2006), including a study of oro-facial verbal dyspraxia (Liégeois et al., 2003), which could be due to reduced sample sizes, distinct activation tasks, and/or distinct clinical subtypes. However, SPECT studies at rest have reported a reversed asymmetry in Wernicke’a area (Chiron et al., 1999) and a more symmetric activation in the temporal lobe (Ors et al., 2005). Furthermore, one study using functional transcranial Doppler ultrasonography during words-to-letter generation reported a lack of leftward dominance of blood flow in adults with persisting SLI, a condition more associated with structural language impairment than transient SLI (Whitehouse and Bishop, 2008).
Our results support the hypothesis of atypical cerebral dominance for literacy and language developmental disorders, although atypical lateralization is not in itself either abnormal or specific. Atypical functional lateralization has been reported in 5% of right-handed and 73–80% of non-right-handed normal subjects (Szaflarski et al., 2002) and in other developmental clinical conditions including speech delay (Bernal and Altman, 2003), stuttering (Brown et al., 2005), autism spectrum disorder (Knaus et al., 2008; Kleinhans et al., 2008), and dyslexia (Maisog et al., 2008; Heim et al., 2010).
Therefore, our study shows that a well-defined form of SLI affecting structural aspects of language is more associated with atypical functional lateralization of core language areas, but we cannot yet affirm that it is a specific marker of T-SLI. As argued by Whitehouse and Bishop (2008), atypical cerebral lateralization may be an indicator, albeit imperfect, of some causal factor that leads together to atypical cerebral lateralization and language impairment.
Left hypoactivation of the pSTG/SMG junction
The second result, provided by the definition task, is the left hypoactivation centred on the junction of the posterior supratemporal plane (STG) and the SMG, extending laterally, deeply into the Sylvian fissure, and superiorly in the parietal operculum and parietal inferior lobule. This region is crucial for language, belonging to the so-called “Wernicke’s area” or “territory” (Blank et al., 2002; Catani et al., 2005).
The central location of the hypoactivation corresponds to the posterior PT/ventral SMG region (Price, 2010) or “area sylvian parietal-temporal” (Hickok and Poeppel, 2007), which may be a sensorimotor interface translating acoustic speech signals from the posterior temporal sulcus into articulatory representation for the premotor cortex and posterior IFG. This region is involved in both complex speech perception and production (e.g. Hickok et al., 2003; Price, 2010), and its lesion may be associated with conduction aphasia, which exhibits phonemic paraphasias with better preserved comprehension (Hillis, 2007). Therefore, on the whole, the putative function of this region is in agreement with the task used here involving both speech reception and (covert) production, and with the linguistic deficit inherent to T-SLI.
In line with our result, SPECT studies of SLI have reported bilateral posterior perisylvian hypoactivation at rest (Lou et al., 1984), and no activation of the left inferior parietal region during phonological discrimination (Tzourio et al., 1994). One fMRI study has also reported a hypoactivation in the left parietal lobe during sentence comprehension (Ellis Weismer et al., 2005). By contrast, using SPECT at rest, Ors et al. (2005) reported a hypoactivation of the right parietal region.
One question is whether our result can be linked to morphological anomalies. Volumetric studies of SLI have shown a reduced volume of the perisylvian temporoparietal region (Jernigan et al., 1991) and reduced left asymmetry of the PT when compared with reading disability (Leonard et al., 2002). However, other authors report normal volume and asymmetry for the PT (leftward) and for the parietal ascending ramus (rightward) (Gauger et al., 1997; Preis et al., 1998), and even an exaggerated leftward asymmetry for the PT (De Fossé et al., 2004; Herbert et al., 2005), without any difference for the parietal opercule and the SMG (De Fossé et al., 2004). Using VBM, Jäncke et al. (2007) found no grey matter differences for core language areas, and Soriano-Mas et al. (2009) reported an increase in grey matter at the right temporoparietal junction. Apart from methodological differences, this heterogeneity may result from the heterogeneity of SLI.
In summary, the left hypoactivation of the pSTG/SMG junction found in T-SLI children during the auditory responsive naming task could reflect a dysfunction of a core region considered as an interface between complex language reception and production (Hickok and Poeppel, 2007; Price, 2010). This result converges with a SPECT study of SLI using a discrimination task involving phonologically-close words (Tzourio et al., 1994).
Right hyperactivation of the anterior insula, adjacent IFG, and head of caudate
The third main result of this study is the right hyperactivation centred on the anterior insula and extending into the adjacent IFG (opercularis and triangularis), as well as into the head of caudate, during the phon-diff task. The anterior insula and adjacent IFG is an important region for language, in continuity with the frontal operculum and, at the left side, Broca’s area (Keller et al., 2009). The head of caudate has already been highlighted in a developmental speech disorder, oro-facial verbal dyspraxia (Vargha-Khadem et al., 2005).
