Abstract
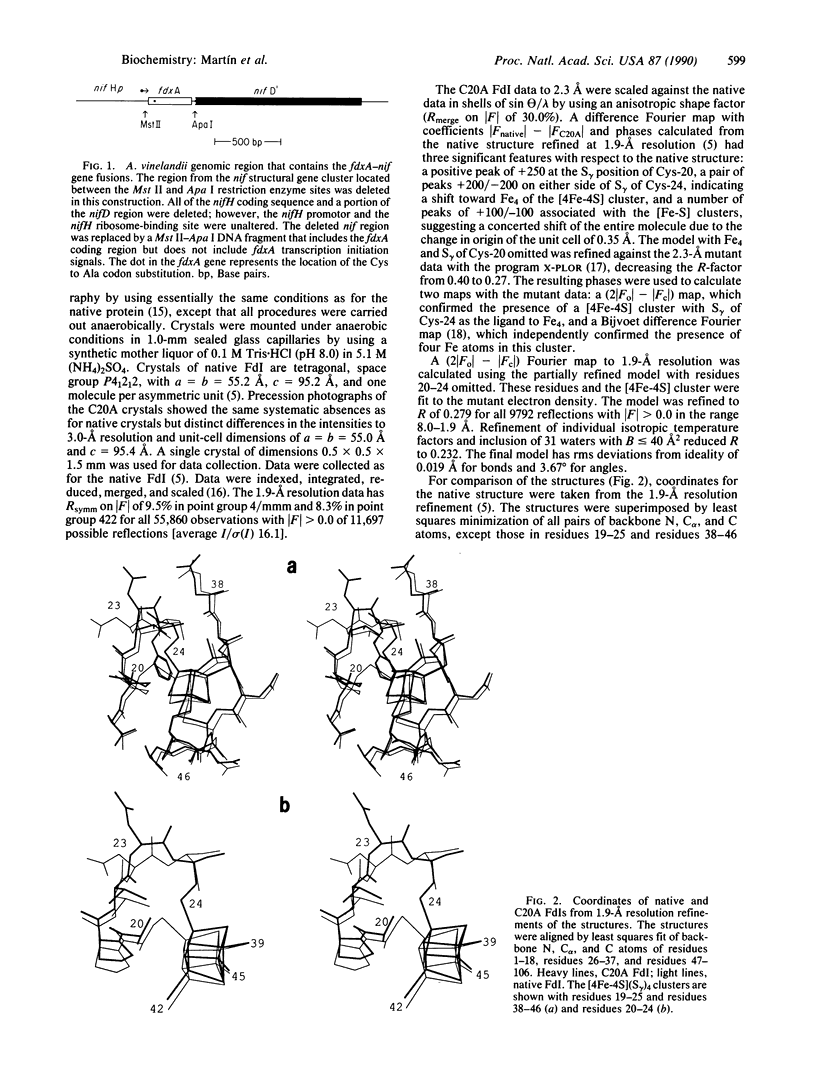
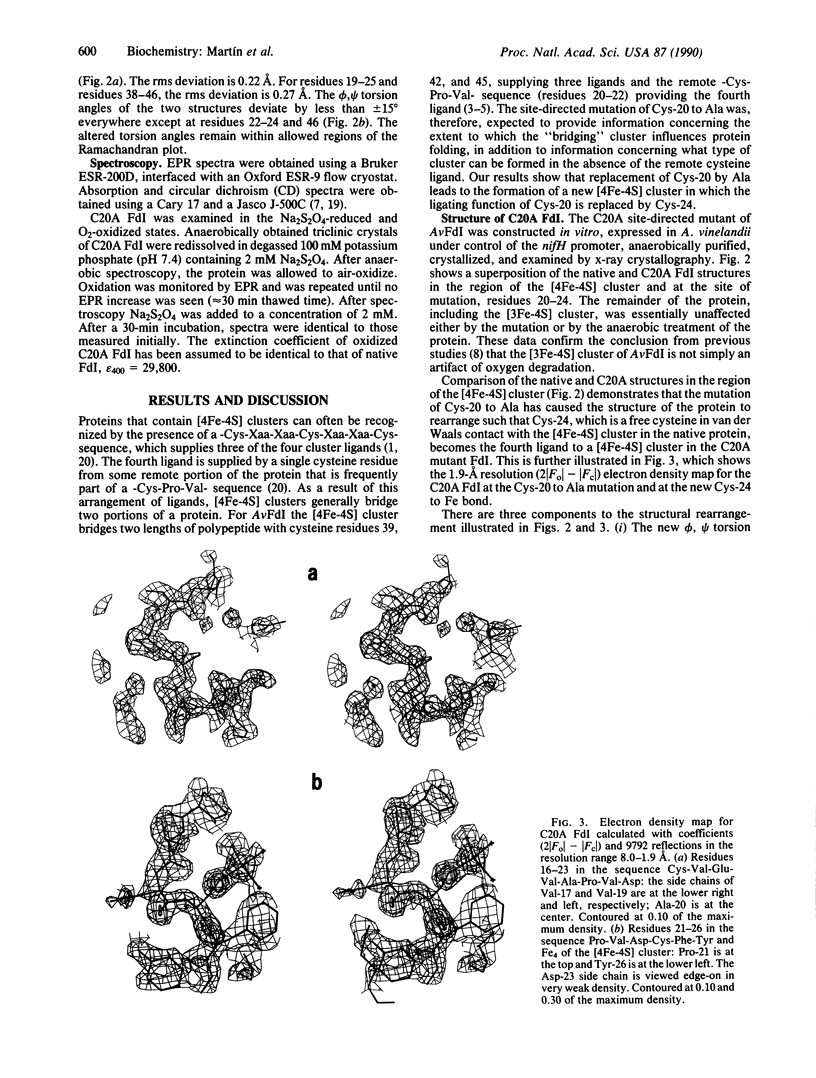
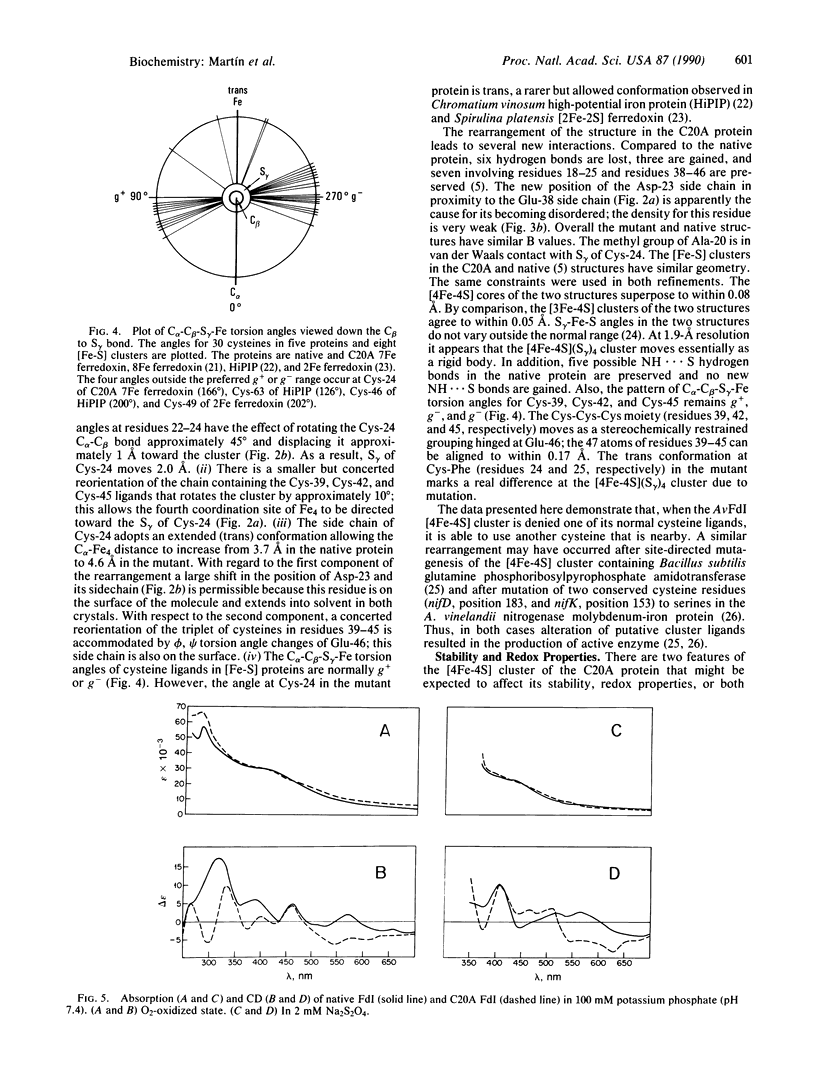
Azotobacter vinelandii ferredoxin I is a small protein that contains one [4Fe-4S] cluster and one [3Fe-4S] cluster. Recently the x-ray crystal structure has been redetermined and the fdxA gene, which encodes the protein, has been cloned and sequenced. Here we report the site-directed mutation of Cys-20, which is a ligand of the [4Fe-4S] cluster in the native protein, to alanine and the characterization of the protein product by x-ray crystallographic and spectroscopic methods. The data show that the mutant protein again contains one [4Fe-4S] cluster and one [3Fe-4S] cluster. The new [4Fe-4S] cluster obtains its fourth ligand from Cys-24, a free cysteine in the native structure. The formation of this [4Fe-4S] cluster drives rearrangement of the protein structure.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman E. T., Siefker L. C., Jensen L. H. Structure of Peptococcus aerogenes ferredoxin. Refinement at 2 A resolution. J Biol Chem. 1976 Jun 25;251(12):3801–3806. doi: 10.2210/pdb1fdx/pdb. [DOI] [PubMed] [Google Scholar]
- Bruschi M., Guerlesquin F. Structure, function and evolution of bacterial ferredoxins. FEMS Microbiol Rev. 1988 Apr-Jun;4(2):155–175. doi: 10.1111/j.1574-6968.1988.tb02741.x. [DOI] [PubMed] [Google Scholar]
