Abstract
Objectives
The surface of pure titanium (Ti) shows decreased histocompatibility over time; this phenomenon is known as biological ageing. UV irradiation enables the reversal of biological ageing through photofunctionalisation, a physicochemical alteration of the titanium surface. Ti implants are sterilised by UV irradiation in dental surgery. However, orthopaedic biomaterials are usually composed of the alloy Ti6Al4V, for which the antibacterial effects of UV irradiation are unconfirmed. Here we evaluated the bactericidal and antimicrobial effects of treating Ti and Ti6Al4V with UV irradiation of a lower and briefer dose than previously reported, for applications in implant surgery.
Materials and Methods
Ti and Ti6Al4V disks were prepared. To evaluate the bactericidal effect of UV irradiation, Staphylococcus aureus 834 suspension was seeded onto the disks, which were then exposed to UV light for 15 minutes at a dose of 9 J/cm2. To evaluate the antimicrobial activity of UV irradiation, bacterial suspensions were seeded onto the disks 0, 0.5, one, six, 24 and 48 hours, and three and seven days after UV irradiation as described above. In both experiments, the bacteria were then harvested, cultured, and the number of colonies were counted.
Results
No colonies were observed when UV irradiation was performed after the bacteria were added to the disks. When the bacteria were seeded after UV irradiation, the amount of surviving bacteria on the Ti and Ti6Al4V disks decreased at 0 hours and then gradually increased. However, the antimicrobial activity was maintained for seven days after UV irradiation.
Conclusion
Antimicrobial activity was induced for seven days after UV irradiation on both types of disk. Irradiated Ti6Al4V and Ti had similar antimicrobial properties.
Cite this article: T. Itabashi, K. Narita, A. Ono, K. Wada, T. Tanaka, G. Kumagai, R. Yamauchi, A. Nakane, Y. Ishibashi. Bactericidal and antimicrobial effects of pure titanium and titanium alloy treated with short-term, low-energy UV irradiation. Bone Joint Res 2017;6:108–112. DOI: 10.1302/2046-3758.62.2000619.
Keywords: UV irradiation, Titanium alloy, Bactericidal effects, Antimicrobial effects
Article focus
To evaluate the bactericidal and antimicrobial effects of treating titanium (Ti) and Ti6Al4V with UV irradiation of a lower and briefer dose than previously reported, for applications in implant surgery.
Key messages
UV irradiation of a short duration and low energy was found to have a bactericidal effect on Ti and Ti6Al4V.
Antimicrobial activity was still induced seven days after UV irradiation on both materials, and Ti and Ti6Al4V had similar antimicrobial activity.
Strengths and limitations
To our knowledge, this is the first study to demonstrate the bactericidal and antimicrobial effects of this briefer and lower energy UV irradiation on Ti and Ti6Al4V.
The principal limitations of our study are that the period of time in which the antimicrobial activity completely disappeared was not clarified, and neither was the relationship between the antimicrobial activity and roughness of Ti surface.
Introduction
Titanium (Ti) implants are often used in orthopaedic and dental surgery. The advantages of Ti are its good bone affinity and osteoconductivity.1,2 To obtain early and strong osseointegration, it is important to minimise the time required for bone cell proliferation and differentiation. To this end, various methods for altering the implant surface, such as modifying the surface of titanium implants by plasma spray coating and changing the amount of surface hydroxyls and roughness with nanostructures, have been investigated.3-5
A surface of pure Ti shows decreased histocompatibility over time, and this phenomenon is known as biological ageing.1 UV irradiation enables the reversal of biological ageing through photofunctionalisation, a physicochemical alteration of the Ti surface1,6,7 that disinfects and improves its osteoblastic and bone affinities.
