Abstract
Background
Both major depressive disorder (MDD) and post-traumatic stress disorder (PTSD) are characterized by alterations in intrinsic functional connectivity. Here we investigated changes in intrinsic functional connectivity across these disorders as a function of cognitive behavioral therapy (CBT), an effective treatment in both disorders.
Methods
53 unmedicated right-handed participants were included in a longitudinal study. Patients were diagnosed with PTSD (n = 18) and MDD (n = 17) with a structured diagnostic interview and treated with 12 sessions of manualized CBT over a 12-week period. Patients received an MRI scan (Siemens 3 T Trio) before and after treatment. Longitudinal functional principal components analysis (LFPCA) was performed on functional connectivity of the bilateral amygdala with the fronto-parietal network. A matched healthy control group (n = 18) was also scanned twice for comparison.
Results
LFPCA identified four eigenimages or principal components (PCs) that contributed significantly to the longitudinal change in connectivity. The second PC differentiated CBT-treated patients from controls in having significantly increased connectivity of the amygdala with the fronto-parietal network following CBT.
Limitations
Analysis of CBT-induced amygdala connectivity changes was restricted to the a priori determined fronto-parietal network. Future studies are needed to determine the generalizability of these findings, given the small and predominantly female sample.
Conclusion
We found evidence for the hypothesis that CBT treatment is associated with changes in connectivity between the amygdala and the fronto-parietal network. CBT may work by strengthening connections between the amygdala and brain regions that are involved in cognitive control, potentially providing enhanced top-down control of affective processes that are dysregulated in both MDD and PTSD.
Keywords: Principal component analysis, Amygdala, Fronto-parietal network, Resting state functional connectivity, Cognitive behavioral therapy
Highlights
-
•
CBT treatment effects found in functional connectivity for combined MDD and PTSD patients.
-
•
Novel longitudinal dimension reduction method characterizes direction of changes.
-
•
Study shows CBT increases amygdala connectivity with the fronto-parietal network.
-
•
CBT mechanism may be to enhance cognitive control region connectivity.
-
•
A post-hoc whole brain voxel-wise analysis independently confirmed the findings.
1. Introduction
Major depressive disorder (MDD) and post-traumatic stress disorder (PTSD) are both associated with similar deficits in functional connectivity (Brown et al., 2014, Dichter et al., 2015, Fonzo et al., 2010, Johnstone et al., 2007, Lanius et al., 2010, Matthews et al., 2008, Oathes et al., 2015) and respond to treatment with cognitive behavioral therapy (CBT). Despite some controversy, the current literature also suggests that CBT is as efficacious as antidepressant medication for the treatment of MDD but with more enduring effects (Hollon et al., 2014, McMain et al., 2015, Weitz et al., 2015). In studies of PTSD, significant empirical support has been demonstrated across sites and within a variety of trauma types for cognitive processing therapy (CPT), a variant of CBT that specifically addresses PTSD (Chard, 2005, Resick et al., 2008). In a study of CPT treatment of rape-related PTSD, CPT demonstrated a significant therapeutic benefit compared with wait-list controls, and these effects remained stable with only 8% of patients meeting criteria for PTSD at a six-month follow-up assessment (Resick and Schnicke, 1992, Resick and Schnicke, 1993).
The current literature implicates functional hypoconnectivity of the fronto-parietal network in patients with both MDD and PTSD compared to healthy control subjects. While CBT has demonstrated considerable efficacy in both MDD and PTSD, it remains unclear how therapeutic changes are encoded within the brain's functional networks. Furthermore, it is unknown whether the neural mechanisms of treatment response are similar in different clinical conditions, resulting in normalization of fronto-parietal hypoconnectivity across these disorders. In the present study, we examined CBT/CPT effects on dimensional MDD and PTSD-associated functional connectivity in a sample of unmedicated patients. Using a fully-exploratory approach, we have previously reported decreased amygdala connectivity with cognitive control regions, including the dorsolateral prefrontal cortex (DLPFC) and inferior frontal gyrus (IFG), in association with depression severity across MDD and PTSD (Satterthwaite et al., 2016). Accordingly, we hypothesized that the reduction in amygdalo-frontal connectivity seen in both MDD and PTSD would show a common pattern of normalization in response to effective CBT. To examine this hypothesis, we used a recently developed multivariate analysis technique (longitudinal functional principal components analysis; LFPCA) (Greven et al., 2010, Shou et al., 2015) that is specifically well suited for the analysis of high-dimensional imaging data acquired longitudinally over time. As described below, we found evidence for resolution of amygdalo-frontal dysconnectivity across both MDD and PTSD following treatment with CBT.