- Burgess B. K., Jacobs D. B., Stiefel E. I. Large-scale purification of high activity Azotobacter vinelandII nitrogenase. Biochim Biophys Acta. 1980 Jul 10;614(1):196–209. doi: 10.1016/0005-2744(80)90180-1. [DOI] [PubMed] [Google Scholar]
- Freer S. T., Alden R. A., Carter C. W., Jr, Kraut J. Crystallographic structure refinement of Chromatium high potential iron protein at two Angstroms resolution. J Biol Chem. 1975 Jan 10;250(1):46–54. [PubMed] [Google Scholar]
- Howard A. J., Nielsen C., Xuong N. H. Software for a diffractometer with multiwire area detector. Methods Enzymol. 1985;114:452–472. doi: 10.1016/0076-6879(85)14030-9. [DOI] [PubMed] [Google Scholar]
- Johnson M. K., Bennett D. E., Fee J. A., Sweeney W. V. Spectroscopic studies of the seven-iron-containing ferredoxins from Azotobacter vinelandii and Thermus thermophilus. Biochim Biophys Acta. 1987 Jan 5;911(1):81–94. doi: 10.1016/0167-4838(87)90273-1. [DOI] [PubMed] [Google Scholar]
- Makaroff C. A., Paluh J. L., Zalkin H. Mutagenesis of ligands to the [4 Fe-4S] center of Bacillus subtilis glutamine phosphoribosylpyrophosphate amidotransferase. J Biol Chem. 1986 Aug 25;261(24):11416–11423. [PubMed] [Google Scholar]
- Morgan T. V., Lundell D. J., Burgess B. K. Azotobacter vinelandii ferredoxin I: cloning, sequencing, and mutant analysis. J Biol Chem. 1988 Jan 25;263(3):1370–1375. [PubMed] [Google Scholar]
