Key Clinical Message
Most of the de novo BRCA1/2 mutations have been identified in patients with early‐onset breast cancer and without family history of the disease. The identification of these alterations could play a prominent role in the prevention and treatment strategies and may influence clinical management of patients.
Keywords: BRCA2 gene, breast cancer, de novo mutation, early onset
Pathogenic variants of BRCA genes confer high cancer risk also in women without a family history of breast/ovarian cancer. Over the years, a large number of distinct mutations and other genetic variants of uncertain significance have been identified in these genes, but very few cases of de novo BRCA1/2 alterations have been described in the literature worldwide. In fact, only nine cases of de novo BRCA1 mutation and six cases of de novo BRCA2 mutation have been reported till date 1, 2, 3, 4, 5. Interestingly, most mutations have been identified in patients with tumors occurring before the age of 40 1, 4, 5. In the present study, we report a patient with a de novo BRCA1 gene mutation (c.5095C>T) who developed early‐onset breast cancer with no family history of the disease. A 32‐year‐old Italian woman with clinical diagnosis of an infiltrating ductal carcinoma of the right breast was referred to genetic counseling. Histological analysis confirmed an invasive ductal carcinoma of 2.5 cm, stage pN3a, and five of 12 lymph nodes were positive. As for tumor hormone receptor status, the carcinoma was ER‐positive (98%), PR‐positive (90%), and HER2/neu‐negative. The patient was treated with radical mastectomy and axillary lymphadenectomy followed by adjuvant chemotherapy and postoperative radiation therapy. The proband's father and mother, aging 63 and 57 years, respectively, were both healthy and had no family history of breast/ovarian cancer (Fig. 1A). After having informed consent, genomic DNA was extracted from peripheral blood lymphocytes using BioRobot EZ1 instrument according to the manufacturer's protocol. Amplification of all coding exons and of corresponding flanking intron(ic) sequences (±30 bp) of BRCA1/2 genes was performed in B‐Pure™ EasySeq™ PCR plates (Nijmegen, The Netherlands) followed by direct DNA sequencing. BRCA1 and BRCA2 mutational analysis revealed a BRCA1 mutation (c.5095C>T), leading to an arginine‐to‐tryptophan change at codon 1699 (Fig. 1B). This mutation has been classified as pathogenic (class 5 according to the IARC classification scheme), using a multifactorial likelihood model 6. Recently, bioinformatics results have demonstrated that c.5095C>T has a deleterious effect and predisposes carriers to BC and OC 7. In detail, the amino acid substitution leads to a folding defect in the BRCT domain of the BRCA1 gene and reduces the proteolytic stability of this domain that interacts with numerous proteins involved in transcription and DNA repair 8. Subsequently, the parents of the patient underwent genetic testing, both resulting negative for the mutation identified in their daughter. The absence of a germ line c.5095C>T BRCA1 mutation in either parents suggested either a “de novo” origin of the alteration during parental germ cell gametogenesis or a nonpaternity event. We ruled out this latter possibility by performing paternity testing on patient's father using the PowerPlex® 16 HS System (Madison, WI, USA), thus reinforcing the hypothesis of a “de novo” origin of the pathogenic mutation identified in the proband. To investigate the possibility of a somatic mosaicism, we analyzed other tissues (hair roots and buccal cells), evidencing in each case the presence of the mutation. Although these results cannot definitively rule out the possibility of postzygotic events, the presence of the same mutation in all the investigated samples is compatible with a “de novo” mutation due to a germinal mosaicism. It is important to emphasize that mosaic events have been revealed using the conventional Sanger sequencing; unfortunately, this technique is able to disclose mosaicism only for rates above 20%. In view of this, the major limitation of this study is the lack of approach of deep sequencing for the detection of low‐rate mosaic mutations. To the best of our knowledge, there is no available information on the frequency of mosaicism for BRCA1 and BRCA2 point mutations. However, the prevalence of these “de novo” mutations could be likely underestimated, based on the typical selection criterion that mainly takes into account the familial history of the proband. The identification of cases of de novo mutations would have a direct impact on the prevention and treatment strategies and may influence clinical management of patients. Furthermore, the presence of these germ line mutations represents a key aspect of genetic counseling, as the carrier's status may contribute to the transmission of spontaneous alterations to the offspring. In the present case, we used early onset of disease as a selection criterion for BRCA1/2 genetic testing in the absence of a familial history of the disease. In fact, the age at onset represents an important feature to predict the presence of “de novo” mutations, because the lack of family history of the disease may be due to different factors including limited family structure (such as families in which there are few women) and the effect of penetrance modifiers that are able to affect the phenotypic outcome of a particular mutation.
Figure 1.
