Abstract
Objective:
The aim of this study was to evaluate the relationship between peripheral artery disease (PAD) severity and complexity, as evaluated by TransAtlantic Inter-Society Consensus-II (TASC-II) classification, and neutrophil-to-lymphocyte (N/L) ratio.
Methods:
A total of 407 patients underwent peripheral angiography due to signs and symptoms of PAD; of these, 64 patients were excluded and the remaining 343 patients were enrolled in this cross-sectional study. Patients with previous peripheral revascularizations, acute coronary syndrome, vasculitis, non-atherosclerotic stenosis, and malignancy were excluded. Patients were divided into 4 groups according to TASC-II classification, and clinical and laboratory data were compared. The chi-square test, Student’s t-test, Mann–Whitney U test, analysis of variance, Kruskal–Wallis test, Spearman’s correlation analysis, multiple logistic regression analysis, and receiver operating characteristic (ROC) curve analysis were used for statistical analysis.
Results:
Lymphocyte count was weakly correlated (r=–0.169, p=0.002) whereas neutrophil count and N/L ratio were moderately correlated with the TASC score (r=0.432, p<0.001 and r=0.470, p<0.001, respectively). Low-density lipoprotein cholesterol [odds ratio (OR)=1.010, 95% confidence interval (CI) 95%=1.003–1.017, p=0.004], high-density lipoprotein cholesterol (OR=0.940, 95% CI=0.894–0.987, p=0.013), and N/L ratio (OR=1.914, 95% CI=1.515–2.418, p<0.001) were the independent factors for predicting a higher TASC class in multiple logistic regression analysis. The cut-off value of the N/L ratio for predicting TASC C&D class was >3.05 (sensitivity=75.0%, specificity=62.9%, area under the curve=0.678, 95% CI=0.688–0.784, p<0.001) in ROC curve analysis.
Conclusion:
The N/L ratio, a marker of inflammation, may be an important predictor of PAD complexity. Therefore, a simple blood count test may provide an important clue about the severity of PAD and risk stratification in patients presenting with intermittent claudication. Additional studies are required to confirm our findings.
Keywords: peripheral artery, coronary, angiography, TASC, neutrophil, lymphocyte
Introduction
Lower extremity peripheral artery disease (PAD) affects a considerable percentage of the population. TransAtlantic Inter-Society Consensus-II (TASC-II) classification is an internationally derived, collaboratively created consensus definition that is used for the assessment of PAD according to the anatomic distribution and number and nature of lesions (stenosis, occlusion) in combination with published outcomes of intervention (1, 2). PAD is generally a consequence of systemic atherosclerotic disease processes that affect multiple arterial circulations. Risk factors for atherosclerosis, such as smoking, diabetes, hyperlipidemia, hypertension, and hyperhomocysteinemia, are common among patients with PAD (1, 2). PAD is associated with an increased risk of cardiovascular and all-cause mortality (3, 4).
Inflammation contributes to the initiation and progression of atherosclerosis as well as to the rupture of atherosclerotic plaques (5). Monocytes and T-lymphocytes are involved in atherogenesis (6). Neutrophils play a central role in atherogenesis and atherothrombosis (6). Neutrophils release superoxide and chemokines that affect endothelial cells and promote or amplify the recruitment of other inflammatory cells (7). Neutrophils also support monocyte adhesion and mobilization to the site of inflammation (8). Neutrophils mediate inflammatory responses by numerous biochemical mechanisms, such as the release of arachidonic acid metabolites, platelet-aggravating factors, cytotoxic oxygen-derived free radicals, and hydrolytic enzymes such as myeloperoxidase, elastase, and acid phosphatases (9). While the neutrophil count increases, the lymphocyte count decreases with atherosclerosis (6). Therefore, an elevated neutrophil-to-lymphocyte (N/L) ratio integrates the predictive risk of these two leukocyte subtypes into a single risk factor. It has been suggested that the N/L ratio is a better predictor of cardiovascular events than white blood cell or neutrophil count, even after controlling for various known risk factors. Thus, the N/L ratio appears to be additive to conventional risk factors and commonly used biomarkers (10). The N/L ratio is marker of coronary artery disease (CAD) complexity and major adverse cardiovascular events (11–13). It is also associated with left ventricular ejection fraction (LVEF) in patients with multivessel CAD (14). In addition, it is a prognostic marker in patients with critical limp ischemia (15). However, data regarding the association of the complexity of PAD with the N/L ratio is lacking.
The aim of this study was to evaluate the relationship between PAD complexity, as evaluated by TASC-II classification, and the N/L ratio.
