Abstract
Objective:
Non-dipper hypertension is associated with an increased cardiovascular morbidity and mortality. Besides this, the left atrial (LA) size and functions are accepted to be prognostic factors in various cardiovascular diseases. In this study, we aimed to evaluate the effect of non-dipper hypertension on LA volume and functions using real-time three-dimensional echocardiography (RT3-DE).
Methods:
Forty dipper and 52 non-dipper hypertensives enrolled in this prospective cross-sectional study. Patients with any comorbidities that have a potential for causing structural cardiac alterations were excluded. Two-dimensional echocardiography (2-DE) and RT3-DE were performed to assess LA volumes and functions. The statistical tests used in this study were Shapiro–Wilk's test, Student's t-test, Mann–Whitney U test, chi-square test, Spearman's test, and Pearson's correlation test.
Results:
LA minimal volume, LA volume before LA contraction, and LA total systolic volume were higher in non-dipper hypertensives than in dipper hypertensives (p<0.001, p=0.003, and p=0.03, respectively). Only, the 2-DE measurements of interventricular septal thickness and E/Em ratio were higher in non-dipper hypertensives (p=0.001 and p=0.03, respectively). There was a moderate correlation between LA minimal volume and LA volume before LA contraction with E/Em (r=0.31, p=0.007 and r=0.32, p=0.005, respectively).
Conclusion:
Although LA volume and passive LA systolic functions measured by RT3-DE are significantly increased in non-dipper hypertensives, the alterations in active LA systolic functions are not prominent. RT-3DE may be used to define LA volume and function alterations in conditions that have capabilities of adverse cardiac remodeling such as systemic hypertension.
Keywords: non-dipper hypertension, left atrial volume, real-time 3-D echocardiography
Introduction
Arterial hypertension is a major health problem mainly affecting the heart, brain, kidneys, and the entire vascular system (1). Systemic blood pressure (BP) has a circadian rhythm that is characterized by higher values in morning hours and a decrease from day-time values of more than 10% during sleep. A nocturnal decrease in systolic and diastolic BPs of less than 10% of the daytime values is referred to as a “non-dipping pattern” (2). Numerous studies have shown significantly increased target organ damage, such as cardiovascular events, stroke, and renal failure, in non-dipper hypertensives (3–6). Autonomic dysfunction and increased inflammation are thought to be responsible for non-dipping patterns; however, the underlying cause for non-dipping BP variation is still unclear (7, 8). Twenty-four hour non-invasive ambulatory BP monitoring (ABPM) allows the detection of the BP circadian rhythm and the dipping or non-dipping pattern.
Numerous studies have shown that left atrial (LA) size and function are prognostic factors in various cardiovascular diseases and that they are associated with cardiovascular outcomes, including atrial fibrillation, congestive heart failure, stroke, and death (9–12). Although several methods, such as cardiac computed tomography, cardiac magnetic resonance, and nuclear scintigraphy, have been used to assess LA function, two-dimensional echocardiography (2-DE) and Doppler imaging are routinely used for this purpose in many laboratories. Although in the past, calculations of the LA size and mechanical functions in patients with non-dipper hypertension were defined in two studies, the number of patients included were limited and the method used to calculate LA volumes were driven only by 2-DE (13, 14).
As the shape of LA is not spherical, measuring the LA volume by 2-DE may result in mistakes. A novel imaging technique, real-time three-dimensional echocardiography (RT3-DE), demonstrated the quantitative assessment of LA size and function more accurately than 2-DE in a recent study (15).
In this study, our aim was to evaluate the effect of a dipping and non-dipping hypertension pattern on LA functions and volume by RT3-DE in hypertensives.
Methods
Study design
This prospective cross-sectional study was performed between May 2014 and September 2014 in the İnönü University Faculty of Medicine, Department of Cardiology. The protocol was approved by the local research ethics committee, and written informed consent was obtained from each participant.
