Key Clinical Message
Bleeding is a rare complication of direct oral anticoagulant potentially associated with high mortality rates. Biological monitoring is necessary for more than 24 h after idarucizumab antidote therapy in case of bleeding with dabigatran therapy.
Keywords: Anticoagulant, antidote, bleeding, dabigatran, idarucizumab, non‐VKA oral anticoagulants
Introduction
The first authorized specific antidote for non‐VKA oral anticoagulants (NOACs) is idarucizumab, a humanized antibody fragment that targets dabigatran 1, 2. We describe three cases in which we used idarucizumab for dabigatran reversal in patients with severe bleeding (Table 1).
Table 1.
Biological and clinical characteristics of the three patients, by comparison with the RE‐VERSE AD trial group A (serious bleeding)
| RE‐VERSE AD group A n = 51 | Case 1 | Case 2 | Case 3 | |
|---|---|---|---|---|
| Age (year), Median (Range) | 77 (48–93) | 77 | 96 | 80 |
| Sex (%) | 63% male | F | F | M |
| Weight (Kg), Median (Range) | 70.5 (42.4–127.5) | 60 | 42 | 79.5 |
| Hemoglobin (g/L) | – | 39 | 98 | 137 |
| CHA2DS2‐VASc | – | 3 | 3 | 5 |
| Creatinine clearance (mL/min) | ||||
| Median (range) | 54 (16–187) | 66.6 | 16 | 64 |
| Dose of dabigatran (%) | ||||
| 150 bid | 27 | |||
| 110 bid | 67 | X | X | X |
| 75 bid | 2 | |||
| other | 4 | |||
| Indication for dabigatran (%) | ||||
| NVAF | 92 | X | X | X |
| VTE | 2 | |||
| Other | 6 | |||
| Type of bleeding (%) | ||||
| Intracranial | 35 | X | ||
| Trauma related | 18 | |||
| Gastro‐intestinal | 39 | X | X | |
| Other | 22 | |||
| Unbound dabigatran at entrance | ||||
| Concentration (ng/mL) Median (Range) | 84.4 (3.30–641) | 330 | 455 | 108 |
| TTd (sec) | 44.9 (29.7–110) | – | 75.61 | 42.81 |
| TT (sec) | – | >150 | 354 | 149 |
| Dabigatran maximal rebounce | ||||
| Time post‐reversion (h) | – | 24 | 43 | 40 |
| Concentration (ng/mL) | – | 104 | 51.23 | <30 |
| TTd (sec) | – | – | 37.6 | 32.6 |
| TT (sec) | – | >150 | 94 | 27.5 |
TTd, for diluted thrombin time; TT, for thrombin time; NVAF: non valvular atrial fibrilation; VTE: venous thrombo‐embolism.
Case 1
A 77‐year‐old Caucasian woman was admitted to the intensive care unit of Beaujon Hospital (Paris, France) for major gastrointestinal bleeding after orthopedic surgery. She was on dabigatran etexilate (110 mg twice a day) for nonvalvular atrial fibrillation (NVAF). She had a CHA2DS2‐VASc score of 3, and no history of bleeding. She had spontaneous gastrointestinal bleeding and clinical melena during hospitalization in an orthopedic rehabilitation center, 14 days after orthopedic surgery for a femoral fracture. On day 13, the hemoglobin was 123 g/L. Spontaneous melena occurred on day 14, with severe anemia (60 g/L) that led to emergency admission in the intensive care unit. On admission, she had hemorrhagic shock and a hemoglobin level of 39 g/L. Her dabigatran level was high (330 ng/mL), as evaluated with the Hemoclot® Thrombin Inhibitor (HTI) assay (Hyphen BioMed, France). Her prothrombin time was elevated (PT, 36.9 sec vs. 8.5 sec for control, PT Innovin, Siemens, Germany), as was her activated partial thromboplastin time (aPTT, 113 sec vs. 30 sec for control, TriniCLOT aPPT, Stago, France), and her thrombin time (TT, >150 sec vs. 17 sec for control, BC Thrombin, Siemens, Germany) on the BCS® XP coagulometer (Siemens, Germany). Calculated creatinine clearance (Cockcroft and Gault) was 66.6 mL/min. Emergency endoscopy revealed a peptic ulcer. Local hemostasis was performed and proton pump inhibitor (PPI) and antibiotic‐based eradication of Helicobacter pylori was initiated. She received 4 red cell units, plasma volume expansion with saline solution and intravenous tranexamic acid. Immediately after admission, she received 5 g of idarucizumab intravenously. One hour after idarucizumab injection, dabigatran was undetectable in plasma and her clotting times normalized (aPTT: 40 sec, PT: 11.6 sec, TT: 19 sec). The bleeding stopped and did not recur over the next 3 days. However, 12 h after idarucizumab injection, the dabigatran concentration rose (40 ng/mL) and the TT increased slightly to 62 sec, whereas the aPTT and PT were unchanged. Twenty‐four hours after idarucizumab injection, the dabigatran concentration was 104 ng/mL, the TT was >150 sec, and a slight increase was noted in the other clotting times (aPTT 49 sec, PT 12.1 sec). Thirty‐six hours after idarucizumab injection, the dabigatran concentration remained high (103 ng/mL) (Fig. 1A). Idarucizumab was not re‐injected, because there was no further bleeding and no further fall in the hemoglobin level. Anticoagulation was resumed with enoxaparin 3 days after the bleeding episode and the patient was transferred to a geriatric center.