The anterior insula is involved in motor aspects of speech, although its specific role for speech remains unclear. Overall, this region has been linked with coordination and motor control of speech articulation, vocal tract and mimic muscles, as well as non-speech orofacial gestures, swallowing and respiratory voluntary regulation (Brown et al., 2005, 2009; Ackermann and Riecker, 2010; Price, 2010). Clinical studies have yielded some controversial results, with one study based on lesion overlap reporting a correlation of left insular lesion with deficits in motor programming of speech (Dronkers, 1996), which was not replicated using neuroimaging at stroke onset (Hillis et al., 2004). Single clinical cases and brain stimulation studies involving the insula have reported aphasic, dysarthric, speech initiation and/or non-speech oro-motor disturbances, and functional neuroimaging studies have revealed the involvement of the anterior insula, predominantly on the left, in motor aspects of speech (Ackermann and Riecker, 2010; Price, 2010). Interestingly, Bohland and Guenther (2006) found increased bilateral activation of the insula/IFG junction in proportion to phonological complexity by requiring triads of syllables of varying complexity (“ta-ta-ta”/”ka-ru-ti”/”stra-stra-stra”/”kla-stri-splu”), which suggests a function of integration of low-level motor aspects, abstract speech sounds and prosodic components in speech planning. The right anterior insula/frontal operculum may specifically mediate supra-segmental aspects of speech (prosody, intonation contour), as well as vocal imitation and musical melodies (Brown et al., 2005; Ackermann and Riecker, 2010).
Therefore, the hyperactivation in the right anterior insula/adjacent IFG during our phonological task could reflect an articulatory and/or prosodic compensatory mechanism of defective structural phonological function in T-SLI. Such a compensation could be inter-hemispheric (i.e. left to right) and possibly intra-hemispheric (i.e. lateral IFG to insula). Compensatory interhemispheric recruitment of the right inferior frontal region is well-known after left acquired lesions associated with aphasia (Crinion and Leff, 2007), and the recruitment of the right insula may compensate dysfunction of the left counterpart (Duffau et al., 2001). An intrahemispheric shift of frontal response towards the anterior insula/frontal operculum, at the right side, has also been reported in subjects with left temporal lobe epilepsy (Voets et al., 2006). If further corroborated, this suggests that the insular structure might be involved in the compensation of speech/language function (Ackermann and Riecker, 2010).
Previous functional neuroimaging studies of SLI yield results that are heterogeneous with respect to the insula. A right hyperactivation has been highlighted in the anterior part during speech sound listening (Hugdhal et al., 2004) and in the posterior part during a non-verbal executive paradigm (Dibbets et al., 2006). This contrasts with hypoactivation on the left during word recognition (Ellis Weismer et al., 2005). While one volumetric study reports a volume reduction of the left insula (Jernigan et al., 1991), no anomaly of the insula has been observed using VBM (Jäncke et al., 2007; Soriano-Mas et al., 2009).
In our study, the right hyperactivation extends into the head of caudate. The caudate nucleus participates in sensorimotor coordination including response selection and initiation, in executive-related processes, and may support the planning and execution of correct strategies required for complex goals (Grahn et al., 2008). As regards speech, the caudate nucleus is involved in the control and selection of articulatory motor sequences, and may initiate cortical phonological and controlled processes when automatic processes are not well-suited (Friederici, 2006b; Booth et al., 2007). Furthermore, the bilateral head of caudate is involved in language-based conflict, suggesting that it participates in the suppression of inappropriate responses in a competitive context (Price, 2010). Similarly, the hyperactivation of the right head of caudate during the phon-diff task in our study could reflect higher compensatory attempts of initiation of phonological processes in the context of phonological conflict (e.g. the minimal difference between pain/bain/main).
Regarding previous neuroimaging studies of SLI, the right caudate has been found to be hyperactive during a non-verbal switch paradigm in four children (Dibbets et al., 2005). Moreover, it was bilaterally reduced in the volumetric study by Jernigan et al. (1991), but increased on the left in the VBM study by Soriano-Mas et al. (2009).
In summary, the right hyperactivation of the anterior insula/adjacent IFG and the head of caudate in T-SLI, which is highlighted when requiring phonological differentiation, could reflect compensatory recruitments of non language-specific functions resulting from the structural phonological deficit. This could be associated with higher recruitment of orofacial and intonative motor functions for the anterior insula/adjacent IFG, and response initiation and selection in the context of interferences for the head of caudate.
Comparison to other developmental disorders
Another question is whether the functional abnormalities reported in our study are specific to structural language disorder (i.e. T-SLI) compared to disorders affecting other aspects of language such as communication, reading or speech.
As regards the left temporoparietal hypoactivation, in autistic spectrum disorder, on the contrary, hyperactivation has been detected in the posterior temporal region during a responsive naming task (Knaus et al. 2008) and other language tasks (Just et al., 2004; Harris et al., 2006). On the other hand, left temporoparietal hypoactivation appears to be a “neural signature” of dyslexia (Shaywitz and Shaywitz, 2008), as obtained during reading, rhyme or semantic tasks (Paulesu et al., 1996; Schulz et al., 2008; Richlan et al., 2009). Phonological disturbances in dyslexia nevertheless concern metalinguistic tasks (i.e. phonological awareness) rather than direct oral language, likely reflecting a phonological-access deficit (Ramus and Szenkovits, 2008), and the differentiation from SLI remains clinically and etiologically justified (Bishop and Snowling, 2004). Finally, as regards speech disorders, developmental stuttering has been associated with hypoactivation of the auditory cortices, but not of the temporoparietal junction (Brown et al., 2005; Watkins et al., 2008), while oro-facial verbal dyspraxia in the KE family was associated with hypoactivation near the left pSTG/SMG junction during covert verb generation (Liégeois et al., 2003). Although the core deficit in this family is dyspraxic, affected members also exhibit phonological and grammatical impairments, so linguistic deficits cannot be ruled out (Vargha-Khadem et al., 2005).
