Abstract
Objective:
The purpose of this study was to investigate the factors predicting the maintenance of sinus rhythm in patients with paroxysmal atrial fibrillation (PAF) who underwent cryoablation of the pulmonary veins (PVs).
Methods:
Fifty-one patients (54.6±10.4 years) with paroxysmal AF who underwent the cryoablation of the PVs were to the prospective trial. The clinical risk factors and echocardiographic parameters [left atrial (LA) diameter, left ventricular ejection fraction and dimensions, left atrial spontaneous echo contrast (LASEC), mitral annulus calcification (MAC), left atrial appendage emptying peak flow velocity (LAAV), and PV flow] were assessed before the cryoablation procedure. Patients with PAF who refused to use any medication because of intolerance or presentation of resistant symptoms, despite the use of at least one antiarrhythmic drug were enrolled to the study, patients with LA/LAA thrombus on echocardiographic examination, severe valvular disease, pericardial fluid, and abnormal thyroid function tests as well as systemic disease were excluded from the study. All parameters were tested for their ability to predict the recurrence of AF during a 1-year follow-up period.
Results:
During the period of follow-up, AF recurred in 16 of 51 patients (31.3%/year). All significant parameters associated with the recurrence of AF were evaluated in multivariate logistic regression analysis. The presence of MAC (p<0.001) as well as LA diameter (p<0.0001), LAAV of <30 cm/s (p<0.0001), PV flow systolic wave velocity (p<0.0001), and LASEC (p<0.0001) were detected as independent predictors of recurrence. In the receiver operating characteristic analysis, LAAV of >30 cm/s had a sensitivity of 85% and a specificity of 95% for predicting success after ablation (AUC=0.813; 95% CI:0.76–0.92; p<0.0001).
Conclusion:
The presence of MAC, increased LA diameter, the existence of LASEC, low LAAV, and low peak PV systolic wave velocity are parameters that can predict the recurrence of AF after cryoablation.
Keywords: atrial fibrillation, cryoablation, recurrence, left atrial appendage peak flow velocity, echocardiography
Introduction
Atrial fibrillation (AF) is the most common and significant form of cardiac arrhythmia (1). Pulmonary veins (PVs) play an important role in the occurrence and progression of AF (2). Catheter ablation of AF is recommended for patients with symptomatic paroxysmal atrial fibrillation (PAF), despite the possible use of antiarrhythmic drugs (3).
Cryothermal ablation of PVs has been performed worldwide as an effective and safe option in the treatment of AF. Although the procedure has a high success rate, occasional recurrence of AF is possible.
Structural changes of the left atrium (LA) after PV ablation (e.g., LA fibrosis and dilatation) play an important role in AF recurrence (4). Concurrently, AF can lead to temporary or permanent dysfunction of the LA. This dysfunction may improve with sinus rhythm after the ablation of PVs. Doppler evaluation of the PVs and left atrium appendage (LAA) flow is used to indicate the reservoir and contractile function of the LA (5). In addition, PV flow depends on left ventricular (LV) and LA function. However, it is unclear if these variables, which are closely associated with left atrial functions, can predict a recurrence after the ablation of PVs thereby indicating the progression of atrial dysfunction.
Therefore, we compared Doppler surrogates of LA function after the cryoablation of PVs in patients with paroxysmal AF recurrence versus matched patients without recurrence. We also investigated the clinical and echocardiographic parameters that may be associated with AF recurrence.
Methods
Study group
This prospective cross-sectional study was conducted by the Department of Cardiology at the Ankara University Faculty of Medicine between December 2011 and September 2012. All patients signed a written informed consent approved by the institutional ethics committee. The original plan was to enroll 56 patients with PAF who refused to use any medication because of intolerance or presentation of resistant symptoms, despite the use of at least one antiarrhythmic drug. Patients with LA/LAA thrombus on echocardiographic examination, severe valvular disease, pericardial fluid, and abnormal thyroid function tests as well as systemic disease were excluded from the study. Five patients with AF were excluded after evaluation. Thus, our electrophysiology team performed cryoablation in 51 patients, all of whom had AF durations shorter than 7 days.
All patients underwent a detailed physical examination. Experienced sonographers (S.A, M.K, and P.A) performed transthoracic echocardiographic (TTE) evaluations. An experienced investigator (D.M.G), who was blinded to patient characteristics, obtained transesophageal echocardiographic (TEE) measurements. Detailed histories were taken from the patients, and European Heart Rhythm Association (EHRA) scores were used for the grading of symptoms (1). Age, sex, existence of hypertension (HT), ischemic heart disease (IHD), congestive heart failure (CHF), and diabetes mellitus (DM) were recorded. Multi-slice computed tomography (MSCT) (Aquilion 64; Toshiba Medical Systems, Japan) was also performed for each patient to assess the anatomy of PVs.