Figure 1.
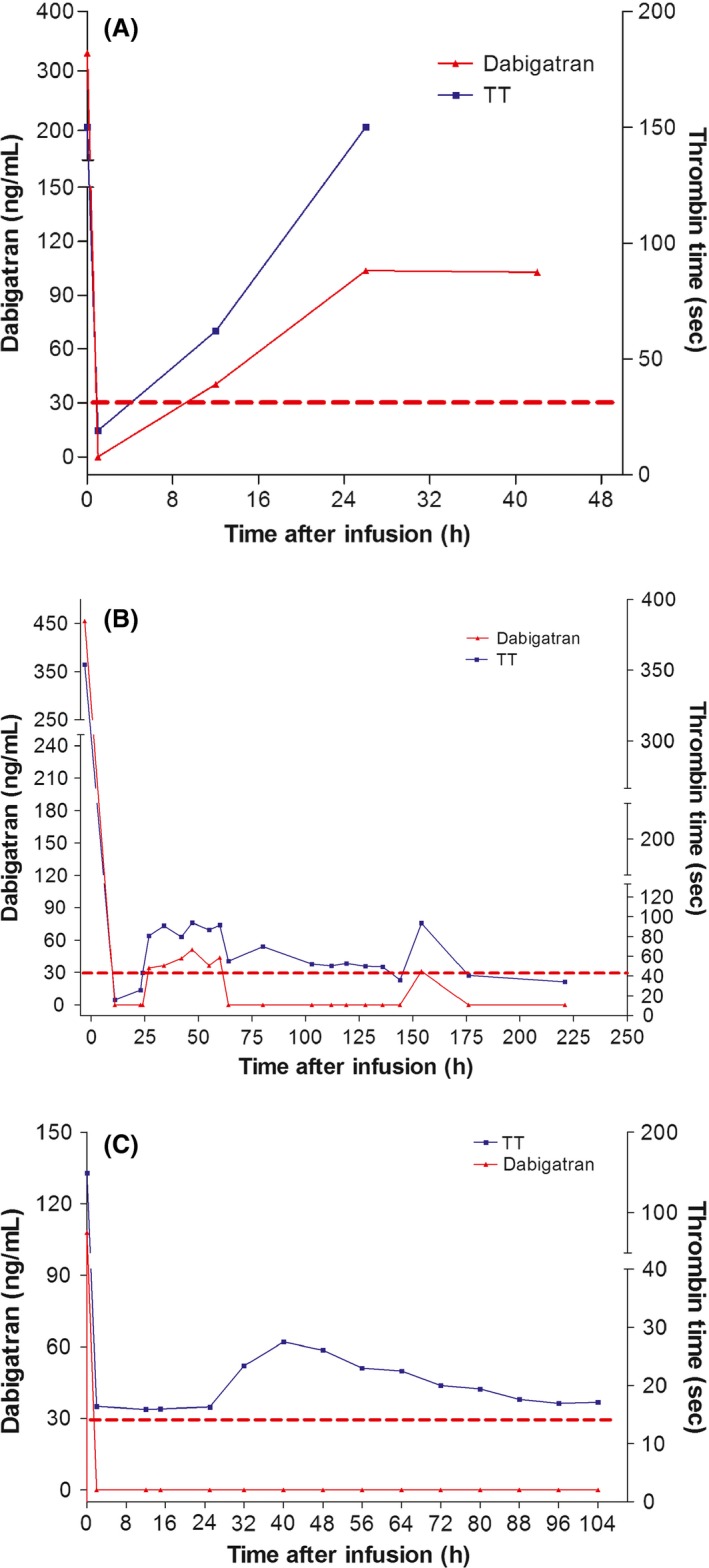
Plasma dabigatran concentration and thrombin time in patients treated with idarucizumab. The Hemoclot® Thrombin Inhibitor assay limit of quantification was defined as 30 ng/mL (red dotted line). (A) Plasma dabigatran concentration and thrombin time in patient 1 (gastrointestinal bleeding). (B) Plasma dabigatran concentration and thrombin time in patient 2 (gastrointestinal bleeding). (C) Plasma dabigatran concentration and thrombin time in patient 3 (intracerebral bleeding).