As regards the right hyperactivation of the anterior insula/adjacent IFG, in autistic spectrum disorder, the insula has been found to be hypoactive during tasks involving social processing (Uddin and Menon, 2009). In dyslexia, hyperactivation of the anterior insula has been observed during reading tasks, either on the left or on the right (Maisog et al., 2008; Richlan et al., 2009). However, one study reports a bilateral hypoactivation in parallel to higher activation of the adjacent frontal operculum during click and speech sound listening (Steinbrink et al., 2009). As regards speech disorders, the anterior insula has been found to be hyperactive at the left side during overt words repetition in oro-facial verbal dyspraxia (Liégeois et al., 2003). Nevertheless, it is noteworthy that hyperactivation of the right frontal operculum/anterior insula during speech production is considered as a “neural signature” of developmental stuttering (Brown et al., 2005; Watkins et al., 2008). This suggests that lower skills for speech result in compensatory hyperactivation of vocal-motor areas, which are right-lateralized because of left dysfunction of the normal dedicated regions (Preibish et al., 2003; Brown et al., 2005).
As regards the right hyperactivation of the caudate, no functional anomaly of the caudate has been found either in autistic spectrum disorder during language tasks (Harris et al., 2006; Knaus et al., 2008), or in dyslexia (Maisog et al., 2008; Richlan et al., 2009). Nevertheless, in studies of oro-facial verbal dyspraxia in the KE family, the caudate was found to be both bilaterally morphologically reduced (Varkha-Khadem et al., 1998; Watkins et al., 2002; Belton et al., 2003), and functionally hyperactive on the left (Vargha-Khadem et al., 1998). In this context, these anomalies come under the hypothesis of a dysfunction of the frontostriatal network (Vargha-Khadem et al., 2005). Finally, the activity of the caudate on both sides has been positively correlated with the severity of developmental stuttering (Giraud et al., 2008), and a dysfunction of the basal ganglia has been speculated to underlie the deficit in the timing of speech motor initiation (Alm, 2004), or to reflect a secondary dysfunction resulting from a left inferior frontal anomaly (Kell et al., 2009).
In summary, the left temporoparietal hypoactivation associated with T-SLI is similar to results obtained by studies of dyslexia (Richlan et al., 2009) and by one study of oro-facial verbal dyspraxia (Liégeois et al., 2003). Moreover, in developmental suttering, the hyperactivation of the right anterior insula is regarded as a “neural signature” (Brown et al., 2005) and the activity of the right caudate is correlated with the severity of impairment (Giraud et al., 2008). As these disorders and the activation tasks used are distinct, further studies are needed to elucidate whether these similarities reflect common dysfunctions, common atypical compensatory modes of resolution of language tasks, and/or more task-specific effects.
Methodological considerations
As described previously (de Guibert et al., 2010), to optimize the feasibility of the procedure for young disordered children, and minimize motion artefacts as well as attentional complications, we implemented four identical block-designed paradigms with a low-level condition as baseline (listening to the noise and fixing a red cross) and without requiring motor responses. These choices reduced the heterogeneity and complexity of the protocol, as the child did not have to understand and achieve supplementary control tasks and also give motor responses. Furthermore, although requiring motor responses is well suited for metalinguistic tasks (i.e. judgment tasks with explicit analysis), which may involve additional non-language functions (Blank et al., 2002; Crinion et al., 2003), it is not appropriate for investigations under more natural conditions such as word production.
However, these choices have some drawbacks. First, the achievement of the tasks cannot be directly assessed. Requiring overt responses would allow online performance monitoring, but speech increases the risk of movement, which is crucial in the case of children, and especially with disordered children (O’Shaughnessy et al., 2008). Therefore, children were intensively prepared before the scanner session using the same order of tasks and stimuli, allowing us to check that they understood and were able to achieve the tasks, and were also questioned after the session. Thus, the design of the tasks and the preparation aimed to ensure high performance for each child during the scanner session. Secondly, low-level control conditions involve more non-language-specific coactivations (Wilke et al., 2006; Holland et al., 2007) and, in some research contexts, it may be important to use high-level control tasks to target more specific functions. Nevertheless, together with the use of several tasks, a low-level control condition makes it possible to map a comprehensive and specific left-lateralized language network in normal children or adults (Ramsey et al., 2001; Tie et al., 2008; de Guibert et al., 2010), and was appropriate in our study for the comparison with language-impaired children. Furthermore, we used a combined tasks analysis to provide a robust assessment of language lateralization.
Moreover, to select a representative sample of the general population, close to the clinical context for language-disordered children, we did not solely recruit right-handed children. In this study, the proportions of left-handed in the T-SLI and control groups are similar and within the normal range estimate. Furthermore, additional analyses excluding left-handed children (n=5) were carried out, which showed no major change of results (e.g. out of 11 LI differences for the whole group, 8 remained significant and 2 were nearly significant [p=0.057] when eliminating left-handers). Moreover, to avoid distortions due to normalization of the childrens’ data with respect to an adult template, we used a tool dedicated to the creation of pair- and group-matched normalized templates based on normative brain data (Wilke et al., 2008).
