Abstract
Objective:
The aim of this study was to evaluate left ventricular (LV) systolic strain by speckle tracking echocardiography (STE) and real-time three-dimensional echocardiography (3-DE) for the early detection of myocardial dysfunction in patients with cardiac syndrome X (CSX).
Methods:
We compared 34 patients with CSX (18 females, mean age 47.9±10.0 years) with 41 healthy persons as a control group (23 females, mean age 50.6±9.9 years). Inclusion criteria for CSX were typical angina, a positive exercise ECG stress test, and angiographically documented normal coronary arteries. Exclusion criteria for both groups were hypertension, valvular heart disease, cardiomyopathies, inflammatory diseases, myocarditis, vasculitis, arthropathies, Tietze's syndrome, gastrointestinal diseases, aortic diseases, hormone replacement therapy, arrhythmias, liver diseases, and alcohol use. All subjects underwent two-dimensional STE and 3-DE to assess resting LV function. STE measures were taken from the basal septum, mid-septum, apical septum, apex, apicolateral, mid-lateral, basal lateral, anteroseptal, anterior, anterolateral, inferolateral, inferior, and inferoseptal walls. Student's t-test, Mann–Whitney U test, and chi-square test were used to statistically analyze data.
Results:
LV echo ejection fraction (EF) and systolic wave peak velocity were similar for both groups. Regional mean longitudinal strain (-17.7±2.5% vs. -19.8±1.8%; p<0.0001) was significantly lower in patients with CSX than in healthy control patients. However, regional mean circumferential strain values (-22.0±1.6% vs. -22.2±2.3%; p=0.78) did not differ significantly between the two groups.
Conclusion:
Significant impairment of LV longitudinal myocardial systolic function was detected with STE in patients with CSX, although normal 3-D EF and tissue Doppler imaging systolic parameters were observed. Arteriosclerosis of small coronary arteries and microvascular dysfunction may affect myocardial longitudinal strain.
Keywords: cardiac syndrome X, speckle tracking echocardiography, strain
Introduction
Cardiac syndrome X (CSX) is an angina-like chest pain with a positive response to exercise stress testing and normal coronary angiographic findings (1, 2). No single underlying mechanism has been identified for the condition. Small vessel coronary artery disease, abnormal coronary vascular resistance, and subendocardial ischemia have been invoked as possible mechanisms, although none of these factors is universally accepted (3, 4). Several studies have found abnormalities consistent with myocardial perfusion in patients with CSX using positron emission tomography (5), scintigraphic myocardial perfusion imaging (6), and nuclear magnetic resonance imaging (7). Although left ventricular (LV) diastolic dysfunction has been shown in studies using conventional and tissue Doppler echocardiography, LV systolic function was found to be normal (8, 9). This discrepancy might have arisen because of the insufficiency of conventional and tissue Doppler imaging (TDI)-derived echocardiographic systolic parameters for determining early abnormalities in systolic function. The recent development of two-dimensional speckle tracking echocardiography (2-D-STE) provides a method for the non-invasive assessment of global and local LV function from two-dimensional (2-D) images (10, 11). Previous studies have indicated that 2-D-STE is more sensitive than conventional echocardiography parameters for detecting subclinical ventricular dysfunction in various clinical disorders (11-13).
The aim of this study was to use STE to evaluate LV systolic strain to detect myocardial dysfunction in patients with CSX.
Methods
Thirty-four consecutive patients diagnosed with CSX who had normal coronary arteries on angiography, which was performed because ischemic signs were observed on an exercise stress test, were included in the present study. Forty-one consecutive healthy subjects with normal coronary arteries on angiography that had no ischemic change during an exercise stress test were included as controls. The inclusion criteria for the CSX group were typical angina, a positive exercise ECG stress test, and angiographically documented normal coronary arteries. The exclusion criteria for the CSX and control groups were as follows: patients who had hypertensive heart disease with left ventricular hypertrophy, valvular heart disease, idiopathic hypertrophic or dilated cardiomyopathy, acute or chronic inflammatory diseases, history of myocarditis and vasculitis, spondyloarthritis, Tietze's syndrome, gastrointestinal tract diseases, diseases of the aorta, hormone replacement therapy, arrhythmias, active hepatobiliary disease, or alcohol consumption. All participants signed their informed consent form before attending the study, and the Local Ethics Committee approved the study protocol. All participants underwent a complete physical examination and routine laboratory analysis before the study.