On the other hand, post-operative infection is one of the most serious complications when metal materials are used in patients. Therefore, to reduce or prevent the initial bacterial adhesion, various methods for altering the implant surface have been studied; such alterations would enable the body’s own cells to attach first, making it difficult for bacteria to form biofilms.8 Pure photocatalytic titanium dioxide (TiO2) substrates have been shown to eliminate organic compounds and to function as disinfectants upon UV irradiation.9 It was previously reported that after being exposed to UV subtype UV-C light, the surface of the Ti alloy Ti6Al4V, a common biomaterial, has a bactericidal effect for both gram-positive and gram-negative bacteria.10 To induce this effect, the Ti6Al4V surface was exposed to a UV-C source for 15 hours, at a dose of 227 J/cm2. In clinical applications, it is difficult to prepare implants with UV irradiation pre-operatively, because the implant size is often decided peri-operatively. Recently, UV irradiation of much lower energy and duration was shown to increase the bioactivity and osteoconductivity of Ti6Al4V.11 The purpose of this study was to evaluate the bactericidal and antimicrobial effects of this briefer and lower energy UV irradiation on Ti and Ti6Al4V, which would be advantageous for clinical applications involving total implant surgery, as they could be applied peri-operatively.
Materials and Methods
Ti and Ti6Al4V disks
Ti and Ti6Al4V disks (20 mm in diameter, 2 mm thick) were prepared by EPSON ATMIX Corp. (Nagano, Japan). The disks were produced in July 2013, and they were used more than three months later. The roughness of the surface was less than 2 μm (in titanium implants used commonly in orthopaedics, the surface roughness of the plasma spray of the stem used in total hip arthroplasty ranges from 20 µm to 50 µm, and that of intramedullary nails used as reduction and internal fixation for fractures of extremities ranges from 0.18 µm to 0.98 µm).12,13 Prior to their use, the disks were placed in a glass laboratory dish and treated with a dry heat steriliser.
Bacterial strains and culture conditions
Staphylococcus aureus strain 834, a clinical sepsis isolate,14 was grown in tryptic soy broth for 12 hours. The bacterial cells were harvested by centrifugation, washed with sterile phosphate-buffered saline (PBS), and diluted with PBS to appropriate cell concentrations as determined by spectrophotometry at 550 nm (OD550). An OD550 of 3 is equivalent to 1 × 109 colony-forming units (CFU)/ml.14
Evaluation of the bactericidal effects of UV irradiation
Adhesion experiments were carried out in a biological safety cabinet. A bacterial suspension (20 μl of 5×107 CFU/ml) was seeded onto each Ti and Ti6Al4V disk (n = 6, each) in glass laboratory dishes, followed by air drying in a safety cabinet for 30 minutes. Bactericidal treatment and photofunctionalisation were then performed by treating the implants with UV light for 15 minutes using a photo device (TheraBeam Affinity, Ushio Inc., Tokyo, Japan) (Fig. 1).11 The light source mounted in the TheraBeam Affinity is a low pressure mercury lamp, which emits predominantly 254 nm UV light. The UV beam was delivered in a continuous wave form. The disks were positioned 1 cm from the light source and centred. Under these conditions, each disk received about 8 mW/cm2 to 10 mW/cm2, which corresponded to a dose of 9 J/cm2. The UV light application was started at room temperature and increased up to 72°C at the end of irradiation.
Fig. 1.

Device used to perform an automatic programme for 15 minutes of UV exposure (TheraBeam Affinity, Ushio Inc., Tokyo, Japan).
The control group was not treated with UV irradiation. After UV irradiation, each disk was placed in a 50 ml centrifuge tube containing 10 ml of PBS (pH 7.2) with 0.05% Tween 80 (Sigma Aldrich, St. Louis, Missouri). The bound bacteria were harvested by two centrifugations, each for ten minutes at 1500 rpm, and ultrasonic vibration for ten minutes, based on the results of our preliminary experiments.
Suspensions of the recovered bacteria were diluted with PBS as a ten-fold dilution series (×10, ×100), and 100 μl of each sample was cultured in tryptic soy agar for 24 hours at 37°C. The number of colonies on the medium was then counted. Therefore, “no colony” is classed as less than 100 CFU on a disk.