Methods
Study design
This cross-sectional retrospective study enrolled 343 patients with PAD who underwent peripheral angiography at Ahi Evren Chest Cardiovascular Surgery Education and Research Hospital cardiology inpatient clinic due to suspected PAD in non-invasive tests between June 2011 and October 2013.
Patient population
Informed consent was obtained from all subjects, and the investigation conformed to the principles outlined in the Declaration of Helsinki. The study protocol was approved by the ethics committee.
We performed 407 peripheral angiographies in our institution between June 2011 and October 2013. Patients with previous peripheral revascularization, acute coronary syndrome, vasculitis, non-atherosclerotic stenosis, malignancy, and no significant peripheral arterial stenosis were excluded from the study. We also excluded patients with known or suspected infectious or inflammatory conditions, those with diabetic foot ulcers, or those needing urgent angiography and intervention. Of the 407 patients, 64 were excluded on the basis of the exclusion criteria. We did not perform invasive peripheral angiography in patients with advanced renal insufficiency and anemia. Patients contraindicated for invasive angiography were not included.
Hypertension was defined by a previous diagnosis of hypertension or the presence of SBP of ≥140 mm Hg or DBP of ≥90 mm Hg (mean of two consecutive measurements). Diabetes was defined as fasting plasma glucose levels of ≥126 mg/dL, plasma glucose levels of ≥200 mg/dL 2 h after the 75-mg oral glucose tolerance test, symptoms of hyperglycemia accompanied by casual plasma glucose levels of ≥200 mg/dL, HbA1C of ≥6.5%, or use of antidiabetic medications.
Hyperlipidemia was defined as low-density lipoprotein (LDL) cholesterol levels of ≥160 mg/dL or statin usage. Chronic renal failure (CRF) was defined as estimated glomerular filtration rate (GFR) of <60 mL/min/1.73 m2 according to the Modification of Diet in Renal Disease (MDRD) formula. Transthoracic echocardiographic assessment (Vivid S5 General Electric, Norway) was performed in all patients and LVEF was measured. Patients who self-reported as having smoked during the previous 6 months were classified as smokers. Venous blood samples were drawn after 12-h overnight fasting. Serum glucose levels were determined spectrophotometrically using the enzymatic hexokinase method (Beckman Coulter, Co. Fullerton, CA, USA). Serum triglyceride and cholesterol levels were determined enzymatically with the Synchron CX auto analyzer (Beckman Coulter, Co. Fullerton, CA, USA). High-density lipoprotein (HDL) cholesterol was determined after specific precipitation and LDL cholesterol was determined by the Friedewald formula. Total and differential leukocyte counts were measured using an automated hematology analyzer. Absolute cell counts were used in these analyses.
Peripheral angiography and TASC-II score
Peripheral angiography was performed with a 6-Fr pigtail catheter using an automatic pump injector. For the evaluation of both lower extremities, the catheter tip was positioned above the aorto-iliac bifurcation. TASC-II score analysis (Table 1) was performed on bilateral aorto-iliac arterial segments (1).
Table 1.
TASC-II classification
| Aorto-iliac lesions | Femoropopliteal lesions | |
|---|---|---|
| TASC A | Single stenosis (<3 cm in length) in the CIA or EIA (unilateral/bilateral) | Single stenosis (<3 cm in length) in the superficial femoral artery or popliteal artery |
| TASC B | 1. Single stenosis (3–10 cm in length) not extending into the CFA 2. A total of 2 stenoses (<5 cm in length) in the CIA and/or EIA and not extending into the CFA 3. Unilateral CIA occlusion |