Echocardiographic analysis
Transthoracic echocardiography was conducted using a Vivid S5 (2–4-MHz phased array transducer; GE, Horten, Norway) system. Standard parasternal long- and short-axis views and apical two- and four-chamber views were obtained in all patients. M-mode echocardiograms were derived from the two-dimensional images, and LV dimension and LA diameter were measured in parasternal long-axis view. LV ejection fraction was calculated using the modified Simpson method (6). The following echocardiographic variables were prospectively measured and calculated: mitral annular calcification (MAC); mitral, aortic, tricuspid and pulmonary valve functions; LA diameter; LV wall motion; and LV ejection fraction. Transmitral flow velocities were measured by placing the sample volume at the tips of the mitral valve leaflets, and peak early diastolic (E) and peak atrial systolic (A) velocities were measured.
Transesophageal echocardiography was performed in all patients using a 5 MHz biplane phased array transducer (Vivid S5; GE, Horten, Norway). All TEE examinations were performed under mild sedation with midazolam during the cryoablation procedure. The measurement of left atrial appendage emptying peak flow velocity (LAAV) (Fig. 1a, b), pulmonary venous flow systolic wave velocity (PVSV), and pulmonary venous flow diastolic wave velocity (PVDV) (Fig. 2) were also obtained using TEE.
Figure 1.
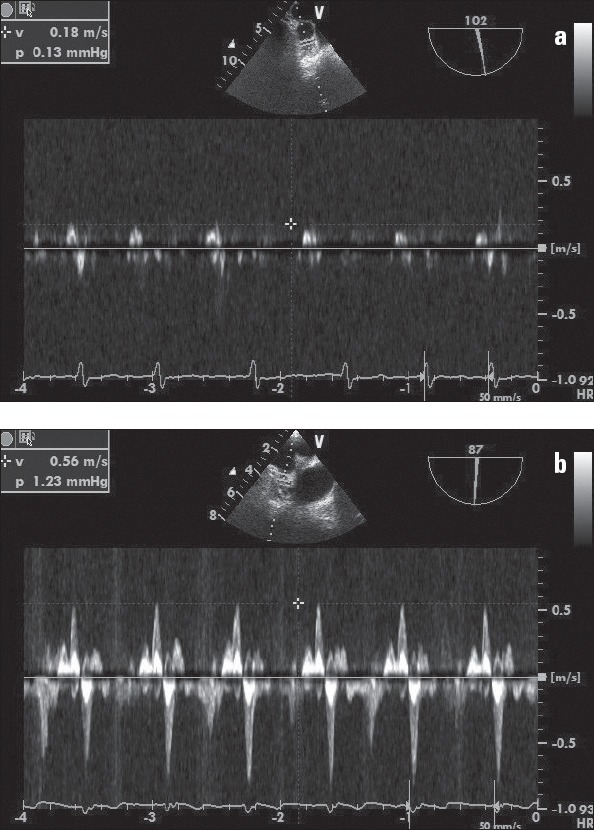
a, b. (a) Pulsed Doppler recording of left atrial appendage flow velocity in a patient with low flow. The mean emptying peak velocities of five consecutive cardiac cycles is <30 cm/s. (b) Pulsed Doppler recording of left atrial appendage flow velocity in a patient without low flow. The mean emptying peak velocities of five consecutive beats is about 56 cm/s.
Figure 2.
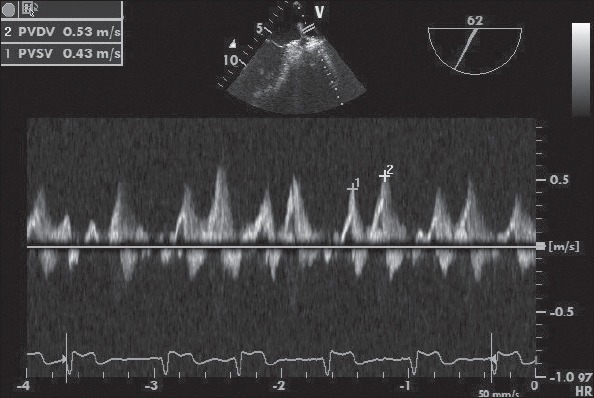
Example of TEE image with Doppler spectra of PV flows. PVDV: pulmonary venous flow diastolic wave velocity; PVSV: pulmonary venous flow systolic wave velocity.