2. Materials and methods
2.1. Participants and clinical assessment
An initial group of 120 patients including 57 with major depressive disorder (MDD) and 63 with post-traumatic stress disorder (PTSD) were recruited. Patients met inclusion and exclusion criteria based on a structured diagnostic interview (DSM-IV-TR criteria using the SCID (First et al., 1996) and the CAPS-IV (First et al., 1996, Blake et al., 1995)), and were scanned at baseline. Participants were excluded from the study due to 1) co-morbid neurological disorders; 2) current alcohol or substance use disorder; 3) history of psychotic disorder, bipolar disorder, or obsessive-compulsive disorder; 4) current suicide risk; 5) recent treatment with any psychotropic or CNS-active drug. Specifically, subjects were required not to have been treated with any psychotropic medication for at least three weeks (five weeks for fluoxetine).
From these, a subset of 65 patients (27 MDD and 38 PTSD) volunteered for a treatment study with CBT yielding a post-treatment sample of 39 (18 MDD and 21 PTSD) who completed treatment and had imaging scans available after 12 weeks of CBT. The final sample included for analysis in this paper, following exclusion criteria for poor image quality (high motion; e.g., mean relative displacement > 0.25 mm) and incomplete data, was comprised of 53 unmedicated participants. These participants were from three groups: 17 patients with MDD, 18 patients with PTSD, and 18 demographically matched HC). Demographic details are provided in Table 1.
Table 1.
Demographics and clinical scores of the study participants by diagnostic groups. MDD and PTSD groups demonstrate improvement in symptom scores after CBT treatment. MDRS T1 represents MDRS scores at the baseline visit and ∆ MDRS was calculated as the change in MDRS between 12 weeks and baseline. Same notations apply to MASQ and PDS scores.
| Overall | HC | MDD | PTSD | P values | |
|---|---|---|---|---|---|
| N (%) | 53 (100%) | 18 (34%) | 17 (32%) | 18 (34%) | |
| Age (SD) | 31.47 (9.21) | 31.05 (10.32) | 31.88 (6.61) | 31.50 (10.57) | 0.97 |
| MDRS T1 | 16.50 (12.54) | 1.67 (2.23) | 28.41 (6.12) | 15.06 (9.13) | < 0.001 |
| ∆ MDRS | − 12.34 (11.85) | − 0.71 (3.04) | − 20.53 (11.05) | − 8.94 (9.11) | < 0.001 |
| MASQ-AA T1 | 28.24 (10.14) | 20.07 (3.83) | 34.07 (10.66) | 30.81 (9.17) | < 0.001 |
| ∆ MASQ-AA | − 6.25 (10.33) | − 1.40 (4.27) | − 9.79 (13.46) | − 6.17 (8.56) | 0.15 |
| PDS T1 | 17.47 (13.06) | 6.50 (7.46) | 18.92 (13.81) | 21.88 (12.07) | 0.02 |
| ∆ PDS | − 8.76 (10.62) | 0.86 (1.86) | − 10.00 (10.23) | − 13.21 (10.52) | 0.009 |
Depression severity was assessed by a trained clinician using the Montgomery Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979). In addition, anxiety severity was assessed using the Mood and Anxiety Symptom Questionnaire (MASQ) Watson et al., (Watson et al., 1995a, Watson et al., 1995b) and PTSD severity was assessed using the PTSD Diagnostic Scale (PDS) (Foa et al., 2016). All participants were right-handed, English-speaking, and aged 18–55. All participants provided written informed consent; the Human Subjects Committees of both Washington University and the University of Missouri-St. Louis approved all study procedures.
2.2. Treatment
CBT: Patients with PTSD or MDD enrolled in the study received manualized individual outpatient CBT sessions weekly over a 12-week period by an expert clinical psychologist (SEB). For MDD patients, the intervention was guided by a standard treatment manual, “Cognitive Therapy of Depression” (CT) (Beck, 1979), which presents the cognitive model of depression and has been used in most recent outcome studies of CBT for depression (Newby et al., 2015, Newby et al., 2016, Amick et al., 2015).
PTSD patients received Cognitive Processing Therapy (CPT), which has been shown to be an effective cognitive behavioral therapy that significantly reduces symptoms associated with PTSD (Chard, 2005, Resick et al., 2008). Both CPT and CBT target faulty cognitions/schemata and emotion regulation, while CPT also assists in processing emotion-laden memories via written exposure.