1. Single stenosis (3–10 cm in length) not involving the distal popliteal artery 2. Heavily calcified stenosis up to 3 cm in length 3. Multiple lesions, each <3 cm in length (stenoses or occlusions) 4. Single or multiple lesions in the absence of continuous tibial runoff to improve inflow for distal surgical bypass |
| TASC C | 1. Bilateral stenosis (5–10 cm in length) in the CIA and/or EIA, not extending into the CFA 2. Unilateral EIA occlusion not extending into the CFA 3. Unilateral EIA stenosis extending into the CFA 4. Bilateral CIA occlusion |
1. Single stenosis or occlusion >5 cm in length 2. Multiple stenoses or occlusions (each 3–5 cm in length) with or without heavy calcification |
| TASC D | 1. Diffuse, multiple unilateral stenosis involving the CIA, EIA, and CFA (usually >10 cm in length) 2. Unilateral occlusion involving both the CIA and EIA 3. Bilateral EIA occlusions 4. Diffuse disease involving the aorta and both iliac arteries 5. Iliac stenosis in a patient with abdominal aortic aneurysm or other lesions requiring aortic or iliac surgery |
Complete CFA or superficial femoral artery occlusion or complete popliteal and proximal trifurcation occlusions |
CIA - common iliac artery; CFA - common femoral artery; EIA - external iliac artery; TASC - Trans Atlantic Inter-Society Consensus-II
Statistical analysis
Analyses were performed using SPSS 17.0 (SPSS for Windows 17.0, Chicago, Illinois). Continuous variables were expressed as mean±standard deviation (SD) (for parameters with normal distribution) or median (inter quartile range, IQR) (for parameters with non-normal distribution), and categorical variables were expressed as percentages. Comparison of categorical variables between groups was performed using the chi-square test. Analysis of normality was performed with the Kolmogorov–Smirnov test. Student’s t-test and Mann–Whitney U test were used for the analysis of continuous variables. Analysis of variance was used for the comparison of multiple variables with normal distribution, and Tukey’s honest significant difference test was used for post hoc analysis. The Kruskal–Wallis test was used for the comparing non-normally distributed multiple variables. Spearman’s correlation analysis was used to analyze the correlates of the TASC-II score. Multiple binary logistic regression analysis was performed to find the independent predictors of a higher TASC-II score. Variables showing significant difference between the groups, including presence of CAD, LDL cholesterol levels, HDL cholesterol levels, and N/L ratio, were entered into the model. Receiver operating characteristic (ROC) curve analysis was performed to find an optimal cut-off value of the N/L ratio for predicting a higher TASC class. The area under curve (AUC) was calculated as a measure of the accuracy of the test. A 2-sided p-value of <0.05 was considered significant within 95% confidence intervals (CIs).
Results
The clinical and demographic characteristics of the study population are given in Table 2. None of the patients had anemia or hepatic dysfunction. The medications of the patients are presented in Table 3. The correlates of TASC-II classification are shown in Table 4. The patients were divided into 4 groups according to TASC-II classification; 162, 97, 53, and 31 patients were classified into TASC A, TASC B, TASC C, and TASC D classes, respectively. Most patients were males, and male predominance was significantly evident with increasing TASC-II class.
Table 2.
Characteristics of patients