The LA and LAA were evaluated for thrombus and spontaneous echo contrast (SEC), which was graded from 0 (none) to 4 (severe) during the TEE procedure (7). The absence of SEC in LA (LASEC) was recorded as SEC negative, whereas the presence of SEC at various degrees was recorded as SEC positive.
Left atrial appendage emptying peak flow velocity was measured with pulsed Doppler by placing the sample volume 1 cm into the orifice of the LAA. The average LAAV was determined by averaging five consecutive cardiac cycles during the TEE procedure immediately prior to ablation. The PV wave values represent the mean of all four PVs.
Ablation procedure
If the patient was receiving oral anticoagulant therapy, this drug was discontinued at least 48–72 hours before the procedure; enoxaparin (1 mg/kg) was subsequently administered when the international normalized ratio (INR) was <2. Cryoablation was performed when INR decreased to <1.5. In addition, antiarrhythmic agents being used continuously were discontinued before the transaction for longer than five times the half-life.
Cryoablation was performed under conscious sedation with midazolam. The anesthesiology team monitored invasive arterial pressure, oxygen saturation, and electrocardiography (ECG) throughout the procedure. Right femoral vein and left femoral artery/vein punctures were performed using the Seldinger technique. Transseptal puncture was performed using a Brockenbrough transseptal needle (BRK-1; St. Jude Medical, Minnetonka, MN, USA) with fluoroscopy and TEE guidance. Subsequently, the carrier 12 F sheath (outer diameter: 15 mm; FlexCath, CryoCath, Montreal, Quebec, Canada) was placed into the LA. Anticoagulation during the procedure was achieved with intravenous unfractionated heparin, with an activated clotting time (ACT) of 300–350 s targeted. The potentials of the PVs were investigated using a circular mapping catheter (Lasso; Biosense Webster, Inc., Diamond Bar, CA, USA) that passed through a single transseptal sheath. Coronary sinus stimulation was performed on separate PV potentials from atrium potentials. The circular mapping catheter was then removed and a 28-mm cryoballoon catheter (Arctic Front ©; Medtronic CryoCath LP, Kirkland, Canada) was directed into the PV through the same transseptal sheath.
After the guidewire entered the target vessel, the balloon was inflated in the LA and then directed towards the PV ostium. Afterwards, a contrast agent diluted to 50% with physiological saline was infused inside the balloon to investigate whether the PV was clogged.
After deciding the best position for the catheter using contrast injection, standard five-minute freeze cycles were performed at least twice for each PV. After ablation of all PVs, a circular mapping catheter was placed into the PVs to determine if the isolation was complete. The disappearance of PV potentials, dissociation of the PV potentials, or the existence of an input block were considered as the primary endpoints. If PV isolation could not be achieved, a re-freezing process was performed with the cryoballoon.
Patients were followed after the procedure with close hemodynamic and ECG monitoring in the intensive care unit. Soon after the procedure, a TTE was performed to search for pericardial fluid. Warfarin, along with enoxaparin therapy, was administered in the first 4–6 hours after the procedure. After reaching the targeted INR level (2–3), enoxaparin was discontinued; the patients were discharged with the use of oral anticoagulants and antiarrhythmic therapy.
Follow-up
All patients were recalled for 3-, 6-, and 12-month follow-up visits, during which they underwent a 12-lead ECG, standard TTE, and 24-h Holter examination. TEE was only repeated on the 12-month follow-up. On the 3-month follow-up visit, use of the antiarrhythmic drug was stopped and the continuation of anticoagulant therapy was decided according to the patients’ CHA2DS2Vasc score.
AF recurrence was based on clinical, ECG, and Holter data. Where AF, atrial flutter, or tachycardia episodes were detected by ECG recorders with durations of >30 s, this was considered recurrence if they appeared after the blind period.
Statistical analysis
The Statistical Package for Social Sciences software, version 16.0 (SPSS Inc, Chicago, IL, USA) was used for statistical analysis. Categorical variables are represented as percentages, whereas numeric variables are shown as arithmetic means ± standard deviation (SD). The x2 test and the Fisher exact test were used to compare categorical variables. Parameters were assessed by Kolmogorov-Smirnov and Shapiro-Wilk tests to determine whether they conformed to the normal distribution. If parameters were normally distributed, they were analyzed using a paired-t test. A Mann-Whitney U test and the Wilcoxon Rank test were applied for comparison of nonparametric variables. To determine the relationship between variables and recurrence, univariate and multivariate logistic regression analyses were performed. Left atrial appendage emptying peak flow velocity has a distinctive feature for recurrence, whether or not it has been tested according to the Receiver Operating Characteristics (ROC) curve analysis. The cut-off value was calculated according to the Youden index for variables with hallmark (8). The results were considered statistically significant if p<0.05.