Study population
A total of 92 consecutive patients (18–70 years old) with essential hypertension were enrolled to the study, and they were divided into two subgroups: 40 dipper (18 males and 22 females, mean age: 52.9±10.6 years) and 52 non-dipper (24 males and 28 females, mean age: 54.1±8.7) hypertensives. Hypertension was defined as systolic BP (SBP) of >140 mm Hg and/or diastolic BP (DBP) of >90 mm Hg in an office setting and SBP of >130 mm Hg and/or DBP of >80 mm Hg based on the mean 24-h circadian ABPM (16). Because it is not easy to make two homogeneous groups in terms of antihypertensive medication, we included patients with newly diagnosed hypertension or treatment-naïve hypertensives. The exclusion criteria were defined as any condition that has a capability to alter cardiac structures and functions: a body mass index over 30 kg/m2, diabetes mellitus, secondary hypertension, history of coronary artery disease, chronic renal failure, liver disease, chronic inflammatory disease, moderate or severe valvular disease, atrial fibrillation, congenital heart disease, left ventricular (LV) systolic dysfunction [ejection fraction (EF) <50%], and obstructive sleep apnea. Patients with poor-quality imaging on 2-DE or RT3-DE were also excluded (three patients) from the study.
Study protocol
A medical history was taken, and a complete physical examination was performed in all patients. A 12-lead resting electrocardiogram (ECG) was obtained. Demographic parameters were recorded. Blood samples were taken from all patients in the morning following a 12-h fasting period. Plasma samples were collected by centrifugation 2 h after collection and were studied on the same day. The serum levels of fasting blood glucose, total cholesterol, triglycerides, and low-density lipoprotein cholesterol were measured using standard methods and recorded.
BP was measured during two separate clinical visits using a sphygmomanometer in an office setting. Following a 5-min resting period, SBP and DBP were recorded at Korotkoff phases I and V, respectively. ABPM was performed using a portable compact digital recorder (Delmar Reynolds, Tracker NIBM2, Hertford, United Kingdom) and data were examined for all the patients. Sleep and awake periods were determined according to information given by the patient. The device was set to obtain BP measurements at 15-min intervals during the day and at 30-min intervals during the night. Non-dipper hypertension defined as night-time BP decrease of less than 10% from the average daytime BP (16).
ECG measurements were performed by one experienced observer blinded to patients groups with a standard technique using the IE-33 machine with a broadband S5-1 transducer probe (Philips Medical Systems, Bothell, WA, USA) with all patients in the supine position. All comprehensive 2-DE measurements including LV end-diastolic and end-systolic diameter (LVEDD and LVESD, mm), LA diameter (mm), interventricular septum (IVS, mm), and posterior wall (PW, mm) thickness in diastole were measured. LV EF was calculated with Simpson's method. Mitral inflow velocities of the early diastole (E) wave (m/s), the late systole (A) wave (m/s), the E/A ratio, E wave deceleration time (DT), and isovolumetric relaxation time (IVRT) were measured with Doppler echocardiography, and LV diastolic dysfunction was assessed according to the recommendation of the American Society of Echocardiography report (17). Tissue Doppler pulsed wave (TDI) sample volume was placed on septal mitral annulus in the apical four-chamber view and myocardial systolic (Sm), peak early diastolic (Em), peak late diastolic (Am) velocities, and Em/Am ratio were obtained. The E/Em and Em/Am ratios were subsequently calculated.
RT3-DE was performed by the same blinded observer using an X3 matrix-array transducer (Philips Medical Systems; 1–3 MHz). The 3-D images were acquired in full volume mode within four consecutive cardiac cycles. Individuals were instructed to hold their breath in expiration. To calculate the LA volume; anatomic landmarks were used and manually identified by marking the mitral annulus at the anterior, inferior, lateral, and septal annuli, and the fifth point at the apex of LA. The left atrial endocardial border of each frame was defined by automated processing and manually set for LA appendage pulmonary vein ostia exclusion. A 3-D model of LA volume was created with this data, and the data were then digitally stored and analyzed using analysis software (QLab-Phillips version 7.1; Philips Medical Systems, Fig. 1). The LA maximal and minimal volumes were automatically calculated by the software. Maximum volume (Vmax) is the time point that is accepted as the volume of LA is maximal just before the mitral valve opens, and minimum volume (Vmin) is the time point that is accepted as the volume of LA is minimal just at the end-diastole before mitral valve closure. Volume before atrial contraction (Vpre A) is the last frame before mitral valve reopening or the same time with P-wave on ECG. Using these three volumes, the other calculations were made and accepted as indices of LA function according to previous studies (18, 19).