2.3. Image acquisition and processing
All subjects were imaged on the same scanner (Siemens 3T Trio) using the same acquisition protocol. High-resolution structural images were acquired using a T1-weighted MPRAGE sequence: TR 2400 ms, TE 3.13 ms, TI 1000 ms, flip angle 8°, slice thickness/gap 1 mm/0 mm, effective voxel resolution 1.0 mm3. Resting-state gradient spin-echo functional images were acquired in two series of 210 volumes (7:42 duration each) using the following parameters: TR 2200 ms, TE 27 ms, flip angle 90°, slice thickness/gap 4 mm/0 mm, effective voxel resolution 4.0 mm3. T1 images were processed as previously described to register and segment the images (Satterthwaite et al., 2016). In order to avoid registration bias and maximize sensitivity to detect regional effects that can be impacted by registration error, a custom template was created with ANTs (Avants et al., 2011a); T1 images were normalized to this population-specific template space using the top-performing SyN diffeomorphic registration method implemented in ANTs (Klein et al., 2009). Structural images were then processed with ‘antsCorticalThickness’ (Tustison et al., 2014), which uses the custom template to guide brain extraction, N4 bias correction (Tustison et al., 2010), and Atropos probabilistic tissue segmentation (Avants et al., 2011b).
Resting-state timeseries data were processed using a validated confound regression procedure that has been optimized to reduce the influence of subject motion (Satterthwaite et al., 2013). After the first 4 volumes of the functional timeseries were removed to allow signal stabilization, functional images were re-aligned using MCFLIRT (Jenkinson et al., 2002), and smoothed with a Gaussian filter at 6 mm FWHM. Confound regression included 9 confounding signals (6 motion parameters + global/WM/CSF) as well as the temporal derivative, quadratic term, and temporal derivative of the quadratic of each (36 regressors total) (Satterthwaite et al., 2013). Finally, timeseries were band-pass filtered to retain frequencies between 0.01 and 0.08 Hz; all motion parameters and confound timecourses were band-pass filtered in an identical fashion as the timeseries data itself in order to prevent frequency mismatch (Hallquist et al., 2013). Functional images were co-registered to the T1 image using boundary-based registration (Greve and Fischl, 2009) and aligned to template space (Satterthwaite et al., 2016). Throughout, all transformations were concatenated so that only one interpolation was performed.
Subject-specific amygdala segmentations were created using multi-atlas label fusion (MALF) that were in turn used for seed-based connectivity analyses. Manually labeled amygdalas from 30 images were registered to each participant's T1 image using ANTs. The derived subject-specific amygdala segmentation was then projected into native fMRI space and used to extract the average amygdala timecourse, which was in turn used to generate a seed connectivity map. For more details see (Satterthwaite et al., 2016).
2.4. Statistical analysis
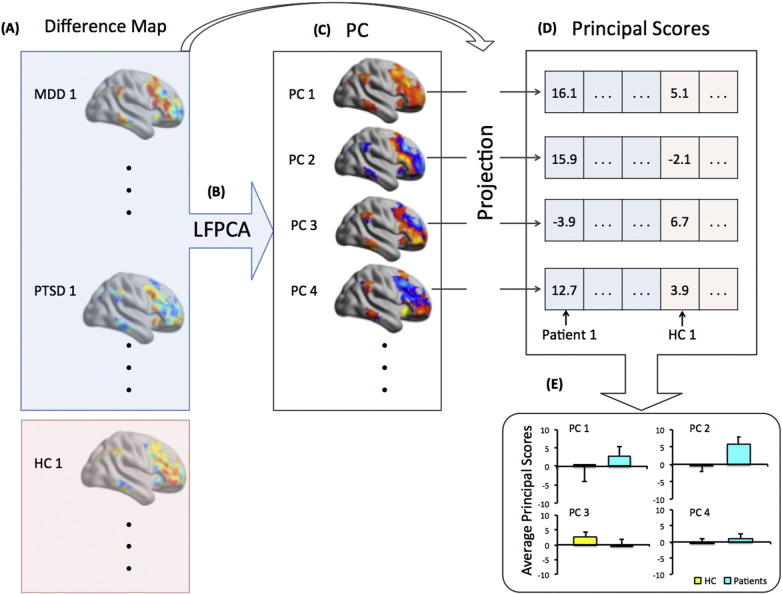
We restricted the analyses to amygdala connectivity with the fronto-parietal network based on our prior work implicating its importance in MDD and PTSD (Satterthwaite et al., 2016). To identify possible regions of CBT treatment effects, we compared the changes between the two time points for amygdala connectivity between patients (both MDD and PTSD) and HCs using dimension reduction approaches. Specifically, we modeled connectivity maps from the two time points using longitudinal functional principal component analysis (Greven et al., 2010, Shou et al., 2015) that identified lower-dimensional patterns while accounting for within-subject correlations simultaneously. The treatment effects could be estimated as a time effect among patients. Hence, it is equivalent to conduct a principal component analysis on the set of difference connectivity maps (Time 2-Time 1). In particular, the steps for our analysis are shown in Fig. 1 and detailed below:
-
(1)
For each participant, we computed the functional connectivity maps (Fisher Z-transformed Spearman's correlation coefficients) separately for unilateral left- and right- amygdala at baseline (Time 1) and after treatment (Time 2); A difference map of amygdala functional connectivity was then computed (Time 2 − Time 1);
-
(2)
Each within-network difference map was rearranged into a vector of connectivity values and then stacked into a matrix across the 35 patients (MDD + PTSD). The singular value decomposition (SVD) was then applied to the matrix and a set of reduced dimensional features was extracted to summarize the complex patterns of amygdala-frontal connectivity change over time in response to treatment. The SVD on the difference maps generated a sequence of population eigenimages (principal components) and subject-specific projections (principal scores) on each eigenimage. The resulting eigenimages can be mapped back to the 3D brain template with loadings on each voxel representing the relative contributions to an overall connectivity pattern that explain most of the variability in the difference maps across subjects. It allows us to identify the regions that change most over time among the diagnostic groups.