Finally, a well-known issue with the category of SLI is its clinical heterogeneity, since it encompasses impairments reflecting structural rule-like deficits (i.e. mainly phonology and morphosyntax), as well as impairments of articulatory, auditory receptive or pragmatic aspects of language. Since this heterogeneity may be a crucial source of inconsistency in the neuroimaging results, we focus here on structural language impairments, known as typical SLI or linguistic dysphasia. Future studies need to investigate whether distinct subtypes of SLI are associated with distinct brain functional anomalies. Secondly, since the current psychometric diagnostic tools are not totally adequate when used in isolation, because they lack clinical congruence, we selected T-SLI children on both psychometric and clinical grounds. The T-SLI children, as a group, failed three subtests (repetition of unfamiliar words, sentence completion, and sentence repetition) that are acknowledged as being especially sensitive to SLI, and all of the subjects had an early history of selective language impairment and had been diagnosed in our specialized hospital Centre.
In conclusion, by using fMRI with a panel of distinct language tasks, this study provides evidence that a well-defined type of SLI affecting structural components of language is associated with a lack of left functional lateralization in core language areas (pars opercularis and triangularis of the IFG; STG; SMG), with hypoactivation of the left superior temporoparietal junction, within Wernicke’s area, as well as with hyperactivation of the right anterior insula, adjacent inferior frontal gyrus and head of caudate. These results are similar to some findings from studies of developmental disorders involving other aspects of language such as dyslexia, stuttering or orofacial verbal dyspraxia, which will require further comparisons.
Acknowledgments
Founding
This work was supported by a “Projet Hospitalier de Recherche Clinique” (PHRC–2007; University Hospital, Pontchaillou, Rennes, France), and the “Association pour la Recherche Clinique sur l’Epilepsie”. This work benefited from a research delegation from INRIA (Institut National de Recherche en Informatique et Automatique) granted to CdG.
We would like to thank the children who participated in the study and their parents, as well as the teams at the Regional Centre for Language and Learning Disorders and at the Department of Neuroradiology, for their contribution.
Abbreviations
- IFG
inferior frontal gyrus
- LI
lateralization index
- PT
planum temporale
- ROI
region of interest
- SMG
supramarginal gyrus
- STG
superior temporal gyrus
- T-SLI
typical specific language impairment
References
- Ackermann H, Riecker A. The contribution(s) of the insula to speech production: a review of the clinical and functional imaging literature. Brain Struct Funct. 2010;214:419–33. doi: 10.1007/s00429-010-0257-x. [DOI] [PubMed] [Google Scholar]
- Alm PA. Stuttering and the basal ganglia circuits: a critical review of possible relations. J Commun Disord. 2004;37:325–69. doi: 10.1016/j.jcomdis.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Annett M. Left, right, hand and brain: the Right Shift Theory. Hillsdale, NJ: Erlbaum; 1985. [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Balsamo LM, Xu B, Grandin CB, Petrella JR, Braniecki SH, Elliott TK, Gaillard WD. A functional magnetic resonance imaging study of left hemisphere language dominance in children. Arch Neurol. 2002;59:1168–74. doi: 10.1001/archneur.59.7.1168. [DOI] [PubMed] [Google Scholar]
- Bates E. Origins of language disorders: a comparative approach. Dev Neuropsychol. 1997;13:447–76. [Google Scholar]
- Belton E, Salmon CH, Watkins KE, Vargha-Khadem F, Gadian DG. Bilateral brain abnormalities associated with dominantly inherited verbal and orofacial dyspraxia. Hum Brain Mapp. 2003;18:194–200. doi: 10.1002/hbm.10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal B, Altman NR. Speech delay in children: a functional MR imaging study. Radiology. 2003;229:651–8. doi: 10.1148/radiol.2293021746. [DOI] [PubMed] [Google Scholar]
- Bishop DV. Uncommon Understanding: Development and Disorders of Comprehension in Children. East Sussex, UK: Hove; 1997. [Google Scholar]
- Bishop DV. How does the brain learn language. Insights from the study of children with and without language Impairment. Dev Med Child Neurol. 2000;42:133–42. doi: 10.1017/s0012162200000244. [DOI] [PubMed] [Google Scholar]
- Bishop DV. What causes specific language impairment in children? Curr Dir Psychol Sci. 2006;15:217–21. doi: 10.1111/j.1467-8721.2006.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DV. Diagnostic dilemmas in specific language impairment. In: Verhoeven L, Van Balkom J, editors. Classification of developmental language disorders. Mahwah, NJ: Erlbaum; 2004. pp. 309–26. [Google Scholar]