Evaluation of the antimicrobial activity of UV irradiation
Ti and Ti6Al4V disks were treated with a dry heat steriliser, subjected to photofunctionalisation with UV light for 15 minutes as described above for the bactericide experiment, and then stored in a darkroom. A bacterial suspension (20 μl of 5×107 CFU/ml) was seeded onto each Ti and Ti6Al4V disk in a glass laboratory dish at 0, 0.5, one, six, 24, and 48 hours (n = 6, each), and three and seven days (n = 3, each), after UV irradiation, and then they were air dried in a safety cabinet for 30 minutes. Each disk was then placed in a 50 ml centrifuge tube containing 10 ml of PBS with 0.05% Tween 80. The bacteria were then harvested, diluted, cultured, and counted as described above for the bactericidal experiment. The control group was not treated with UV irradiation.
Statistical analysis
Differences in bactericidal effects and antimicrobial activity between Ti and Ti6Al4V were examined using the Mann-Whitney U test. Differences in antimicrobial activity between untreated and photofunctionalised disks were examined by analysis of variance. Significance was set at a p-value < 0.05. Data are reported as mean and standard deviation (sd).
Results
Bactericidal effects of UV irradiation
No colonies were observed when UV irradiation was applied after the bacterial suspension was added to either the Ti or Ti6Al4V disks (Fig. 2). Without UV irradiation, the average number of surviving bacteria was 52.7 (sd 26.8) × 104 CFU on Ti disks, and 49.1 (sd 18.5) × 104 CFU on Ti6Al4V (Fig. 3). There was no significant difference between Ti and Ti6Al4V. These results confirmed the bactericidal effect of UV irradiation.
Fig. 2.
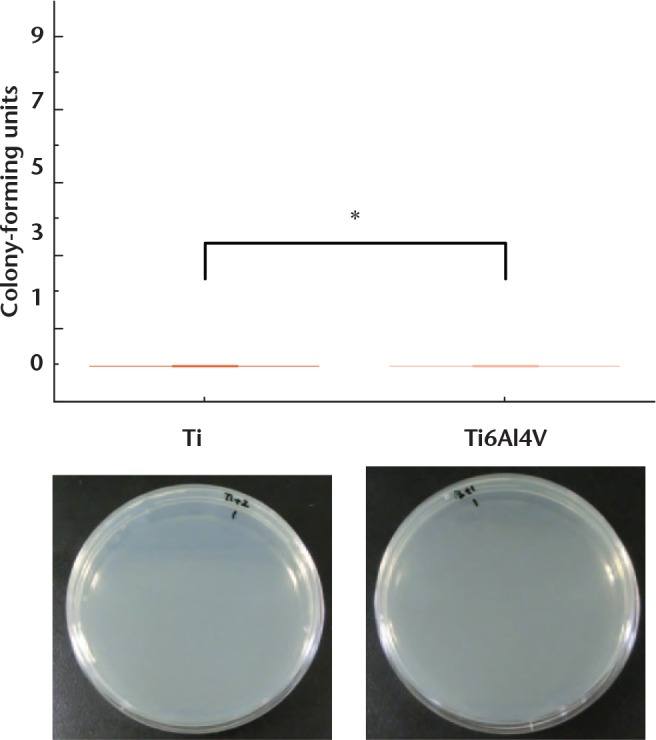
Number of colonies when bacterial seeding was followed by UV irradiation. No colonies were observed on the nutrient medium for either substrate (titanium (Ti), n = 6; Ti6Al4V, n = 6) (*not significant).
Fig. 3.