| Variable | TASC-A n=162 | TASC-B n=97 | TASC-C n=53 | TASC-D n=31 | P* |
|---|---|---|---|---|---|
| Age, year | 64.60±10.09 | 65.14±9.69 | 63.87±9.37 | 67.87±7.50 | 0.292 |
| Male, n % | 126 (77.8) | 93 (95.6) | 53 (100) | 31 (100) | <0.001 |
| CAD, n % | 77 (47.5) | 56 (57.7) | 35 (66.0) | 24 (77.4) | 0.005 |
| Smoking, n % | 73 (45.1) | 47 (48.5) | 27 (50.9) | 18 (58.1) | 0.571 |
| HT, n % | 77 (47.5) | 52 (53.6) | 29 (54.7) | 16 (51.6) | 0.722 |
| HL, n % | 49 (30.2) | 31 (32.0) | 23 (43.4) | 11 (35.5) | 0.353 |
| LDL, mg/dL | 134.72±34.35 | 140.20±33.19 | 162.55±52.14 | 139.87±53.61 | <0.001 |
| HDL, mg/dL | 38.79±6.89 | 37.62±6.11 | 35.38±5.31 | 36.77±9.51 | 0.012 |
| DM, n % | 56 (34.6) | 24 (29.3) | 18 (34.0) | 9 (29.0) | 0.928 |
| CRF, n % | 41 (25.3) | 17 (17.5) | 9 (17) | 3 (9.7) | 0.140 |
| LVEF, % | 58.90±10.43 | 57.16±9.57 | 58.55±10.37 | 56.23±8.97 | 0.386 |
| Glucose, mg/dL | 105 (33.25) | 105 (28.5) | 105 (27.5) | 103 (20) | 0.661 |
| Creatinine, mg/dL | 1.00 (0.33) | 0.96 (0.36) | 1.00 (0.38) | 1.04 (0.18) | 0.410 |
| GFR, ml/min/1.73 m2 | 80.55±28.10 | 85.74±31.33 | 82.65±35.12 | 77.43±16.24 | 0.437 |
| Neutrophil | 4.19±1.22 | 5.21±1.30 | 5.48±1.36 | 5.70±1.21 | <0.001 |
| Lymphocyte | 1.66±0.53 | 1.61±0.50 | 1.49±0.60 | 1.36±0.48 | 0.012 |
| N/L ratio | 2.45 (0.89) | 3.28 (1.50) | 3.57 (2.96) | 4.23 (2.09) | <0.001 |
Chi-square, analysis of variance and Kruskal-Wallis test
CAD - coronary artery disease; CRF - chronic renal failure (Glomerular filtration rate<60 ml/min/1.73 m2); DM - diabetes mellitus; GFR - estimated glomerular filtration rate; HDL - high density lipoprotein cholesterol; HL - hyperlipdemia; HT - hypertension; LDL - low density lipoprotein cholesterol; LVEF - left ventricle ejection fraction; N/L ratio - neutrophil/lymphocyte ratio; PAD - peripheral artery disease; TASC - Trans Atlantic Inter-Society Consensus II classification
Post hoc analysis:
LDL-TASC-A vs. TASC-B p=0.699, TASC-A vs. TASC-C p<0.001, TASC-A vs. TASC-D p=.909, TASC-B vs. TASC-C p=0.005, TASC-B vs. TASC-D p=1.000, TASC-C vs. TASC-D p= 0.054
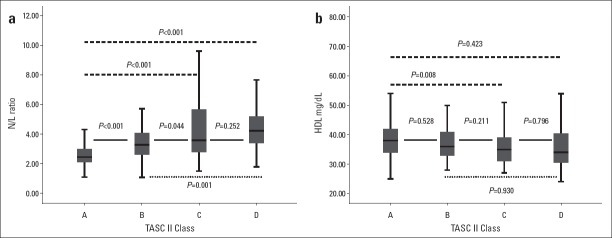
HDL-TASC-A vs. TASC-B p=0.528, TASC-A vs. TASC-C p=0.008, TASC-A vs. TASC-D p=0.423, TASC-B vs. TASC-C p=0.211, TASC-B vs. TASC-D p=0.930, TASC-C vs. TASC-D p=0.796
Neutrophil-TASC-A vs. TASC-B p<0.001, TASC-A vs. TASC-C p<0.001, TASC-A vs. TASC-D p<0.001, TASC-B vs. TASC-C p=0.582, TASC-B vs. TASC-D p=0.238, TASC-C vs. TASC-D p=0.874
Lymphocyte-TASC-A vs. TASC-B p=0.827, TASC-A vs. TASC-C p=0.19, TASC-A vs. TASC-D p=0.018, TASC-B vs. TASC-C p=0.570, TASC-B vs. TASC-D p=0.106, TASC-C vs. TASC-D p= 0.690
N/L ratio- TASC-A vs. TASC-B p<0.001, TASC-A vs. TASC-C p<0.001, TASC-A vs. TASC-D p<0.001, TASC-B vs. TASC-C p=0.044, TASC-B vs. TASC-D p=0.001, TASC-C vs. TASC-D p= 0.252
Table 3.
The baseline medications of the patients
| Variable | TASC-A n=162(%) | TASC-B n=97(%) | TASC-C n=53(%) | TASC-D n=31(%) | P* |
|---|---|---|---|---|---|
| Antidiabetic | 56 (34.6) | 31 (32.0) | 18 (34.0) | 9 (29.0) | 0.928 |
| Statin | 49 (30.2) | 31 (32.0) | 23 (43.4) | 11 (35.5) | 0.353 |
| ACE-i/ARB | 66 (40.7) | 43 (44.3) | 26 (49.1) | 12 (38.7) | 0.698 |
| CCB | 62 (38.3) | 43 (44.3) | 24 (45.3) | 14 (45.2) | 0.684 |
| Beta blocker | 67 (41.4) | 40 (41.2) | 18 (34.0) | 8 (25.8) | 0.332 |
| Pentoxifylline | 92 (56.8) | 59 (60.8) | 31 (58.5) | 18 (58.1) | 0.939 |
| Cilastazol | 65 (40.1) | 49 (50.5) | 27 (50.9) | 15 (48.4) | 0.307 |
Chi-square test
ACE-i/ARB - angiotensin converting enzyme inhibitor/angiotensin receptor blocker; CCB - calcium channel blocker; TASC - Trans Atlantic Inter-Society Consensus II class
Table 4.
