Abstract
Background
Patients with gastroenteropancreatic neuroendocrine tumors often present with metastases. Identification of the primary tumor is important for operative management, and therefore we sought to determine our success at identifying primary tumors with diagnostic testing and operative exploration.
Methods
A clinical neuroendocrine tumor database was reviewed to identify patients presenting with metastases and primary tumor in situ. Results of radiologic, endoscopic, and operative procedures were evaluated to determine which correctly identified the primary tumor.
Results
There were 197 patients presenting with metastases and unresected primaries, 134 who had an operation and 63 managed nonoperatively. Primaries were identified preoperatively in 168 (84%), at operative exploration in 7, and were not found in 22 patients. Computed tomography found 150/197 primary tumors, somatostatin-receptor scintigraphy 88/155, and endoscopy 43/107. The sensitivity of computed tomography surpassed scintigraphy (76% vs 57%, P < .01). The primary was removed in 130/134 (97%) patients, and hepatic debulking was performed in 67%. Median survival for operative patients with small bowel and pancreatic tumors was 145 and 71 months, respectively.
Conclusion
Imaging and endoscopy identified the primary tumor in most patients, and the majority of the others were found at exploration. Preoperative testing facilitated operative planning, allowing for resection of the primary and hepatic debulking in most patients.
Neuroendocrine tumors (NETs) may occur throughout the body, and up to 55% occur in the gastrointestinal tract.1 These tumors may secrete hormones, which give rise to symptoms, especially in the setting of metastatic disease. It is estimated that 50–60% of patients with gastroenteropancreatic NETs (GEPNETs) present with regional or distant metastases,2 with the liver being the site of metastasis in 85% of patients presenting with distant disease.3
It has been shown that resection of primary tumors in patients with GEPNETs may reduce the risk of future complications and improve overall survival, even in those with metastatic disease.4–9 Several studies have suggested an improvement in survival of up to 3-fold with resection of the primary tumor in patients with metastases.6,7,10 Therefore, identification of the primary tumor site is an important part of the workup for patients with metastatic GEPNETs to facilitate operative exploration. Liver directed operation also has the potential to improve survival and ameliorate symptoms in patients with metastatic GEPNETs.9–13
Several modalities are commonly used to identify GEPNETs preoperatively, including computed tomography (CT) scan, somatostatin receptor scintigraphy (SRS; Octreoscan, Mallinckrodt Pharmaceuticals, St. Louis, MO), and endoscopy, each with varying rates of sensitivity, depending on how successful localization is defined. Despite the fact that most patients have multiple diagnostic tests performed, it is not always a simple task to identify the primary site, and a subset of patients will not have their primary tumor identified after preoperative workup and operative exploration.2,14–16
Recent reports evaluating the success of identifying the primary NET when patients present with metastases have varied widely, but have been limited by small numbers. Massimino et al15 found 17% of primary NETs by preoperative testing, which improved to 79% with operative exploration. Similar studies by Wang et al14 and Bartlett et al16 reported primary tumor identification rates of 54% and 79% preoperatively, increasing to 89% and 87% with exploration, respectively. Given the wide variation in these studies, we sought to define our experience in finding the site of primary tumors in a larger group of patients presenting with metastatic GEPNETs.
METHODS
Patients
Individuals presenting to the University of Iowa NET Clinic between 1999 and 2016 were consented to participate in an Institutional Review Board-approved tumor registry. Information regarding demographics, symptoms, laboratory values, radiologic, or endoscopic results, findings at operation, pathologic information, and status at last follow-up were collected and entered into the registry. From this database, patients presenting with biopsy-proven or suspected neuroendocrine liver metastases (by virtue of increased biochemical markers and imaging characteristics) and a primary tumor still in place evaluated by a single surgeon were selected for additional study. These patients generally had a variety of tests performed at multiple institutions, and after careful review of these studies, as well as a history and physical examination, discussions were carried out with patients regarding the pros and cons of resecting their primary tumors and debulking of liver metastases.
