Abstract
Objective:
Recent conflicting studies on the renal effects of N-acetyl cysteine (NAC) after cardiac surgery have been published. The aim of this study was to evaluate the renal effects of NAC using neutrophil gelatinase-associated lipocalin (NGAL) blood levels in elderly patients undergoing coronary artery bypass grafting (CABG).
Methods:
This randomized, double-blinded, placebo-controlled study was conducted among geriatric patients (>65 years) scheduled to undergo CABG. A total of 60 consecutive patients were randomly assigned to 2 groups. The first group received I.V. NAC (n=30) and the second group received placebo (n=30) at induction of anesthesia and then for 20 h. NGAL values were determined and conventional renal function tests were performed. Statistical analysis was performed using SPSS 17.0 (IL, Chicago, USA). A p value of <0.05 was considered statistically significant
Results:
Plasma creatinine levels at 24 h postoperatively were significantly higher in the placebo group than in the NAC group (1.41±0.63 vs. 1.13±0.35; p<0.05). The mean serum NGAL levels at 3 h postoperatively were higher in the placebo group than in the NAC group (104.94±30.51 vs. 87.82±25.18; p<0.05). NGAL levels were similar between the groups at all other measurement time points. Plasma creatinine levels of ≥1.5 mg/dL or >25% of the baseline value at any time during the study period were observed in 27% of patients in the NAC group and 37% of patients in the placebo group; the difference was statistically significant (p<0.05).
Conclusion:
In the present study, we found that I.V. NAC infusion in elderly patients undergoing CABG reduced the incidence of acute kidney injury as determined by blood NGAL and creatinine levels.
Keywords: geriatric, coronary artery surgery, NAC, NGAL, kidney injury
Introduction
Patients undergoing coronary artery bypass grafting (CABG), particularly with cardiopulmonary bypass (CPB), have a considerably high risk (7.7%) of developing acute kidney injury (AKI) (1). Increased serum creatinine levels and decreased urine output are the most commonly observed findings of AKI. High rates of postoperative morbidity and mortality and increased in-hospital costs are very important negative outcomes of AKI after CABG surgery (2).
The major factors leading to AKI after CABG are preoperative renal impairment (increased serum creatinine levels), heart failure (reduced ejection fraction), diabetes mellitus, and the duration of CPB, which might be considered as independent determinants (3, 4). Advanced age has a particular importance among other risk factors, because being older in itself means having more chronic illnesses, such as diabetes mellitus, hypertension, peripheral arterial disease, and renal disease, resulting in a higher risk of postoperative complication rates for CABG, as is the case with any other surgical intervention (5).
N-acetyl cysteine (NAC) has antioxidant properties owing to its thiol group (6). NAC also acts as a vasodilatator (7). Dimari et al. (8) suggested that NAC refills glutathione stores, augments superoxide dismutase activity, interferes with autocatalytic lipid peroxidation, and scavenges free hydroxyl radicals. It has been suggested that NAC plays an important role as a renoprotective agent in ischemic and toxic acute renal failure in experimental models (9–11). Some recent clinical studies have demonstrated that NAC might prevent contrast nephropathy (12–14). However, the effect of NAC on renal function in patients undergoing CABG, particularly elderly patients, has not yet been completely defined. NAC has some side effects, such as cutaneous eruptions, wheezing, hemolysis, and moderate neutropenia, which must be monitored closely.
Early detection of AKI is very important for effective prevention and treatment. Effective treatment of AKI is dependent on early biomarkers. Serum creatinine level is currently used for the diagnosis of renal failure. However, it is not a reliable indicator during acute changes in renal function (15) because the levels can be within the normal range even in patients with >50% kidney damage (16). Therefore, there is an urgent need for additional early biomarkers of CABG-related AKI. When compared with creatinine, neutrophil gelatinase-associated lipocalin (NGAL) may be considered as a reliable diagnostic biomarker for the early detection of kidney injury (17).
In this double-blinded, randomized, placebo-controlled study, we aimed to evaluate whether NAC administration has renoprotective effects in elderly patients undergoing CABG, a high-risk group. The protective mechanism of NAC on renal functions may be related to the amelioration of tubular injury caused by oxidative stress (18). To detect the effect of NAC on renal function, we measured the serum levels of NGAL and creatinine.
