Abstract
Objective:
We investigated whether the inhibitory effect of the immunosuppressant everolimus (RAD001) on vascular smooth muscle cell (VSMC) proliferation is mediated by p27/kip1 gene promoter activity.
Methods:
In this experimental study, cultured rat VSMCs were transiently transfected with a recombinant plasmid (pXp27) containing p27/kip1 gene promoter sequence and a chloramphenicol acetyltransferase (CAT) reporter gene. After stimulation with the mitogen platelet-derived growth factor (PDGF-BB, 10 ng/mL) in the presence or absence of RAD001 (10 nM), the promoter activity, mRNA expression, and protein expression of p27/kip1 were examined by CAT assay, RT–PCR, and immunoblotting, respectively. Cell cycle–related changes were detected by flow cytometry. DNA synthesis was determined using 3H-TdR incorporation.
Results:
Compared with the non-stimulation group, PDGF-BB stimulation induced a significant proliferative response in the VSMCs as indicated by decreased p27/kip1 gene promoter activity, decreased p27/kip1 mRNA and protein expression, increased S-phase and G2/M-phase cells, and increased DNA synthesis. RAD001 intervention increased p27/kip1 gene promoter activity 3.5-fold, promoted p27/kip1 mRNA and protein expression, increased G0-phase cells, reduced DNA synthesis, and, overall, inhibited PDGF-BB–stimulated cell proliferation.
Conclusion:
RAD001 inhibits PDGF-BB–stimulated proliferation of cultured VSMCs by upregulating p27/kip1 gene promoter activity and increasing p27/kip1 mRNA and protein expression.
Keywords: p27/kip1, everolimus, promoter, vascular smooth muscle cell, proliferation
Introduction
Vascular smooth muscle cell (VSMC) proliferation and migration are important contributing factors in restenosis after percutaneous coronary intervention (PCI) for coronary heart disease (1). The immunosuppressant everolimus (RAD001) significantly inhibits VSMC proliferation and migration, which contributes to the prevention and treatment of clinical coronary heart disease and restenosis after PCI (2, 3). However, the underlying mechanism of this inhibition is not clear.
Cyclin-dependent kinase inhibitors (CKIs) are naturally occurring gene products that inhibit the activity of cyclin/cyclin-dependent kinase, leading to G1 arrest. p27/kip1 is a CKI that is constitutively expressed in normal arteries and acts as a “molecular switch” in cell cycle regulation (4). p27/kip1 is downregulated after arterial injury, becomes upregulated during the later phases of arterial repair, and is inversely correlated with VSMC proliferation (4). A deficiency of p27/kip1 in mice leads to benign hyperplasia in multiple organs, but it does not directly produce tumors (4).
In this study, a plasmid (pXp27) carrying the p27/kip1 gene promoter was constructed and used to transfect cultured rat VSMCs. RAD001 was then used to interfere with platelet-derived growth factor (PDGF)-BB–stimulated proliferation of the transfected VSMCs. By examining p27/kip1 gene promoter activity and the expression of the corresponding mRNA and protein, the mechanism of RAD001 inhibition of VSMC proliferation was explored.
Methods
Cell culture
Rat aortic VSMCs were obtained by the tissue adhesion method reported by McMurray et al. (5). In brief, Wistar rats were killed by cervical dislocation, and the aortas were isolated under sterile conditions. After removal of vascular adventitia and blunt curettage of the intima, the membrane of blood vessels was shredded into 1 mm3 pieces, which were transferred to the bottom wall of a cell culture flask at a distance of app- roximately 0.3–0.5 cm from each other. The flask was turned upside down and filled with 3–5-mL Dulbecco's modified Eagle medium (DMEM; Invitrogen, Carlsbad, CA, USA) containing 20% fetal bovine serum (FBS; Invitrogen) and cultured at 37°C in a 5% CO2 incubator for 2–4 h. After the tissue pieces dried and adhered to the flask, the flask was slowly turned over so that the tissue pieces were completely immersed in the tissue culture medium. Culturing was continued for approximately 1 week for the VSMCs to move out of the tissue blocks. The VSMCs were purified by the differential adherence method. The purified cells were verified by light microscopy and a-smooth muscle actin (a-SM-actin) immunocytochemistry. VSMCs of the fifth and sixth passages were used in the experiments. The study protocol was approved by the Ethics Committee of our hospital.
Plasmid construction
A pMOSBlue plasmid carrying the p27/kip1 gene promoter (GE Healthcare, Little Chalfont, United Kingdom) and a pXp1 plasmid with a chloramphenicol acetyltransferase (CAT) reporter gene but no promoter (Promega, Madison, WI, USA) were digested with the Hind III restriction endonuclease, ligated with T4 ligase (Hua Mei), and used to transfect Escherichia coli strain DH5a. Positive clones were picked, plasmids from conventional mini-preparation were digested with Hind III restriction enzyme, PCR identified, and further sequenced by Invitrogen Corporation to obtain pXp27 plasmids carrying the p27/kip1 gene promoter and the reporter.
Transient transfection
VSMCs were plated in 6-well plates at 3x105 cells each well and cultured in 2 mL DMEM containing 10% FBS at 37°C in a 5% CO2 incubator for 24 h to reach 50–80% confluence. The cells were transfected with pXp27 plasmid using 1,2-Di-(9Z-octadecenoyl)-3-trimethylammonium propane methylsulfate (DOTAP) transfection reagent (Roche Applied Science, Indianapolis, IN). For a negative control, the cells were transfected with 5-µg pXp1 reporter plasmid with or without promoter activity; for a positive control, the cells were transfected with plasmid pGL2 containing CAT (Promega); and as an internal control, the cells were transfected with plasmid pSVAP2 with alkaline phosphatase (ALP) expression (SINO-AMERICAN BIOTECHNOLOGY COMPANY, Luoyang, Henan, China). Six hours after transfection, pXp27-transfected cells were fed 1 mL of 10% FBS culture medium, 1 mL of medium with 10 ng/mL PDGF-BB (R&D), or 1 mL of medium with 10 ng/mL PDGF-BB+10 nM RAD001 (Novartis Pharma AG, Basel, Switzerland) and cultured for an additional 24 h.
Experiment grouping design
After transient transfection, the cells were divided into the following experimental groups: control group, VSMCs were transfected with 5-µg pXp1 reporter plasmid with pXp27 promoter activity; negative control group, VSMCs were transfected with 5-µg pXp1 reporter plasmid without pXp27 promoter activity; positive control group, VSMCs were transfected with 5-µg pGL2CAT expression plasmid; PDGF-BB+pXp27 group, control group cultured with 10-ng/mL PDGF-BB for an additional 24 h; and PDGF-BB+pXp27+RAD001 group: control group cultured with 10-ng/mL PDGF-BB and 10 nM RAD001for an additional 24 h.
Measurement of p27/kip1 gene promoter activity
p27/kip1 gene promoter activity was measured by CAT activity assay (Beyotime Biotechnology, Shanghai, China). Cultured cells were washed three times with phosphate-buffered saline (PBS) pre-cooled on ice and lysed with lysis buffer. Protein concentration (Bradford method), ALP activity (ALP kit), and CAT expression (CAT-ELISA; Promega) were determined. Each experiment was performed in duplicate and repeated three times. CAT expression and ALP activity were normalized to the protein content in the corresponding sample. The p27/kip1 gene promoter activity data were expressed as multiples of the pGL2 group.
RT–PCR
VSMCs in six-well plates were cultured to 50–80% confluence in DMEM containing 10% FBS and then switched to fresh medium alone, fresh medium with PDGF-BB, or fresh medium with PDGF-BB and RAD001 and cultured for an additional 24 h before collecting cells. Total RNA was extracted using a TriPure kit (Roche; Invitrogen), and p27/kip1 mRNA expression was detected using an RT-PCR Kit (TaKaRa Biotechnology Co. Ltd, Dalian, China). PCR products were subjected to electrophoresis, and the optical densities of the relevant bands were quantified and normalized to that of the internal reference 3-glyceraldehyde phosphate dehydrogenase (GAPDH). The P27/kip1 primers (Shanghai GeneCore BioTechnologies Co., Ltd., Shanghai, China) were: forward 5’-CGT GCG AGT GTC TAA CGG-3’, reverse 5’-CGG ATC AGT CTT TGG GTC-3’; amplicon size 453 bp. GAPDH PCR was used as an internal reference (amplicon size 254 bp) (5).
Western blotting
VSMCs were rinsed twice with ice-cold PBS and lysed with 300-µL pre-cooled cell lysis buffer. The scraped cells were collected in 1.5-mL centrifuge tubes, incubated on ice for 20 min, and centrifuged at 12.000 xg at 4°C for 15 min. The supernatant was collected and protein content was determined using a nucleic acid/protein analyzer. Protein samples were mixed with 2x sample buffer, boiled for 5 min, separated on a 10% SDS-polyacrylamide gel with 100-µg protein in each well and transferred to a nitrocellulose membrane. The membrane was first blocked with tris-buffered saline–Tween (TBST) with 5% skim milk powder at room temperature for 2 h and then incubated in primary antibody (mouse anti-rat p27/kip1 monoclonal antibody, 1: 200 in TBST) at room temperature for 2 h. After three washes in TBST, the membrane was incubated with secondary antibody solution at room temperature for 2 h, developed with ECL reagent, and exposed on film. The data of the PDGF-BB and PDGF-BB+RAD001 groups were expressed as multiples of the control group.
Flow cytometry
Flow cytometry was used to determine the percentage of cells in each cell cycle phase. VSMCs were cultured to 80% confluence in DMEM containing 10% FBS, switched to serum-free medium (DMEM) for 24 h, and then switched back to fresh 10% FBS containing medium alone, or fresh medium with PDGF-BB, or fresh medium with PDGF-BB and RAD001, and cultured for an additional 24 h. The cells were collected and prepared as a single cell suspension, fixed in 70% ethanol, stained with PI, and analyzed in a flow cytometer with an excitation wavelength of 488 nm. Fractions of the cell population in the G0/G1 and S/G2/M cell cycle phases were evaluated and expressed in percentages.
3H-TdR incorporation
3H-TdR incorporation methodology was used to determine DNA synthesis. The experimental grouping and treatments were the same as for the flow cytometry above. VSMCs were plated in 24-well plates with 1x105 cells per well; three wells were set up for each experimental condition. The cells were cultured at 37°C in a 5% CO2 incubator for 24 h, in serum-free medium for 24 h, and then transfected and given intervention factors for 24 h. Then, 3H-TdR was added at a final concentration of 3.7x104 Bq/mL, and the culture continued for 16 more hours. The cells were collected after conventional digestion, vacuum filtered through a microporous membrane, and the cell membranes were broken with 10% trichloroacetic acid. The microporous membrane was dried and placed in a scintillation bottle with scintillation fluid; the radioactivity was measured with a liquid scintillation counter.
Determination of cell viability
Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyl tetrazolium bromide (MTT) assay. The experimental grouping and treatments were the same as for the flow cytometry. VSMCs were seeded in a 96-well plate at 3x103 per well; three wells were set up for each experimental condition. After adherence, the cells were cultured in serum-free medium for 24 h and then transfected with reporter plasmids, and the final volume of each well was 200 µL. Upon detection, 20 µL of MTT solution (5 mg/mL) was added to each well, and the cells were incubated at 37°C for 4 h. The culture supernatant was removed, and 150 µL of DMSO was added. The plate was shaken for 10 min to completely dissolve the crystals, and then the absorbance value (OD value) at 490 nm was determined using a plate reader.
Statistical analysis
All data are expressed as the mean±SD. Statistical analysis was performed using SPSS 19.0 software. The independent samples t test was used with p <0.05 set as a significant difference and p <0.01 as a very significant difference.
Results
RAD001 increased p27/kip1 gene promoter activity in VSMCs stimulated with PDGF-BB
VSMCs were transfected with 5-µg pXp1 reporter plasmid with or without promoter activity (negative control), or with 5-µg pGL2 CAT expression plasmid (positive control). Six hours after transfection, the pXp27-transfected cells were fed 1 mL of fresh 10% FBS culture medium alone, medium containing 10 ng/mL PDGF-BB, or medium containing 10-ng/mL PDGF-BB + 10 nM RAD001 and cultured for 24 h. p27/kip1 gene promoter activity was expressed as the relative CAT expression level normalized to protein concentration and ALP activity.
CAT expression in the untreated pXp27 transfection group (pXp27 group) significantly increased compared with the negative control group (pXp1) (p<0.01). CAT expression significantly decreased by 2/3 in the PDGF-BB–stimulated VSMCs compared with the pXp27 group (p<0.01). CAT expression increased 2.5-fold from RAD001 intervention in PDGF-BB–stimulated VSMCs (p<0.01) (Fig. 1 and Table 1).
Figure 1.

Effect of RAD001 onp27/kip1 gene promoter activity in VSMCs stimulated with PDGF-BB. N=6 in each group. **P<0.01 vs. pXp27, ##P<0.01 vs. PDGF-BB+pXp27. pXp27: VSMCs were transfected with 5-μg pXp1 reporter plasmid with pXp27 promoter activity; PDGFBB+ pXp27:pXp27group cultured with 10 ng/mL PDGF-BB for additional 24 h; PDGF-BB+pXp27+RAD001: pXp27group cultured with 10 ng/mL PDGF-BB and 10 nM RAD001for additional 24 h; pGL2: VSMCs were transfected with 5-μg pGL2 CAT expression plasmid; pXp1: VSMCs were transfected with 5-μg pXp1 reporter plasmid without pXp27 promoter activity
Table 1.
Baseline characteristics results of experimental units and statistical test result
| pXp27 | PDGF-BB+pXp27 | PDGF-BB+pXp27+RAD001 | pGL2 | pXp1 | P | |
|---|---|---|---|---|---|---|
| CAT relative expression | 1.69±0.21 | 0.57±0.19** | 1.43±0.18## | 1.00±0.21 | 0.10±0.16** | <0.01 |
| p27/kip1 protein expression | 1.00±0.20 | 0.07±0.22** | 0.86±0.20## | <0.01 | ||
| Percent of cells in G0-G1, % | 83.01±8.66 | 55.92±5.05** | 75.02±7.53## | <0.01 | ||
| Percent of cells in S/G2/M, % | 15.99±1.34 | 44.08±4.95** | 24.98±2.47## | <0.01 | ||
| 3H-TdR incorporation, cpm | 5616±478 | 16819±728** | 8816±502## | <0.01 | ||
| Absorbance (OD value) | 0.23±0.09 | 0.68±0.03** | 0.46±0.12## | <0.01 |
N=6 in each group.
P<0.01 vs. pXp27;
P<0.01 vs. PDGF - BB+pXp27. pXp27 - VSMCs were transfected with 5 µg reporter plasmids pXp1 with pXp27 promoter activity;
PDGF - BB+pXp27-group pXp27 cultured with 10 ng/mL PDGF-BB for additional 24 h; PDGF-BB+pXp27+RAD001 - group pXp27 cultured with 10 ng/mL PDGF - BB and 10 nM RAD001 for additional 24 h; pGL2-VSMCs were transfected with 5 µg CAT expression pGL2 plasmid; pXp1-VSMCs were transfected with 5 µg reporter plasmids pXp1 without pXp27 promoter activity
RAD001 increased p27/kip1 mRNA expression in VSMCs stimulated by PDGF-BB
As shown in Figure 2, the p27/kip1 gene mRNA expression level in the pXp27 group was 0.81; the expression was significantly decreased to 0.16 after stimulation with 10 ng/mL PDGF-BB; intervention with 10 nM RAD001 reversed the decrease and restored the expression level to 0.56.
Figure 2.
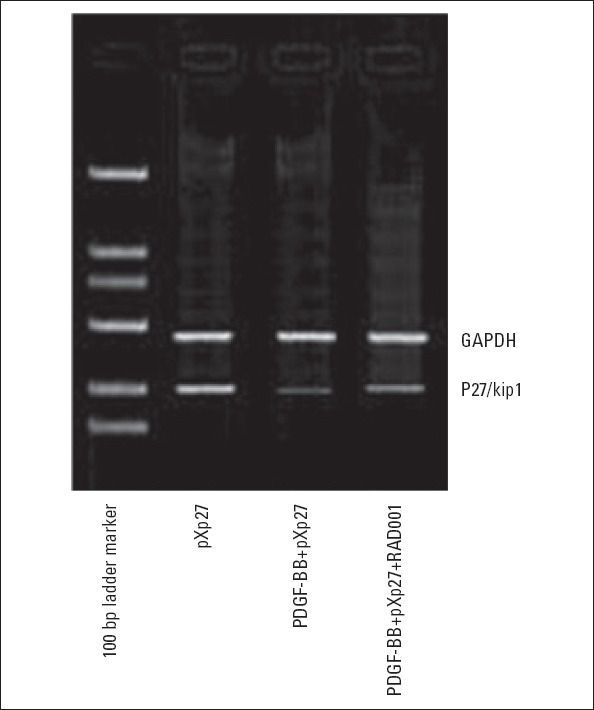
Effect of RAD001 on the RNA expression of p27 mRNA in VSMCs stimulated with PDGF-BB. pXp27: VSMCs were transfected with 5-μg pXp1 reporter plasmid with pXp27 promoter activity; PDGFBB+ pXp27:group pXp27 cultured with 10-ng/mL PDGF-BB for additional 24 h; PDGF-BB+pXp27+RAD001: group pXp27 cultured with 10-ng/mL PDGF-BB and 10 nM RAD001for additional 24 h
RAD001 increased the protein expression of p27/kip1 in VSMCs stimulated by PDGF-BB
RAD001 affected p27/kip1 protein expression in VSMCs stimulated by PDGF-BB similarly to p27/kip1 mRNA expression. Compared with the normal pXp27 group, PDGF-BB stimulated a significant reduction in p27/kip1 protein expression, while RAD001 intervention increased p27/kip1 protein expression (Fig. 3 and Table 1).
Figure 3.
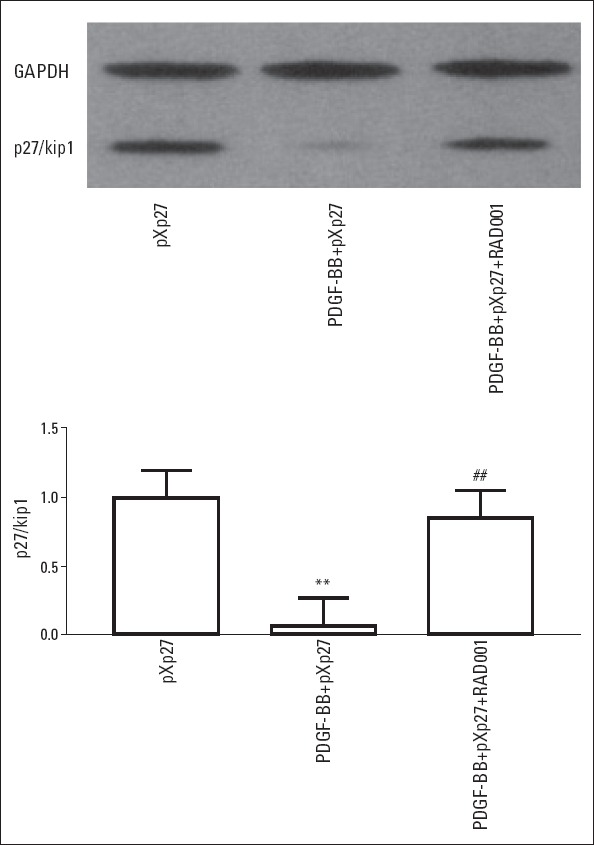
Effect of RAD001 on the protein expression of p27/kip1 in VSMCs stimulated with PDGF-BB. N=6 in each group. **P<0.01 vs. pXp27, ##P<0.01 vs. PDGF-BB+pXp27. pXp27: VSMCs were transfected with 5-μg pXp1 reporter plasmid with pXp27 promoter activity; PDGFBB+ pXp27: group pXp27 cultured with 10 ng/mL PDGF-BB for additional 24 h; PDGF-BB+pXp27+RAD001: group pXp27 cultured with 10 ng/mL PDGF-BB and 10 nM RAD001for additional 24 h
RAD001 induced cell cycle changes in PDGF-BB–stimulated VSMCs
In the pXp27 group, VSMCs were mainly in G0/G1 phase. Upon PDGF-BB stimulation, cells in G0/G1 phase were significantly fewer (p<0.01), while the fractions of cells in G2/M phase and S phase were significantly greater (p<0.01); RAD001 intervention significantly increased the fraction of cells in G0/G1 phase and decreased the fractions of cells in G2/M phase and S phase compared with the PDGF-BB+pXp27 group (Fig. 4 and Table 1).
Figure 4.
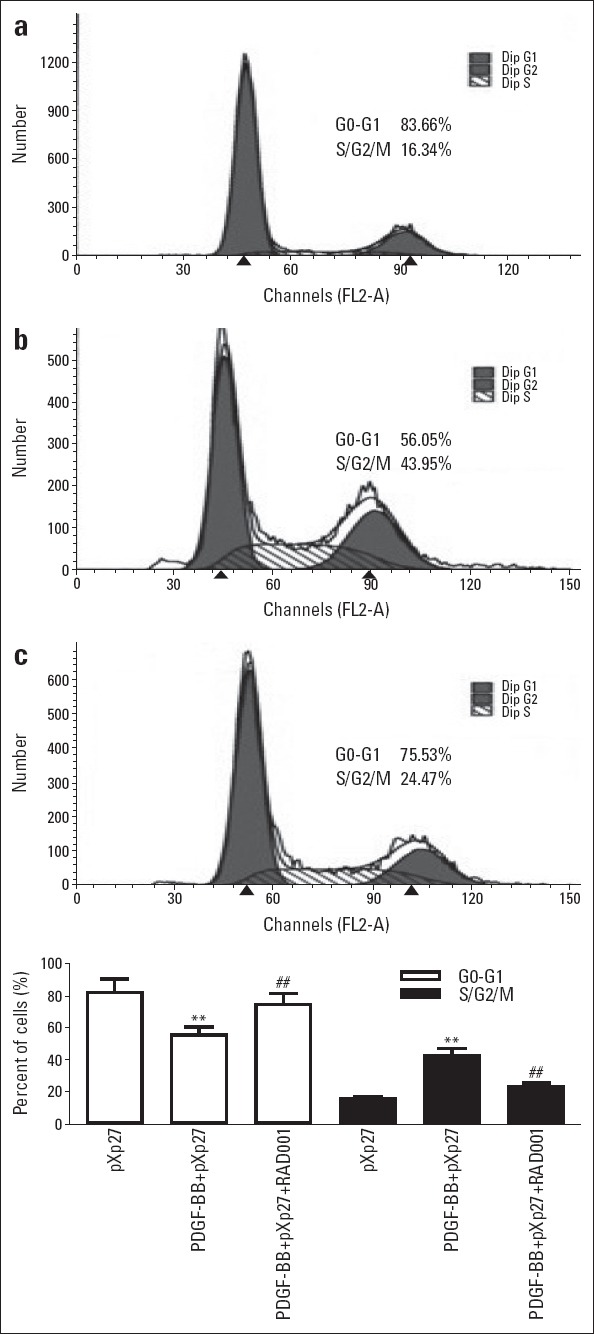
Effect of RAD001 on cell cycle-related changes in VSMCs stimulated with PDGF-BB. (a) pXp27; (b) PDGF-BB+pXp27; (c) PDGFBB+ pXp27+RAD001. Fractions of the cell population in the G0/G1 and S/G2/M cell cycle phases were evaluated and expressed in percentages (%). N=6 in each group. **P< 0.01 vs. pXp27, ##P<0.01 vs. PDGF-BB+pXp27. pXp27: VSMCs were transfected with 5-μg pXp1 reporter plasmid with pXp27 promoter activity; PDGF-BB+pXp27: pXp27 group cultured with 10 ng/mL PDGF-BB for additional 24 h; PDGF-BB+pXp27+RAD001: pXp27 group cultured with 10 ng/mL PDGF-BB and 10 nM RAD001for additional 24 h
RAD001 decreased DNA synthesis by PDGF-BB–stimulated VSMCs
DNA synthesis was estimated by measuring 3H-TdR incorporation and absorbance (Fig. 5 and Table 1). After PDGF-BB stimulation, the 3H-TdR incorporation and absorbance values significantly increased about three-fold (p<0.01), indicating remarkably increased DNA synthesis. RAD001 intervention significantly reduced 3H-TdR incorporation and absorbance (p<0.01).
Figure 5.
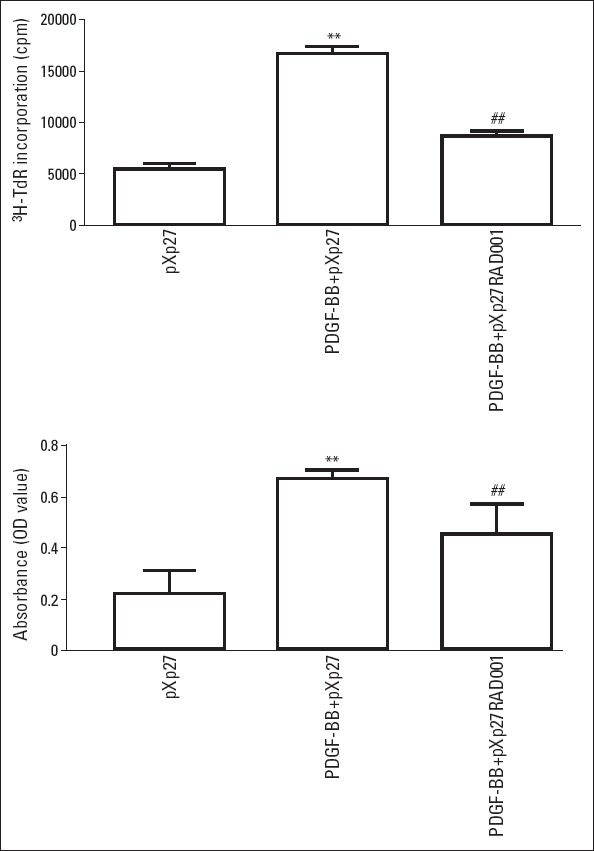
RAD001 decreased DNA synthesis by PDGF-BB–stimulated VSMCs. N=6 in each group. **P<0.01 vs. pXp27; ##P<0.01 vs. PDGFBB+ pXp27. pXp27: VSMCs were transfected with 5-μg pXp1 reporter plasmid with pXp27 promoter activity; PDGF-BB+pXp27: pXp27 group cultured with 10 ng/mL PDGF-BB for additional 24 h; PDGFBB+ pXp27+RAD001: pXp27 group cultured with 10 ng/mL PDGF-BB and 10 nM RAD001for additional 24 h
Discussion
In this study, the plasmid pXp27 carrying the p27/kip1 gene promoter was constructed and used to transiently transfect rat VSMCs using liposomes. The VSMCs were stimulated with PDGF-BB to induce proliferation and then treated with RAD001. The results showed that PDGF-BB stimulation significantly decreased p27/kip1 gene promoter activity compared with the normal control group and significantly decreased p27/kip1 gene mRNA and protein expression levels; PDGF-BB stimulation significantly decreased the cell fraction in G0/G1 phase while significantly increasing the fractions in G2/M phase and S phase, and it increased DNA synthesis. The results suggest that PDGF-BB significantly inhibited p27/kip1 gene promoter activity, leading to VSMC proliferation. RAD001 intervention significantly increased p27/kip1 gene promoter activity, although it was still lower than normal levels. RAD001 intervention significantly increased p27/kip1 gene mRNA and protein expression, decreased DNA synthesis, and significantly inhibited VSMC proliferation compared with the PDGF-BB stimulation group.
Rapamycin and its derivatives, including everolimus (RAD001), are inhibitors of the mammalian target of rapamycin (mTOR) and are effective at suppressing cell growth and inhibiting mTOR kinase activity. RAD001 is less toxic for humans than are other anti-VEGFR inhibitors and has been used as an immunosuppressive agent. RAD001 is widely used in anti-tumor therapies for cancers such as liver cancer (6) and ovarian cancer (7), as well as to prevent transplant rejection (8) and to induce endothelial cell senescence (9), among other uses. Currently, RAD001 has been increasingly used in the treatment of cardiovascular diseases (10). Studies have shown that rapamycin and its derivatives can stimulate increased expression of the cell cycle protein p27/kip1, reduce mitogen stimulation-induced p27/kip1 expression, and inhibit VSMC proliferation and migration in culture and in vivo (11). The p27/kip1 gene promoter is critical for p27/kip1 mRNA and protein expression. Therefore, we hypothesized that RAD001 inhibits VSMC proliferation and migration by affecting the activity of the p27/kip1 gene promoter.
PDGF-BB inhibition of p27/kip1 gene promoter activity may involve specific cell signal transduction. It was reported that p27/kip1 is a target gene in c-myc transcription inhibition. When using p27/kip1 gene promoter and c-myc expression vectors to co-transfect WEHI 231 cells, p27/kip1 gene promoter activity was found to be significantly inhibited (12). In rat VSMCs, c-myc inhibition of the activity of a p27/kip1 promoter lacking the TATA box has also been observed. Additionally, the sequence mediating P27/kip1 gene transcription activity and c-myc inhibition was positioned –20 to +20 bp of the p27/kip1 gene. PDGF induces c-myc transcription by transcriptional and post-transcriptional mechanisms and decreases p27/kip1 gene transcription and accumulation of the corresponding p27/kip1 mRNA (13). Therefore, we speculate that PDGF, by promotion of c-myc gene transcription, affects the sequence related to c-myc gene transcriptional inhibition of the p27/kip1 gene, thus inhibiting p27/kip1 gene promoter activity and leading to reduced p27/kip1 gene mRNA and protein levels.
RAD001 can significantly increase p27/kip1 gene promoter activity, and the mechanism is closely related to the transcription factor SP1. Existing studies have shown that the sequences GGGCGG (–545 to –539 bp) and CCAAT (–525 to –520 bp) are required for the induction of p27/kip1 gene expression (14). SP1 binds to the sequence GGGCGG (15), demonstrating a clear link between p27/kip1 gene expression and SP1. In VSMC culture, mutations at the SP1 binding site of the p27/kip1 gene greatly reduce p27/kip1 gene promoter activity, demonstrating that SP1 is required for effective p27/kip1 gene transcription (16). Using gene knockout technology, it was found that the start codon corresponding to the region –774 to –435 bp in the p27/kip1 gene may contain a basic transcription factor binding site (17), and the region 170 bp upstream of the start site of p27/kip1 gene promoter transcription is sufficient to ensure the maximal p27/kip1 gene promoter transcriptional activity. Sequence analysis of this 170 bp region showed that several GC-rich regions are clearly SP1 binding sites. By anchoring one or two GC-rich regions, SP1 activates the p27/kip1 domain (18, 19). Therefore, we speculate that RAD001 binds to its intracellular receptor FKBP-12, inhibits the P70 (S6) kinase, interferes with p27/kip1 gene promoter signal transduction pathways, promotes the expression of transcription factor SP1 (20), and thus affects the SP1 binding sites in the p27/kip1 gene promoter and facilitates p27/kip1 gene promoter activity. Our study and recent studies reported an antiproliferative effect of everolimus through upregulation p27Kip1 (3, 21, 22), although Ferri's article previously suggested that p27Kip1 was not altered by everolimus alone (23). Therefore, more studies may be needed to identify the mechanism.
Study limitations
Because of limitations of research grant and methods, we did not give an inhibitor of everolimus to demonstrate other influencing factors in the study. Further studies should be done to uncover the mechanisms underlying the inhibitory effect of everolimus on proliferation of rat VSMCs.
Conclusion
In summary, this study showed that RAD001 significantly inhibits PDGF-BB–induced VSMC proliferation, and this inhibition is closely related to an increase in p27/kip1 gene promoter activity. The result provides a theoretical basis for clinical applications to interfere with the p27/kip1 gene for therapy of coronary heart disease and restenosis after PCI.
Footnotes
Conflict of interest: This manuscript has no conflict of interest.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept- B.R., W.L., Y.L.; Design- B.R., W.L., Y.L.; Supervision- B.R., W.L., Y.L.; Funding-Y.L., W.L., Q.L.; Materials- Y.L., W.L., Y.P.; Data collection &/or processing – Y.L., W.L., Y.P.; Analysis and/or interpretation– Q.L., Y.F., Q.T.; Literature search- Q.L., Y.F., Y.Y.; Writing – Q.T., Y.Y., Y.P.; Critical review- Q.T., Y.Y., Y.P.
References
- 1.Marx SO, Totary-Jain H, Marks AR. Vascular smooth muscle cell proliferation in restenosis. Circ Cardiovasc Interv. 2011;4:104–11. doi: 10.1161/CIRCINTERVENTIONS.110.957332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mead H, Zeremski M, Guba M. Mtor signaling in angiogenesis;Mtor pathway and mtor inhibitors in cancer therapy. Springer. 2010:49–74. [Google Scholar]
- 3.Tang B, Dong X, Wei Z, Qiao H, Jiang H, Liu B, et al. Enhanced autophagy by everolimus contributes to the antirestenotic mechanisms in vascular smooth muscle cells. J Vas Res. 2014;51:259–68. doi: 10.1159/000365927. [DOI] [PubMed] [Google Scholar]
- 4.Tanner FC, Boehm M, Akyürek LM, San H, Yang ZY, Tashiro J, et al. Differential effects of the cyclin-dependent kinase inhibitors p27(kip1), p21(cip1), and p16(ink4) on vascular smooth muscle cell proliferation. Circulation. 2000;101:2022–5. doi: 10.1161/01.cir.101.17.2022. [DOI] [PubMed] [Google Scholar]
- 5.McMurray HF, Parrott DP, Bowyer DE. A standardised method of culturing aortic explants, suitable for the study of factors affecting the phenotypic modulation, migration and proliferation of aortic smooth muscle cells. Atherosclerosis. 1991;86:227–37. doi: 10.1016/0021-9150(91)90219-s. [DOI] [PubMed] [Google Scholar]
- 6.Buitrago-Molina LE, Pothiraju D, Lamle J, Marhenke S, Kossatz U, et al. Rapamycin delays tumor development in murine livers by inhibiting proliferation of hepatocytes with DNA damage. Hepatology. 2009;50:500–9. doi: 10.1002/hep.23014. [DOI] [PubMed] [Google Scholar]
- 7.Mabuchi S, Altomare DA, Cheung M, Zhang L, Poulikakos PI, Hensley HH, et al. Rad001 inhibits human ovarian cancer cell proliferation, enhances cisplatin-induced apoptosis, and prolongs survival in an ovarian cancer model. Clin Cancer Res. 2007;13:4261–70. doi: 10.1158/1078-0432.CCR-06-2770. [DOI] [PubMed] [Google Scholar]
- 8.Semsroth S, Stigler RG, Bernecker OY, Ruttmann-Ulmer E, Troppmair J, Macfelda K, et al. Everolimus attenuates neointimal hyperplasia in cultured human saphenous vein grafts. Eur J Cardiothorac Surg. 2009;35:515–20. doi: 10.1016/j.ejcts.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 9.Ota H, Eto M, Ako J, Ogawa S, Iijima K, Akishita M, et al. Sirolimus and everolimus induce endothelial cellular senescence via sirtuin 1 down-regulation: Therapeutic implication of cilostazol after drug-eluting stent implantation. J Am Coll Cardiol. 2009;53:2298–305. doi: 10.1016/j.jacc.2009.01.072. [DOI] [PubMed] [Google Scholar]
- 10.Mueller MA, Beutner F, Teupser D, Ceglarek U, Thiery J. Prevention of atherosclerosis by the mtor inhibitor everolimus in ldlr-/- mice despite severe hypercholesterolemia. Atherosclerosis. 2008;198:39–48. doi: 10.1016/j.atherosclerosis.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Daniel C, Pippin J, Shankland SJ, Hugo C. The rapamycin derivative rad inhibits mesangial cell migration through the cdk-inhibitor p27/kip1. Lab Invest. 2004;84:588–96. doi: 10.1038/labinvest.3700078. [DOI] [PubMed] [Google Scholar]
- 12.Wierstra I, Alves J. Cyclin e/cdk2, p/caf, and e1a regulate the transactivation of the c-myc promoter by foxm1. Biochem Biophys Res Commun. 2008;368:107–15. doi: 10.1016/j.bbrc.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 13.Kim TJ, Yun YP. Antiproliferative activity of nq304, a synthetic 1,4-naphthoquinone, is mediated via the suppressions of the pi3k/akt and erk1/2 signaling pathways in pdgf-bb-stimulated vascular smooth muscle cells. Vascul Pharmacol. 2007;46:43–51. doi: 10.1016/j.vph.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Andres V, Urena J, Poch E, Chen D, Goukassian D. Role of sp1 in the induction of p27 gene expression in vascular smooth muscle cells in vitro and after balloon angioplasty. Arterioscler Thromb Vasc Biol. 2001;21:342–7. doi: 10.1161/01.atv.21.3.342. [DOI] [PubMed] [Google Scholar]
- 15.Wei Q, Miskimins WK, Miskimins R. The sp1 family of transcription factors is involved in p27(kip1)-mediated activation of myelin basic protein gene expression. Mol Cell Biol. 2003;23:4035–45. doi: 10.1128/MCB.23.12.4035-4045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang CS, Ho WL, Lee WS, Sheu MT, Wang YJ, Tu SH, et al. Sp1-regulated p27/kip1 gene expression is involved in terbinafine-induced human a431 cancer cell differentiation: An in vitro and in vivo study. Biochem Pharmacol. 2008;75:1783–96. doi: 10.1016/j.bcp.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Inoue T, Kamiyama J, Sakai T. Sp1 and nf-y synergistically mediate the effect of vitamin D(3) in the p27(kip1) gene promoter that lacks vitamin d response elements. J Biol Chem. 1999;274:32309–17. doi: 10.1074/jbc.274.45.32309. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Lin SC. Molecular characterization of the cyclin-dependent kinase inhibitor p27 promoter. Biochim Biophys Acta. 1997;1353:307–17. doi: 10.1016/s0167-4781(97)00063-8. [DOI] [PubMed] [Google Scholar]
- 19.Chen F, Kim E, Wang CC, Harrison LE. Ciglitazone-induced p27 gene transcriptional activity is mediated through sp1 and is negatively regulated by the mapk signaling pathway. Cell Signal. 2005;17:1572–7. doi: 10.1016/j.cellsig.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Buerke M, Guckenbiehl M, Schwertz H, Buerke U, Hilker M, Platsch H, et al. Intramural delivery of sirolimus prevents vascular remodeling following balloon injury. Biochim Biophys Acta. 2007;1774:5–15. doi: 10.1016/j.bbapap.2006.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juengel E, Kim D, Makarević J, Reiter M, Tsaur I, Bartsch G, et al. Molecular analysis of sunitinib resistant renal cell carcinoma cells after sequential treatment with RAD001 (everolimus) or sorafenib. J Mol Cell Cardiol. 2015;19:430–41. doi: 10.1111/jcmm.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huynh H, Chow KH, Soo KC, Toh HC, Choo SP, Foo KF, et al. RAD001 (everolimus) inhibits tumour growth in xenograft models of human hepatocellular carcinoma. J Mol Cell Cardiol. 2009;13:1371–80. doi: 10.1111/j.1582-4934.2008.00364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferri N, Granata A, Pirola C, Torti F, Pfister PJ, Dorent R, et al. Fluvastatin synergistically improves the antiproliferative effect of everolimus on rat smooth muscle cells by altering p27Kip1/Cyclin E Expression. Mol Pharmacol. 2008;74:144–53. doi: 10.1124/mol.108.046045. [DOI] [PubMed] [Google Scholar]