-
(3)
Principal components selection was based on the screen plot, where an inflection point in the slope appeared after the 4th PC, suggesting that the first 4 components were separable in their importance from the rest of the PCs. Thus, our analyses were focused on the first 4 PCs, which explained 20% of the variance.
-
(4)
For each patient and control, a principal score was computed for each of these 4 PCs by projecting the difference map computed in step 1 onto a given PC. The principal score reflects the amount of longitudinal change in functional connectivity for each subject that can be explained by a particular PC.
-
(5)
For each of the 4 selected PCs, we performed linear regression comparing the corresponding principal scores between the patient (MDD and PTSD combined) and HC group, adjusting for potential confounding factors including age, gender and in-scanner head motion. The components with principal scores showing significantly differential distributions (under Bonferroni correction for multiple comparisons of the 4 PCs) between patients and HC are identified as the lower dimensional features that reflect the longitudinal treatment effect among patients. Post-hoc tests of the identified PC scores between individual diagnostic groups (MDD vs. HC and PTSD vs. HC) were also conducted separately using linear regressions.
Fig. 1.
Procedures for Longitudinal Functional Principal Components Analysis (LFPCA). (A) For the set of 53 participants included in the analysis, calculate the difference maps of amygdala functional connectivity (Δ Amygdala FC) between time 2 and time 1 for each individual, within the fronto-parietal network as defined by Yeo et al. (Yeo et al., 2011). (B) Aggregate the data across all patients (18 MDD and 17 PTSD) and conduct singular value decomposition (SVD) within the fronto-parietal network. (C) Obtain the eigenimages in the Yeo fronto-parietal network template where the value of each voxel represents the loadings of the principal components (PC) within the fronto-parietal network. The color maps indicate the relative loadings that contribute to the corresponding principal component, with red for positive loadings and blue for negative loadings. Colors are based on the actual PCA results. Voxels with higher absolute values are regions that most reflect the longitudinal changes in amygdala connectivity. (D) Project each amygdala connectivity difference map (including patients and controls) onto the identified eigenimage and obtain a principal score for each subject and each eigenimage. (E) Perform statistical testing on the principal scores to identify the PC that best differentiates patients from healthy controls.
To confirm the LFPCA findings of group differences, we implemented traditional whole brain voxel-wise analyses via linear mixed effects models comparing the changes of amygdala connectivity before and after CBT in patients. Covariates including age, sex and motion were adjusted in the mixed effects regression. Results were corrected within the study specific grey matter mask using cluster correction via Gaussian Random Field theory (Zhang et al., 2009): z > 2.33, p < 0.05. For regions identified, we followed up with linear regressions adjusting for age, sex and motion for compared the baseline average connectivity between patients and controls by using the baseline scans to fully understand the mechanistic changes in the connectivity of the ROI.