Methods
This randomized, double-blinded, placebo-controlled study was performed after approval of the local ethics committee of the university. It was supported by the University Scientific Research Projects Unit. Written informed consent was obtained from the 60 patients included in the study. The patients were evaluated between May 2013 and October 2014 in 2 different cardiovascular surgery clinics (cardiovascular surgery departments at Afyon Kocatepe University and Antalya Education and Research Hospital). Seventy-eight eligible geriatric patients (>65 years) were scheduled for CABG with CPB. G-power 3.0.10 was used to determine the size of the treatment and control groups. When accepting the effect size as large, for the 30 treatment and 30 control patients, the power was found as 86%.
Eighteen of them were excluded from the study for the following reasons: preoperative plasma creatinine levels of >1.4 mg/dL, chronic renal replacement therapy, kidney transplantation, any concomitant operation, reoperation, patients with chronic pulmonary disease, ejection fraction of <35%, emergency operations, hepatic failure, autoimmune disease, systemic inflammatory disease, history of cerebrovascular disease within the last 6 months, and known or suspected allergy to NAC. The flow diagram of the study participants is given in Figure 1.
Figure 1.
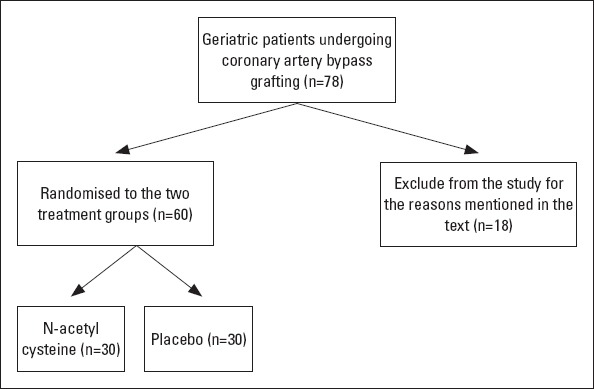
Flow diagram of the study participants
Before induction of anesthesia, 60 patients were randomly divided to receive NAC (n=30) or placebo (n=30). Allocation into the treatment or the placebo group and preparation of the study drug was performed by a person unrelated to this study. The patients were randomly assigned to the groups using envelopes. Study personnel, patients, and individuals participating in data collection and data analysis were blinded to the treatment assignment. NAC (Asist, Hüsnü Arsan Pharmacy Ltd, İstanbul, TURKEY) was administered in 0.9% saline as a loading dose of 150 mg/kg over 15 min, followed by 50 mg/kg over the next 4 h and 100 mg/kg over 16 h. The placebo group received similar volumes of 0.9% saline (1).
Preoperatively, all patients received their standard cardiac medications except for angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists. Acetylsalicylic acid and non-steroidal anti-inflammatory drugs were stopped 5 days and 1 day before surgery, respectively. The same anesthesia protocol was performed for all study patients. The patients were monitored (Datex-Ohmeda Avance, GE Healthcare, Helsinki, Finland) by continuous electrocardiography, pulse oximetry, capnography, entropy (state and response), central venous pressure (CVP), and invasive blood pressure. Median sternotomy was performed. All patients underwent conventional CABG with CPB by the same surgical teams in each center. The patients were anticoagulated with 300 U/kg of heparin to provide an activated clotting time (ACT) longer than 400 s. CPB was started after cannulation of the aorta and the right atrium. Membrane oxygenators (Hilite 7000, Medos, Stolberg, Germany) were primed with 1000 mL of Ringer’s lactate and 100 mL of 20% mannitol to maintain a hematocrit level of 26%±2%. None of the patients received aprotinin. Another 100 mL of 20% mannitol was given just before declamping of the aorta. Non-pulsatile pump flow was set at 2.4 L/min/m2 to maintain mean arterial pressure (MAP) between 50 and 70 mm Hg. CPB was performed at mild hypothermia with a core temperature of 32°C. Intermittent antegrade crystalloid cardioplegia with blood at a ratio of 4:1 or 8:1 was used for myocardial protection. Protamine sulfate was used to antagonize heparin. The patients were rewarmed to a temperature of 37°C. During CPB, when required, Isolyte-S (Ecz.Baxter drugs, Isolyte-S, pH 7.4, PVC 1000 mL) was added to the CPB circuit to keep the volume above the minimal level of the venous reservoir.
Intraoperative hemodynamic parameters, i.e., MAP, heart rate (HR), CVP, and oxygen saturation measured by pulse oximetry (SaO2, %), were recorded and analyzed statistically. Each of these was recorded before administration of the study medication, before CPB, during CPB, and at the end of surgery. Any hypotension (MAP: <60 mm Hg) or bradycardia (HR: <60/min) episode longer than 10 min in the intensive care unit (ICU) follow-up period and inotropic drug need or intra-aortic balloon pump (IABP) support were also recorded.