- Morgan T. V., Stephens P. J., Devlin F., Stout C. D., Melis K. A., Burgess B. K. Spectroscopic studies of ferricyanide oxidation of Azotobacter vinelandii ferredoxin I. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1931–1935. doi: 10.1073/pnas.81.7.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. C., Burgess B. K., Dean D. R. Activity, reconstitution, and accumulation of nitrogenase components in Azotobacter vinelandii mutant strains containing defined deletions within the nitrogenase structural gene cluster. J Bacteriol. 1986 Apr;166(1):180–186. doi: 10.1128/jb.166.1.180-186.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. C., Dean D. R., Burgess B. K. Iron-molybdenum cofactor biosynthesis in Azotobacter vinelandii requires the iron protein of nitrogenase. J Biol Chem. 1987 Oct 15;262(29):14327–14332. [PubMed] [Google Scholar]
- Saeki K., Wakabayashi S., Zumft W. G., Matsubara H. Pseudomonas stutzeri ferredoxin: close similarity to Azotobacter vinelandii and Pseudomonas ovalis ferredoxins. J Biochem. 1988 Aug;104(2):242–246. doi: 10.1093/oxfordjournals.jbchem.a122450. [DOI] [PubMed] [Google Scholar]
- Stephens P. J., Thomson A. J., Dunn J. B., Keiderling T. A., Rawlings J., Rao K. K., Hall D. O. Circular dichroism and magnetic circular dichroism of iron-sulfur proteins. Biochemistry. 1978 Oct 31;17(22):4770–4778. doi: 10.1021/bi00615a026. [DOI] [PubMed] [Google Scholar]