Treadmill exercise stress testing
All treadmill exercise tests were conducted according to the Bruce protocol using a T600 Treadmill (Spacelabs Burdick, WI 53531, USA). Three ECG leads (V2, V5, and aVF) were continuously monitored during these tests. A standard 12-lead ECG print was obtained. Total exercise times were recorded in all cases. Electrocardiographic recovery was also continuously monitored until the depressed ST-segment returned to baseline levels. A positive treadmill test was defined by the occurrence of ischemic ST-segment depression (≥1.0 mm horizontal or downsloping depression at 80 ms from the J point) in at least two contiguous leads (usually II, III, aVF, or V3–6) on ECG during the treadmill exercise test.
Cardiac catheterization
Coronary angiography was performed using the Judkins technique in all patients (Philips Medical Systems Integris H 3500 and 5000). Coronary arteries were classified as normal based on the absence of any luminal irregularity in a visual assessment. To exclude the possibility of coronary artery vasospasm, all patients underwent a hyperventilation test during coronary angiography, which was performed by asking the patients to breathe quickly and deeply for 5 min.
Echocardiography
A transthoracic echocardiographic study was performed using a Philips IE-33 machine with a broadband S5-1 transducer (Philips, Bothell, USA). Left atrial (LA), LV end-systolic, and LV end-diastolic diameters were obtained from 2-D images. LV ejection fraction (LVEF) was determined using Simpson's rule. Transmitral pulsed-wave Doppler velocities were obtained from the apical 4-chamber view with the sample volume placed just below the mitral leaflet tips. We measured early (E) and late (A) wave velocities, E/A ratio, E deceleration time (DT), and isovolumetric relaxation time (IVRT). TDI of the mitral annulus was obtained by placing the sample volume at the septal mitral annulus, and peak myocardial systolic (Sm), peak early diastolic (Em), and peak late diastolic (Am) myocardial velocities, as well as E/Em and Em/Am ratios, were measured.
2-D speckle tracking analyses were performed on grayscale images of the left ventricle obtained in the apical 4-chamber views and short-axis mid-ventricular views. Three consecutive end-expiratory cardiac cycles using high frame rate (≥50 Hz) harmonic imaging in each echocardiographic view were acquired. Resultant data were analyzed using 2-D speckle-tracking software (TMQA, Q-lab, Philips). A single cardiac cycle in which an apical 4-chamber view in the end-diastolic frame was used for analysis. Septal and lateral references points on the mitral annulus and apical endocardium were manually selected. After initialization, the program positioned those references points at equal distances on the endocardial surface of the LV cavity. Later, the target area was analyzed automatically via the software according to a 7-segment model. Visual assessment of the speckle tracking together with manual fine adjustments was performed if necessary. The software automatically divided the target area into equal segments from which mean longitudinal and circumferential strains were determined. Longitudinal strain was measured from the basal septum, mid-septum, apical septum, apex, apicolateral, mid-lateral, and basal lateral wall segments. Circumferential strain was measured from the anteroseptal, anterior, anterolateral, inferolateral, inferior, and inferoseptal walls.
Three-dimensional echocardiography
LVEF was measured using real-time three-dimensional echocardiography (3-DE) by obtaining a matrix-array full-volume dataset (X3, ie33, Philips Medical Systems, MA, USA). Using an offline analysis program (Qlab, Philips Medical Systems), conventional 4-chamber, 2-chamber, and short-axis views were derived from this dataset. After the selection of two annular and apical reference points, a 3-DE endocardial shell was built using semi-automated contour tracing.