The correlates of TASC II class
| Variable | R | P* |
|---|---|---|
| Age | 0.030 | 0.578 |
| Gender (male 88.3%) | 0.306 | <0.001 |
| Estimated glomerular filtration rate | 0.026 | 0.634 |
| Low density lipoprotein cholesterol | 0.134 | 0.013 |
| High density lipoprotein cholesterol | -0.203 | <0.001 |
| Glucose | -0.061 | 0.257 |
| Creatinin | 0.048 | 0.377 |
| Chronic renal failure | -0.122 | 0.024 |
| Left ventricular ejection fraction | -0.117 | 0.030 |
| Coronary artery disease | 0.188 | <0.001 |
| Hypertension | 0.054 | 0.314 |
| Diabetes mellitus | -0.027 | 0.618 |
| Hyperlipidemia | 0.075 | 0.168 |
| Smoking | 0.070 | 0.193 |
| Neutrophil | 0.432 | <0.001 |
| Lymphocyte | -0.169 | 0.002 |
| Neutrophil/Lymphocyte ratio | 0.470 | <0.001 |
Spearman correlation analysis. TASC - Trans Atlantic Inter-Society Consensus
Patients in TASC C class had significantly higher LDL cholesterol and lower HDL cholesterol levels than those in other classes. The neutrophil count increased with increasing TASC class and patients in TASC A class had a significantly lower neutrophil count than those in other classes. Patients in TASC D class had a significantly lower lymphocyte count than those in other classes. The N/L ratio increased significantly with increasing TASC-II class (Fig. 1).
Figure 1.
(a) A box plot showing the N/L ratio according to the TASC-II class. (b) A box plot showing HDL cholesterol levels according to the TASC-II class
Lymphocyte count, HDL cholesterol levels, and LVEF were inversely and weakly correlated with the TASC-II class (r=–0.169, p=0.002; r=–0.203, p<0.001; and r=–0.117, p=0.030, respectively). LDL cholesterol levels and presence of CAD were weakly correlated with the TASC class (r=0.134, p=0.013 and r=0.188, p<0.001, respectively). Neutrophil count and N/L ratio were moderately correlated with the TASC-II class (r=0.432, p<0.001 and r=0.470, p<0.001, respectively).
We regrouped patients as TASC A&B and TASC C&D. The features of the patients are shown in Table 5. Male sex, presence of CAD, LDL cholesterol levels, HDL cholesterol levels, neutrophil count, lymphocyte count, and N/L ratio were significantly different between the groups.
Table 5.
The characteristics of patients
| Variable | TASC A&B (n=259) | TASC C&D (n=84) | P* |
|---|---|---|---|
| Age, year | 64.80±9.93 | 65.35±8.90 | 0.656 |
| Male, n % | 219 (84.6%) | 84 (100%) | <0.001 |
| CAD, n % | 133 (51.4%) | 59 (70.2%) | 0.002 |
| Smoking, n % | 120 (46.3%) | 45 (53.6%) | 0.249 |
| HT, n % | 129 (49.8%) | 45 (53.6%) | 0.549 |
| HL, n % | 80 (30.9%) | 34 (40.5%) | 0.105 |
| LDL, mg/dL | 136.77±33.96 | 154.18±53.51 | 0.006 |
| HDL, mg/dL | 38.35±6.62 | 35.89±7.12 | 0.004 |
| DM, n % | 87 (33.6%) | 27 (32.1%) | 0.807 |
| CRF, n % | 58 (22.4%) | 12 (14.3%) | 0.109 |
| LVEF, % | 58.25±10.14 | 57.69±9.88 | 0.658 |
| Glucose, mg/dL | 105 (31) | 105 (24.5) | 0.560 |
| Creatinine, mg/dL | 0.99 (0.34) | 1.02 (0.26) | 0.131 |
| GFR, ml/min/1.73m2 | 82.49±29.40 | 80.73±29.57 | 0.634 |
| Neutrophil | 4.57±1.34 | 5.56±1.30 | <0.001 |
| Lymphocyte | 1.64±0.52 | 1.44±0.56 | 0.003 |
| N/L ratio | 2.61 (1.30) | 3.99 (2.70) | <0.001 |
Chi-square test, student’s t- test and Mann-Whitney U test. CAD - coronary artery disease; CRF - chronic renal failure (Glomerular filtration rate<60 ml/min/1.73 m2); DM - diabetes mellitus; GFR - estimated glomerular filtration rate; HDL - high density lipoprotein cholesterol; HL - hyperlipdemia; HT - hypertension; LDL - low density lipoprotein cholesterol; LVEF - left ventricle ejection fraction; N/L ratio - Neutrophil/Lymphocyte ratio; PAD - peripheral artery disease; TASC - Trans Atlantic Inter-Society Consensus II classification
Sex, presence of CAD, LDL cholesterol levels, HDL cholesterol levels, and N/L ratio were entered into multiple logistic regression analysis (Table 6). Male sex was not included in the model because all patients in the TASC C&D group were male. LDL cholesterol levels [odds ratio (OR)=1.010, 95% CI=1.003–1.017, p=0.004], HDL cholesterol levels (OR=0.940, 95% CI=0.894–0.987, p=0.013), and N/L ratio (OR=1.914, 95% CI=1.515–2.418, p<0.001) were the independent factors for predicting a higher TASC class (TASC C&D) in multivariate logistic regression analysis.