Results
Among 51 patients, 26 were females and 25 were males; mean age was 54.6±10.4 (range: 20–75 years). During inquiry for complaints, EHRA score (2–4) was detected as the median. Sixteen of the patients (31.3%) were receiving warfarin before the procedure, and the remaining 35 patients (68.6%) were using aspirin. The comorbid conditions of patients were examined and results showed that 32 (63.6%) had HT, 17 (33.3%) had IHD, 12 (24.2%) had CHF, and 6 (12.1%) had DM (Table 1).
Table 1.
Baseline patient characteristics.
| Average age, years ± SD | 54.6±10.4 |
|---|---|
| Age range | 20–75 |
| Male, % | 25 (49.0%) |
| Hypertension, % | 32 (62.7%) |
| Ischemic heart disease, % | 17 (33.3%) |
| Congestive heart failure, % | 12 (23.5%) |
| Diabetes mellitus, % | 6 (11.7%) |
Echocardiographic records with TTE and TEE were taken before cryoablation. Using TTE, MAC was detected in 12 patients (24.2%); SEC was detected in eight patients (15.1%) using TEE. The cryoballoon used in all patients was 28 mm in size. Acute procedural success (isolation of all PVs) was achieved in all patients. A median of two cryoballoon procedures (range: 2–5) was applied per PV. Average procedure and fluoroscopy times were measured as 72.5±5.3 (range: 50–90) min and 14±3.5 (range: 12–24) min, respectively.
In terms of complications, cardiac tamponade developed in one (0.02%) patient, and groin complications developed in one (0.02%) patient. Phrenic nerve paralysis was not observed in any of the patients.
In total, 16 (31.3%) patients developed recurrence after 3 months, whereas sinus rhythm (SR) was maintained in 35 cases (68.6%). Radiofrequency ablation (RF) was performed for three patients (18.7%) with recurrence during the follow-up period. The remaining 13 (81.2%) patients were followed up using antiarrhythmic drug therapy because they were asymptomatic and some did not accept the reablation.
Patient characteristics
Patients were divided into two subgroups: the recurrence (+) group and recurrence (-) group. No significant difference was observed between the two groups when they were compared in terms of age, sex, HT, DM, and CHF. Clinical findings for both groups are shown in Table 2.
Table 2.
Patient characteristics in the recurrence (+) group and recurrence (-) groups.
| Recurrence (+) group (n=16) | Recurrence (–) group (n=35) | P | |
|---|---|---|---|
| >60 years old | 8 | 13 | NS |
| Female/male | 10/6 | 16/19 | NS |
| Ischemic heart disease | 7 | 10 | NS |
| Hypertension | 15 | 17 | NS |
| Congestive heart failure | 5 | 7 | NS |
| Diabetes mellitus | 2 | 4 | NS |
Transthoracic echocardiography and TEE parameters
There were no significant differences between the recurrence (+) and recurrence (-) groups in the echocardiographic measurements of left ventricular ejection fraction (LVEF) and pulmonary venous flow diastolic wave velocity (PVDV). However, the frequency of MAC was significantly higher (p<0.001) and LA diameter was significantly greater in the recurrence (+) group (p<0.000) (Table 3).
Table 3.
Echocardiographic data in the recurrence (+) and recurrence (-) groups
| Recurrence (+) group (n=16) | Recurrence (–) group (n=35) | P | |
|---|---|---|---|
| LA diameter, cm | 4.1±0.5 | 3.4±0.5 | <0.0001 |
| LVEDD, mm | 45.7±6.9 | 46.2±6.1 | 0.30 |
| LVESD, mm | 28±4.8 | 29±3.4 | 0.90 |
| LVEF, % | 57.8±13.7 | 59.9±10.4 | 0.28 |
| MAC | 10 | 2 | <0.001 |
| LAAV, cm/s | 25.00±9.16 | 56±26.72 | <0.0001 |
| LASEC | 6 | 2 | <0.0001 |
| PVSV, cm/s | 40.8±21.4 | 57.6±19.4 | <0.0001 |
| PVDC, cm/s | 48.0±22.0 | 52.0±16.0 | 0.10 |
LA - left atrium; LAAV - left atrial appendage emptying peak flow velocity; LASEC - left atrial spontaneous echo contrast; LVEDD - LV end-diastolic diameter; LVEF - left ventricle ejection fraction; LVESD - LV end-systolic diameter; MAC - mitral annular calcification; PVDV - pulmonary venous flow diastolic wave velocity; PVSV - pulmonary venous flow systolic wave velocity.