After CPB and in the ICU follow-up period, additional fluid (Isolyte-S or fresh frozen plasma) was administered according to routine postoperative care. Red blood cells were transfused when the hemoglobin concentration fell below 6 mg/dL during extracorporeal circulation or below 8.5 mg/dL after surgery.
Blood NGAL levels were evaluated preoperatively (baseline) and at 3 h, 12 h, and 24 h postoperatively. Plasma creatinine levels were determined preoperatively (baseline) and at 3 h, 12 h, 24 h, and 48 h postoperatively. Estimated glomerular filtration rate (eGFR) for all study patients were also calculated using the Modification of Diet in Renal Disease (MDRD) formula.
Plasma samples were stored at –20°C and analyzed at the Central Hospital Laboratory of our University. NGAL levels were measured with a human lipocalin-2/NGAL Elisa kit (Biovendor Laboratorni medicina a.s., Czech Republic). The within-series coefficient of variation (CV) was 7.03% and 8.38% at 68.19 and 23.63 ng/mL, respectively, and the between-series CV was 9.73% and 9.77% at 32.55 and 38.14 ng/mL, respectively. Plasma creatinine and blood urea nitrogen levels were respectively analyzed with the enzymatic CREA-plus assay method and kinetic UV assay for urea nitrogen from BEN Biochemical Enterprise (BEN Biochemical Enterprise, Milano, Italy) on a ChemWell 2910 analyzer (Awareness Technology, Inc. Palm City, USA).
Serum NGAL levels of >149 ng/mL and plasma creatinine levels of ≥1.5 mg/dL of >25% of the baseline were the outcome measures (19). Mortality within 30 days after surgery, renal replacement therapy, and length of ICU stay were also recorded.
Statistical analysis
The Shapiro–Wilk test was used as the normality test for the distribution of continuous variables. Student’s t-test was used for normally distributed variables and the results were given as mean±standard deviation (SD). The Mann–Whitney U test was used for non-normally distributed variables and the results were given as median (25%–75%). The chi-square test was used for the comparison of categorical variables and the results were given as percentiles (%). Statistical analysis was performed using SPSS 17.0 (IL, Chicago, USA). A p value of <0.05 was considered statistically significant.
Results
A total of 60 patients were included, 30 in the placebo group (group I) and 30 in the NAC group (group II). While the mean body mass index (BMI) was 26.06±4.97 in the NAC group, it was 28.83±3.44 in the placebo group. This difference was statistically significant (p<0.05). Except for the difference in BMI, the demographic features were similar between the 2 study groups (Table 1).
Table 1.
Demographic features and perioperative variables of the 2 groups
| Variables | Placebo (group I, n=30) | NAC (group II, n=30) | P |
|---|---|---|---|
| Age, years | 70.50 (68.00–73.25) | 71.50 (69.00–73.50) | 0.212 |
| Male, n, % | 73 | 60 | 0.281 |
| BMI, kg/m2 | 28.27 (26.75–30.40) | 25.03 (22.84–28.20) | 0.015 |
| LVEF, % | 56.50 | 60.00 | 0.424 |
| DM, n, % | 47 | 50 | 0.800 |
| HT, n, % | 57 | 43 | 0.310 |
| PAD, n, % | 13 | 10 | 0.694 |
| Smoking, n, % | 53 | 37 | 0.201 |
| ACE Inh., n, % | 23 | 27 | 0.770 |
| Duration of | |||
| surgery, min | 240 (235–270) | 220 (210–250) | 0.434 |
| CPB time, min | 99.33±22.50 | 91.47±22.06 | 0.362 |
| Cross-clamp | |||
| time, min | 54.00 (43.25–60.00) | 49.00 (39.25–62.00) | 0.626 |
| Bleeding, mLa | 545 (380–713) | 565 (438–700) | 0.506 |
| Red blood cells, | |||
| Unitsb | 1.70±0.84 | 1.30±1.01 | 0.073 |
| Urine output, mL | |||
| PO day 1 | 2913±471 | 2982±534 | 0.599 |