Table 6.
Results of multiple binary regression analysis
| Variable | OR | P | CI 95% |
|---|---|---|---|
| Coronary artery disease | 0.844 | 0.623 | 0.430–1.658 |
| Low density lipoprotein cholesterol | 1.010 | 0.004 | 1.003–1.017 |
| High density lipoprotein cholesterol | 0.940 | 0.013 | 0.894–0.987 |
| Neutrophil -to- lymphocyte ratio | 1.914 | <0.001 | 1.515–2.418 |
CI - confidence interval; OR - odds ratio
The cut-off value of the N/L ratio for predicting TASC C&D was >3.05 (sensitivity=75.0%, specificity=62.9%, AUC=0.678, 95% CI=0.688–0.784, p<0.001) in ROC curve analysis (Fig. 2).
Figure 2.
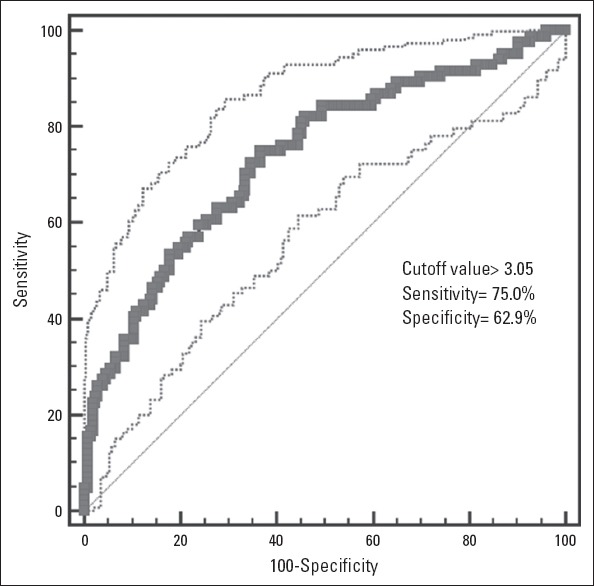
An ROC curve showing the sensitivity and specificity of the N/L ratio for predicting a higher TASC-II class
Discussion
In the present study, we showed that the N/L ratio, a marker of chronic inflammation, was an independent predictor of the TASC-II class. This finding indicates that the degree of inflammation correlates with the complexity of PAD.
Hirsh et al. (16) have reported that lower extremity PAD affects 29% of the population and that the prevalence of PAD increases with increasing age. As a consequence of coexisting coronary and cerebrovascular disease, there is an increased risk of myocardial infarction (MI), stroke, and cardiovascular death in patients with lower extremity PAD. There is a 20%–60% increased risk of MI and a 2–6-fold increased risk of death due to CAD in such patients (17, 18). The annual mortality rate derived from epidemiological studies of patients with lower extremity PAD is 4%–6% and it is highest in those with the most severe disease (19–22). Inflammatory and immune mechanisms play a pivotal role in the development and progression of atherosclerotic plaques (5). Neutrophil infiltration in atherosclerotic plaques has a major role in CAD (6, 23–25). Accordingly, we found that the neutrophil count was associated with the severity and complexity of PAD. Inflammatory biomarkers may be helpful in risk stratification and selection of the most efficient therapy. Neutrophils are an important predictor of cardiovascular outcomes in patients with symptomatic PAD (26). This may be attributable to a link between increased inflammation and increased PAD complexity, and the N/L ratio may be a feasible marker for the detection of this linkage. In addition, presence of CAD was higher among patients with more complex PAD in the present study, which supported our hypothesis (27, 28).
Age, sex, serum cholesterol levels, hypertension, smoking, diabetes, and CAD are associated with an increased risk of developing PAD. Elevated LDL cholesterol levels and decreased HDL cholesterol levels are associated with an increased risk of PAD (2). We found that LDL cholesterol levels were positively correlated, whereas HDL cholesterol levels were negatively correlated with the TASC-II class. In multivariate regression analysis, HDL cholesterol and LDL cholesterol levels were independent predictors of the TASC class. Male sex, increasing age, and smoking conferred a 1.5-fold increased risk of developing PAD (1, 2). Complexity of PAD was significantly increased among male patients in this study.