Statistical analysis
All statistical analyses were performed using SPSS software, version 17.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were presented as means±standard deviation (SD), and categorical variables were presented as numbers and percentages. All data were tested for normality with the Shapiro–Wilk test. Between-group comparisons of categorical data were achieved with chi-square or Fisher's exact tests. Between-group comparisons of parametric and nonparametric continuous data were conducted using unpaired Student's t-test and Mann–Whitney U test, respectively. A two-tailed p value of <0.05 was considered statistically significant.
Results
Baseline clinical characteristics and laboratory findings for the 34 CSX and 41 control subjects are presented in Table 1. There was no statistically significant difference between the groups with regard to age, gender, dyslipidemia, and body mass index. LV end-diastolic diameter, 3-D EF, Sm, IVRT, E/Em, and Em/Am were also similar between the groups. DT (191.62±15.70 ms vs. 178.54±10.20 ms, p<0.000) and IVRT (88.38±7.45 vs. 76.59±8.24) were prolonged in patients with CSX compared with the control subjects, whereas the E/A ratio was significantly lower (1.32±0.13 vs. 1.45±0.24, p=0.005) in the patients with CSX. The LV longitudinal and circumferential systolic strain parameters of patients with CSX and control subjects are shown in Table 2. An example of STE analysis is shown in Figures 1 and 2. In the patients with CSX, all segments showed a significant decrease in longitudinal strain than the healthy controls. However, there was no significant difference in regional circumferential strain between the two groups. As a result, the mean longitudinal strain (-17.7±2.5% vs. -19.8±1.8%; p<0.000) was significantly impaired in the CSX group relative to the control group; however, the mean circumferential strain was similar between the groups (-22.0±1.6% vs. -22.2±2.3%; p=0.78).
Table 1.
Baseline characteristics of the study population
| CSX (n=34) | Controls (n=41) | *P | |
|---|---|---|---|
| Age, years | |||
| Total | 47.9±10.0 | 50.6±9.9 | 0.23 |
| Women | 49.9±9.5 | 52.4±7.7 | 0.36 |
| Men | 45.6±10.3 | 48.4±11.9 | 0.46 |
| Women/men | 18/16 | 23/18 | 0.81 |
| BMI, kg/m2 | 26.3±4.2 | 26.5±4.1 | 0.79 |
| Plasma glucose, mg/dL | 97.2±9.0 | 96.3±8.5 | 0.68 |
| Total cholesterol, mg/dL | 187.1±35.5 | 191.9±40.6 | 0.59 |
| 3-D LV EF, % | 67.12±3.51 | 66.85±3.19 | 0.88 |
| Left atrial diameter, mm | 35.0±2.1 | 34.5±1.9 | 0.35 |
| LVEDD, mm | 44.9±1.8 | 44.3±3.0 | 0.33 |
| E/A | 1.32±0.13 | 1.45±0.24 | 0.005 |
| DT, ms | 191.6±15.7 | 178.5±10.2 | <0.0001 |
| IVRT, ms | 88.3±7.4 | 76.5±8.2 | <0.0001 |
| Sm, cm/s | 10.7±1.6 | 10.9±1.8 | 0.51 |
| E/Em | 7.20±1.55 | 7.01±1.09 | 0.19 |
| Em/Am | 1.74±1.84 | 1.79±2.34 | 0.43 |
Data are presented as mean±SD values
Unpaired student's t-test or chi-square test
A - late transmitral wave velocity; Am - peak late diastolic myocardial velocity; BMI - body mass index; CSX - cardiac syndrome X; DT - deceleration time; E - early transmitral wave velocity; EF - ejection fraction; Em - peak early diastolic myocardial velocity; IVRT - isovolumetric relaxation time; LV - left ventricle; LVEDD - left ventricular end-diastolic diameter; Sm - peak myocardial systolic myocardial velocity
Table 2.