Figure 1.
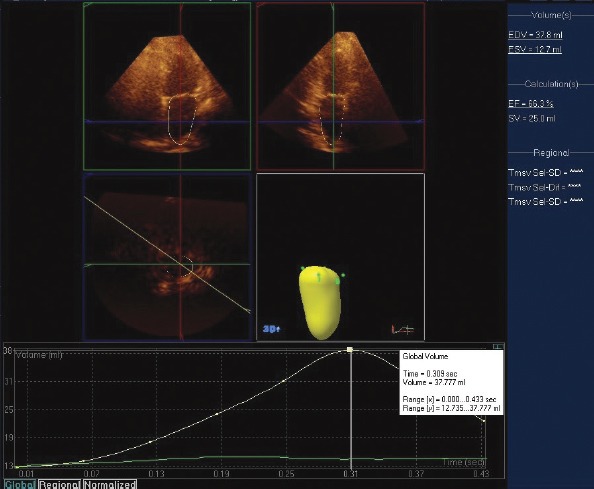
Analysis of left atrial (LA) volumes using real-time threedimensional echocardiography (RT3-DE) with QLAB software and the time–volume curve with LA end-diastolic (EDV) and LA end-systolic (ESV) volume
EF - ejection fraction; SV - stroke volume
Detailed three-dimensional echocardiographic measurements and abbreviations
Left atrial total systolic volume (TSV)=Vmax-Vmin, left atrial total emptying fraction (TEF)=TSV/Vmax x 100, left atrial active stroke volume (ASV)=Vpre A–Vmin, left atrial active emptying fraction (AEF)=ASV/Vpre A x 100, left atrial expansion index (EI)=TSV/Vmin x 100, left atrial passive emptying fraction (PEF)=(Vmax-Vpre A)/Vmax x 100, left atrial maximum volume index (LA Max VI)=Vmax/body surface area.
Statistical analysis
Posthoc power analyses revealed 0.92 power considering an LA minimal volume difference between the two groups of 6.1, standard deviations of 6.9 and 10, and type one error of 0.05. Statistical analyses were performed by IBM SPSS statistics version 21. Continuous variables were expressed as mean±standard deviation. The parameters were evaluated for normality and distribution using the Shapiro–Wilk's test. Differences between the variables were tested with independent samples using the Student's t-test, Mann–Whitney U test, and chi-square test when appropriate. Pearson's correlation coefficient was used to evaluate the association between LA volume indices and the other echocardiographic parameters. Spearman test was performed for the correlations of intra-observer variability. Statistical significance was accepted as p value less than 0.05.
Results
Ninety-two patients (52 non-dipper hypertensives, mean age 54.1±8.7 and 40 dipper hypertensives, mean age 52.9±10.6) were included the study. The clinical characteristics for the dipper and non-dipper hypertensive patient groups are listed in Table 1. Twenty-four hour and night-time average SBP (135.3±22.1 vs. 146.9±17.8, p=0.009; 118.6±24.1 vs. 145.5±19.2, p=0.0001, respectively) and DBP (77.1±16.4 vs. 83.1±9.4, p=0.044; 68.6±10.3 vs. 80.2±10.8, p=0.0001, respectively) were significantly higher in non-dipper hypertensives than in dipper hypertensive group. There were no significant differences in any other demographic, clinical, and laboratory data parameters between the two groups.
Table 1.