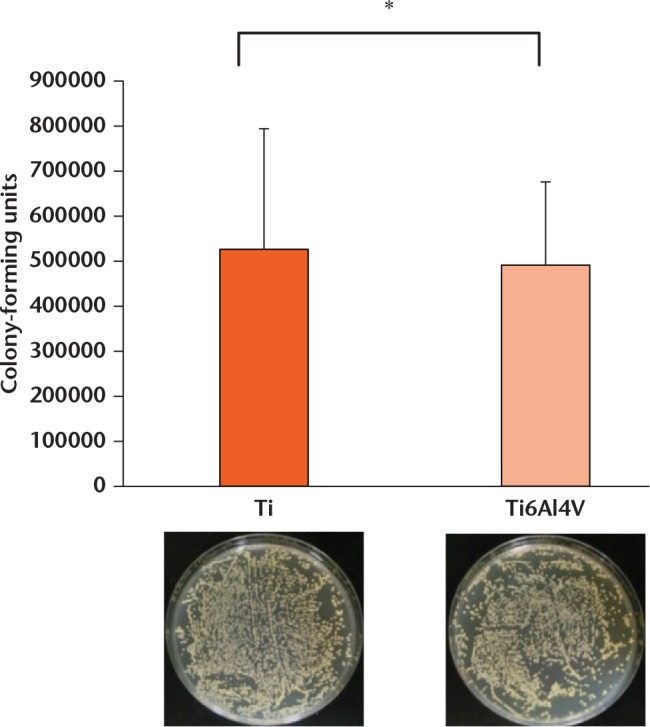
Number of colonies without UV irradiation. The mean was 52.7 (standard deviation (sd) 26.8) × 104 colony forming units (CFU) on titanium (Ti) disks and 49.1 (sd 18.5) × 104 CFU from Ti6Al4V in the absence of UV irradiation. Error bars represent sd of the mean (*not significant, Mann-Whitney U test).
Antimicrobial activity of UV irradiation
The number of surviving bacteria on Ti disks when the bacteria were seeded 0, 0.5, 1, 6, 24, and 48 hours, and three and seven days after UV irradiation are shown in Figure 4. The number of surviving bacteria on the Ti6Al4V disks are also shown in Figure 4. On both materials, the number of colonies increased in a time-dependent manner after UV irradiation. There were no significant differences in the number of colonies between the Ti and Ti6Al4V disks at any time point. The average number of surviving bacteria was 45.3 sd 25.7 × 104 CFU on Ti disks and 42.5 sd 19.3 × 104 CFU on Ti6Al4V disks without UV irradiation. The number of surviving bacteria on the Ti and Ti6Al4V disks at all time points after UV irradiation was significantly lower than that on untreated disks. These results confirmed the antimicrobial activity of UV irradiation.
Fig. 4.
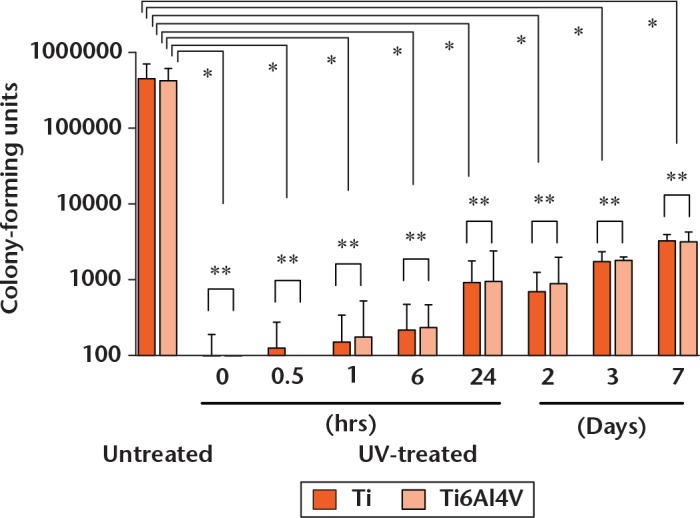
Time course of antimicrobial activity (n = 6 each at 0, 0.5, one, six, 24, and 48 hours after UV irradiation, and n = 3 each at three and seven days after UV irradiation) (logarithmic scale). The number of colonies increased in a time-dependent manner. Error bars represent standard deviations of the mean, p < 0.05 (*statistically significant; **not significant, analysis of variance).