- Bishop DV, Hayiou-Thomas ME. Heritability of specific language impairment depends on diagnostic criteria. Genes Brain Behav. 2008;7:365–72. doi: 10.1111/j.1601-183X.2007.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DV, Snowling MJ. Developmental dyslexia and specific language impairment: same or different? Psychol Bull. 2004;130:858–86. doi: 10.1037/0033-2909.130.6.858. [DOI] [PubMed] [Google Scholar]
- Blank SC, Scott SK, Murphy K, Warburton E, Wise RJ. Speech production: Wernicke, Broca and beyond. Brain. 2002;125:1829–38. doi: 10.1093/brain/awf191. [DOI] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32:821–41. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- Booth JR, Wood L, Lu D, Houk JC, Bitan T. The role of the basal ganglia and cerebellum in language processing. Brain Res. 2007;1133:136–44. doi: 10.1016/j.brainres.2006.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Ingham RJ, Ingham JC, Laird AR, Fox PT. Stuttered and fluent speech production: an ALE meta-analysis of functional neuroimaging studies. Hum Brain Mapp. 2005;25:105–17. doi: 10.1002/hbm.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Laird AR, Pfordresher PQ, Thelen SM, Turkeltaub P, Liotti M. The somatotopy of speech: phonation and articulation in the human motor cortex. Brain Cogn. 2009;70:31–41. doi: 10.1016/j.bandc.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Chevrie-Muller C, Simon AM, Fournier S. Attention (L2MA) Paris: Editions du Centre de Psychologie Appliquée; 1997. Batterie Langage oral et écrit. Mémoire. [Google Scholar]
- Chevrie-Muller C, Plaza M. Nouvelles Epreuves pour l’Examen du Langage (N-EEL) Paris: Editions du Centre de Psychologie Appliquée; 2001. [Google Scholar]
- Chiron C, Pinton F, Masure MC, Duvelleroy-Hommet C, Leon F, Billard C. Hemispheric specialization using SPECT and stimulation tasks in children with dysphasia and dystrophia. Dev Med Child Neurol. 1999;41:512–20. doi: 10.1017/s0012162299001139. [DOI] [PubMed] [Google Scholar]
- Conti-Ramsden G, Crutchley A, Botting N. The extent to which psychometric tests differentiate subgroups of children with SLI. J Speech Lang Hear Res. 1997;40:765–77. doi: 10.1044/jslhr.4004.765. [DOI] [PubMed] [Google Scholar]
- Conti-Ramsden G. Processing and linguistic markers in young children with specific language impairment (SLI) J Speech Lang Hear Res. 2003;46:1029–37. doi: 10.1044/1092-4388(2003/082). [DOI] [PubMed] [Google Scholar]
- Crinion JT, Leff AP. Recovery and treatment of aphasia after stroke: functional imaging studies. Curr Opin Neurol. 2007;20:667–73. doi: 10.1097/WCO.0b013e3282f1c6fa. [DOI] [PubMed] [Google Scholar]
- Crinion JT, Lambon-Ralph MA, Warburton EA, Howard D, Wise RJ. Temporal lobe regions engaged during normal speech comprehension. Brain. 2003;126:1193–201. doi: 10.1093/brain/awg104. [DOI] [PubMed] [Google Scholar]
- De Fossé L, Hodge SM, Makris N, Kennedy DN, Caviness VS, McGrath L, et al. Language-association cortex asymmetry in autism and specific language impairment. Ann Neurol. 2004;56:757–66. doi: 10.1002/ana.20275. [DOI] [PubMed] [Google Scholar]
- de Guibert C, Maumet C, Ferré JC, Jannin P, Biraben A, Allaire C, et al. fMRI language mapping in children: a panel of language tasks using visual and auditory stimulation without reading or metalinguistic requirements. Neuroimage. 2010;51:897–909. doi: 10.1016/j.neuroimage.2010.02.054. [DOI] [PubMed] [Google Scholar]
- Deblaere K, Backes WH, Hofman P, Vandemaele P, Boon PA, Vonck K, et al. Developing a comprehensive presurgical functional MRI protocol for patients with intractable temporal lobe epilepsy: a pilot study. Neuroradiology. 2002;44:667–73. doi: 10.1007/s00234-002-0800-4. [DOI] [PubMed] [Google Scholar]
- Dibbets P, Bakker K, Jolles J. Functional MRI of task switching in children with Specific Language Impairment (SLI) Neurocase. 2006;12:71–9. doi: 10.1080/13554790500507032. [DOI] [PubMed] [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384:159–61. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Duffau H, Bauchet L, Lehéricy S, Capelle L. Functional compensation of the left dominant insula for language. Neuroreport. 2001;12:2159–63. doi: 10.1097/00001756-200107200-00023. [DOI] [PubMed] [Google Scholar]
- Dunn M, Flax J, Sliwinksi M, Aram D. The use of spontaneous language measures as criteria for identifying children with specific language impairment: an attempt to reconcile clinical and research incongruence. J Speech Lang Hear Res. 1996;39:643–54. doi: 10.1044/jshr.3903.643. [DOI] [PubMed] [Google Scholar]
- Ellis Weismer E, Plante E, Jones M, Tomblin JB. A functional magnetic resonance imaging investigation of verbal working memory in adolescents with specific language impairment. J Speech Lang Hear Res. 2005;48:405–25. doi: 10.1044/1092-4388(2005/028). [DOI] [PubMed] [Google Scholar]
- Fisher SE, Lai CS, Monaco AP. Deciphering the genetic basis of speech and language disorders. Ann Rev Neurosci. 2003;26:57–80. doi: 10.1146/annurev.neuro.26.041002.131144. [DOI] [PubMed] [Google Scholar]