Clinical characteristics and laboratory data of study patients
| Dippers (n=40) | Non-dippers (n=52) | P | |
|---|---|---|---|
| Age, years | 52.9±10.6 | 54.1±8.7 | 0.22 |
| Sex, female/male | 18/22 | 24/28 | 0.82 |
| BMI, kg/m2 | 25.4±3 | 26.7±3.6 | 0.1 |
| 24-h average SBP, mm Hg | 135.3±22.1 | 146.9±17.8 | 0.009 |
| 24-h average DBP, mm Hg | 77.1±16.4 | 83.1±9.4 | 0.044 |
| Daytime SBP, mm Hg | 147.8 ±6.5 | 149.6±7.9 | 0.28 |
| Daytime DBP, mm Hg | 86.7 ±12.9 | 90.2±9.1 | 0.14 |
| Night-time average SBP, mm Hg | 118.6±24.1 | 145.5±19.2 | <0.001 |
| Night-time average DBP, mm Hg | 68.6±10.3 | 80.2±10.8 | <0.001 |
| Heart rate, beats/min | 77.2±11.7 | 76.3±13.4 | 0.76 |
| Total cholesterol, mg/dL | 203.9±36.7 | 205.4±41.4 | 0.87 |
| LDL cholesterol, mg/dL | 122.4±26.7 | 132.3±31.5 | 0.13 |
| HDL cholesterol, mg/dL | 38.5±10.4 | 38.4±7.4 | 0.96 |
| Blood glucose, mg/dL | 88±10.7 | 90.4±8.7 | 0.64 |
| Blood urea nitrogen, mg/dL | 28.9±4.5 | 33.4±5.5 | 0.51 |
| Creatinine, mg/dL | 0.96±0.22 | 1.02±0.19 | 0.45 |
BMI - body mass index; DBP - diastolic blood pressure; HDL - high-density lipoprotein; LDL - low-density lipoprotein; SBP - systolic blood pressure. Student's t-test and chi-square test were used
2-DE findings are shown in Table 2. The interventricular septal thickness and E/Em ratio were significantly higher in the non-dipper hypertensive group (9.7±1.8 vs. 11.1±1.5, p=0.001 and 6.7±2.3 vs. 7.9±2.6, p=0.03, respectively). There was no significant difference between the groups in terms of other 2-DE and Doppler parameters (Table 2).
Table 2.
Two dimensional echocardiographic and Doppler parameters of the study population
| Dippers | Non-dippers | P | |
|---|---|---|---|
| LVEDD, mm | 45±3.9 | 46.3±3.3 | 0.1 |
| LVESD, mm | 31.5±2.8 | 31.6±3.3 | 0.68 |
| Ejection fraction, % | 64.1±3.5 | 62.9±4.2 | 0.92 |
| Left atrial diameter, mm | 34.9±3.1 | 36.8±2.9 | 0.16 |
| IVST, mm | 9.7±1.8 | 11.1±1.5 | 0.001 |
| Posterior wall thickness, mm | 9.2±1 | 9.8±2 | 0.1 |
| E/A* | 0.88 (0.63) | 0.86 (0.63) | 0.58 |
| DT, ms | 200±57.8 | 204.9±47.8 | 0.65 |
| IVRT, ms | 100.5±29.6 | 104.7±23.7 | 0.47 |
| Sm, cm/s | 8.8±2.3 | 9.5±3.0 | 0.22 |
| E/Em | 6.7±2.3 | 7.9±2.6 | 0.03 |
| Em/Am* | 0.88 (0.81) | 0.65 (0.33) | 0.41 |
| Peak E’velocity, cm/s | 10.3±3.5 | 9.6±4.1 | 0.23 |
| Peak A’velocity, cm/s | 10.7±3 | 12.4±4.6 | 0.06 |
Median (interquartile range)
DT - deceleration time; E/A - peak velocity of early diastolic flow/peak velocity of late diastolic flow; E/Em - peak velocity of early diastolic flow across mitral valve/peak septal annular velocity of early diastole; IVRT - isovolumetric relaxation time; IVST - interventricular septal thickness; LVEDD - left ventricular end-diastolic diameter; LVESD - left ventricular end-systolic diameter. Student's t-test and Mann–Whitney U test were used
Three dimensional echocardiographic results are shown in Table 3. LA minimal volume, LA volume before LA contraction, and LA total systolic volume were significantly higher in non-dippers than in dippers (p<0.001, p=0.003, and p=0.03, respectively, Fig. 2). However, the LA maximal volume, LA total emptying fraction, LA active stroke volume, LA active emptying fraction, LA expansion index, LA passive emptying fraction, an LA maximum volume index values between the two groups were similar. Additionally, there was a moderate correlation between the LA minimal volume and LA volume before LA contraction with E/Em representing LV filling pressure (r=0.31 and p=0.007 and r=0.32 and p=0.005, respectively). There was no correlation between the LA total systolic volume and E/Em. Further, we could not see any correlation with the IVST and 3-D measurements.