Case 2
A 96‐year‐old Caucasian woman was admitted to the emergency department of Georges Pompidou Hospital (Paris, France) with a nine‐day history of rectorrhagia. She had no recent history of constipation, diarrhea or abdominal pain. She had NVAF with a CHA2DS2‐VASc score of 3, treated with dabigatran etexilate (110 mg twice a day). Calculated creatinine clearance (Cockcroft and Gault) was 16 mL/min. On admission, her hemoglobin was 98 g/L, the dabigatran level was 455 ng/mL, and her PT (27.1 s vs. 14.1 sec for control, STA neoplastin CI, Stago, France), aPTT (59 sec vs. 30 sec for control, CK‐Prest 5 aPPT, Stago, France) and thrombin time (354 sec vs. 17 sec for control, STA Thrombin 10, Stago, France) were elevated and explored with a STA‐R® (Stago, France) coagulometer (same for cases 2 and 3, Georges Pompidou hospital). She received plasma volume expansion with saline solution plus 5 g of idarucizumab intravenously, immediately after admission. Upper GI endoscopy was normal. Twelve hours after idarucizumab injection, plasma dabigatran was undetectable and her clotting times normalized (aPTT: 26 sec, PT: 14.7 sec, TT: 16 sec). The bleeding stopped without an interventional procedure. Twenty‐two hours after idarucizumab injection, the dabigatran concentration rose to 34 ng/mL and the TT rose slightly to 81 sec. The aPTT and PT also rose during the next 24 h, and then all three clotting values normalized. The dabigatran concentration rose again to 37 ng/mL 150 h after idarucizumab injection, and the TT increased to 87 sec (Fig. 1B). No further idarucizumab was administered because the bleeding did not recur and the hemoglobin showed no further fall. Coloscopy revealed rectal carcinoma. The DDimer level (Vidas, bioMérieux, France) increased from 283 to 1777 ng/mL between admission and the 27th hour after idarucizumab injection, then slowly fell to 923 ng/mL on day 10, when the patient was transferred to a geriatric center. After discussion with cardiologists, anticoagulation was not restarted and no recurrence of bleeding was observed.
Case 3
An 80‐year‐old Caucasian man was admitted to the emergency department of Georges Pompidou Hospital for a head injury following a fall without loss of consciousness. He reported right lower limb pain for the past 3 days. He had NVAF, a CHA2DS2‐VASc score of 5, and was treated with dabigatran etexilate (110 mg twice a day). He also had heart failure, diabetes, hypertension, external ventricular derivation 14 years previously for hydrocephalus, and prostate cancer treated with radiotherapy 10 years previously. Calculated creatinine clearance (Cockcroft and Gault) was 64 mL/min. Physical examination showed a conscious patient without disorientation, no anomalies of the cranial pairs, and no meningeal or intracranial hypertension syndrome. Distal deep venous thrombosis was suspected (with calf pain, edema, and calf cramp). Doppler examination confirmed superficial venous thrombosis of the small right saphenous vein, which occurred on dabigatran etexilate.