3. Results
3.1. CBT-induced changes in amygdala intrinsic functional connectivity with the fronto-parietal network
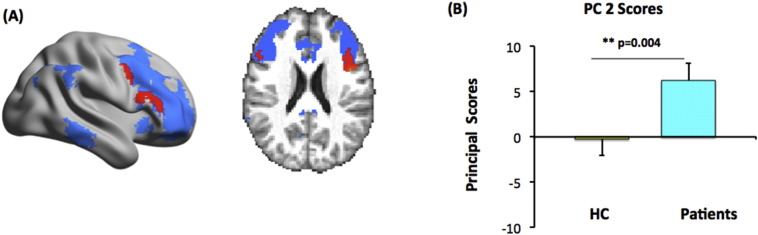
The first four eigenimages derived from the patients' difference connectivity maps via LFPCA together explain 20% of the total variance in the data. Among the corresponding four sets of principal scores obtained as in step (4) of the LFPCA method, scores from the second PC showed a significant differential distributions (under Bonferroni correction for multiple comparisons of the 4 PCs) between patients and HC. Hence, we focused on the spatial pattern of the second eigenimage. The voxels with the highest loadings (shown in red in Fig. 2A) were located in IFG (Yeo et al., 2011). A test comparing the second principal scores indicated a significantly larger increase in connectivity between the right amygdala and fronto-parietal network (a more positive principal score) for CBT-treated (combining MDD and PTSD) participants than for controls (Fig. 2B: p = 0.004). No significant results were observed for the CBT-induced left amygdala functional connectivity changes in patients. The post-hoc tests between individual diagnostic groups (MDD vs. HC and PTSD vs. HC) showed that both MDD and PTSD demonstrated increased amygdala connectivity within the identified IFG regions after CBT, although MDD had a larger effect size (p = 0.009) than PTSD (p = 0.012) with HC being the reference group. The findings remained consistent even with smaller numbers of patients as compared to the results from the combined patients comparison. The details are shown in Supplement Fig. S.1.
Fig. 2.
CBT is associated with increased functional connectivity between the amygdala and fronto-parietal cortex in across MDD and PTSD. (A) The top 10% highest loading voxels of the second eigenimage (in red) were superimposed on the fronto-parietal network (blue background) and displayed on a surface map (left) and a slice view (right), MNI coordinates x,y,z = 53.70,50; (B) Bar plots of the average principal scores of the second eigenimage for all patients (combining MDD and PTSD: light blue bar) and healthy controls (HC: yellow bar).
3.2. CBT normalizing the baseline connectivity differences between groups
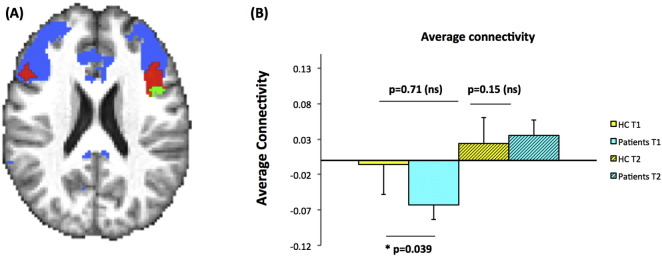
As a final step, we validated the findings from LFPCA using traditional whole brain voxel-wise regression among patients. Z maps from a linear mixed effects model comparing pre- and post-treatment connectivity after adjusting for age, sex and motion at the two time points indicated an average increased connectivity in the IFG (Z = 2.33, p < 0.05) within the fronto-parietal network after treatment. Within the identified IFG region, we compared the average connectivity between HC and patients at baseline and at the second time points 12 weeks later, using linear regression adjusting for age, gender and motion. Results indicated that at baseline, patients had deficient connectivity within the identified region compared with HC (p-value 0.039), and that no difference was found after 12 weeks (p-value 0.15) (Fig. 3).
Fig. 3.
A region in IFG showed a significant increase in connectivity with amygdala using whole brain voxel-wise regression. (A) Spatial map on the same axial slice as in Fig. 2A demonstrating the voxel-wise determined ROI in IFG (bright green) with significant increase in connectivity after treatment. The ROI is overlaid on top of the regions with the highest 10% PC 2 scores (in red) as in Fig. 2A. The blue background again indicates the pre-selected fronto-parietal network based on Yeo's 7 network template; (B) Bar plot showing the distribution of the average connectivity of the identified IFG ROI (green in A) for HC and patients at two time points.
Such findings confirm our hypothesis that CBT normalized the baseline deficient amygdala FC within the IFG region among patients. The average T1 and T2 connectivity levels within the green ROI for MDD and PTSD separately are shown in Supplement Fig. S.2. Results show similarly directions of group differences and treatment effects for both MDD and PTSD as compared to HC separately.