Circumferential and longitudinal strain values assessed by speckle tracking echocardiography
| CSX (n=34) | Controls (n=41) | *P | |
|---|---|---|---|
| Longitudinal strain, % | |||
| Basal septum | -14.7±2.9 | -16.8±3.0 | 0.004 |
| Mid septum | -17.4±4.0 | -19.7±3.1 | 0.008 |
| Apical septum | -21.1±3.4 | -23.6±3.1 | 0.002 |
| Basal lateral | -15.7±2.7 | -17.5±2.8 | 0.006 |
| Mid lateral | -17.1±3.3 | -19.1±3.2 | 0.011 |
| Apical lateral | -19.8±3.0 | -22.0±2.8 | 0.003 |
| Apex | -21.2±2.6 | -23.3±2.4 | 0.001 |
| Mean longitudinal strain | -17.7±2.5 | -19.8±1.8 | 0.0001 |
| Circumferential strain, % | |||
| Anteroseptal | -22.7±3.0 | -22.4±3.6 | 0.62 |
| Anterior | -20.9±3.6 | -22.4±4.3 | 0.12 |
| Anterolateral | -21.8±3.9 | -21.0±4.0 | 0.45 |
| Inferolateral | -22.5±2.8 | -22.9±4.0 | 0.54 |
| Inferior | -22.6±3.4 | -21.3±4.1 | 0.15 |
| Inferoseptal | -21.9±2.9 | -22.4±4.2 | 0.52 |
| Mean circumferential strain | -22.0±1.6 | -22.1±2.3 | 0.78 |
Data are presented as mean±SD values
Unpaired student's t-test
CSX - cardiac syndrome X
Figure 1.
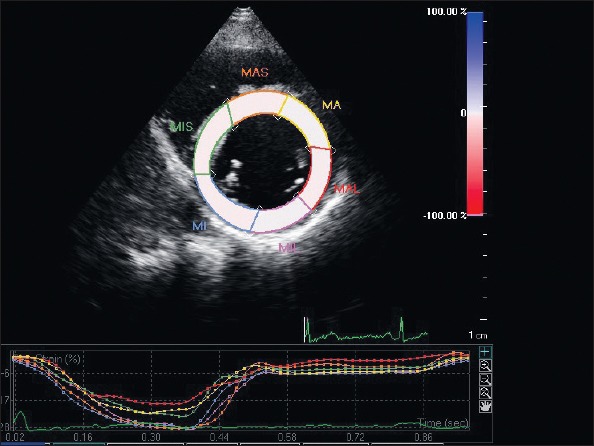
Regional circumferential strain on left ventricular short-axis view
Figure 2.
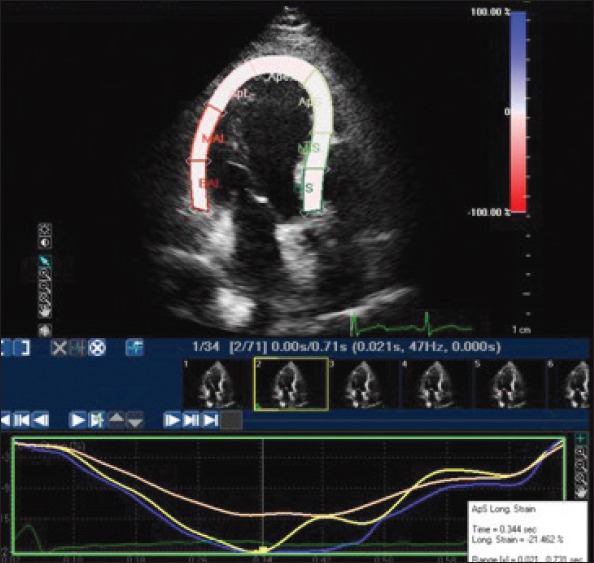
Apical longitudinal strain on left ventricular apical 4-chamber view
Discussion
The main finding of our study was the detection of a significant impairment of LV longitudinal myocardial systolic function in patients with CSX by speckle tracking echocardiography, despite 3-D EF and several TDI echocardiographic systolic parameters not being significantly different between the CSX and control groups.