- Stout C. D. 7-Iron ferredoxin revisited. J Biol Chem. 1988 Jul 5;263(19):9256–9260. doi: 10.2210/pdb3fd1/pdb. [DOI] [PubMed] [Google Scholar]
- Stout C. D. Refinement of the 7 Fe ferredoxin from Azotobacter vinelandii at 1.9 A resolution. J Mol Biol. 1989 Feb 5;205(3):545–555. doi: 10.1016/0022-2836(89)90225-8. [DOI] [PubMed] [Google Scholar]
- Stout C. D. Two crystal forms of Azotobacter ferredoxin. J Biol Chem. 1979 May 10;254(9):3598–3599. [PubMed] [Google Scholar]
- Stout G. H., Turley S., Sieker L. C., Jensen L. H. Structure of ferredoxin I from Azotobacter vinelandii. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1020–1022. doi: 10.1073/pnas.85.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahs G., Kraut J. Low-resolution electron-density and anomalous-scattering-density maps of Chromatium high-potential iron protein. J Mol Biol. 1968 Aug 14;35(3):503–512. doi: 10.1016/s0022-2836(68)80010-5. [DOI] [PubMed] [Google Scholar]
- Sweeney W. V., Rabinowitz J. C., Yoch D. C. High and low reduction potential 4Fe-4S clusters in Azotobacter vinelandii (4Fe-4S) 2ferredoxin I. Influence of the polypeptide on the reduction potentials. J Biol Chem. 1975 Oct 10;250(19):7842–7847. [PubMed] [Google Scholar]
- Yoch D. C., Arnon D. I. Two biologically active ferredoxins from the aerobic nitrogen-fixing bacteriu, Azotobacter vinelandii. J Biol Chem. 1972 Jul 25;247(14):4514–4520. [PubMed] [Google Scholar]