Study limitations
The present study has several limitations. First, the N/L ratio is considerably affected by many factors, including dehydration, overhydration, diluted blood specimens, and in vitro blood specimen handling. TASC is an anatomic classification. Addition of clinical classifications, including the Rutherford classification, may provide additional information. The Rutherford classification is used to evaluate critical limp ischemia according to the presence of ischemic signs and symptoms. Requirement of perfusion is determined according to the Rutherford category. However, the reperfusion strategy (percutaneous or surgery) is directed by the TASC-II class. We evaluated patients with invasive peripheral angiography. Addition of BT or MR angiography may provide further information such as the plaque composition. Furthermore, we did not measure other inflammatory markers, including TNF-a, CRP, high-sensitivity CRP, interleukins, and chemokines, in this study.
Conclusion
The N/L ratio, a marker of inflammation, may be an important predictor of PAD complexity. Therefore, a simple blood count test may provide an important clue about the severity of PAD and risk stratification in patients presenting with intermittent claudication. Additional studies are required to confirm our findings.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Author contributions: Concept- A.Ç.A., E.H., C.Y.K.; Design- A.Ç.A., D.A.A.; Supervision- D.A.A., S.Ç., E.K.; Funding- D.A.A., S.Ç., E.K.; Materials- A.Ç.A., D.A.A., C.Y.K., R.Z., E.H.; Data collection &/or processing – A.Ç.A., C.Y.K., R.Z., S.Ç., T.G.; Analysis and/or interpretation–A.Ç.A.; Literature search- A.Ç.A., D.A.A.; Writing – A.Ç.A.; Critical review- S.Ç., E.K., C.Y.K., R.Z., E.H., D.A.A, T.G.; Other-D.A.A., T.G.
References
- 1.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33(Suppl 1):1–75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:1425–43. doi: 10.1161/CIR.0b013e31828b82aa. [DOI] [PubMed] [Google Scholar]
- 3.Megnien JL, Simon A, Gariepy J, Denarie N, Cocaul M, Linhart A, et al. Preclinical changes of extracoronary arterial structures as indicators of coronary atherosclerosis in men. J Hypertens. 1998;16:157–63. doi: 10.1097/00004872-199816020-00005. [DOI] [PubMed] [Google Scholar]
- 4.Khoury Z, Schwartz R, Gottlieb S, Chenzbraun A, Stern S, Keren A. Relation of coronary artery disease to atherosclerotic disease in the aorta, carotid and femoral arteries evaluated by ultrasound. Am J Cardiol. 1997;80:1429–33. doi: 10.1016/s0002-9149(97)00701-7. [DOI] [PubMed] [Google Scholar]
- 5.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–51. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ionita MG, van den Borne P, Catanzariti LM, Moll FL, de Vries JP, Pasterkamp G, et al. High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of rupture-prone lesions. Arterioscler Thromb Vasc Biol. 2010;30:1842–8. doi: 10.1161/ATVBAHA.110.209296. [DOI] [PubMed] [Google Scholar]
- 7.Baetta R, Corsini A. Role of polymorphonuclear neutrophils in atherosclerosis: current state and future perspectives. Atherosclerosis. 2010;210:1–13. doi: 10.1016/j.atherosclerosis.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 8.Soehnlein O, Zernecke A, Weber C. Neutrophils launch monocyte extravasation by release of granule proteins. Thromb Haemost. 2009;102:198–205. doi: 10.1160/TH08-11-0720. [DOI] [PubMed] [Google Scholar]
- 9.Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653–7. doi: 10.1016/j.amjcard.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Gül M, Uyarel H, Ergelen M, Uğur M, Işık T, Ayhan E, et al. Predictive value of neutrophil to lymphocyte ratio in clinical outcomes of non-ST elevation myocardial infarction and unstable angina pectoris: a 3-year follow-up. Clin Appl Thromb Hemost. 2014;20:378–84. doi: 10.1177/1076029612465669. [DOI] [PubMed] [Google Scholar]
- 11.Sönmez O, Ertaş G, Bacaksız A, Tasal A, Erdoğan E, Asoğlu E, et al. Relation of neutrophil-to-lymphocyte ratio with the presence and complexity of coronary artery disease: an observational study. Anadolu Kardiyol Derg. 2013;13:662–7. doi: 10.5152/akd.2013.188. [DOI] [PubMed] [Google Scholar]