There were no significant differences in E, A, or the E/A ratio between the two groups. Mitral E was 53.1±25.7 and 52.9±25.6 cm/s, whereas mitral A was 33.9±13.6 and 33.8±13.7 cm/s, in the recurrence (+) group and recurrence (-) group respectively (p=NS for both). The E/A ratio was 1.3±0.8 in both groups.
PVSV was higher in the recurrence (-) group (57.6±19.4) than in the recurrence (+) group (40.8±21.4; p<0.000). Recurrence was significantly higher in patients with LASEC (p<0.000).
The average LAAV (cm/s) was lower in the recurrence (+) group (25.00±9.16 cm/s) than in the recurrence (-) group (56.00±26.72 cm/s; p<0.000) (Table 3). To determine the best cut-off value of LAAV for predicting recurrence, ROC analysis was performed. ROC curve analysis data indicated that when a <30 cm/s cut-off value was used, the LAAV for predicting recurrence could achieve a sensitivity of 85% and a specificity of 95%. The area under the ROC curve for LAAV, which was used to show recurrence, was calculated as 0.813 (p<0.000). Figure 3 shows ROC analysis for LAAV. When assessing patients in terms of LAAV, AF recurred in three patients (18.7%) with LAAV of ≥30 cm/s and in 13 patients (81.2%) with LAAV of <30 cm/s. Recurrence occurred significantly less often in patients with LAAV of >30 cm/s (p<0.000) (Fig. 4). AF recurred in three patients (18.7%) with PVSV of ≥35 cm/s and in 13 patients (81.2%) with PVSV of <35 cm/s. Recurrence occurred significantly less often in patients with PVSV of ≥35 cm/s (p<0.000).
Figure 3.
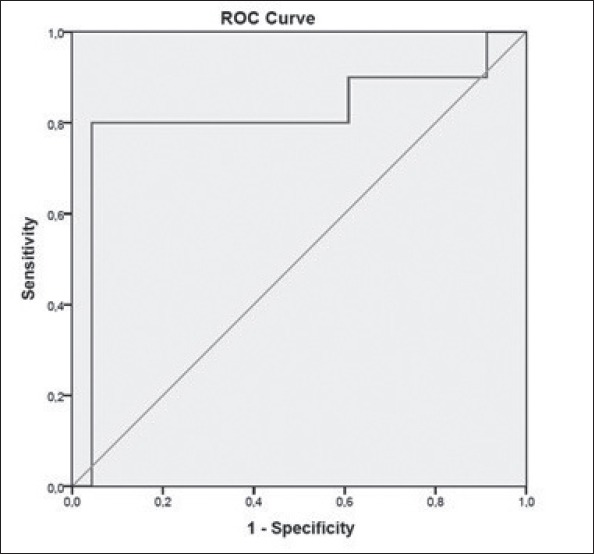
Receiver operating characteristic curves for LAAV (AUC: 0.813; 95% CI: 0.76–0.92; p<0.000).
Figure 4.
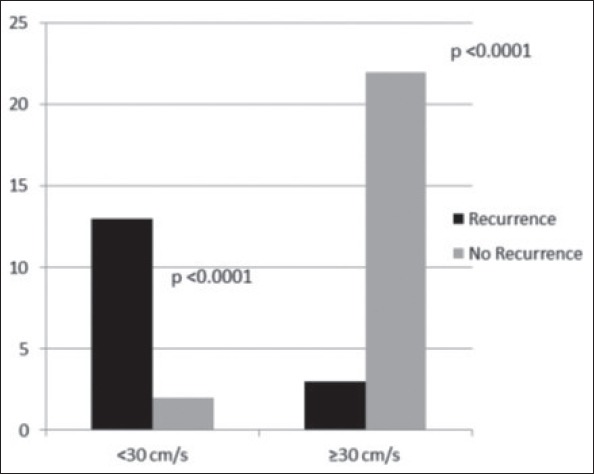
The number of patients with LAAV of <30 cm/s and ≥30 cm/s with and without AF recurrence.