Table 3.
Three-dimensional echocardiographic data of the study population
| Variable | Dippers (n=40) | Non-dippers (n=52) | P |
|---|---|---|---|
| LA maximal volume, mL | 41.1±16.1 | 45.6±22.8 | 0.08 |
| LA minimal volume, mL | 18.3±6.9 | 24.4±10 | 0.001 |
| LA volume before LA contraction, mL | 26.5±9.6 | 33.5±11.6 | 0.003 |
| LA total systolic volume, mL | 22.8±9.5 | 26.8±7.9 | 0.03 |
| LA total emptying fraction, % | 55.1±4.2 | 53.1±7.2 | 0.12 |
| LA active stroke volume, mL | 8.2±3.1 | 9±3.1 | 0.24 |
| LA active emptying fraction,% | 31.2±5.4 | 27.6±7.1 | 0.4 |
| LA expansion index | 126.5±23.2 | 120.2±32.4 | 0.3 |
| LA passive emptying fraction, % | 34.3±6.2 | 35.2±4.8 | 0.22 |
| LA maximum volume index | 24.4±9.7 | 25.6±12.6 | 0.32 |
LA - left atrial
Figure 2.
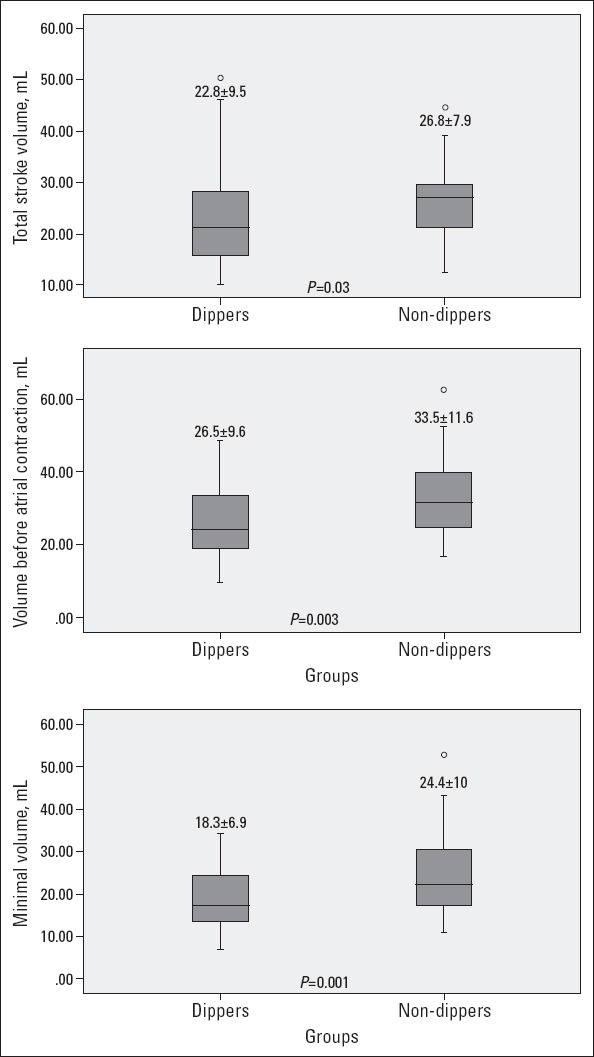
Comparison of two hypertensive groups in terms of left atrial (LA) minimal volume, LA volume before LA contraction, and LA total systolic volume measured by real-time three-dimensional echocardiography (RT3-DE)
The intra-observer correlation for 20 patients was good for 3-D measurements of LA volume including minimal, maximal, and preA (r=0.67 and p=0.02, r=0.77 and p=0.01, r=0.84 and p=0.008, respectively).