4. Discussion
In the present study, CBT effects on depression in MDD- and PTSD-associated functional connectivity were examined in unmedicated patients with a primary diagnosis of either MDD or PTSD. We focused on a priori amygdala connectivity with the fronto-parietal network based on previous work showing reduced resting-state connectivity of the amygdala with the DLPFC and IFG (Satterthwaite et al., 2016) in order to determine the effect of CBT on dysconnectivity. Here we found that this pattern of dysconnectivity was normalized in response to CBT across MDD and PTSD using recently developed multivariate techniques (LFPCA).
In previous studies of MDD, resting-state hypoconnectivity between the amygdala and regions involved in affective processing is a commonly-reported characteristic of the disorder (Dunlop and Mayberg, 2014, Kaiser et al., 2015). The present literature is mixed, however, regarding the role of CBT in effecting connectivity changes in MDD (Franklin et al., 2016). An early study of resting-state metabolic activity with PET scanning in MDD patients associated CBT response with decreases in glucose metabolic activity of several prefrontal areas, including the DLPFC (Goldapple et al., 2004). More recent meta-analyses, however, implicated the DLPFC as an area showing reduced functional connectivity in MDD (Murrough et al., 2016) and one of the main areas to show increases in functional activity following psychotherapy (Quide et al., 2012). Similarly, hypoconnectivity of the IFG with amygdala also predicted responsiveness to treatment by differentiating CBT responders from non-responders (Dichter et al., 2015). Divergent findings may be due to heterogeneity among MDD subtypes (Dunlop and Mayberg, 2014).
Studies in PTSD have highlighted several instances of hypoactivation in prefrontal circuitry, including fronto-parietal regions such as the DLPFC and IFG (Hayes et al., 2012, MacNamara et al., 2016). Furthermore, hypoactivation of the DLPFC during cognitive control tasks has been related to increased severity of PTSD symptoms (Aupperle et al., 2012). In conjunction with this finding, a recent meta-analysis assessing treatment changes in PTSD highlighted increases in DLPFC activity following psychotherapy across a number of studies (Thomaes et al., 2014), indicating that aberrant DLPFC activity normalizes with treatment. Using a network approach, prior work has implicated fronto-parietal network hypoconnectivity in PTSD, with patients exhibiting decreased connectivity between the basolateral amygdala and the IFG (Brown et al., 2014). Thus, converging evidence suggests that CBT generates meaningful changes in brain activity by increasing the capacity for “top-down” emotion regulation (Franklin et al., 2016, Brooks and Stein, 2015), and recruitment of the fronto-parietal network may be especially crucial to this process.
An important function of the prefrontal cortex (PFC) is to exert cognitive control, representing and maintaining context for responding and achieving goals, which in turn biases processing in associated areas to support task appropriate responding (Miller and Cohen, 2001). Cognitive dysfunction in neuropsychiatric disorders can be conceptualized as reflecting deficits in these processes (Snyder, 2013), consistent with reproducible findings of reduced attention, concentration and executive function (Snyder et al., 2015, Barch, 2013). Prior literature on CBT has highlighted a descriptive role for functional network connectivity in characterizing symptoms and likelihood of recovery. Our results expand upon these findings by demonstrating a network-based mechanism for recovery with CBT. In particular, the current imaging study, conducted before and after CBT treatment, implicates changes in dysfunctional cognitive control network connectivity as an important mechanism for CBT efficacy across both MDD and PTSD.
One caveat in the study is that MDD and PTSD patients underwent slightly different therapies. As part of CPT, PTSD subjects had an element of exposure to the prior trauma, through written trauma accounts, in addition to exercises emphasizing reframing and refocusing, elements common to both CBT and CPT. Considering that baseline fronto-parietal network hypoconnectivity is a common neural underpinning for both MDD and PTSD, however, the present findings serve to elucidate important similarities between the disorders before, during, and after recovery. These commonalities may be representative of a shared neural basis for recovery that operates through the primary focus of the treatment regimen on enhancing cognitive control. Other caveats include the restriction of CBT-induced amygdala connectivity changes to the a priori determined fronto-parietal network, the relatively small sample size and the majority of the participants were female since the PTSD patients were survivors of interpersonal violence. Thus future studies are needed to determine the generalizability of these findings.
In summary, an important aspect of this study was the novel method for examining longitudinal change in functional connectivity. Our principal component based analysis, LFPCA, as compared to the conventional voxel-wise approach, reduced the number of tests to be conducted and automatically identified the important latent patterns of connectivity changes that differed between patients and controls. The network-based approach focused on a widely-used parcellation template (Yeo et al., 2011). We restricted analysis to the fronto-parietal cognitive control network given our a priori hypotheses and limited sample size. Further, the validity and specificity was justified using a subsequent whole brain search. Despite the similarity in the identified regions between LFPCA and voxel-wise analysis, the voxel-wise GLM after multiple comparison adjustment produced more conservative results with fewer voxels showing treatment effects compared with LFPCA, since the latter identifies global patterns within the network of interest. Therefore LFPCA both draws strength across measurements and alleviates the need for overly conservative multiple testing adjustments to maintain the same confidence level. Thus, LFPCA may be a more powerful method for detecting longitudinal change; however this possibility awaits replication.