Multiple pathophysiologic abnormalities, such as small vessel coronary artery disease, abnormal coronary vascular resistance, and subendocardial ischemia have been reported and invoked as possible mechanisms for CSX, although none of these is universally accepted as causative factor. Recently, noninvasive imaging techniques have demonstrated abnormal myocardial perfusion and abnormal LV function in this cohort of the study population. The presence of coronary microvascular abnormalities in patients with CSX has been supported by the detection of reversible myocardial perfusion defects during stress myocardial scintigraphy (14-16) and, perhaps more so, by evidence for an impaired response to vasodilator stimuli of coronary blood flow and/or resistance (6, 7, 17, 18). A reduced coronary flow reserve in patients with normal epicardial arteries suggests a dysfunction of the coronary microvasculature. Panting et al. (7) used gadolinium cardiovascular magnetic resonance imaging to show an impairment of myocardial perfusion in subendocardial layers in response to adenosine, thereby providing supporting evidence for the presence of coronary microvascular dysfunction in patients with CSX. They found consistent evidence for an abnormality of myocardial perfusion limited to the subendocardium on cardiac MR imaging in patients with CSX, which was compatible with subendocardial ischemia, despite the absence of LV wall motion abnormalities in a significant proportion of the patients with CSX. Sun et al. (19) also showed that there was impairment in LV function in the follow-up of patients with CSX who had an abnormal thallium scan.
Different results have been obtained from 2-D echocardiographic studies that evaluated LV function in patients with CSX (8, 9, 20). In general, LV systolic functions were determined as normal in these studies, but impairment of diastolic parameters was detected. Masseri et al. (21) documented the difficulty in identifying LV dysfunction, as well as ischemic metabolites, using routine diagnostic methods; their results imply that, in patients with CSX, microvascular dysfunction has a patchy distribution in the myocardial wall rather than a diffuse distribution. Thus, normal segments may easily obscure diminished LV function in the small, involved segments. For this reason, routine 2-D echocardiographic measurements of LV functions may be insensitive. In addition, these measurements have major disadvantages such as angle dependence, limited spatial resolution, and deformation analysis in one dimension. The recent development of 2-D-STE overcomes some of these limitations, and 2-D-STE can be used for the quantitative assessment of global and local LV function from 2-D images (11, 12).
Regarding regional systolic function, despite normal LV ejection fraction and TDI systolic peak velocities in our CSX population, a significant reduction in global longitudinal strain was detected. Patients with microvascular angina might have diffuse subendocardial ischemia, and subendocardial dysfunction could potentially affect ventricular longitudinal functions. Ventricular subendocardial fibers are predominantly longitudinal in orientation, and early manifestations of cardiac abnormalities are usually observed in the subendocardial layer (22). In previous studies, longitudinal functions have been shown to deteriorate earlier than radial and circumferential functions in myocardial systolic dysfunction (23, 24). For this reason, longitudinal systolic functions deteriorate before the development of overt heart failure. In our study, all study patients had normal LV ejection fractions, and we detected impairment in longitudinal systolic functions despite the preservation of circumferential functions, which suggests early myocardial involvement. Subendocardial ischemia due to microvascular dysfunction may affect myocardial longitudinal fibers and may lead to the deterioration of longitudinal systolic functions.
Study limitations
The major limitation of our study is the small sample size; further studies with larger populations will help us define the eventual role of STE in the determination of LV functions in CSX. Additionally, the accuracy of STE largely depends on image quality; however, 8 patients were excluded from our study because of inadequate image quality.
Conclusion
In our study, we detected impaired longitudinal systolic strain on STE despite normal systolic parameters being measured by TDI and 3-D EF echocardiography in the patients with CSX. Therefore, our findings demonstrate that 2-D-STE is a feasible technique that allows the evaluation of LV regional systolic function in patients with CSX with higher sensitivity than TDI and conventional parameters. Despite normal LV EF and TDI systolic peak velocities in our patients with CSX, a significant reduction in global longitudinal strain was detected. The present study helps to further our understanding of the relationship between fiber architecture and myocardial function in patients with CSX as well as in other clinical circumstances, but further studies are still required.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Author contributions: Concept - J.Y.; Design - J.Y., N.A.; Supervision - M.C.; Materials - J.Y., N.E.; Data collection &/or processing - N.E., J.Y.; Analysis &/or interpretation - N.E., J.Y.; Literature search - J.Y., Y.K.; Writing - E.K., J.Y.; Critical review - E.K.