Operative management
Patients were divided into groups based on whether they underwent operative procedures at our institution or had nonoperative management. Patients who did not have operation were divided into 3 groups based on why this was not performed: 1) those who had other treatments recommended (medical management, embolization, peptide radioreceptor therapy) or were lost to follow-up; 2) those deemed to be at high-risk for operative intervention (due to medical comorbidities or extensive liver replacement) or who died prior to making a decision about operation; 3) those patients who elected not to undergo operative exploration despite being offered operation.
Data analysis
In all patients, the tests performed in the diagnostic workup were reviewed carefully to determine whether the site of the primary could be identified, and the frequency with which it was identified with each testing modality. These modalities included CT scans, SRS, endoscopy (colonoscopy, upper endoscopy [EGD], endoscopic ultrasound [EUS]), and operative exploration. Although 68Ga-DOTATOC PET scans were available during the later years of this study, they were not included due to the low proportion of patients having them and the limited availability of this imaging modality elsewhere. CT scans were considered positive if they identified a discrete mass or suspicious thickening of the stomach, intestine or pancreas, or if multiple enlarged nodes were seen within the mesentery indicative of a small bowel primary. SRS was considered positive if there was uptake in the pancreas, stomach, or intestine distinct from normal background activity, or uptake in mesenteric nodes in the case of small bowel NETs (SBNETs). Endoscopy was considered positive if a submucosal mass was identified in the intestine, with or without pathologic confirmation. EUS was considered positive if it identified a submucosal mass in the stomach, duodenum, or rectum, or a mass within the pancreas. In patients having operative exploration, finding the primary allowed for confirmation of diagnostic testing, although this was not possible in those who were not explored. Sensitivity was calculated for each test by the number of patients with positive tests divided by the total number of patients examined by that modality. Statistical comparisons were made using Welch’s t test, Fisher exact test, Kaplan-Meier, and McNemar’s test.
RESULTS
During this period, 332 patients presented to one surgical oncologist with metastatic neuroendocrine tumors. Of this number, the primary tumor had not been resected in 197 patients. The others either had their primaries removed at an outside hospital or had incomplete records and the status of the primary tumor could not be determined. Of the 197 patients with intact primaries, 134 went on to have an operation, while 63 patients were evaluated in the clinic but were not operated on in our hospital. The diagnosis of metastatic neuroendocrine tumor was made by liver or lymph node biopsy in 185 cases, and the finding of liver lesions suspicious for NETs in conjunction with clinical symptoms and elevated NET markers in the other 12. The demographics and diagnostic tests used in operative and nonoperative groups were similar (Table I).
Table I.
Demographics and diagnostic studies in operative and nonoperative patient groups
| Operative (n = 134) | Nonoperative (n = 63) | Total (n = 197) | |
|---|---|---|---|
| Mean age (y) | 63.1 | 62.1 | 62.8 |
| Sex | |||
| Male (%) | 78 (58.2) | 38 (60.3) | 116 (58.9) |
| Female (%) | 56 (41.8) | 25 (39.7) | 81 (41.1) |
| Imaging studies | |||
| CT (%) | 134 (100) | 63 (100) | 197 (100) |
| SRS (%) | 105 (78.4) | 50 (79.4) | 155 (78.7) |
| Endoscopy (%) | 73 (54.5) | 34 (54.0) | 107 (54.3) |
| Mean number of modalities | 2.3 | 2.3 | 2.3 |
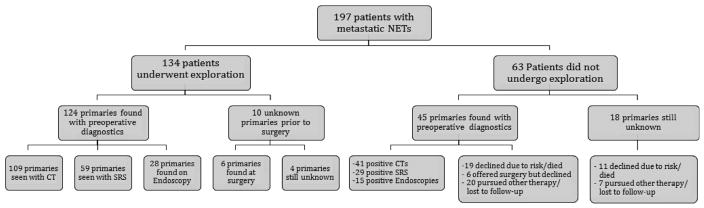
In the 134 patients who underwent operation, the primary tumor was identified with preoperative diagnostic studies in 124 patients (92.5%, all confirmed at operation), 6 (4.5%) were not identified but found at operation, and in 4 (3.0%) the primary was not found by preoperative testing or at exploration (Fig). The primary tumor was found to be in the small bowel in 90 patients (67.2%), the pancreas in 33 (24.6%), the stomach in 3 (2.2%), the duodenum in 3 (2.2%), and 1 patient (0.7%) had a rectal carcinoid (Table II).