CT revealed a 5‐mm subdural hematoma on the right tentorium of the cerebellum. On admission, the dabigatran concentration was 108 ng/mL and clotting times were elevated (PT = 17.8 sec; aPTT = 44.1 sec and TT = 149 sec). His last dabigatran etexilate dose was taken 12 h previously. Intracranial bleeding was treated with 4000 IU of prothrombin complex concentrate (PCC, 63 IU/kg) and 5 g of idarucizumab intravenously. Two hours after idarucizumab injection, plasma dabigatran was undetectable and the TT, PT and aPTT normalized. Clotting times and the dabigatran concentration were evaluated at least twice a day for the next 5 days. No increase in dabigatran (Fig. 1C) or clotting times (PT and aPTT) was observed, while the TT was slightly elevated from 24 to 72 h following idarucizumab injection. Between admission and day 5, the DDimer rose from 753 to 1943 ng/mL. Repeat CT showed stabilization of the subdural hematoma. The patient was discharged after 5 days. Anticoagulation was stopped for at least 1 month, despite the superficial venous thrombosis that had occurred while on dabigatran etexilate.
In the three cases described here, we observed an immediate normalization of clotting times upon idarucizumab administration, but dabigatran levels rebounded after 24 h in the two patients with the highest levels at admission (cases 1 and 2). Close monitoring of the dabigatran level is thus necessary during the first 72 h after idarucizumab injection. Idarucizumab re‐administration was not necessary, owing to a favorable clinical course. The recently published ISTH guideline 3 recommends the use of a NOAC antidote for immediate anticoagulant neutralization, to prevent hemorrhage expansion and to facilitate interventions to stem the bleeding source in specific situations. The guideline recommends the use of an antidote in case of life‐threatening bleeding, as in case 3 (intracranial bleeding) and in case of uncontrolled bleeding, as in case 1. Idarucizumab infusion in case 2 is open to discussion, as the guideline does not recommend the use of an antidote in case of gastrointestinal bleeding that responds to supportive measures. Indeed, in case 2, the gastrointestinal bleeding stopped without hemostatic measures, anemia was moderate, and the dabigatran level was not considered to show overdose. Classical clinical management might have been sufficient to manage this major bleeding episode. Despite recent data showing the safety of restarting dabigatran etexilate 24 h after idarucizumab reversal in healthy volunteers 4, we have no clinical data for NVAF patients. After treatment of a bleeding episode that occurs on dabigatran, anticoagulant resumption in NVAF patients is a difficult issue. If the bleeding has stopped, the observed re‐increase in the dabigatran concentration 24 h after antidote injection might provide sufficient anticoagulation, allowing few hours for therapeutic decision‐making. Apixaban is the only commercial NOAC that was not associated with an increased risk of gastrointestinal bleeding in phase III trials 5, 6. Apixaban is also the only other available “twice‐a‐day” treatment, along with dabigatran etexilate, allowing the patient to switch drugs without changing their treatment schedule. Renal status and age may favor the use of a VKA in NVAF patients, but the past history must be taken into account. In the two patients with gastrointestinal bleeding described here, LMWH was chosen as first‐line treatment before switching back to oral anticoagulation, while no oral anticoagulation was reintroduced for the patient with intracranial bleeding. No procoagulant effect was detected during clinical evaluation of idarucizumab 7. In our patients 2 and 3, a rise in DDimers was observed, but it probably resulted from concomitant PCC administration (and superficial venous thrombosis in case 3). Despite idarucizumab use in two of these three cases, co‐administration of non‐specific hemostatic agents was decided (tranexamic acid and PCC in cases 1 and 3, respectively) and these agents may have prothrombotic effects. The increased DDimer level could also be a consequence of anticoagulant withdrawal after complete neutralization of dabigatran in the procoagulant context of NVAF. Further studies are needed to understand this DDimer rebound, according to the clinical context and anticoagulant resumption.
These case studies suffer from technical limitations. First, DDimers were not assayed in patient 1 because we have no remaining plasma samples. Second, the HTI has a lower limit of quantification (LOQ) (according to the manufacturer, it is 50 ng/mL). However, the LOQ of the HTI assay used in our laboratory is 30 ng/mL. We adapted with a local calibration curve the method for the measurements of low concentrations of dabigatran. In our laboratories, we use this LOQ of 30 ng/mL because the French working group on perioperative hemostasis 8 recommends a dabigatran concentration of ≤30 ng/mL for urgent surgery with a hemorrhagic risk. Thus, results below this threshold have been expressed as <30 ng/mL and this is why we determined the thrombin time in parallel. A normal TT reasonably excludes a clinically relevant dabigatran concentration 9. The LC‐MS/MS should be best method to evaluate plasma concentration. However, this method is not routinely available for emergency use. The main purpose of our report is to describe routine use. Finally, the patients we describe were treated in two different centers, using different commercial thromboplastins (PT Innovin and STA neoplastin CI), which explains the different PT control values. TT assay is not standardized and is affected by many variables (type of thrombin, clot detection method). In Beaujon Hospital Laboratory, BC Thrombin was used for TT assay, with an upper limit of quantification of 150 sec. In Georges Pompidou European Hospital, the STA thrombin 10 kit was used for the TT assay, which measures TT values up to 900 sec, as in the REVERSE AD trial 2.