- Friederici AD. The neural basis of language development and its impairment. Neuron. 2006a;52:941–52. doi: 10.1016/j.neuron.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Friederici AD. What’s in control of language? Nat Neurosci. 2006b;9:991–2. doi: 10.1038/nn0806-991. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiack RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, McKinney C, Papero PH, Weinstein S, et al. fMRI language task panel improves determination of language dominance. Neurology. 2004;63:1403–8. doi: 10.1212/01.wnl.0000141852.65175.a7. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Moore EN, Weber DA, Ritzl EK, Berl MM. Functional magnetic resonance imaging in normal and pathological language development. In: Riva D, Rapin I, Zardini G, editors. Language: normal and pathological development. John Libbey Eurotext; 2006. pp. 105–20. [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, et al. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum Brain Mapp. 2003;18:176–85. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauger LM, Lombardino LJ, Leonard CM. Brain morphology in children with specific language impairment. J Speech Lang Hear Res. 1997;40:1272–84. doi: 10.1044/jslhr.4006.1272. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology. I. A hypothesis and a program for research. Arch Neurol. 1985;42:428–59. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Neumann K, Bachoud-Levi AC, von Gudenberg AW, Euler HA, Lanfermann H, Preibisch C. Severity of dysfluency correlates with basal ganglia activity in persistent developmental stuttering. Brain Lang. 2008;104:190–9. doi: 10.1016/j.bandl.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86:141–55. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Hardyck C, Petrinovich LF. Left-handedness. Psychol Bull. 1977;84:385–404. [PubMed] [Google Scholar]
- Harris GJ, Chabris CF, Clark J, Urban T, Aharon I, Steele S, et al. Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain Cogn. 2006;61:54–68. doi: 10.1016/j.bandc.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Heim S, Grande M, Meffert E, Eickhoff SB, Schreiber H, Kukolja J, et al. Cognitive levels of performance account for hemispheric lateralisation effects in dyslexic and normally reading children. Neuroimage. 2010;53:1346–58. doi: 10.1016/j.neuroimage.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Kenet T. Brain abnormalities in language disorders and in autism. Pediatr Clin North Am. 2007;54:563–83. doi: 10.1016/j.pcl.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Kennedy DN, Filipek PA, et al. Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain. 2005;128:213–26. doi: 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- Hickok G, Buchsbaum B, Humphries C, Muftuler T. Auditory–motor interaction revealed by fMRI: Speech, music, and working memory in area Spt. J Cogn Neurosci. 2003;15:673–82. doi: 10.1162/089892903322307393. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hillis AE. Aphasia: progress in the last quarter of a century. Neurology. 2007;69:200–13. doi: 10.1212/01.wnl.0000265600.69385.6f. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127:1479–87. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- Holland SK, Vannest J, Mecoli M, Jacola LM, Tillema JM, Karunanayaka PR, et al. Functional MRI of language lateralization during development in children. Int J Audiol. 2007;46:533–51. doi: 10.1080/14992020701448994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K, Gundersen H, Brekke C, Thomsen T, Rimol LM, Ersland L, Niemi J. fMRI brain activation in a finnish family with specific language impairment compared with a normal control group. J Speech Lang Hear Res. 2004;47:162–72. doi: 10.1044/1092-4388(2004/014). [DOI] [PubMed] [Google Scholar]
- Jäncke L, Siegenthaler T, Preis S, Steinmetz H. Decreased white-matter density in a left-sided fronto-temporal network in children with developmental language disorder: evidence for anatomical anomalies in a motor-language network. Brain Lang. 2007;102:91–8. doi: 10.1016/j.bandl.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Hessenlink JR, Sowell E, Tallal PA. Cerebral structure on magnetic resonance imaging in language-and-learning-impaired children. Arch Neurol. 1991;48:539–45. doi: 10.1001/archneur.1991.00530170103028. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–21. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kell CA, Neumann K, von Kriegstein K, Posenenske C, von Gudenberg AW, Euler A, Giraud AL. How the brain repairs stuttering. Brain. 2009;132:2747–60. doi: 10.1093/brain/awp185. [DOI] [PubMed] [Google Scholar]
- Keller SS, Crow T, Foundas A, Amunts K, Roberts N. Broca’s area: nomenclature, anatomy, typology and asymmetry. Brain Lang. 2009;109:29–48. doi: 10.1016/j.bandl.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Müller RA, Cohen DN, Courchesne E. Atypical functional lateralization of language in autism spectrum disorders. Brain Res. 2008;1221:115–25. doi: 10.1016/j.brainres.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus TA, Silver AM, Lindgren KA, Hadjikhani N, Tager-Flusberg H. fMRI activation during a language task in adolescents with ASD. J Int Neuropsychol Soc. 2008;14:967–79. doi: 10.1017/S1355617708081216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach JL, Holland SK. Functional MRI in children: clinical and research applications. Pediatr Radiol. 2010;40:31–49. doi: 10.1007/s00247-009-1452-x. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Lombardino LJ, Walsh K, Eckert MA, Mockler JL, Rowe LA, et al. Anatomical risk factors that distinguish dyslexia from SLI predict reading skill in normal children. J Commun Disord. 2002;35:501–31. doi: 10.1016/s0021-9924(02)00120-x. [DOI] [PubMed] [Google Scholar]