Fig.
Patients presenting with NET liver metastases and intact primary tumors to a single surgeon. Results of imaging, endoscopy, and operative exploration are shown. Reasons for nonoperative management also are listed.
Table II.
Primary tumor sites in patients with metastatic GEPNETs who underwent operative exploration
| Site | Number | Percent |
|---|---|---|
| Small bowel | 90 | 67.2 |
| Pancreas | 33 | 24.6 |
| Unknown | 4 | 3.0 |
| Stomach | 3 | 2.2 |
| Duodenum | 3 | 2.2 |
| Rectum | 1 | 0.8 |
The 63 patients evaluated in clinic with metastases and intact primaries that did not undergo operation could be divided into 3 groups. The first group was patients who were deemed poor operative candidates due to either coexisting medical problems or a high degree of liver replacement (>50–70%) leading to concerns for postoperative liver failure (30 patients). The second included those electing to pursue other therapy and/or who were lost to follow-up (27 patients), and the third group consisted of 6 patients who elected to not pursue exploration despite this being recommended. Preoperative testing demonstrated the likely origin of the primary in 44 of the 63 patients, and 1 patient had a subsequent exploratory laparotomy performed for obstruction at an outside hospital where the primary was found (for an overall primary identification rate of 71%). In the patients who had their primary site identified by diagnostic testing (or operation in 1 case), it was found in the pancreas in 30 patients, small bowel in 12 patients, and 1 patient each had gastric, rectal, and colonic NETs (Table III).
Table III.
Primary tumor sites in patients with metastatic GEPNETs in the nonoperative group
| Site | Number | Percent |
|---|---|---|
| Pancreas | 30 | 47.6 |
| Unknown | 18 | 28.6 |
| Small bowel | 12 | 19.0 |
| Stomach | 1 | 1.6 |
| Rectum | 1 | 1.6 |
| Colon | 1 | 1.6 |
The most useful imaging modality was CT scan, which identified the primary site in 150 of 197 patients (76%). SRS localized the primary in 88 of 155 (56%), and endoscopy (EGD, colonoscopy, or EUS) in 43 of 107 patients (40%). The sensitivities of CT, SRS, and endoscopic procedures found in the operative, nonoperative, and combined groups are shown in Table IV. The sensitivity of CT scan was significantly greater in the operative group than the nonoperative group (P = .02), while that of SRS and endoscopy were similar in both groups. Within the operative subgroup, CT found the primary in significantly more cases than SRS (P <.01). However, tumors were identified in 8 patients with SRS that CT failed to identify, and in 30 patients CT revealed the primary site while SRS did not. The mean sizes of tumors detected by these 2 imaging modalities were not significantly different, at 3.3 cm for CT and 3.5 cm SRS (P = .53). There were significant differences in the size of tumors detected versus those not detected by CT, SRS, and endoscopy, with smaller tumors in the group not detected (P < .01 for CT and SRS, P = .02 for endoscopy; Table V). There was no correlation between preoperative or postoperative laboratory values (chromogranin, serotonin, pancreastatin) and the rate of detection of the primary tumor. Patients underwent a mean of 2.3 diagnostic testing modalities.
Table IV.