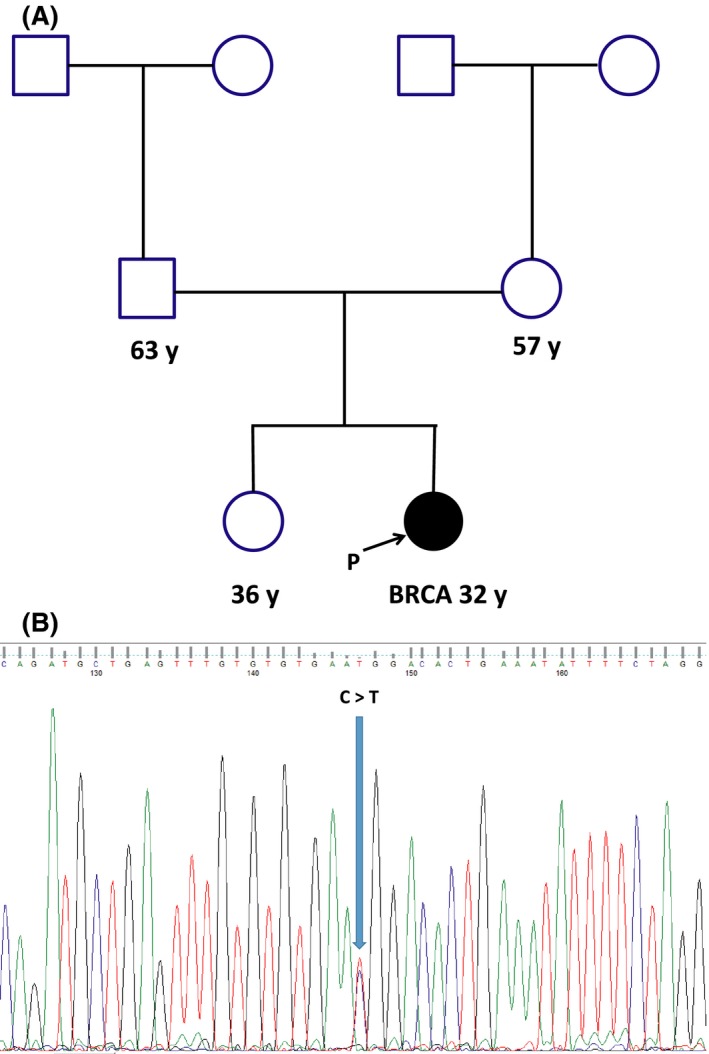
(A) Pedigree of patient carrying the de novo mutation in BRCA1 gene; (B) electropherogram showing missense mutation.
Conflict of Interest
We declare no conflict of interests.
References
- 1. Edwards, E. , Yearwood C., Sillibourne J., Baralle D., and Eccles D.. 2009. Identification of a de novo BRCA1 mutation in a woman with early onset bilateral breast cancer. Fam. Cancer 8:479–482. [DOI] [PubMed] [Google Scholar]
- 2. Friedman, E. , Efrat N., Soussan‐Gutman L., Dvir A., Kaplan Y., Ekstein T., et al. 2015. Low‐level constitutional mosaicism of a de novo BRCA1 gene mutation. Br. J. Cancer 112:765–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Golmard, L. , Delnatte C., Laugé A., Moncoutier V., Lefol C., Abidallah K., et al. 2015. Breast and ovarian cancer predisposition due to de novo BRCA1 and BRCA2 mutations. Oncogene 35:1324–1327. [DOI] [PubMed] [Google Scholar]
- 4. Kwong, A. , Ng E. K., Tang E. Y., Wong C. L., Law F. B., Leung C. P., et al. 2011. A novel de novo BRCA1 mutation in a Chinese woman with early onset breast cancer. Fam. Cancer 10:233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marshall, M. , Solomon S., and Lawrence Wickerham D.. 2009. Case report: de novo BRCA2 gene mutation in a 35‐year‐old woman with breast cancer. Clin. Genet. 76:427–430. [DOI] [PubMed] [Google Scholar]
- 6. Easton, D. F. , Deffenbaugh A. M., Pruss D., Frye C., Wenstrup R. J., Allen‐Brady K., et al. 2007. A systematic genetic assessment of 1,433 sequence variants of unknown clinical significance in the BRCA1 and BRCA2 breast cancer‐predisposition genes. Am. J. Hum. Genet. 81:873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laraqui, A. , Uhrhammer N., Lahlou‐Amine I., El Rhaffouli H., El Baghdadi J., Dehayni M., et al. 2013. Mutation screening of the BRCA1 gene in early onset and familial breast/ovarian cancer in Moroccan population. Int. J. Med. Sci. 10:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laraqui, A. , Uhrhammer N., Rhaffouli H. E., Sekhsokh Y., Lahlou‐Amine I., Bajjou T., et al. 2015. BRCA genetic screening in Middle Eastern and North African: mutational spectrum and founder BRCA1 mutation (c.798_799delTT) in North African. Dis. Markers 2015:194293. [DOI] [PMC free article] [PubMed] [Google Scholar]