Discussion
In the study, the most important finding was that the LA minimal volume, LA volume before LA contraction, and LA total systolic volume were significantly higher in the non-dipper hypertensives than in the dipper hypertensives. This means that increases in LA volumes are more prominent in non-dipper hypertension than in dipper hypertension and that passive LA functions are more altered than active functions. Although we could not detect any relationship of 2-D derived parameters with the other LA volume indices, it is not wrong to say that the correlation of E/Em with minimal LA volume and Vpre A indicates that volume changes are related to LV filling pressure.
LA has three important functions in the normal cardiac cycle: a “reservoir” for pulmonary venous blood during ventricular systole phase, a “conduit” for that blood during the early diastole phase, and a “muscular pump” to complete the process of LV filling (20). Hypertension is the leading cause of LV hypertrophy and impaired LV diastolic functions. It is well known that blood flow from LA toward LV deteriorates when LV stiffness increases and LV enlargement capacity decreases and in turn, LA volumes and left atrial reservoir function increase (15, 21, 22).
Numerous studies have shown an increased LV mass index, impaired diastolic function, and higher diastolic LV filling pressure in non-dippers than in dippers (12, 13, 23). In this study, we showed impaired LV diastolic functions and more hypertrophy in non-dippers too.
During early diastole, the passive emptying volume reduces on account of increased LV stiffness and deteriorated diastolic relaxation (21). The impairment of LA passive emptying volume also contributes to a larger residual LA volume before its active contraction. The increased volume of passive LA emptying indicates a larger residual volume of LA before its active pumping. The increase in presystolic LA volume and fiber length results in augmented LA contraction forces (Frank–Starling mechanism). LA systolic functions play a pivotal role during LV filling as suggested by the increased LA active emptying volume and emptying fraction in non-dipper hypertensives.
2-DE linear measurements and calculations are easy to perform and are routinely used for calculating LA size and function in many laboratories and clinical studies. However, these may lead to an error because their calculation methods are based on geometric assumptions such cube or spherical models in 2-DE. Because of the oblique position of the interatrial septum, the presence of the LA appendage, and the asymmetric enlargement of LA, calculations as a symmetric shape may result in mistakes (24). Although the gold standard to measure LA size is magnetic resonance imaging, RT3-DE allows the determination of the endocardial borders of LA more accurately than 2-D; therefore, RT3-DE correlates with more accurate results than 2-DE for the detection of LA volume (25).
LA volumes were assessed in various studies, and minimum LA volume, in particular, has been shown as a predictor of adverse cardiovascular events. In a previous study, it was reported that the increase in minimum LA volume was more specific than maximal LA volume to demonstrate worsening diastolic function, even if in the early stage of diastolic dysfunction (26). In addition, the RT3-DE minimum LA volume was the best independent predictor of major adverse cardiovascular events (27). Furthermore, an increased LA minimum volume shows decreased contraction function of the atrium in the late diastole (28). In our study, the LA minimal volume was significantly higher in non-dipper hypertensives, and this may be an indicator of more adverse events in these patients.
Study limitations
The study had several potential limitations. First, the sample size was relatively small, and the findings should be supported by data from prospectively designed studies with large populations. Second, ventricular elastance or diastolic strain/strain rate measurements were not performed; these parameters could provide more accurate LV diastolic functions. Third, we did not measure atrial natriuretic peptide hormone levels. As a response to atrial enlargement, atrial natriuretic peptide is secreted from the atria and the usage of this parameter to compare measurements of LA by RT3-DE may be more significant. Finally, the LA appendage plays an important role in the LA reservoir function, during an increase in LA pressure and volume in particular. However, we did not include the LA appendage for the calculation of LA volume and function. Studies including the LA appendage for the evaluation of volume and function will probably give more accurate results.