Sensitivity of different diagnostic procedures
| Study | Operative | Nonoperative | Total |
|---|---|---|---|
| CT | 109/134 (81.3%) | 41/63 (65.1%) | 150/197 (76.1%) |
| SRS | 59/105 (56.2%) | 29/50 (58.0%) | 88/155 (56.8%) |
| Endoscopy | 28/73 (38.3%) | 15/34 (44.1%) | 43/107 (40.2%) |
Table V.
Mean size of tumors detected by each diagnostic test versus those not detected (cm)
| Study | Detected (n) | Not detected (n) | P value |
|---|---|---|---|
| CT | 3.25 (109) | 1.84 (25) | <.01 |
| SRS | 3.51 (59) | 2.30 (46) | <.01 |
| Endoscopy | 3.28 (28) | 2.08 (45) | .02 |
In operative patients with SBNETs, the sensitivity of CT was 82% (positive in 74/90), and SRS 54% (39/72), 29% (14/48) were identified at colonoscopy. CT revealed a discrete mass or thickening of the small bowel in 39 patients, 33 of who also had mesenteric adenopathy. The other 35 patients were diagnosed as having SBNETs on CT by the observation of mesenteric adenopathy without signs of small bowel thickening or a discrete mass. The SBNET patients that had positive CT scans had significantly larger tumors than those that had negative scans (2.15 cm vs 1.69 cm; P = .03), as did patients who had a positive SRS (2.2 cm vs 1.7 cm; P = .021). Colonoscopy also tended to detect larger tumors than when they are not found (2.3 cm vs 1.7 cm), but the difference was not statistically significant (P = .17). There were 45 operative patients with multifocal SBNETs, which was only suggested by CT in 2 cases.
In those operative patients with pancreatic NETs (PNETs), the sensitivity of CT was 97% (positive in 32/33) and SRS 69% (18/26). EUS was positive in 10/16 patients (63%). With all 3 diagnostic modalities, the tumors detected were slightly larger than those not detected (CT: 5.7 vs 4.0 cm; SRS: 6.3 vs 4.7 cm; EUS: 4.7 vs 4.4 cm), but none of these differences were statistically significant.
At operative exploration, 6 of 10 (60%) primaries not identified on preoperative imaging were found. The mean size of these tumors was significantly smaller than the tumors discovered on imaging (1.83 vs 3.12 cm, P = .04). These included 5 small bowel and 1 pancreatic tumor. All 4 patients who underwent an operation but in whom the primaries were not found were thoroughly explored with the bowel run from ligament of Treitz to the ileocecal valve, the colon inspected from cecum to rectosigmoid, and the pancreas palpated. Three patients had no abnormal findings beside their liver metastases, and all 3 underwent liver directed operation. Another patient had biliary obstruction and was found to have a hard, unresectable peripancreatic mass, but transduodenal biopsy of this was negative. In addition to the removal of the primary in 130 of 134 patients, 90 patients also had liver debulking procedures, including 3 of 4 patients explored but in whom the primaries were not found. The median overall survival for patients explored operatively was 145 months for metastatic SBNETs and 71 months for PNETs. In comparison, the median overall survival for those patients who did not undergo operation at our institution was 70 months for SBNETs and 50 months for PNETs (P values of 0.44 and 0.16, respectively, when compared with operative patients).