Discussion
The time from Ti implant production to use can be months or years due to shortcomings in the distribution system. Biological ageing of the Ti implant surface is known to occur, during which Ti surfaces constantly absorb hydrocarbons from the atmosphere, water, and cleaning solutions after the implants are manufactured,15 and become hydrophobic. Therefore, the potential for early and strong osseointegration, as well as bactericidal and antimicrobial activity, decreases over time. Recently, the UV photofunctionalisation of Ti has enabled the recovery of osteoconductivity and bioactivity by disinfecting the Ti surface.1,6,7,10 UV treatment removes carbon deposition and converts the surfaces of bulk titanium, which consists of a layer of titanium dioxide, from hydrophobic to superhydrophilic.16 -18 The amount of surface hydroxyls is a key factor determining the biocompatibility at the tissue-material interface, although the degree of hydrophilicity does not correlate with the level of bioactivity of titanium surfaces.6,7 Treatment of titanium surfaces with UV light has demonstrated increased biocompatibility and osteoconductivity, and such treatments are now required for the biomaterials used in orthopaedics.
The thickness and detachment resistance of the biofilms that form on metal materials vary with the material. Ti has advantages with regards to infectious incidence.19 The biofilm formed on Ti, compared with that on stainless steel, is more easily removed with antibiotics because photofunctionalisation of the anatase form of TiO2 has bactericidal effects and antimicrobial activity.19 The photon energy from UV light excites valence electrons and generates electron-hole pairs that diffuse and become trapped on the TiO2 surface. These excited electrons and holes have strong reducing and oxidising properties, and react with H2O or hydroxide ions to generate hydroxyl radicals (OH) and superoxide ions (O2).20,21 Electron holes, OH and O2 are extremely reactive upon contact with organic matter, which they can completely oxidise to carbon dioxide.22,23 Reactive oxygen species are reported to attack polyunsaturated phospholipids in bacteria and to catalyse site-specific DNA damage.9,22 -24 In addition, it has been proposed that the bactericidal effect of direct UV irradiation is due to the formation of chemical bonds between adjacent thymine bases in bacteria, which obstruct DNA replication.
In the present study, we evaluated the bactericidal effects and antimicrobial activity of UV irradiation using a UV-C source for 15 minutes, with a dose of 9 J/cm2. This UV irradiation duration and dose were much lower than those used in previous studies.10 In our analysis of the bactericidal effect of direct UV irradiation after the application of bacteria, the number of surviving bacteria decreased to 0 CFU on both types of disk; thus, a clear bactericidal effect was observed even though the duration and energy of the UV irradiation were low. Regarding the antimicrobial activity, our findings showed that it was still present seven days after UV irradiation on both types of disks. Our results also indicated that Ti6Al4V and Ti have similar antimicrobial properties. However, the mechanisms by which the antimicrobial activity is maintained on the irradiated surface after turning off the UV lamp are not completely understood. In ordinary semiconductors, after activation, the electron-hole pairs are recombined very quickly. In contrast, this process in TiO2 is relatively slow. For this reason, the TiO2 surface continues to be stable after photoactivation, which is why TiO2 is widely used as a photocatalytic support.10,25 In addition, Hogt et al26 reported that the adhesion of staphylococci to more hydrophilic cellulose acetate is low. It is possible that the antimicrobial activity is maintained on Ti because the surface charge and hydrophilicity simultaneously persist on the TiO2 surface of Ti and Ti6Al4V after turning off the UV lamp.