All significant echocardiographic parameters were re-evaluated together in a multivariate logistic regression analysis (Table 4), which was used to evaluate the independent correlates of the recurrence. The variables with an unadjusted p<0.02 in univariate analysis were adjusted to the full model. In multivariate logistic regression, the presence of MAC, as well as LA diameter, LAAV <30 cm/s, PVSV, and LASEC were included in the analysis. As shown in Table 4, after adjustment for confounding variables in the multivariate logistic regression analysis, LAAV of <30 cm/s (OR 1.129, 95% CI 1.115–1.228, p=0.0004) and the LA diameter (OR 1.015, 95% CI 0.998–1.049, p=0.018) were independently associated with recurrence. Thus, an LAAV value of <30 cm/s increased the risk of recurrence 1.129-fold.
Table 4.
Independent predictors of recurrence in multivariate logistic regression analysis.
| Variables | Odds ratio | 95% CI | P |
|---|---|---|---|
| LA diameter | 1.015 | 0.998–1.049 | 0.018 |
| MAC | 0.875 | 0.776–0.981 | 0.045 |
| LAAV<30 | 1.129 | 1.115–1.228 | 0.004 |
| LASEC | 0.966 | 0.874–0.993 | 0.031 |
| PVSV | 0.910 | 0.852–0.987 | 0.046 |
Multivariate logistic regression analysis was used. CI - confidence interval; LA - left atrium; LAAV - left atrial appendage emptying peak flow velocity; LASEC - left atrial spontaneous echo contrast; MAC - mitral annular calcification; PVSV - pulmonary venous flow systolic wave velocity.
Discussion
We demonstrated that the presence of MAC, an increase in LA diameter, the existence of SEC in LA, low LAAV, and low peak PV systolic wave velocity were significantly and independently associated with the recurrence of AF after cryoablation. The most important finding of our study was that LAAV levels were significantly higher in patients without recurrence than in those in the recurrence group. In addition, LAAV levels of >30 cm/s predicted the success of ablation with a sensitivity of 85% and a specificity of 95% in our study population.
Cryoballoon ablation is an effective and sometimes curative treatment option in patients with AF. In sustained treatment of PAF (STOP-AF), which was the first randomized cryoballoon study, patients were randomized into antiarrhythmic drug and cryoablation groups. During a 12-month follow-up period, AF episodes were not observed in 69.9% of the cryoballoon group and in 7.3% of the antiarrhythmic drug group (9). According to the results obtained in our study, AF episodes did not occur in 68.6% of the patients with paroxysmal AF during the 12-month follow-up period. This rate of recurrence is similar to other studies in which antiarrhythmic medication was stopped 3 months after each ablation (9, 10). On the other hand, the limited experience of our operators played a major role in this recurrence rate. According to the recent EHRA Expert Consensus Statement on the ablation of AF, an operator should have previously performed a minimum of 30–50 AF ablations; however, this was our first cryoablation case series (3). Therefore, our operators did not meet this criterion because they had previously performed only 20 AF ablations. A single primary operator obtained the data for this study.
In patients who underwent PV ablations with RF, the parameters that may predict the recurrence of AF have previously been investigated in numerous studies (11-14). However, there are few studies in the literature regarding the predictive echocardiographic and laboratory parameters of AF recurrence after cryoablation (15-18).
In our study, an increase in LA diameter, the presence of MAC on TTE and TEE views, LAAV of <30 cm/s, reduced PVSV, and the existence of LASEC were found to be independent predictors for the recurrence of PAF after cryoballoon ablation.
The increase in the duration of AF causes atrial enlargement leading to more circlets of atrial re-entry. Also, atrial myocardial fibrosis and inflammation reduce the intra-atrial conduction velocity, which results in longer and more frequent episodes (19). In our study, LA diameters of >4.0 cm were associated with frequent recurrence. Theoderakis et al. (20) reported an increase in the cases of recurrence after cardioversion in patients with LA diameters of >4.5 cm. Aytemir et al. (21) showed that LA diameter was significantly larger in AF patients with recurrence after cryoablation.