DISCUSSION
Patients presenting with GEPNETs and metastatic disease pose several challenges. One is the identification of where the metastases came from, which is important for determining what operative approach will be taken and can inform choices for medical management. Localization of primary tumors can be difficult, especially when they are small and because they often lie within the wall of a hollow viscus. The reported sensitivity of diagnostic tests varies greatly between studies, and even with multiple tests, some patients will require operative exploration to identify the site of the primary.6,7,9,15,16 Therefore, exploration may be both diagnostic and therapeutic, and in many cases, liver directed operation for hepatic metastases can also be performed to improve symptoms and survival.9,12,13
We were able to identify the site of the primary tumor with preoperative diagnostic tests in 85% (168/197) of patients using a combination of CT scan, SRS, and endoscopy. This is substantially greater than the rates described in similar series of metastatic GEPNETS by Massimino et al15 and Bartlett et al,16 who reported preoperative primary identification rates of 17% and 54% with 61 and 63 patients, respectively. A comparable study by Wang et al14 localized the primary tumor preoperatively in a similar proportion of patients (56/71; 79%); these patients most commonly had CT, SRS, and FDG-PET, and a few patients had MRI, small bowel series, or capsule endoscopy.
The sensitivities reported for localizing unknown primaries by CT were 6.7% in Massimino et al,15 34.6% in Wang et al,14 38% in Bartlett et al,16 and 76% for both operative and nonoperative cases in this series (Table VI). None of these other studies reported considering mesenteric lymphadenopathy as being indicative of a SBNET. However, Bartlett et al16 described that when this finding was present in nonlocalized patients, the primary was found in 16/17 cases (it was likely, but not specified that these were all SBNETs), but their overall rate of finding SBNETs by CT was 52%. In the series of Wang et al,14 GI primaries were only found 35% of the time by CT (33 SBNETS, 15 colorectal, 1 gastric NET). We think that the finding of mesenteric lymphadenopathy on CT (especially when associated with calcifications) is strongly suggestive of SBNET primaries. In an earlier study, we described that 33/56 (60%) SBNETs had mesenteric lymphadenopathy, while only 27/56 (48%) had a mass or bowel wall thickening observed. Using both criteria together, 44/56 (79%) SBNETs were localized by CT.17 In this study, 68/90 (76%) patients with SBNETs who were explored had mesenteric lymphadenopathy seen on CT, and mesenteric lymphadenopathy was the only indicator of an SBNET in 35/90 (39%) cases. Therefore, this feature should be looked for carefully in all preoperative CTs done in patients with metastatic GEPNETs and occult primaries. This idea is reinforced by the study of Chambers et al,18 who found that 34/65 (52%) patients with occult GI NETs (59 SBNETs) had mesenteric lymphadenopathy seen on CT. Looking further at those patients who had mesenteric lymphadenopathy seen on CT (68/90) and those patients who did not have any mesenteric lymphadenopathy seen on CT (22/90), there was a difference in the pathologic size of the mesenteric masses/nodes (3.33 vs 2.32 cm, respectively; P = .026), but there was no difference in the pathologic size of the primary tumors (2.08 vs 2.05 cm; P = .91). CT enterography (CTE) may further improve the sensitivity of CT, because the use of water as intraluminal negative contrast accentuates enhancing lesions in the bowel wall, while radioopaque contrast tends to obscure them.19 Only one person in this series definitively had CTE, but we think that identification of small bowel lesions could have been further improved by greater use of CTE for SBNETs.
Table VI.
Sensitivity of diagnostic testing for finding primary tumors in studies of patients presenting with metastatic NETs
| Year | n | CT | SRS | Endoscopy | Operation | |
|---|---|---|---|---|---|---|
| Wang et al14 | 2010 | 79 | 0.35 | 0.26 | 0.33* | 0.87 |
| Massimino et al15 | 2012 | 63 | 0.07 | 0.02 | 0.12† | 0.79 |
| Bartlett et al16 | 2013 | 61 | 0.38 | 0.22 | 0.56‡ | 0.89 |
| Present study | 2016 | 197 | 0.76 | 0.57 | 0.38§ | 0.97 |
1/21 upper endoscopy and 20/42 colonoscopy.
0/24 upper endoscopy and 6/26 colonoscopy.
3/5 EUS and 2/4 capsule endoscopy.
13/19 upper endoscopy and 15/54 colonoscopy.