On the other hand, the surface oxide on Ti is extremely thin (2 nm to 5 nm), and is often in an amorphous state and unable to crystallise. In addition, under normal oxidation processes, a rutile-type TiO2 structure is more easily generated, and there is relatively little anatase-form TiO2-mediated photocatalyst activity, and thus little sterilisation effect of OH radicals.21 Gallardo-Moreno et al10 reported that Ti6Al4V does not undergo oxidation after UV irradiation. Sakata3 reported that anatase-form TiO2 has enhanced osteoblastic biocompatibility due to photofunctionalisation effects. Thus, future studies will aim to increase the photocatalyst activity by altering the Ti surface.
In conclusion, short-duration and low-energy UV radiation was found to have a bactericidal effect on pure Ti and Ti alloy. Antimicrobial activity was still induced seven days after UV irradiation on both materials, and Ti6Al4V and Ti had similar antimicrobial activity.
Footnotes
Author Contribution: T. Itabashi: Corresponding author, Writing the paper, Data collection, Data analysis.
K. Narita: Second author, Data collection, Data analysis, Final approval of the version to be submitted and any revised version.
A. Ono: Approval of the version to be submitted and any revised version.
K. Wada: Approval of the version to be submitted and any revised version.
T. Tanaka: Approval of the version to be submitted and any revised version.
G. Kumagai: Final approval of the version to be submitted and any revised version.
R. Yamauchi: Approval of the version to be submitted and any revised version.
A. Nakane: Final approval of the version to be submitted and any revised version.
Y. Ishibashi: Final approval of the version to be submitted and any revised version.
ICMJE Conflicts of Interest: None declared.
Funding Statement
None declared
We thank USHIO Inc., and Y. Morimoto for technical advice of UV irradiation, and EPSON ATMIX Corp. for providing samples of Ti disks. We also are grateful for the helpful discussions with members of the Department of Orthopaedic Surgery, and Microbiology and Immunology at Hirosaki University.
References
- 1. Att W, Hori N, Takeuchi M, et al. Time-dependent degradation of titanium osteoconductivity: an implication of biological aging of implant materials. Biomaterials 2009;30:5352-5363. [DOI] [PubMed] [Google Scholar]
- 2. Xie Y, Liu X, Huang A, Ding C, Chu PK. Improvement of surface bioactivity on titanium by water and hydrogen plasma immersion ion implantation. Biomaterials 2005;26:6129-6135. [DOI] [PubMed] [Google Scholar]
- 3. Sakata M. The effects of anatase-formed titanium-dioxide on photofunctionalization and osteoblastic compatibility of titanium. Hokkaido J Dent Sci 2012;32:193-201. (In Japanese) [Google Scholar]
- 4. Homsy CA, Cain TE, Kessler FB, Anderson MS, King JW. Porous implant systems for prosthesis stabilization. Clin Orthop Relat Res 1972;89:220-235. [PubMed] [Google Scholar]
- 5. Goldman M, Juodzbalys G, Vilkinis V. Titanium surfaces with nanostructures influence on osteoblasts proliferation: a systematic review. J Oral Maxillofac Res 2014;5:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Att W, Hori N, Iwasa F, et al. The effect of UV-photofunctionalization on the time-related bioactivity of titanium and chromium-cobalt alloys. Biomaterials 2009;30:4268-4276. [DOI] [PubMed] [Google Scholar]
- 7. Aita H, Hori N, Takeuchi M, et al. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials 2009;30:1015-1025. [DOI] [PubMed] [Google Scholar]
- 8. Aita H, Att W, Ueno T, et al. Ultraviolet light-mediated photofunctionalization of titanium to promote human mesenchymal stem cell migration, attachment, proliferation and differentiation. Acta Biomater 2009;5:3247-3257. [DOI] [PubMed] [Google Scholar]
