Abstract
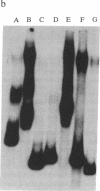
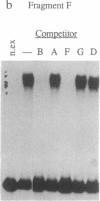
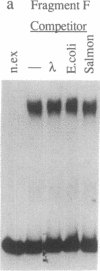
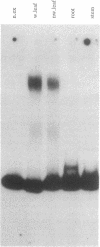
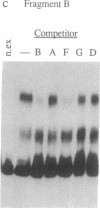
Deletion analysis from the 3' to the 5' end of the promoter region of the wound-inducible potato proteinase inhibitor IIK gene has identified a 421-base sequence at -136 to -557 that is necessary for expression. Utilizing DNA band-shift assays, a 10-base sequence within the 421-base region was found to bind a nuclear protein from wounded tomato leaves. This 10-base sequence is adjacent to an 8-base consensus sequence at -147 to -155 that is present in the promoter region of several elicitor-inducible genes from various other plants. The evidence suggests that a complex set of cis- and trans-acting elements within the -136 to -165 region of the potato IIK gene may be involved with the signaling mechanisms that regulate the inducibility of this gene in response to pest and pathogen attacks.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G. Development of plant promoter expression vectors and their use for analysis of differential activity of nopaline synthase promoter in transformed tobacco cells. Plant Physiol. 1986 May;81(1):86–91. doi: 10.1104/pp.81.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An G., Mitra A., Choi H. K., Costa M. A., An K., Thornburg R. W., Ryan C. A. Functional analysis of the 3' control region of the potato wound-inducible proteinase inhibitor II gene. Plant Cell. 1989 Jan;1(1):115–122. doi: 10.1105/tpc.1.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. D., Pearce G., Bryant J. E., Ryan C. A. Isolation and characterization of the proteinase inhibitor-inducing factor from tomato leaves. Identity and activity of poly- and oligogalacturonide fragments. J Biol Chem. 1984 Nov 10;259(21):13172–13177. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C., Hoffmann H., Schulz W., Hahlbrock K. Structure and elicitor or u.v.-light-stimulated expression of two 4-coumarate:CoA ligase genes in parsley. EMBO J. 1987 May;6(5):1189–1195. doi: 10.1002/j.1460-2075.1987.tb02353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Pearce G., Ryan C. A. In vitro phosphorylation of plant plasma membrane proteins in response to the proteinase inhibitor inducing factor. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1539–1542. doi: 10.1073/pnas.86.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinbaum R. L., Ausubel F. M. Transcriptional regulation of the Arabidopsis thaliana chalcone synthase gene. Mol Cell Biol. 1988 May;8(5):1985–1992. doi: 10.1128/mcb.8.5.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T. R., Ryan C. A. Wound-Induced Proteinase Inhibitor in Plant Leaves: A Possible Defense Mechanism against Insects. Science. 1972 Feb 18;175(4023):776–777. doi: 10.1126/science.175.4023.776. [DOI] [PubMed] [Google Scholar]
- Green T. R., Ryan C. A. Wound-induced Proteinase Inhibitor in Tomato Leaves: Some Effects of Light and Temperature on the Wound Response. Plant Physiol. 1973 Jan;51(1):19–21. doi: 10.1104/pp.51.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha S. B., An G. Identification of upstream regulatory elements involved in the developmental expression of the Arabidopsis thaliana cab1 gene. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8017–8021. doi: 10.1073/pnas.85.21.8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann A., Schulz W., Hahlbrock K. Two alleles of the single-copy chalcone synthase gene in parsley differ by a transposon-like element. Mol Gen Genet. 1988 Apr;212(1):93–98. doi: 10.1007/BF00322449. [DOI] [PubMed] [Google Scholar]
- Keil M., Sanchez-Serrano J., Schell J., Willmitzer L. Primary structure of a proteinase inhibitor II gene from potato (Solanum tuberosum). Nucleic Acids Res. 1986 Jul 25;14(14):5641–5650. doi: 10.1093/nar/14.14.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Brown W. E., Graham J. S., Pearce G., Fox E. A., Dreher T. W., Ahern K. G., Pearson G. D., Ryan C. A. Molecular characterization and phylogenetic studies of a wound-inducible proteinase inhibitor I gene in Lycopersicon species. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7277–7281. doi: 10.1073/pnas.83.19.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois R., Dietrich A., Hahlbrock K., Schulz W. A phenylalanine ammonia-lyase gene from parsley: structure, regulation and identification of elicitor and light responsive cis-acting elements. EMBO J. 1989 Jun;8(6):1641–1648. doi: 10.1002/j.1460-2075.1989.tb03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plunkett G., Senear D. F., Zuroske G., Ryan C. A. Proteinase inhibitors I and II from leaves of wounded tomato plants: purification and properties. Arch Biochem Biophys. 1982 Feb;213(2):463–472. doi: 10.1016/0003-9861(82)90572-0. [DOI] [PubMed] [Google Scholar]
- Ryan C. A. Oligosaccharide signalling in plants. Annu Rev Cell Biol. 1987;3:295–317. doi: 10.1146/annurev.cb.03.110187.001455. [DOI] [PubMed] [Google Scholar]
- Thornburg R. W., An G., Cleveland T. E., Johnson R., Ryan C. A. Wound-inducible expression of a potato inhibitor II-chloramphenicol acetyltransferase gene fusion in transgenic tobacco plants. Proc Natl Acad Sci U S A. 1987 Feb;84(3):744–748. doi: 10.1073/pnas.84.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmitzer L., Wagner K. G. The isolation of nuclei from tissue-cultured plant cells. Exp Cell Res. 1981 Sep;135(1):69–77. doi: 10.1016/0014-4827(81)90300-1. [DOI] [PubMed] [Google Scholar]
- Winberg G., Hammarskjöld M. L. Isolation of DNA from agarose gels using DEAE-paper. Application to restriction site mapping of adenovirus type 16 DNA. Nucleic Acids Res. 1980 Jan 25;8(2):253–264. doi: 10.1093/nar/8.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]