CT is very useful for finding pancreatic primaries, localizing 32/33 (97%) patients who had confirmation at operative exploration in this study. The one patient not localized had a tumor in the tail, and we have noted that tumors at the junction of the pancreatic tail and spleen have been missed by CT in other PNET patients. In the study by Bartlett et al,16 all 7 PNETs were found by CT, and in Wang et al,14 all 43 of their PNETs were identified with CT (where the mean size was 8 cm).
SRS was the next most commonly performed imaging test performed in all of these series, with sensitivities of 2%,15 22%,16 and 26%14 vs 57% in the present series (Table VI). One of the difficulties with studies reporting results of SRS historically has been what constitutes a positive scan. An early study by Krenning et al20 reported uptake on SRS in 96% of carcinoid cases (69/72), but this report likely considered any uptake (including liver, bowel, and nodes) to be a positive scan. In studies looking for occult primaries these criteria are not appropriate, but requiring that the scan detect diminutive primaries within the small bowel when there is often substantial background activity in the bowel may also not be fair. The utility of uptake being seen in mesenteric nodes is similar to what has been discussed with CT scans, and uptake reported to be in the small bowel by SRS often is actually in the mesenteric nodes. In a prior study we found that when the criterion of any nonhepatic, intra-abdominal uptake was used, that 35/ 47 (74%) operatively confirmed SBNETs were positive by SRS preoperatively.17 Another study of SRS in SBNETs found that the only factor significantly correlating with SRS positivity in SBNETs was tumor size >2 cm, but not multifocality, number of positive nodes, or SSTR2 gene expression in tumors relative to normal small bowel tissue.21
Endoscopy is another frequently used procedure in the workup of patients with occult GI NET primaries. Its utility depends on the site of the tumor, and often both EGD and colonoscopy are used, and for pancreatic lesions, EUS. Colonoscopy will detect infrequent colorectal NETs fairly reliably (3/3 in this study), but for lesions in the distal ileum, it requires that the endoscopist pass through the ileocecal valve and then find a submucosal lesion and/or perform deep biopsies. In this series, only 15/57 (26%) patients with occult SBNETs had their primaries identified by colonoscopy. In the 7 gastroduodenal tumors in this study, 3 were determined by EGD and 4 by CT. EUS is a good test for evaluating PNETs, as it gives information regarding size, location, relationships to major vascular structures, and also allows for biopsy. EUS successfully localized 23/34 (68%) PNETs in this study, but was usually performed after a CT suggested a pancreatic mass. Capsule endoscopy is another promising test for patients with occult GI primaries, as it can survey the entire GI tract. Small lesions may be missed by this modality, and when large lesions are present, there is the potential for the capsule to not pass. Four patients had capsule endoscopy in this series, which successfully identified 3 of 4 SBNETs. Double balloon enteroscopy is another endoscopic test that is being used with increasing frequency for the diagnosis of occult SBNETs, and allows for surveillance of the entire small bowel if done both from above and below.
In our patients where the primary tumor was not localized preoperatively, it was found at operative exploration in 60% (6/10). Five were in the small bowel and 1 in the pancreas, and these were significantly smaller than those localized preoperatively (mean size of 1.83 cm vs 3.12 cm; P = .04). Operative exploration also may have been helpful in 18 patients in the nonoperative group who were not localized, but 11 were not offered operation due to extensive disease or comorbidities, while the other 7 patients elected to pursue other therapy or were lost to follow-up. In other series, Bartlett et al16 identified 23/28 previously occult primaries at operative exploration, but 16 of 18 had lymphadenopathy seen on CT. Wang et al14 found 13/15 nonlocalized primaries at operative exploration, all of which were SBNETs, and Massimino et al15 found 39/52 at exploration. It is unclear from the latter studies how many of these patients would have been counted as having positive localization by the finding of mesenteric lymphadenopathy on CT.