The presence of LASEC and low LAAV measurement demonstrate low flow in LA and functional abnormalities of LA. Both peak LAAV and peak PVSV have previously been validated as indicators of contractile and reservoir function, respectively (11). We found that AF recurrence was more frequent in patients with LAAV of <30 cm/s (p<0.0001). Combes et al. (13) evaluated 40 patients who had RF ablation of persistent long-standing AF. Similar to our findings, they showed that LAAV of <30 cm/s was the only independent predictor of recurrence. Likewise, Isobe et al. (15) showed that LAAV was the best predictor of long-term recurrence in patients who underwent the cryoablation of chronic AF during cardiac surgery. Other studies report that LAAVs of >31 cm/s (22) and >40 cm/s (23) are independent predictors of cardioversion success. Our study showed that reduced PVSV was also related to the recurrence of AF. In a manner similar to the study by Verma et al. (11) involving RF ablation, we detected more recurrences in patients with low PVSV (p<0.05).
Although studies to evaluate the role of LAAV in the recurrence of AF after RF ablation or in patients with chronic AF have been conducted, there are currently no reports of trials in which the recurrence of AF after cryoablation in patients with PAF was studied. In addition, PVSV as well as the presence of LASEC or MAC have previously not been studied as predictors of recurrence after the cryoablation of PAF. To the best of our knowledge, the present study is the first to show that high LAAV and low PVSV predict the recurrence of PAF over a long-term period after cryoablation. Hence, with this finding, our work differs from existing studies in the literature.
Study limitations
Our study has some limitations as follows. First, our sample size was small. Second, as is usual in AF studies, ECG and periodic Holter monitoring may have led to some degree of underestimation of recurrence rates. Third, because this was the first case series of our center, the experience of our operators was limited. Fourth, a less accurate LA diameter rather than LA volume was used in our analysis. Fifth, the lack of >24 h Holter monitoring during the follow-up is another limitation. Finally, because of small number of recurrences and patients, the results cannot be generalized to the wider community and also we did not perform intra and inter-observer variability for TTE parameters.
Conclusion
In patients who underwent cryoablation because of PAF, the presence of MAC, an increase in LA dilatation, low PVSV, reduced LAAV, and the presence of LASEC as echocardiographic parameters may predict AF recurrence. Wider comunity studies are necessary.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Author contributions: Concept - B.C., D.M.G.; Design - C.K.; Supervision - C.K.; Materials - S.M.A.; Data collection &/or processing - S.M.A., A.A.; Analysis and/or interpretation - Ö.U.Ö., B.C.; Literature search - D.M.G., H.G.; Writing - D.M.G., H.G.; Critical review - V.K.V., Ç.E.
References
- 1.Stewart S, Hart CL, Hole DJ, McMurray JJ. Population prevalence incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart. 2001;86:516–21. doi: 10.1136/heart.86.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 3.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 4.Fleck T, Wolf F, Bader T, Lehner R, Aigner C, Stix G, et al. Atrial function after ablation procedure in patients with chronic atrial fibrillation using steady-state free precession magnetic resonance imaging. Ann Thorac Surg. 2007;84:1600–4. doi: 10.1016/j.athoracsur.2007.05.063. [DOI] [PubMed] [Google Scholar]
- 5.Oki T, Tabata T, Yamada H, Wakatsuki T, Fukuda K, Abe M, et al. Evaluation of left atrial filling using systolic pulmonary venous flow velocity measurements in patients with atrial fibrillation. Clin Cardiol. 1998;21:169–74. doi: 10.1002/clc.4960210306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography: American Society of Echocardiography committee on standards, subcommittee on quantitation of two-dimensional echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 7.Fatkin D, Kelly R, Feneley M. Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J Am Coll Cardiol. 1994;23:961–9. doi: 10.1016/0735-1097(94)90644-0. [DOI] [PubMed] [Google Scholar]
- 8.Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology. 2005;16:73–81. doi: 10.1097/01.ede.0000147512.81966.ba. [DOI] [PubMed] [Google Scholar]