- Liégeois F, Baldeweg T, Connelly A, Gadian DG, Mishkin M, Vargha-Kadem F. Language fMRI abnormalities associated with FOXP2 gene mutation. Nat Neurosci. 2003;6:1230–37. doi: 10.1038/nn1138. [DOI] [PubMed] [Google Scholar]
- Lou HC, Henriksen L, Bruhn P. Focal cerebral hypoperfusion in children with dysphasia and/or attention deficit disorder. Arch Neurol. 1984;41:825–9. doi: 10.1001/archneur.1984.04050190031010. [DOI] [PubMed] [Google Scholar]
- Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF. A meta-analysis of functional neuroimaging studies of dyslexia. Ann N Y Acad Sci. 2008;1145:237–59. doi: 10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- Medina LS, Bernal B, Ruiz J. Role of functional MR in determining language dominance in epilepsy and nonepilepsy populations: a Bayesian analysis. Radiology. 2007;242:94–100. doi: 10.1148/radiol.2421050677. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ors M, Ryding E, Lindgren M, Gustafsson P, Blennow G, Rosen I. SPECT findings in children with specific language impairment. Cortex. 2005;41:316–26. doi: 10.1016/s0010-9452(08)70269-7. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy ES, Berl MM, Moore EN, Gaillard WD. Pediatric functional magnetic resonance imaging (fMRI): issues and applications. J Child Neurol. 2008;23:791–801. doi: 10.1177/0883073807313047. [DOI] [PubMed] [Google Scholar]
- Parisse C, Maillart C. Specific language impairment as systemic developmental disorders. J Neuroling. 2009;22:109–22. [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RS, Frith CD. Is developmental dyslexia a disconnection syndrome? Evidence from PET scanning. Brain. 1996;119:143–57. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- Plante E, Swisher L, Vance R. fMRI findings in boys with specific language impairment. Brain Lang. 1991;41:52–66. doi: 10.1016/0093-934x(91)90110-m. [DOI] [PubMed] [Google Scholar]
- Preibisch C, Neumann K, Raab P, Euler HA, von Gudenberg AW, Lanfermann H, et al. Evidence for compensation for stuttering by the right frontal operculum. Neuroimage. 2003;20:1356–64. doi: 10.1016/S1053-8119(03)00376-8. [DOI] [PubMed] [Google Scholar]
- Preis S, Jäncke L, Schittler P, Huanq Y, Steinmetz H. Normal intrasylvian anatomical asymmetry in children with developmental language disorder. Neuropsychologia. 1998;36:849–55. doi: 10.1016/s0028-3932(98)00033-5. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Ramsey NF, Sommer I, Rutten GJ, Kahn R. Combined analysis of language tasks in fMRI improves assessment of hemispheric dominance for language functions in individual subjects. Neuroimage. 2001;13:719–33. doi: 10.1006/nimg.2000.0722. [DOI] [PubMed] [Google Scholar]
- Ramus F, Szenkovits G. What phonological deficit? Q J Exp Psychol. 2008;61:129–41. doi: 10.1080/17470210701508822. [DOI] [PubMed] [Google Scholar]
- Rapin I, Allen DA. Developmental language disorders: nosological considerations. In: Kirk U, editor. Neuropsychology of language, reading and spelling. New York: Academic Press; 1983. pp. 155–84. [Google Scholar]
- Rapin I, Dunn M, Allen DA. Developmental language disorders. In: Segalowitz SJ, Rapin I, editors. Handbooks of neuropsychology. Part II. Vol. 8. Amsterdam: Elsevier; 2003. pp. 593–630. [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Hum Brain Mapp. 2009;30:3299–308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux FE, Boulanouar K, Lotterie JA, Mejdoubi M, Le Sage JP, Berry I. Language functional magnetic resonance imaging in preoperative assessment of language areas: correlation with direct cortical stimulation. Neurosurgery. 2003;52:1335–47. doi: 10.1227/01.neu.0000064803.05077.40. [DOI] [PubMed] [Google Scholar]
- Rutten GJ, Ramsey NF, van Rijen PC, van Veelen CW. Reproducibility of fMRI-determined language lateralization in individual subjects. Brain Lang. 2002;80:421–37. doi: 10.1006/brln.2001.2600. [DOI] [PubMed] [Google Scholar]
- Schulz E, Maurer U, van der Mark S, Bucher K, Brem S, Martin E, Brandeis D. Impaired semantic processing during sentence reading in children with dyslexia: combined fMRI and ERP evidence. Neuroimage. 2008;41:153–68. doi: 10.1016/j.neuroimage.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Sharot T, De Martino B, Dolan RJ. How choice reveals and shapes expected hedonic outcome. J Neurosci. 2009;29:3760–5. doi: 10.1523/JNEUROSCI.4972-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA. Paying attention to reading: the neurobiology of reading and dyslexia. Dev Psychopathol. 2008;20:1329–49. doi: 10.1017/S0954579408000631. [DOI] [PubMed] [Google Scholar]