- 9. Maness PC, Smolinski S, Blake DM, et al. Bactericidal activity of photocatalytic TiO(2) reaction: toward an understanding of its killing mechanism. Appl Environ Microbiol 1999;65:4094-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gallardo-Moreno AM, Pacha-Olivenza MA, Fernández-Calderón MC, et al. Bactericidal behaviour of Ti6Al4V surfaces after exposure to UV-C light. Biomaterials 2010;31:5159-5168. [DOI] [PubMed] [Google Scholar]
- 11. Minamikawa H, Ikeda T, Att W, et al. Photofunctionalization increases the bioactivity and osteoconductivity of the titanium alloy Ti6Al4V. J Biomed Mater Res A 2014;102:3618-3630. [DOI] [PubMed] [Google Scholar]
- 12. Hayes JS, Vos DI, Hahn J, Pearce SG, Richards RG. An in vivo evaluation of surface polishing of TAN intermedullary nails for ease of removal. Eur Cell Mater 2009;18:15-26. [DOI] [PubMed] [Google Scholar]
- 13. Lombardi AV, Jr, Berend KR, Mallory TH, Skeels MD, Adams JB. Survivorship of 2000 tapered titanium porous plasma-sprayed femoral components. Clin Orthop Relat Res 2009;467:146-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nakane A, Okamoto M, Asano M, Kohanawa M, Minagawa T. Endogenous gamma interferon, tumor necrosis factor, and interleukin-6 in Staphylococcus aureus infection in mice. Infect Immun 1995;63:1165-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kilpadi DV, Lemons JE, Liu J, et al. Cleaning and heat-treatment effects on unalloyed titanium implant surfaces. Int J Oral Maxillofac Implants 2000;15:219-230. [PubMed] [Google Scholar]
- 16. Takeuchi M, Sakamoto K, Martra G, Coluccia S, Anpo M. Mechanism of photoinduced superhydrophilicity on the TiO2 photocatalyst surface. J Phys Chem B 2005;109:15422-15428. [DOI] [PubMed] [Google Scholar]
- 17. Wang R, Hashimoto K, Fujishima A. Light-induced amphiphilic surfaces. Nature 1997;388:431-432. [Google Scholar]
- 18. Takeuchi M, Martra G, Coluccia S, et al. Verification of the photoadsorption of H2O molecules on TiO2 semiconductor surfaces by vibrational absorption spectroscopy. J Phys Chem C 2007;111:9811-9817. [Google Scholar]
- 19. Adachi K, Tsurumoto T, Yonekura A, et al. New quantitative image analysis of staphylococcal biofilms on the surfaces of nontranslucent metallic biomaterials. J Orthop Sci 2007;12:178-184. [DOI] [PubMed] [Google Scholar]
- 20. Matsunaga T, Tomoda R, Nakajima T, Nakamura N, Komine T. Continuous-sterilization system that uses photosemiconductor powders. Appl Environ Microbiol 1988;54:1330-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hanawa T. Properties and problems of metallic materials used for treatment of spinal reconstitution. Sekitsuisekizui 2010;23:19-25. (In Japanese) [Google Scholar]
- 22. Jacoby WA, Maness PC, Wolfrum EJ, et al. Mineralization of bacterial cell mass on a photocatalytic surface in air. Environ Sci Technol 1998;32:2650-2653. [Google Scholar]
- 23. Legrini O, Oliveros E, Braun AM. Photochemical processes for water treatment. Chem Rev 1993;93:671-698. [Google Scholar]
- 24. Hirakawa K, Mori M, Yoshida M, Oikawa S, Kawanishi S. Photo-irradiated titanium dioxide catalyzes site specific DNA damage via generation of hydrogen peroxide. Free Radic Res 2004;38:439-447. [DOI] [PubMed] [Google Scholar]
- 25. Yatskiv VI, Korzhak AV, Granchak VM, et al. Peculiarities of the behaviour of porous titanium dioxide in the photocatalytic evolution of molecular hydrogen from aqueous ethanolic solutions. Theor Exp Chem 2003;39:172-176. [Google Scholar]
- 26. Hogt AH, Dankert J, de Vries JA, Feijen J. Adhesion of coagulase-negative staphylococci to biomaterials. J Gen Microbiol 1983;129:2959-2968. [DOI] [PubMed] [Google Scholar]