References
- 1.Cannon RO, III, Epstein SE. Microvascular angina as a cause of chest pain with angiographically normal coronary arteries. Am J Cardiol. 1988;61:1338–43. doi: 10.1016/0002-9149(88)91180-0. [DOI] [PubMed] [Google Scholar]
- 2.Kaski JC. Pathophysiology and management of patients with chest pain and normal coronary arteriograms (Cardiac syndrome X) Circulation. 2004;109:568–72. doi: 10.1161/01.CIR.0000116601.58103.62. [DOI] [PubMed] [Google Scholar]
- 3.Vinereanu D, Fraser AG, Robinson M, Lee A, Tweddel A. Adenosine provokes diastolic dysfunction in microvascular angina. Postgrad Med J. 2002;78:40–2. doi: 10.1136/pmj.78.915.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan O, Meriç M, Acar Z, Kale A, Demircan S, Yılmaz O, et al. The effect of exercise and antioxidant enzyme levels in syndrome X and coronary slow flow phenomenon: an observational study. Anatol J Cardiol. 2013;13:641–6. doi: 10.5152/akd.2013.186. [DOI] [PubMed] [Google Scholar]
- 5.Marroquin OC, Holubkov R, Edmundowicz D, Rickens C, Pohost G, Buchthal S, et al. Heterogeneity of microvascular dysfunction in women with chest pain not attributable to coronary artery disease: Implications for clinical practice. Am Heart J. 2003;145:628–35. doi: 10.1067/mhj.2003.95. [DOI] [PubMed] [Google Scholar]
- 6.Zeiher AM, Krause T, Schächinger V, Minners J, Moser E. Impaired endothelium-dependent vasodilation of coronary resistance vessels is associated with exercise-induced myocardial ischemia. Circulation. 1995;91:2345–52. doi: 10.1161/01.cir.91.9.2345. [DOI] [PubMed] [Google Scholar]
- 7.Panting JR, Gatehouse PD, Yang GZ, Grothues F, Firmin DN, Collins P, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346:1948–53. doi: 10.1056/NEJMoa012369. [DOI] [PubMed] [Google Scholar]
- 8.Yazıcı HU, Şen N, Tavil Y, Hizal F, Turfan M, Poyraz F, et al. Left ventricular functions in patients with cardiac syndrome X: a tissue Doppler study. Anatol J Cardiol. 2009;9:467–72. [PubMed] [Google Scholar]
- 9.Moreno R, Garcia-Fernandez MA, Moreno M, Puerta P, Bermejo J, Ortega A, et al. Regional diastolic function in microvascular angina studied by pulsed-wave Doppler tissue imaging. Echocardiography. 1999;16:239–44. doi: 10.1111/j.1540-8175.1999.tb00808.x. [DOI] [PubMed] [Google Scholar]
- 10.Teske AJ, De Boeck BW, Melman PG, Sieswerda GT, Doevendans PA, Cramer MJ. Echocardiographic quantification of myocardial function using tissue deformation imaging, a guide to image acquisition and analysis using tissue Doppler and speckle tracking. Cardiovasc Ultrasound. 2007;5:27. doi: 10.1186/1476-7120-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yağmur J, Şener S, Açıkgöz N, Cansel M, Ermiş N, Karıncaoğlu Y, et al. Subclinical left ventricular dysfunction in Behcet's disease assessed by two-dimensional speckle tracking echocardiography. Eur J Echocardiogr. 2011;12:536–41. doi: 10.1093/ejechocard/jer088. [DOI] [PubMed] [Google Scholar]