- Soriano-Mas C, Pujol J, Ortiz H, Deus J, López-Sala A, Sans A. Age-related brain structural alterations in children with specific language impairment. Hum Brain Mapp. 2009;30:1626–36. doi: 10.1002/hbm.20620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrink C, Ackermann H, Lachmann T, Riecker A. Contribution of the anterior insula to temporal auditory processing deficits in developmental dyslexia. Hum Brain Mapp. 2009;30:2401–11. doi: 10.1002/hbm.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stothard SE, Snowling MJ, Bishop DV, Chipchase BB, Kaplan CA. Language-impaired preschoolers: a follow-up into adolescence. J Speech Lang Hear Res. 1998;41:407–18. doi: 10.1044/jslhr.4102.407. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Binder JR, Possing ET. Language lateralization in left-handed and ambidextrous people. fMRI data. Neurology. 2002;59:238–44. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- Tie Y, Whalen S, Suarez RO, Golby AJ. Group independent component analysis of language fMRI from word generation tasks. Neuroimage. 2008;42:1214–25. doi: 10.1016/j.neuroimage.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin JB, Records NL, Zhang W. A system for the diagnosis of specific language impairment in kindergarten children. J Speech Lang Hear Res. 1996;39:1284–94. doi: 10.1044/jshr.3906.1284. [DOI] [PubMed] [Google Scholar]
- Tzourio N, Heim H, Zilbovicius M, Gerard C, Mazoyer BM. Abnormal regional CBF reponse in left hemisphere of dysphasic children during a language task. Pediatr Neurol. 1994;10:20–6. doi: 10.1016/0887-8994(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Menon V. The anterior insula in autism: under-connected and under-examined. Neurosci Biobehav Rev. 2009;33:1198, 203. doi: 10.1016/j.neubiorev.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lely HKJ. Domain-specific cognitive systems: insight from grammatical specific language impairment. Trends Cogn Sci. 2005;9:53–9. doi: 10.1016/j.tics.2004.12.002. [DOI] [PubMed] [Google Scholar]
- van Hout A. Acquired aphasia in childhood. In: Segalowitz SJ, Rapin I, editors. Handbooks of neuropsychology. Part II. Vol. 8. Amsterdam: Elsevier; 2003. pp. 631–58. [Google Scholar]
- Vargha-Khadem F, Gadian DG, Copp A, Mishkin M. FOXP2 and the neuroanatomy of speech and language. Nat Rev Neurosci. 2005;6:131–8. doi: 10.1038/nrn1605. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Watkins KE, Price CJ, Ashburner J, Alcock KJ, Connelly A, et al. Neural basis of an inherited speech and language disorder. Proc Natl Acad Sci U S A. 1998;95:12695–700. doi: 10.1073/pnas.95.21.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets NL, Adcock JE, Flitney DE, Behrens TEJ, Hart Y, Stacey R, et al. Distinct right frontal lobe activation in language processing following left hemisphere injury. Brain. 2006;129:754–66. doi: 10.1093/brain/awh679. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Smith SM, Davis S, Howell P. Structural and functional abnormalities of the motor system in developmental stuttering. Brain. 2008;131:50–9. doi: 10.1093/brain/awm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Vargha-Khadem F, Ashburner J, Passingham R, Connelly A, Friston K, et al. MRI analysis of an inherited speech and language disorder: Structural brain abnormalities. Brain. 2002;125:465–78. doi: 10.1093/brain/awf057. [DOI] [PubMed] [Google Scholar]
- Webster RI, Shevell MI. Neurobiology of specific language impairment. J Child Neurol. 2004;19:471–81. doi: 10.1177/08830738040190070101. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manuel de l’échelle d’intelligence pour adultes––3è édition (WAIS-III) Paris: ECPA; 2000. [Google Scholar]
- Wechsler D. Manuel du WISC-IV. Paris: ECPA; 2005. [Google Scholar]
- Whitehouse JO, Bishop DV. Cerebral dominance for language function in adults with specific language impairment or autism. Brain. 2008;131:3193–200. doi: 10.1093/brain/awn266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Holland SK, Altaye M, Gaser C. Template-O-Matic: a toolbox for creating customized pediatric templates. NeuroImage. 2008;41:903–13. doi: 10.1016/j.neuroimage.2008.02.056. [DOI] [PubMed] [Google Scholar]
- Wilke M, Lidzba KJ. LI-tool: a new toolbox to assess lateralization in functional MR-data. J Neurosci Methods. 2007;163:128–36. doi: 10.1016/j.jneumeth.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Wilke M, Lidzba KJ, Staudt M, Buchenau K, Grodd W, Krägeloh-Mann I. An fMRI task battery for assessing hemispheric language dominance in children. Neuroimage. 2006;32:400–10. doi: 10.1016/j.neuroimage.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ, Holland SK. Normative pediatric brain data for spatial normalization and segmentation differs from standard adult data. Magn Reson Med. 2003a;50:749–57. doi: 10.1002/mrm.10606. [DOI] [PubMed] [Google Scholar]
- Wilke M, Sohn JH, Byars AW, Holland SK. Bright spots: correlations of gray matter volume with IQ in a normal pediatric population. Neuroimage. 2003b;20:202–15. doi: 10.1016/s1053-8119(03)00199-x. [DOI] [PubMed] [Google Scholar]