We found and were able to resect the primary tumor in all 124 primary tumors identified by preoperative tests and in 6 that were not localized, for an overall success rate of 97% (130/134). This number compares favorably with other studies, which have reported success rates of 79–100%.4,14–16 A significant proportion of our patients (67%), also underwent liver directed operation. These interventions led to a median overall survival of 145 months in the operative group with metastatic SBNETs and 71 months for those with metastatic PNETs. These are greater than comparable patients from the SEER database, where median survival for patients with metastatic SBNETs was 56 months and 24 months for PNETs.2 Patients having nonoperative management had shorter survival, which was 70 months for SBNETs and 50 months in PNETs. This survival difference between operative and nonoperative patients did not reach statistical significance for SBNETs and PNETs (P = .44 and .16, respectively). One explanation for this is that nonoperative patients usually receive long-acting somatostatin analogs, and may receive multiple other treatments, including hepatic embolization, peptide receptor radiotherapy, and/or medical therapy.
These data reveal that CT scan and SRS remain important modalities for the identification of primary tumors in patients presenting with metastatic GEPNETs, and endoscopy is also a useful adjunct to these studies. In circumstances where preoperative workup fails to identify the site of a primary tumor, operative exploration should be considered strongly. This provides an opportunity for identification of the primary site, which can inform future treatments for the metastatic disease. Furthermore, it allows for resection of the primary tumor, which has been shown to confer a survival advantage even in the setting of metastatic disease.6,7,22 Operative debulking of liver metastases also may be performed at the same time, which may further improve survival.12,13,23
Acknowledgments
Supported by the National Institutes of Health T32 Surgical Oncology Training Grant #T32CA148062 and National Institutes of Health Neuroendocrine Specialized Program of Research Excellence Grant #P50CA174521.
References
- 1.Akerstrom G, Hellman P. Surgical aspects of neuroendocrine tumours. Eur J Cancer. 2009;45(Suppl 1):237–50. doi: 10.1016/S0959-8049(09)70039-5. [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 3.Pape UF, Berndt U, Muller-Nordhorn J, Bohmig M, Roll S, Koch M, et al. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer. 2008;15:1083–97. doi: 10.1677/ERC-08-0017. [DOI] [PubMed] [Google Scholar]
- 4.Norton JA, Kivlen M, Li M, Schneider D, Chuter T, Jensen RT. Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors. Arch Surg. 2003;138:859–66. doi: 10.1001/archsurg.138.8.859. [DOI] [PubMed] [Google Scholar]
- 5.Makridis C, Oberg K, Juhlin C, Rastad J, Johansson H, Lorelius LE, et al. Surgical treatment of mid-gut carcinoid tumors. World J Surg. 1990;14:377–83. doi: 10.1007/BF01658532. discussion 84–5. [DOI] [PubMed] [Google Scholar]
- 6.Hill JS, McPhee JT, McDade TP, Zhou Z, Sullivan ME, Whalen GF, et al. Pancreatic neuroendocrine tumors: the impact of surgical resection on survival. Cancer. 2009;115:741–51. doi: 10.1002/cncr.24065. [DOI] [PubMed] [Google Scholar]
- 7.Givi B, Pommier SJ, Thompson AK, Diggs BS, Pommier RF. Operative resection of primary carcinoid neoplasms in patients with liver metastases yields significantly better survival. Surgery. 2006;140:891–7. doi: 10.1016/j.surg.2006.07.033. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 8.Boudreaux JP, Putty B, Frey DJ, Woltering E, Anthony L, Daly I, et al. Surgical treatment of advanced-stage carcinoid tumors: lessons learned. Ann Surg. 2005;241:839–45. doi: 10.1097/01.sla.0000164073.08093.5d. discussion 45–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers AJ, Pasieka JL, Dixon E, Rorstad O. The palliative benefit of aggressive surgical intervention for both hepatic and mesenteric metastases from neuroendocrine tumors. Surgery. 2008;144:645–51. doi: 10.1016/j.surg.2008.06.008. discussion 51–3. [DOI] [PubMed] [Google Scholar]