In conclusion, idarucizumab normalized clotting times within a few hours in the three cases described here. Further real‐world data are needed to refine the use of idarucizumab for dabigatran reversal in different situations and patient groups.
Authorship
NG, ALFP, AMF, CLS, ALLL, and DMS: involved in the patients’ care at the European Georges Pompidou Hospital. IJ, EDR, and VB: involved in the patients’ care at the Beaujon Hospital. NG, ALLL and DMS: wrote the manuscript. All authors involved in the editing of the manuscript.
Conflicts of Interest
NG, ALFP, IJ, EDR, and VB have nothing to declare. AMF declares consulting fees from Bayer. ALL declares consulting fees from Sanofi. DMS declares consulting fees from Bayer, BMS, Boehringer Ingelheim and Léo Pharma.
Acknowledgments
Nicolas Gendron was supported by a grant from AP‐HP (Assistance publique – hopitaux de paris). David M. Smadja is supported by Paris Descartes University, The Conny‐Maeva Charitable Foundation and Région Ile de France‐CORDDIM (Domaine d'intérêt majeur Cardiovasculaire Obésité Rein Diabète).
References
- 1. Schiele, F. , van Ryn J., Canada K., Newsome C., Sepulveda E., Park J., et al. 2013. A specific antidote for dabigatran: functional and structural characterization. Blood 121:3554–3562. [DOI] [PubMed] [Google Scholar]
- 2. Pollack, C. V. Jr , Reilly P. A., Eikelboom J., Glund S., Verhamme P., Bernstein R. A., et al. 2015. Idarucizumab for dabigatran reversal. N. Engl. J. Med. 373:511–520. [DOI] [PubMed] [Google Scholar]
- 3. Levy, J. H. , Ageno W., Chan N. C., Crowther M., Verhamme P., and Weitz J. I.. 2016. When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J. Thromb. Haemost. 14:623–627. [DOI] [PubMed] [Google Scholar]
- 4. Glund, S. , Moschetti V., Norris S., Stangier J., Schmohl M., van Ryn J., et al. 2015. A randomised study in healthy volunteers to investigate the safety, tolerability and pharmacokinetics of idarucizumab, a specific antidote to dabigatran. Thromb. Haemost. 113:943–951. [DOI] [PubMed] [Google Scholar]
- 5. Granger, C. B. , Alexander J. H., McMurray J. J., Lopes R. D., Hylek E. M., Hanna M., et al. 2011. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 365:981–992. [DOI] [PubMed] [Google Scholar]
- 6. Di Minno, A. , Spadarella G., Spadarella E., Tremoli E., and Di Minno G.. 2015. Gastrointestinal bleeding in patients receiving oral anticoagulation: current treatment and pharmacological perspectives. Thromb. Res. 136:1074–1081. [DOI] [PubMed] [Google Scholar]
- 7. Glund, S. , Stangier J., Schmohl M., Gansser D., Norris S., van Ryn J., et al. 2015. Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebo‐controlled, double‐blind phase 1 trial. Lancet 386:680–690. [DOI] [PubMed] [Google Scholar]
- 8. Pernod, G. , Albaladejo P., Godier A., Samama C. M., Susen S., Gruel Y., et al. 2013. Management of major bleeding complications and emergency surgery in patients on long‐term treatment with direct oral anticoagulants, thrombin or factor‐Xa inhibitors: proposals of the working group on perioperative haemostasis (GIHP) ‐ March 2013. Arch. Cardiovasc. Dis. 106:382–393. [DOI] [PubMed] [Google Scholar]
- 9. Lessire, S. , Douxfils J., Baudar J., Bailly N., Dincq A. S., Gourdin M., et al. 2015. Is Thrombin Time useful for the assessment of dabigatran concentrations? An in vitro and ex vivo study. Thromb. Res. 136:693–696. [DOI] [PubMed] [Google Scholar]