- 12.Arbel Y, Finkelstein A, Halkin A, Birati EY, Revivo M, Zuzut M, et al. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis. 2012;225:456–60. doi: 10.1016/j.atherosclerosis.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Park JJ, Jang HJ, Oh IY, Yoon CH, Suh JW, Cho YS, et al. Prognostic value of neutrophil to lymphocyte ratio in patients presenting with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2013;111:636–42. doi: 10.1016/j.amjcard.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Doğdu O, Akpek M, Yarlıoğlueş M, Kalay N, Ardıç I, Elçik D, et al. Relationship between hematologic parameters and left ventricular systolic dysfunction in stable patients with multi-vessel coronary artery disease. Turk Kardiyol Dern Ars. 2012;40:706–13. doi: 10.5543/tkda.2012.82429. [DOI] [PubMed] [Google Scholar]
- 15.Gary T, Pichler M, Belaj K, Hafner F, Gerger A, Froehlich H, et al. Neutrophil-to-lymphocyte ratio and its association with critical limb ischemia in PAOD patients. PLoS One. 2013;8:e56745. doi: 10.1371/journal.pone.0056745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–24. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 17.Smith GD, Shipley MJ, Rose G. Intermittent claudication, heart disease risk factors, and mortality. The Whitehall Study. Circulation. 1990;82:1925–31. doi: 10.1161/01.cir.82.6.1925. [DOI] [PubMed] [Google Scholar]
- 18.Kornitzer M, Dramaix M, Sobolski J, Degre S, De Backer G. Ankle/arm pressure index in asymptomatic middle-aged males: an independent predictor of ten-year coronary heart disease mortality. Angiology. 1995;46:211–9. doi: 10.1177/000331979504600304. [DOI] [PubMed] [Google Scholar]
- 19.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–6. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 20.McKenna M, Wolfson S, Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis. 1991;87:119–28. doi: 10.1016/0021-9150(91)90014-t. [DOI] [PubMed] [Google Scholar]
- 21.Howell MA, Colgan MP, Seeger RW, Ramsey DE, Sumner DS. Relationship of severity of lower limb peripheral vascular disease to mortality and morbidity: a six-year follow-up study. J Vasc Surg. 1989;9:691–6. doi: 10.1067/mva.1989.vs0090691. [DOI] [PubMed] [Google Scholar]
- 22.Valentine RJ, Grayburn PA, Eichhorn EJ, Myers SI, Clagett GP. Coronary artery disease is highly prevalent among patients with premature peripheral vascular disease. J Vasc Surg. 1994;19:668–674. doi: 10.1016/s0741-5214(94)70040-0. [DOI] [PubMed] [Google Scholar]
- 23.Naruko T, Ueda M, Haze K, van der Wal AC, van der Loos CM, Itoh A, et al. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation. 2002;106:2894–900. doi: 10.1161/01.cir.0000042674.89762.20. [DOI] [PubMed] [Google Scholar]
- 24.Kalaycıoğlu E, Gökdeniz T, Aykan AC, Gül I, Boyacı F, Gürsoy OM, et al. Comparison of neutrophil to lymphocyte ratio in patients with coronary artery ectasia versus patients with obstructive coronary artery disease. Kardiol Pol. 2014;72:372–80. doi: 10.5603/KP.a2013.0349. [DOI] [PubMed] [Google Scholar]
- 25.Balta S, Demirkol S, Çelik T, Küçük U, Ünlü M, Arslan Z, et al. Association between coronary artery ectasia and neutrophil-lymphocyte ratio. Angiology. 2013;64:627–32. doi: 10.1177/0003319713480424. [DOI] [PubMed] [Google Scholar]
- 26.Haumer M, Amighi J, Exner M, Mlekusch W, Sabeti S, Schlager O, et al. Association of neutrophils and future cardiovascular events in patients with peripheral artery disease. J Vasc Surg. 2005;41:610–7. doi: 10.1016/j.jvs.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Aykan AÇ, Gül I, Gökdeniz T, Hatem E, Arslan AO, Kalaycıoğlu E, et al. Ankle brachial index intensifies the diagnostic accuracy of epicardial fat thickness for the prediction of coronary artery disease complexity. Heart Lung Circ. 2014;23:764–71. doi: 10.1016/j.hlc.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Aykan AÇ, Hatem E, Karabay CY, Gül I, Gökdeniz T, Kalaycıoğlu E, et al. Complexity of lower extremity peripheral artery disease reflects the complexity of coronary artery disease. Vascular. 2015;23:366–73. doi: 10.1177/1708538114550738. [DOI] [PubMed] [Google Scholar]