- 12.Binnetoğlu FK, Yıldırım Ş, Topaloğlu N, Tekin M, Kaymaz N, Aylanç H, et al. Early detection of myocardial deformation by 2D speckle tracking echocardiography in normotensive obese children and adolescents. Anatol J Cardiol. 2015;15:151–7. doi: 10.5152/akd.2014.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erdoğan E, Akkaya M, Bacaksız A, Tasal A, Turfan M, Kul S, et al. Subclinical left ventricular dysfunction in women with polycystic ovary syndrome: an observational study. Anatol J Cardiol. 2013;13:784–90. doi: 10.5152/akd.2013.196. [DOI] [PubMed] [Google Scholar]
- 14.Çavuşoğlu Y, Entok E, Timuralp B, Vardareli E, Kudaiberdieva G, Birdane A, et al. Regional distribution and extent of perfusion abnormalities, and the lung to heart uptake ratios during exercise thallium-201 SPECT imaging in patients with cardiac syndrome X. Can J Cardiol. 2005;21:57–62. [PubMed] [Google Scholar]
- 15.Kaski JC, Rosano G, Collins P, Nihoyannopoulos P, Maseri A, Poole-Wilson PA. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol. 1995;25:807–14. doi: 10.1016/0735-1097(94)00507-M. [DOI] [PubMed] [Google Scholar]
- 16.Tweddel AC, Martin W, Hutton I. Thallium scans in syndrome X. Br Heart J. 1992;68:48–50. doi: 10.1136/hrt.68.7.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galassi AR, Crea F, Araujo LI, Lammertsma AA, Pupita G, Yamamoto Y, et al. Comparison of regional myocardial blood flow in syndrome X and one-vessel coronary artery disease. Am J Cardiol. 1993;72:134–9. doi: 10.1016/0002-9149(93)90148-6. [DOI] [PubMed] [Google Scholar]
- 18.Vermeltfoort IA, Bondarenko O, Raijmakers PG, Odekerken DA, Kuijper AF, Zwijnenburg A, et al. Is subendocardial ischemia present in patients with chest pain and normal coronary angiograms?. A cardiovascular MR study. Eur Heart J. 2007;28:1554–8. doi: 10.1093/eurheartj/ehm088. [DOI] [PubMed] [Google Scholar]
- 19.Sun SS, Huang JL, Tsai SC, Ho YJ, Kao CH. The higher likelihood of developing cardiomegaly during follow-up in patients with syndrome X and abnormal thallium-201 myocardial perfusion SPECT. Int J Cardiovasc Imaging. 2001;17:271–8. doi: 10.1023/a:1011661300903. [DOI] [PubMed] [Google Scholar]
- 20.Nihoyannopoulos P, Kaski JC, Crake T, Maseri A. Absence of myocardial dysfunction during stress in patients with syndrome X. J Am Coll Cardiol. 1991;18:1463–70. doi: 10.1016/0735-1097(91)90676-z. [DOI] [PubMed] [Google Scholar]
- 21.Maseri A, Crea F, Kaski JC, Crake T. Mechanisms of angina pectoris in syndrome X. J Am Coll Cardiol. 1991;17:499–506. doi: 10.1016/s0735-1097(10)80122-6. [DOI] [PubMed] [Google Scholar]
- 22.Greenbaum RA, Ho SY, Gibson DG, Becker AE, Anderson RH. Left ventricular fibre architecture in man. Br Heart J. 1981;45:248–63. doi: 10.1136/hrt.45.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Shanks M, et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol. 2009;104:1398–401. doi: 10.1016/j.amjcard.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 24.Mizuguchi Y, Oishi Y, Miyoshi H, Iuchi A, Nagase N, Oki T. The functional role of longitudinal, circumferential, and radial myocardial deformation for regulating the early impairment of left ventricular contraction and relaxation in patients with cardiovascular risk factors: a study with two-dimensional strain imaging. J Am Soc Echocardiogr. 2008;21:1138–44. doi: 10.1016/j.echo.2008.07.016. [DOI] [PubMed] [Google Scholar]