- 9.Packer DL, Kowal RC, Wheelan KR, Irwin JM, Champagne J, Guerra PG, et al. STOP AF Cryoablation Investigators. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61:1713–23. doi: 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- 10.Schade A, Langbein A, Spehl S, Barth S, Deneke T, Groschup G, et al. Recurrence of paroxysmal atrial fibrillation after cryoisolation of the pulmonary veins. Is a “redo” procedure using the cryoballoon useful? J Interv Card Electrophysiol. 2013;36:287–95. doi: 10.1007/s10840-012-9725-y. [DOI] [PubMed] [Google Scholar]
- 11.Verma A, Marrouche NF, Yamada H, Grimm RA, Cummings J, Burkhardt JD, et al. Usefulness of intracardiac Doppler assessment of left atrial function immediately post–pulmonary vein antrum isolation to predict short-term recurrence of atrial fibrillation. Am J Cardiol. 2004;94:951–4. doi: 10.1016/j.amjcard.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 12.Donal E, Grimm R, Yamada H, Kim YJ, Marrouche N, Natale A, et al. Usefulness of Doppler assessment of pulmonary vein and left atrial appendage flow following pulmonary vein isolation of chronic atrial fibrillation in predicting recovery of left atrial function. Am J Cardiol. 2005;95:941–7. doi: 10.1016/j.amjcard.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Combes S, Jacob S, Combes N, Karam N, Chaumeil A, Guy-Moyat B, et al. Predicting favourable outcomes in the setting of radiofrequency catheter ablation of long-standing persistent atrial fibrillation: a pilot study assessing the value of left atrial appendage peak flow velocity. Arch Cardiovasc Dis. 2013;106:36–43. doi: 10.1016/j.acvd.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Kumagai K, Sakamoto T, Nakamura K, Hayano M, Yamashita E, Oshima S. Pre-procedural prediction of termination of persistent atrial fibrillation by catheter ablation as an indicator of reverse remodeling of the left atrium. Circ J. 2013;77:1416–23. doi: 10.1253/circj.cj-12-0934. [DOI] [PubMed] [Google Scholar]
- 15.Isobe N, Taniguchi K, Oshima S, Kamiyama H, Ezure M, Kaneko T, et al. Left Atrial appendage outflow velocity is superior to conventional criteria for predicting of maintenance of sinus rhythm after simple cryoablation of pulmonary vein orifices. Circ J. 2005;69:446–51. doi: 10.1253/circj.69.446. [DOI] [PubMed] [Google Scholar]
- 16.Evranos B, Aytemir K, Oto A, Okutucu S, Karakulak U, Şahiner L, et al. Predictors of atrial fibrillation recurrence after atrial fibrillation ablation with cryoballoon. Cardiol J. 2013;20:294–303. doi: 10.5603/CJ.2013.0075. [DOI] [PubMed] [Google Scholar]
- 17.Canpolat U, Aytemir K, Yorgun H, Şahiner L, Kaya EB, Çay S, et al. Usefulness of serum uric acid level to predict atrial fibrillation recurrence after cryoballoon-based catheter ablation. Europace. 2014;16:1731–7. doi: 10.1093/europace/euu198. [DOI] [PubMed] [Google Scholar]
- 18.Gürses KM, Yalçın MU, Koçyiğit D, Evranos B, Ateş AH, Yorgun H, et al. Red blood cell distribution width predicts outcome of cryoballoon-based atrial fibrillation ablation. J Interv Card Electrophysiol. 2015;42:51–8. doi: 10.1007/s10840-014-9959-y. [DOI] [PubMed] [Google Scholar]
- 19.Narayam M, Cain M, Smith J. Atrial fibrilation. Lancet. 1997;350:943–50. doi: 10.1016/S0140-6736(97)06359-9. [DOI] [PubMed] [Google Scholar]
- 20.Theodorakis GN, Markionos M, Kouroubetsis CK, Livanis EG, Paraskevaidis IA, Kremastinos DT. Clinical, adrenergic and heart endocrine measures in chronic atrial fibrilation as predictors of conversion and maintenance of sinus rhythm after direct current cardioversion. Eur Heart J. 1996;17:550–6. doi: 10.1093/oxfordjournals.eurheartj.a014908. [DOI] [PubMed] [Google Scholar]
- 21.Aytemir K, Gürses KM, Yalçın MU, Koçyiğit D, Dural M, Evranos B. Safety and efficacy outcomes in patients undergoing pulmonary vein isolation with second-generation cryoballoon. Europace. 2015;17:379–87. doi: 10.1093/europace/euu273. [DOI] [PubMed] [Google Scholar]
- 22.Pálinkás A, Antonielli E, Picano E, Pizzuti A, Varga A, Nyúzó B, et al. Clinical value of left atrial appendage flow velocity for predicting of cardioversion success in patients with non-valvular atrial fibrillation. Eur Heart J. 2001;22:2201–8. doi: 10.1053/euhj.2001.2891. [DOI] [PubMed] [Google Scholar]
- 23.Antonielli E, Pizzuti A, Pálinkás A, Tanga M, Gruber N, Michelassi C, et al. Clinical value of left atrial appendage flow for prediction of long-term sinus rhythm maintenance in patients with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2002;39:1443–9. doi: 10.1016/s0735-1097(02)01800-4. [DOI] [PubMed] [Google Scholar]