- 10.Norlen O, Stalberg P, Oberg K, Eriksson J, Hedberg J, Hessman O, et al. Long-term results of surgery for small intestinal neuroendocrine tumors at a tertiary referral center. World J Surg. 2012;36:1419–31. doi: 10.1007/s00268-011-1296-z. [DOI] [PubMed] [Google Scholar]
- 11.Schurr PG, Strate T, Rese K, Kaifi JT, Reichelt U, Petri S, et al. Aggressive surgery improves long-term survival in neuroendocrine pancreatic tumors: an institutional experience. Ann Surg. 2007;245:273–81. doi: 10.1097/01.sla.0000232556.24258.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que FG. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197:29–37. doi: 10.1016/S1072-7515(03)00230-8. [DOI] [PubMed] [Google Scholar]
- 13.Maxwell JE, Sherman SK, O’Dorisio TM, Bellizzi AM, Howe JR. Liver-directed surgery of neuroendocrine metastases: what is the optimal strategy? Surgery. 2016;159:320–35. doi: 10.1016/j.surg.2015.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang SC, Parekh JR, Zuraek MB, Venook AP, Bergsland EK, Warren RS, et al. Identification of unknown primary tumors in patients with neuroendocrine liver metastases. Arch Surg. 2010;145:276–80. doi: 10.1001/archsurg.2010.10. [DOI] [PubMed] [Google Scholar]
- 15.Massimino KP, Han E, Pommier SJ, Pommier RF. Laparoscopic surgical exploration is an effective strategy for locating occult primary neuroendocrine tumors. Am J Surg. 2012;203:628–31. doi: 10.1016/j.amjsurg.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Bartlett EK, Roses RE, Gupta M, Shah PK, Shah KK, Zaheer S, et al. Surgery for metastatic neuroendocrine tumors with occult primaries. J Surg Res. 2013;184:221–7. doi: 10.1016/j.jss.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Dahdaleh FS, Lorenzen A, Rajput M, Carr JC, Liao J, Menda Y, et al. The value of preoperative imaging in small bowel neuroendocrine tumors. Ann Surg Oncol. 2013;20:1912–7. doi: 10.1245/s10434-012-2836-y. [DOI] [PubMed] [Google Scholar]
- 18.Chambers AJ, Pasieka JL, Dixon E, Rorstad O. Role of imaging in the preoperative staging of small bowel neuroendocrine tumors. J Am Coll Surg. 2010;211:620–7. doi: 10.1016/j.jamcollsurg.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Pilleul F, Penigaud M, Milot L, Saurin JC, Chayvialle JA, Valette PJ. Possible small-bowel neoplasms: contrast-enhanced and water-enhanced multidetector CT enteroclysis. Radiology. 2006;241:796–801. doi: 10.1148/radiol.2413051429. [DOI] [PubMed] [Google Scholar]
- 20.Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WA, Kooij PP, Oei HY, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993;20:716–31. doi: 10.1007/BF00181765. [DOI] [PubMed] [Google Scholar]
- 21.Maxwell JE, Sherman SK, Menda Y, Wang D, O’Dorisio TM, Howe JR. Limitations of somatostatin scintigraphy in priy small bowel neuroendocrine tumors. J Surg Res. 2014;190:548–53. doi: 10.1016/j.jss.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed A, Turner G, King B, Jones L, Culliford D, McCance D, et al. Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocr Relat Cancer. 2009;16:885–94. doi: 10.1677/ERC-09-0042. [DOI] [PubMed] [Google Scholar]
- 23.Mayo SC, de Jong MC, Pulitano C, Clary BM, Reddy SK, Gamblin TC, et al. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol. 2010;17:3129–36. doi: 10.1245/s10434-010-1154-5. [DOI] [PubMed] [Google Scholar]