Conclusions
In this study, the increased LA volume and passive systolic functions in the non-dipping pattern compared to that in the dipping pattern high BP was confirmed by RT3-DE measurements. RT3-DE may be used to define atrial volume and function alterations in conditions that have adverse cardiac remodeling capabilities such as systemic hypertension.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept- N.E.; Design- N.E.; Materials- J.Y.; Data collection &/or processing – A.A., Ş.H.; Analysis and/or interpretation– N.E.; Literature search- N.E.; Writing – Y.Ö.O.; Critical review- N.A., M.C., M.C.Ç.
References
- 1.Lovic D, Erdine S, Çatakoğlu AB. How to estimate left ventricular hypertrophy in hypertensive patients. Anadolu Kardiyol Derg. 2014;14:389–95. doi: 10.5152/akd.2014.5115. [DOI] [PubMed] [Google Scholar]
- 2.Verdecchia P, Schillaci G, Guerrieri M, Gatteschi C, Benemio G, Boldrini F, et al. Circadian blood pressure chang¬es and left ventricular hypertrophy in essential hypertension. Circulation. 1990;81:528–36. doi: 10.1161/01.cir.81.2.528. [DOI] [PubMed] [Google Scholar]
- 3.Ulu SM, Ahsen A, Akcı Ö, Yaman F, Demir K, Yaman G, et al. The relationship between dipping-non-dipping arterial blood pressure pattern and frequency of restless leg syndrome with related factors. Anatol J Cardiol. 2015;15:284–8. doi: 10.5152/akd.2014.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183–9. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Cuspidi C, Meani S, Salerno M, Valerio C, Fusi V, Severgnini B, et al. Cardiovascular target organ damage in essential hypertensives with or without reproducible nocturnal fall in blood pressure. J Hypertens. 2004;22:273–80. doi: 10.1097/00004872-200402000-00010. [DOI] [PubMed] [Google Scholar]
- 6.ErmişN Yağmur J, Açıkgöz N, Cansel M, Cuğlan B, Pekdemir H, et al. Serum gamma-glutamyl transferase (GGT) levels and inflammatory activity in patients with non-dipper hypertension. Clin Exp Hypertens. 2012;34:311–5. doi: 10.3109/10641963.2011.577485. [DOI] [PubMed] [Google Scholar]
- 7.Tosu AR, Demir S, Selçuk M, Aya Y, Akyol A, Özdemir M, et al. Comparison of inflammatory markers in non-dipper hypertension vs. dipper hypertension and in normotensive individuals: uric acid, C-reactive protein and red blood cell distribution width readings. Postepy Kardiol Interwencyjnej. 2014;10:98–03. doi: 10.5114/pwki.2014.43514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherwood A, Steffen PR, Blumenthal JA, Kuhn C, Hinderliter AL. Night-time blood pressure dipping: The role of the sympathetic nervous system. Am J Hypertens. 2002;15:111–8. doi: 10.1016/s0895-7061(01)02251-8. [DOI] [PubMed] [Google Scholar]
- 9.Suh IW, Song JM, Lee EY, Kang SH, Kim MJ, Kim JJ, et al. Left atrial volume measured by Real-Time 3-Dimensional echocardiography predicts clinical outcomes in patients with severe left ventricular dysfunction and in sinus rhythm. J Am Soc Echocardiogr. 2008;21:439–45. doi: 10.1016/j.echo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Benjamin EJ, D’Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835–41. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 11.Tsang TSM, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90:1284–9. doi: 10.1016/s0002-9149(02)02864-3. [DOI] [PubMed] [Google Scholar]
- 12.Laukkanen JA, Kurl S, Eranen J, Huttunen M, Salonen JT. Left atrium size and the risk of cardiovascular death in middle-aged men. Arch Intern Med. 2005;165:1788–93. doi: 10.1001/archinte.165.15.1788. [DOI] [PubMed] [Google Scholar]