Conflict of interest
All authors report no conflict of interest.
Author contributions
HS conducted LFPCA analyses and wrote the paper; ZY conducted data analysis and wrote the paper; TDS conducted the original a priori data analysis, contributed to interpretation of the data and edited the paper; PC wrote scripts for data analysis, conducted data analysis and edited the paper; SEB recruited patients, conducted patient evaluations and administered CBT; RTS conducted statistical analysis, assisted in data interpretation and edited the paper; BR wrote the paper; YIS obtained funding for the study, designed the study, evaluated patients and wrote the paper. All authors approved the final article.
Acknowledgements
This work was supported by the following grants: RC MH089704 (YIS), R01MH064821-A2 (YIS), K24MH098260 (YIS); R01MH107703 (TDS); K23 MH090366 (SEB); RO1NS085211 (RTS); R21NS093349 (RTS).
Footnotes
The funding sources had no role in study design, collection, analysis and interpretation of data, in writing the article or the decision to submit the article for publication.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2017.01.030.
Appendix A. Supplementary data
Supplementary figures
References
- Amick H.R. Comparative benefits and harms of second generation antidepressants and cognitive behavioral therapies in initial treatment of major depressive disorder: systematic review and meta-analysis. BMJ. 2015;351:h6019. doi: 10.1136/bmj.h6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle R.L. Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Arch. Gen. Psychiatry. 2012;69(4):360–371. doi: 10.1001/archgenpsychiatry.2011.1539. [DOI] [PubMed] [Google Scholar]
- Avants B.B. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 2011;54(3):2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B.B. An open source multivariate framework for n-tissue segmentation with evaluation on public data. Neuroinformatics. 2011;9(4):381–400. doi: 10.1007/s12021-011-9109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch D.M. Brain network interactions in health and disease. Trends Cogn. Sci. 2013;17(12):603–605. doi: 10.1016/j.tics.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T. Guilford press; 1979. Cognitive therapy of depression. [Google Scholar]
- Blake D.D. The development of a clinician-administered PTSD scale. J. Trauma. Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Brooks S.J., Stein D.J. A systematic review of the neural bases of psychotherapy for anxiety and related disorders. Dialogues Clin. Neurosci. 2015;17(3):261–279. doi: 10.31887/DCNS.2015.17.3/sbrooks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown V.M. Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology. 2014;39(2):351–359. doi: 10.1038/npp.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chard K.M. An evaluation of cognitive processing therapy for the treatment of posttraumatic stress disorder related to childhood sexual abuse. J. Consult. Clin. Psychol. 2005;73(5):965–971. doi: 10.1037/0022-006X.73.5.965. [DOI] [PubMed] [Google Scholar]
- Dichter G.S., Gibbs D., Smoski M.J. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J. Affect. Disord. 2015;172:8–17. doi: 10.1016/j.jad.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop B.W., Mayberg H.S. Neuroimaging-based biomarkers for treatment selection in major depressive disorder. Dialogues Clin. Neurosci. 2014;16(4):479–490. doi: 10.31887/DCNS.2014.16.4/bdunlop. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B. Biometrics Research; New York, New York State Psychiatric Institute: 1996. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), Version 2. [Google Scholar]
- Foa E.B. Psychometric properties of the posttraumatic diagnostic scale for DSM-5 (PDS-5) Psychol. Assess. 2016;28(10):1166–1171. doi: 10.1037/pas0000258. [DOI] [PubMed] [Google Scholar]
- Fonzo G.A. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol. Psychiatry. 2010;68(5):433–441. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin G., Carson A.J., Welch K.A. Cognitive behavioural therapy for depression: systematic review of imaging studies. Acta Neuropsychiatr. 2016;28(2):61–74. doi: 10.1017/neu.2015.41. [DOI] [PubMed] [Google Scholar]
- Goldapple K. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch. Gen. Psychiatry. 2004;61(1):34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Greve D.N., Fischl B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 2009;48(1):63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greven S. Longitudinal functional principal component analysis. Electron. J. Stat. 2010;4:1022–1054. doi: 10.1214/10-EJS575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist M.N., Hwang K., Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage. 2013;82C:208–225. doi: 10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J.P., Hayes S.M., Mikedis A.M. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol. Mood Anxiety Disord. 2012;2:9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollon S.D. Effect of cognitive therapy with antidepressant medications vs antidepressants alone on the rate of recovery in major depressive disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71(10):1157–1164. doi: 10.1001/jamapsychiatry.2014.1054. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jenkinson M. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnstone T. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J. Neurosci. 2007;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R.H. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72(6):603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius R.A. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr. Scand. 2010;121(1):33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- MacNamara A., DiGangi J., Phan K.L. Aberrant spontaneous and task-dependent functional connections in the anxious brain. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2016;1(3):278–287. doi: 10.1016/j.bpsc.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews S.C. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. J. Affect. Disord. 2008;111:13–20. doi: 10.1016/j.jad.2008.05.022. [DOI] [PubMed] [Google Scholar]
- McMain S. Cognitive behavioral therapy: current status and future research directions. Psychother. Res. 2015;25(3):321–329. doi: 10.1080/10503307.2014.1002440. [DOI] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Murrough J.W. Reduced global functional connectivity of the medial prefrontal cortex in major depressive disorder. Hum. Brain Mapp. 2016;37(9):3214–3223. doi: 10.1002/hbm.23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby J.M. Systematic review and meta-analysis of transdiagnostic psychological treatments for anxiety and depressive disorders in adulthood. Clin. Psychol. Rev. 2015;40:91–110. doi: 10.1016/j.cpr.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Newby J.M., Mewton L., Andrews G. Transdiagnostic versus disorder-specific internet-delivered cognitive behaviour therapy for anxiety and depression in primary care. J. Anxiety Disord. 2016 doi: 10.1016/j.janxdis.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Oathes D.J. Neurobiological signatures of anxiety and depression in resting-state functional magnetic resonance imaging. Biol. Psychiatry. 2015;77(4):385–393. doi: 10.1016/j.biopsych.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quide Y. Differences between effects of psychological versus pharmacological treatments on functional and morphological brain alterations in anxiety disorders and major depressive disorder: a systematic review. Neurosci. Biobehav. Rev. 2012;36(1):626–644. doi: 10.1016/j.neubiorev.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Resick P., Schnicke M. Vol. 4. Sage; 1993. Cognitive Processing Therapy for Rape Victims: A Treatment Manual. [Google Scholar]
- Resick P.A., Schnicke M.K. Cognitive processing therapy for sexual assault victims. J. Consult. Clin. Psychol. 1992;60(5):748–756. doi: 10.1037//0022-006x.60.5.748. [DOI] [PubMed] [Google Scholar]
- Resick P.A. A randomized clinical trial to dismantle components of cognitive processing therapy for posttraumatic stress disorder in female victims of interpersonal violence. J. Consult. Clin. Psychol. 2008;76(2):243–258. doi: 10.1037/0022-006X.76.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage. 2013;64(0):240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D. Dimensional depression severity in women with major depression and post-traumatic stress disorder correlates with fronto-amygdalar hypoconnectivty. Mol. Psychiatry. 2016;21(7):894–902. doi: 10.1038/mp.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou H. Structured functional principal component analysis. Biometrics. 2015;71(1):247–257. doi: 10.1111/biom.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H.R. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol. Bull. 2013;139(1):81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H.R., Miyake A., Hankin B.L. Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Front. Psychol. 2015;6:328. doi: 10.3389/fpsyg.2015.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaes K. Can pharmacological and psychological treatment change brain structure and function in PTSD? A systematic review. J. Psychiatr. Res. 2014;50:1–15. doi: 10.1016/j.jpsychires.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Tustison N.J. N4ITK: improved N3 bias correction. IEEE Trans. Med. Imaging. 2010;29(6):1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison N.J. Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. NeuroImage. 2014;99:166–179. doi: 10.1016/j.neuroimage.2014.05.044. [DOI] [PubMed] [Google Scholar]
- Watson D. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J. Abnorm. Psychol. 1995;104(1):15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- Watson D. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J. Abnorm. Psychol. 1995;104(1):3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Weitz E.S. Baseline depression severity as moderator of depression outcomes between cognitive behavioral therapy vs pharmacotherapy: an individual patient data meta-analysis. JAMA Psychiatry. 2015;72(11):1102–1109. doi: 10.1001/jamapsychiatry.2015.1516. [DOI] [PubMed] [Google Scholar]
- Yeo B.T. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Nichols T.E., Johnson T.D. Cluster mass inference via random field theory. NeuroImage. 2009;44(1):51–61. doi: 10.1016/j.neuroimage.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures