Abstract
Objective:
The aim of this study was to evaluate the expression levels of cardiac-related circulating microRNAs (miRNAs) in ST-elevation myocardial infarction (STEMI) patients.
Methods:
This study has a prospective experimental cohort design. A total of 12 consecutive patients with acute chest pain within 12 h admitted to emergency department (STEMI group) and 13 adult patients with normal coronary angiography during the same period were enrolled (control group) in this study. Changes in the expression of miR-122, miR-208, miR-375, miR-22, miR-133b, miR-92b, miR-21, miR-133a, miR-423-5p, miR-27b, miR-30a-3p, miR-17, miR-30d, miR-642, and miR-95 were analyzed using quantitative reverse transcription-polymerase chain reaction. Blood samples were collected before angiography and 24 h after angiography. Data were analyzed using the Statistical Package for the Social Sciences v19.
Results:
The STEMI group included 12 patients (7 males) with an average age of 56.5±8.3 (range, 44–69) years. The control group included 13 patients (9 males) with an average age of 59±11 (range, 42–80) years. When fold differences were calculated for the miRNA expression values, only miR-30d and miR-423-5p expression levels in STEMI patients showed significant differences in expression levels compared with control patients. The miRNA levels were 2.3-fold higher for miR-30d (p=0.034) and 6.9-fold higher for miR-423-5p (p=0.017). There was no significant correlation between troponin I and miR-30d or miR-423-5p levels (p>0.05).
Conclusion:
In this study, the expression levels of miRNAs related to cardiac disease were evaluated in peripheral blood. The circulating miR-423-5p and miR-30d levels in peripheral blood were found to be higher in STEMI cases than in the control group. Further studies should be conducted to evaluate their potential use as biomarkers in STEMI cases. (Anatol J Cardiol 2016; 16; 392-6)
Keywords: circulating microRNAs, miR-423-5p, miR-30d, ST-elevation myocardial infarction, diagnosis
Introduction
Coronary artery disease is the most common cause of mortality worldwide, particularly in developed countries (1). Acute coronary syndromes may present as ST-elevation myocardial infarction (STEMI), non-STEMI, and unstable angina pectoris (2). Any delay in the time of reperfusion after arrival at the hospital is associated with a higher adjusted risk of mortality for acute STEMI patients (3).
The miRNAs, composed of approximately 22 nucleotides, are negative regulators of gene expression by binding to the 3’UTR of mRNA (4). Recent studies discovered that miRNAs are stably present in human serum/plasma (5). Most importantly, these circulating miRNAs can serve as potential biomarkers for various diseases such as cancer, tissue injury, autoimmune diseases, and other common clinical diseases (6). More recent studies have demonstrated that miRNAs play an important role in acute STEMI and could be used as biomarkers for the diagnosis (7).
The possibility of using miRNAs as a novel myocardial biomarker has been previously raised; however, studies on the detection of blood miRNAs in acute myocardial infarction (AMI) patients are limited. In this study, we examined the expression levels of miRNA related to cardiac diseases in circulating blood among STEMI patients versus a control group to identify miRNA candidates that can play an important role as a diagnostic marker to predict acute STEMI.
Methods
Study design
This study has a prospective experimental cohort design. Approval was obtained from the local Ethics Committee before initiation of the study, and written informed consent was obtained from all patients. All patients were prospectively followed until the time of discharge. A total of 12 consecutive patients with acute chest pain within 12 h admitted to our center, between January 2012 and December 2012 (STEMI group) and 13 patients with normal coronary angiography findings as controls (control group). All patients who underwent angiography were given acetylsalicylic acid and adenosine diphosphate receptor antagonists just before coronary angiography and treated with beta blockers (if no contraindications existed), renin angiotensin receptor blockers (if tolerated), and statins during the follow up.
Inclusion and exclusion criteria
The inclusion criteria regarding acute STEMI were based on the third universal definition of the myocardial infarction expert consensus document (8). The following patients were excluded: those who experienced chest pain caused by trauma or drugs, those who required medical intervention, and those with renal failure who required dialysis or received intravenous thrombolytic therapy before the initial blood samples were obtained.
Measurements of laboratory parameters and quantification of microRNA expression
Changes in the expression levels of miR-122, miR-208, miR-375, miR-22, miR-133b, miR-92b, miR-21, miR-133a, miR-423-5p, miR-27b, miR-30a-3p, miR-17, miR-30d, miR-642, and miR-95 were analyzed using quantitative reverse transcription-polymerase chain reaction (qRT-PCR). The first peripheral blood samples were collected before angiography and stored at –80°C for miRNA analysis. The second sample was taken 24 h after angiography. These two samples were collected from both acute STEMI and control groups.
A total of 10 mL blood was obtained and transferred to sterile PAXgene® blood RNA tubes. The extraction of miRNA from blood was performed using the mirVana™ miRNA isolation kit (Applied Biosystems, MA, USA) following the manufacturer's instructions. A total of 300 µL of blood was used for extraction and mixed with 300 µL of binding buffer. The extracted RNA was eluted in 50 µL of elution buffer. The expression levels of 14 mRNAs were elucidated using qRT-PCR. U6 small nuclear RNA was used as a control for normalization of the results. Reverse transcription was performed using 5 µL of extracted miRNA using TaqMan small RNA assay (Applied Biosystems, MA, USA), as advised by the manufacturer. qRT-PCR was performed using 20x fluorescent-labeled primers and the TaqMan small RNA assay with Universal PCR Master Mix II (Applied Biosystems, MA, USA), as advised by the manufacturer. The amplification was performed using a StepOnePlus real-time PCR system (Applied Biosystems, MA, USA) and the PCR conditions as per manufacturer's instructions. Cycle threshold (Ct) values were defined as the fractional cycle number at which the fluorescence exceeded the given threshold. All results were expressed as Ct and normalized to the calculated median Ct of each sample (ΔCt). The relative expression was calculated using the comparative Ct method (2–ΔΔCt).
Statistical analyses
Data were analyzed using the IBM Statistical Package for the Social Sciences v19 (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean±standard deviation (SD) or range and categorical variables as frequencies and percentages. Comparisons of parametric values between the two groups were performed using an independent samples t test. Pearson's correlation analysis was used to show the correlation between troponin and miRNA levels. The chi-square test was used to compare categorical variables. A two-tailed p value <0.05 was considered statistically significant.
Results
A total of 25 (16 males) patients were included in the present study. The STEMI group included 12 patients (7 males) with an average age of 56.5±8.3 (range, 44–69) years. The control group included 13 patients (9 males) with an average age of 59±11 (range, 42–80) years. With respect to baseline characteristics such as demographic findings, medications, and laboratory parameters, no significant differences were found between the groups (Table 1).
Table 1.
Baseline characteristics of two patients groups
| Characteristic | Control group (n=13) | STEMI group (n=12) | P* |
|---|---|---|---|
| Age, years | 59±11 | 56.5±8.3 | NS |
| Sex, male/female | 9/4 | 7 | NS |
| Current smokers | 10 | 6 | NS |
| Hypertension | 6 | 5 | NS |
| Hyperlipidemia | 6 | 4 | NS |
| Diabetes mellitus | 4 | 2 | NS |
| Family history | 2 | 4 | NS |
| Body mass index, kg/m2 | 29.50±5.61 | 27.47±4.25 | NS |
| Waist circumference, cm | 100.08±10.63 | 89.33±6.61 | ***0.006 |
| Beta blocker use | 8 | 8 | NS |
| **RAS blocker use | 10 | 8 | NS |
| Statin use | 12 | 7 | NS |
| Aspirin use | 13 | 12 | NS |
| *ADP receptor antagonists | 12 | 13 | NS |
ADP - adenosine diphosphate;
RAS - renin-angiotensin system
independent samples t-test used
When fold differences were calculated for the miRNA expression values, only miR-30d and miR-423-5p expression levels in STEMI patients showed significant differences in expression levels than in control patients (Fig. 1). The miRNA levels were 2.3-fold higher for miR-30d (p=0.034) and 6.9-fold higher for miR-423-5p (p=0.017) (Fig. 2). In contrast, we did not detect any difference in the level of miR-122, miR-208, miR-375, miR-22, miR-133b, miR-92b, miR-21, miR-133a, miR-27b, miR-30a-3p, miR-17, miR-642, and miR-95 between the STEMI and control groups (Table 2). There was no significant correlation between troponin and miR-30d or miR-423-5p levels (p>0.05).
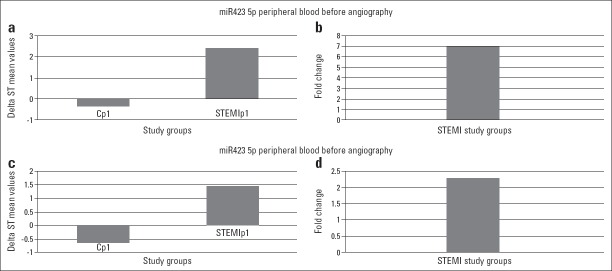
Figure 1.
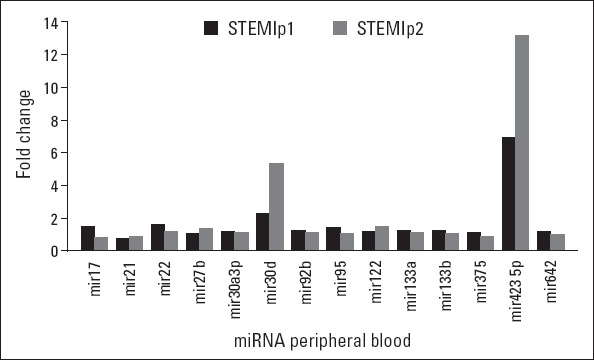
Fold changes of the miRNAs studied when the values from STEMI patients and control patients were compared. Only two miRNAs showed more than a 2-fold higher expression in both pre- and postangiography samples. STEMIp1 indicates the results obtained in the samples before angiography, and STEMIp2 indicates the results obtained in the samples taken 24 h after angiography
Figure 2.
Fold changes of miR-30d and miR-423-5p, the only miRNAs that had >2-fold increase among 14 miRNAs tested. Statistically, both changes were significant for initial samples, and P values were P=0.034 and P=0.017 for miR-30d and miR-423-5p, respectively. Cp1-control patients’ peripheral blood sample, STEMIp1-STEMI peripheral blood sample. Panels a and c show delta ST value differences between control and STEMI patients for miR-423-5p and miR-30d, respectively. Panels b and d show fold changes
Table 2.
miRNA expression values in peripheral blood from control patients and STEMI patients. The first samples (Control 1 and STEMI 1) were taken before angiography, and the second samples (Control 2 and STEMI 2) 24 h after angiography. The given fluorescence values are ΔΔCt values and are normalized by U6 small nuclear RNA results
| *miRNAs | ΔΔCT values | |||
|---|---|---|---|---|
| Control 1 | Control 2 | **STEMI 1 | STEMI 2 | |
| miR-17 | –0.81933 | –1.37214 | –1.21508 | –1.11383 |
| miR-21 | –2.66039 | –2.1953 | –2.07787 | –1.90472 |
| miR-22 | 2.778293 | 3.478508 | 4.472264 | 4.099575 |
| miR-27b | 5.602938 | 4.438248 | 5.652115 | 5.994638 |
| miR-30a3p | 6.829403 | 6.590719 | 7.855644 | 7.201007 |
| miR-30d | –0.63113 | –0.2469 | 1.430998 | 1.311748 |
| miR-92b | 6.398251 | 6.474871 | 7.969774 | 7.305626 |
| miR-95 | 7.274697 | 8.105195 | 10.38862 | 8.657185 |
| miR-122 | 6.870229 | 5.450016 | 7.819091 | 8.045629 |
| miR-133a | 6.656118 | 6.836686 | 8.308413 | 7.616045 |
| miR-133b | 6.615982 | 7.064376 | 8.294853 | 7.603616 |
| miR-375 | 7.888059 | 8.286711 | 8.780971 | 7.317476 |
| miR-423 5p | –0.34913 | –0.16859 | 2.420079 | 2.218406 |
| miR-642 | 7.359747 | 7.223507 | 8.438755 | 7.032296 |
miRNA - micro RNA;
STEMI - ST segment elevation myocardial infarction
Discussion
The main aim of the present study was to detect the differences between miRNA expressions in the STEMI group versus the control group in peripheral blood. The key finding of this study was that there was a relationship between circulating miRNAs and STEMI. Our study showed that miR-423-5p and miR-30d levels are elevated, and this elevation persisted after 24 h in STEMI patients.
After the discovery that miRNAs are present in not only cells and tissues but also in circulating blood, the idea of using these circulating miRNAs as biomarkers of disease developed, and several studies to demonstrate relations between circulating miRNAs and diseases were subsequently conducted. Some miRNAs are documented to be involved in cell differentiation, hypoxia, fibrosis, and development in response to AMI (9, 10). It suggested that miRNAs played critical roles in AMI pathophysiological processes. In the present study, among the studied miRNAs, circulating miR-423-5p and miR-30d were upregulated in the peripheral blood of STEMI patients.
Previous studies have identified several serum miRNAs, including miR-1, miR-133, and miR-208, whose levels were significantly increased in AMI patients and experimental animals (11, 12). In the present study, the levels of both miR-423-5p and miR-30d were found to be increased in STEMI patients. miR-30d showed a 2.3-fold increase and miR-423-5p showed a 6.9-fold increase in STEMI cases.
A high expression level of miR-423-5p in STEMI patients was consistent with the previous studies (13–16). Nabiałek et al. (13) showed a significantly higher number of miR-423-5p copies in STEMI patients before the coronary intervention. After 6, 12, and 24 h post-procedure, the expression level was similar to the control group and significantly lower than the baseline level. They stated that the expression of miR-423-5p in plasma was significantly increased in acute MI with subsequent normalization within 6 h. A global miRNA screening approach in patients with heart failure identified miR-423-5p to be increased in heart failure patients (14). Tijsen et al. (14) elaborately explored the diagnostic role of plasmatic miRNAs in patients presenting with dyspnea. miR-423-5p demonstrated an excellent discriminatory ability for separating heart failure patients from healthy controls and from non-heart failure cases of dyspnea. Goren et al. (15) evaluated 186 circulating miRNAs in chronic systolic heart failure and control patients. An miRNA score based on cumulative levels of four miRs (including miR-423-5p) was able to discriminate between patients and controls with an overall accuracy of 90% (15). Goldraich et al. (16) observed a positive transcoronary gradient of plasmatic miR-423-5p expression in heart failure patients. In addition, the transcoronary gradient of miR-423-5p was correlated with the B-type natriuretic peptide transcoronary gradient. These findings suggest cardiac release and/or synthesis of miR-423-5p in stable outpatients with heart failure. In contrast with the abovementioned studies, Bauters et al. (17) stated that miR-423-5p is not a useful biomarker of left ventricular remodeling after myocardial infarction. Tıjsen et al. (14) reported that miR423-5p was specifically enriched in the blood of HF cases, and receiver-operator-characteristics curve analysis showed miR423-5p to be a diagnostic predictor of HF.
Çakmak et al. (18) evaluated the expression levels of miRNAs in patients with chronic congestive heart failure and revealed a decrease in the expression of miR-30d. Li et al. (19) showed that miR-30d regulates cardiomyocyte pyroptosis; the upregulation of miR-30d increases the pyroptosis of cardiomyocytes, and its inhibition attenuates pyroptosis. Duisters et al. (20) showed that both miR-133 and miR-30 directly downregulate connective tissue growth factor, a key profibrotic protein, and thereby establish an important role for these miRNAs in the control of structural changes in the extracellular matrix of the myocardium.
Study limitations
There were some limitations of our study. Firstly, the size of our series was relatively small. Secondly, we could not record the exact onset time of STEMI in our patients. Thirdly, some details of history and factors that may influence the outcome may not be completely documented. Finally, this was a single-institution study. Because of these restrictions, associations should be interpreted with caution.
Conclusion
The high level of circulating miR-423-5p and miR-30d levels in peripheral blood indicates their potential use as biomarkers in STEMI cases. However, there was no significant correlation between troponin and these two miRNA levels. Large-scale studies are required to study the utility of these miRNAs as biomarkers.
Acknowledgements
Part of this study was supported by the University of Adnan Menderes Scientific Research Projects Commission (TPF-12036).
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept- Ç.A.; Design- U.E.; Supervision- B.B.; Funding-B.B., N.B., Ö.Y.; Materials- U.E., Ç.A.; Data collection &/or processing – U.E., Ç.A.; Analysis and/or interpretation– İ.K.Ö.; Literature search- U.E., B.B.; Writing – U.E., B.B.; Critical review- Ç.A.
References
- 1.Kontos MC, Diercks DB, Kirk JD. Emergency department and office-based evaluation of patients with chest pain. Mayo Clin Proc. 2010;85:284–99. doi: 10.4065/mcp.2009.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeh RW, Go AS. Rethinking the epidemiology of acute myocardial infarction: challenges and opportunities. Arch Intern Med. 2010;170:759–64. doi: 10.1001/archinternmed.2010.88. [DOI] [PubMed] [Google Scholar]
- 3.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined-a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–69. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Jia Y, Zheng R, Guo Y, Wang Y, Guo H, et al. Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clin Chem. 2010;56:1830–8. doi: 10.1373/clinchem.2010.147850. [DOI] [PubMed] [Google Scholar]
- 7.Adachi T, Nakanishi M, Otsuka Y, Nishimura K, Hirokawa G, Goto Y, et al. Plasma microRNA 499 as a biomarker of acute myocardial infarction. Clin Chem. 2010;56:1183–5. doi: 10.1373/clinchem.2010.144121. [DOI] [PubMed] [Google Scholar]
- 8.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Glob Heart. 2012;7:275–95. doi: 10.1016/j.gheart.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Oglesby IK, McElvaney NG, Greene CM. MicroRNAs in inflammatory lung disease--master regulators or target practice? Respir Res. 2010;11:148. doi: 10.1186/1465-9921-11-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79:581–8. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 11.Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J, et al. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J. 2010;31:659–66. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- 12.Ai J, Zhang R, Li Y, Pu J, Lu Y, Jiao J, et al. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem Biophys Res Commun. 2010;391:73–7. doi: 10.1016/j.bbrc.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Nabiałek E, Wańha W, Kula D, Jadczyk T, Krajewska M, Kowalówka A, et al. Circulating microRNAs (miR-423-5p, miR-208a and miR-1) in acute myocardial infarction and stable coronary heart disease. Minerva Cardioangiol. 2013;61:627–37. [PubMed] [Google Scholar]
- 14.Tijsen AJ, Creemers EE, Moerland PD, de Windt LJ, van der Wal AC, Kok WE, et al. MiR423-5p as a circulating biomarker for heart failure. Circ Res. 2010;106:1035–9. doi: 10.1161/CIRCRESAHA.110.218297. [DOI] [PubMed] [Google Scholar]
- 15.Goren Y, Kushnir M, Zafrir B, Tabak S, Lewis BS, Amir O. Serum levels of microRNAs in patients with heart failure. Eur J Heart Fail. 2012;14:147–54. doi: 10.1093/eurjhf/hfr155. [DOI] [PubMed] [Google Scholar]
- 16.Goldraich LA, Martinelli NC, Matte U, Cohen C, Andrades M, Pimentel M, et al. Transcoronary gradient of plasma microRNA 423-5p in heart failure: evidence of altered myocardial expression. Biomarkers. 2014;19:135–41. doi: 10.3109/1354750X.2013.870605. [DOI] [PubMed] [Google Scholar]
- 17.Bauters C, Kumarswamy R, Holzmann A, Bretthauer J, Anker SD, Pinet F, et al. Circulating miR-133a and miR-423-5p fail as biomarkers for left ventricular remodeling after myocardial infarction. Int J Cardiol. 2013;168:1837–40. doi: 10.1016/j.ijcard.2012.12.074. [DOI] [PubMed] [Google Scholar]
- 18.Çakmak HA, Çoşkunpınar E, İkitimur B, Barman HA, Karadağ B, Tiryakioğlu NO, et al. The prognostic value of circulating microRNAs in heart failure: preliminary results from a genome-wide expression study. J Cardiovasc Med (Hagerstown) 2015;16:431–7. doi: 10.2459/JCM.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Du N, Zhang Q, Li J, Chen X, Liu X, et al. MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis. 2014;5:e1479. doi: 10.1038/cddis.2014.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, van der Made I, et al. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res. 2009;104:170–8. doi: 10.1161/CIRCRESAHA.108.182535. [DOI] [PubMed] [Google Scholar]