- 13.Aydın M, Özeren A, Bilge M, Dursun A, Cam F, Elbey MA. Effects of dipper and non-dipper status of essential hypertension on left atrial mechanical functions. Int J Cardiol. 2004;96:419–24. doi: 10.1016/j.ijcard.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Açar G, Bulut M, Arslan K, Alizade E, Özkan B, Alıcı G, et al. Comparison of left atrial mechanical function in nondipper versus dipper hypertensive patients: a speckle tracking study. Echocardiography. 2013;30:164–70. doi: 10.1111/echo.12023. [DOI] [PubMed] [Google Scholar]
- 15.Anwar AM, Soliman OI, Geleijnse ML, Nemes A, Vletter WB, ten Cate FJ. Assessment of left atrial volume and function by real-time three dimensional echocardiography. Int J Cardiol. 2008;123:155–61. doi: 10.1016/j.ijcard.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 17.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 18.Bayramoğlu A, Taşolar H, Otlu YÖ, Hidayet Ş, Kurt F, Doğan A, et al. Assessment of left atrial volume and mechanical functions using real-time three-dimensional echocardiography in patients with mitral annular calcification. Anatol J Cardiol. 2016;16:42–7. doi: 10.5152/akd.2015.5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aktürk E, Ermiş N, Yağmur J, Açıkgöz N, Kurtoğlu E, Cansel M, et al. Early left atrial mechanics and volume abnormalities in subjects with prehypertension: a real time three-dimensional echocardiography study. Echocardiography. 2012;29:1211–7. doi: 10.1111/j.1540-8175.2012.01795.x. [DOI] [PubMed] [Google Scholar]
- 20.Patel DA, Lavie CJ, Milani RV, Shah S, Gilliland Y. Clinical implications of left atrial enlargement: a review. Ochsner. 2009;9:191–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Kagawa K, Arakawa M, Miwa H, Noda T, Nishigaki K, Ito Y, et al. Left atrial function during left ventricular diastole evaluated by left atrial angiography and left ventriculography. J Cardiol. 1994;24:317–25. [PubMed] [Google Scholar]
- 22.Rizzo V, Di Maio F, Campbell SV, Tallarico D, Petretto F, Lorido A, et al. Left ventricular function, cardiac dysrhythmias, atrial activation, and volumes in nondipper hypertensive individuals with left ventricular hypertrophy. Am Heart J. 2000;139:529–36. doi: 10.1016/s0002-8703(00)90098-x. [DOI] [PubMed] [Google Scholar]
- 23.Aydın M, Özeren A, Bilge M, Atmaca H, Ünalacak M, Dursun A, et al. Left ventricular diastolic function and circadian variation of blood pressure in essential hypertension. Tex Heart Inst. 2005;32:28–34. [PMC free article] [PubMed] [Google Scholar]
- 24.Lupu S, Mitre A, Dobreanu D. Left atrium function assessment by echocardiography - physiological and clinical implications. Med Ultrason. 2014;16:152–9. doi: 10.11152/mu.201.3.2066.162.sl1am2. [DOI] [PubMed] [Google Scholar]
- 25.Mor-Avi V, Yodwut C, Jenkins C, Kühl H, Nesser HJ, Marwick TH, et al. Real-time 3-D echocardiographic quantification of left atrial volume: multicenter study for validation with CMR. JACC Cardiovasc Imaging. 2012;5:769–77. doi: 10.1016/j.jcmg.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, et al. Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: impact of left ventricular systolic function. Heart. 2012;98:813–20. doi: 10.1136/heartjnl-2011-301388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoit BD. Assessment of echocardiographic left atrial size: how accurate do we need to be? JACC Cardiovasc Imaging. 2012;5:778–80. doi: 10.1016/j.jcmg.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Caselli S, Canali E, Foschi ML, Santini D, Di Angelantonio E, Pandian NG, et al. Long-term prognostic significance of three-dimensional echocardiographic parameters of the left ventricle and left atrium. Eur J Echocardiogr. 2010;11:250–6. doi: 10.1093/ejechocard/jep198. [DOI] [PubMed] [Google Scholar]