| PO day 2 | 2816±353 | 2674±436 | 0.170 |
| Volume, mLc | 3330 (3088–3515) | 3100 (2900–3500) | 0.346 |
| Bradycardiad, n, % | 10.00 | 6.67 | 0.647 |
| Hypotensiond, n, % | 20 | 20 | 1.000 |
| ICU stay, days | 1.57±0.50 | 1.63±0.61 | 0.648 |
| Inotropic drug, n, % | 27 | 20 | 0.549 |
| IABP, n, % | 6.67 | 3.33 | 0.561 |
| Hospital stay, days | 5.93±1.17 | 6.20±1.16 | 0.379 |
| Preoperative | |||
| creatinine, mg/dL | 0.98±0.19 | 0.93±0.24 | 0.402 |
| NGAL, ng/mL | 64.13±20.78 | 55.24±24.69 | 0.137 |
ACE Inh. - angiotensin-converting enzyme inhibitors; BMI - body mass index; CPB - cardiopulmonary bypass; DM - diabetes mellitus; HT - hypertension; IABP - intra-aortic balloon pump; ICU - intensive care unit; LVEF - left ventricular ejection fraction; NAC - N-acetyl cysteine; NGAL - neutrophil gelatinase-associated lipocalin; PAD - peripheral arterial disease; PO - postoperative;
total bleeding during the first 24 h postoperatively;
total units of packed red blood cell transfusion during intensive care unit follow-up;
total amount of volume received during the first 24 h postoperatively;
an episode longer than 10 min
Student’s t-test, Mann–Whitney U test, and chi-square test were used for the comparison of groups
There were no statistically significant differences between the 2 groups with respect to CPB time, aortic cross-clamp time, and duration of surgery. The p-values of these variables are also shown in Table 1. Inotropic drug support in the postoperative period was required for 27% patients in the placebo group and 20% patients in the NAC group. This difference between the 2 groups was not statistically significant (p=0.087). There were no statistical differences between the 2 groups with respect to the amount of bleeding (573±195 mL in the placebo group and 612±253 mL in the NAC group) and volume intake (3339±413 mL in the placebo group and 3233±44 mL in the NAC group) during the first 24 h. The occurrence of hypotension, defined as MAP of <60 mm Hg over 10 min (20% in each group), and bradycardia, defined as <60 beats/min over 10 min (10% in the placebo group and 6.67% in the NAC group), was similar between the 2 groups. Two patients in the placebo group and 1 patient in the NAC group suffered from low-output syndrome requiring an IABP. Baseline renal function, as measured by preoperative plasma creatinine levels, was similar between the 2 groups (Fig. 2).
Figure 2.
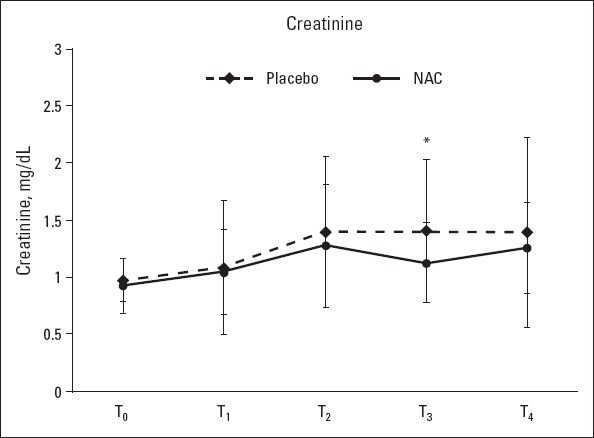
Plasma creatinine levels at T0 (baseline), T1 (3 h postoperatively), T2 (12 h postoperatively), T3 (postoperative day 1), and T4 (postoperative day 2); *: P<0.05
Plasma creatinine levels at 24 h postoperatively were significantly higher in the placebo group than in the NAC group (1.41±0.63 vs. 1.13±0.35, p<0.05). At all other measurement time points, there were no significant differences between the 2 groups with respect to the mean plasma creatinine levels and the total daily amount of urine output (p>0.05) (Table 1, Fig. 2). The mean serum NGAL levels at 3 h postoperatively were higher in the placebo group than in the NAC group (104.94±30.51 vs. 87.82±25.18 ng/mL), and this difference was statistically significant (p<0.05). NAG levels were similar between the groups at all other measurement time points (baseline and 12 and 24 h postoperatively) (Fig. 3). One patient in the placebo group required acute renal replacement therapy during hospital stay. The mean preoperative (baseline) eGFR in the NAC group was significantly lower than that in the placebo group (74.38±16.23 vs. 84.15±20.37, p=0.045) (Fig. 4).
Figure 3.
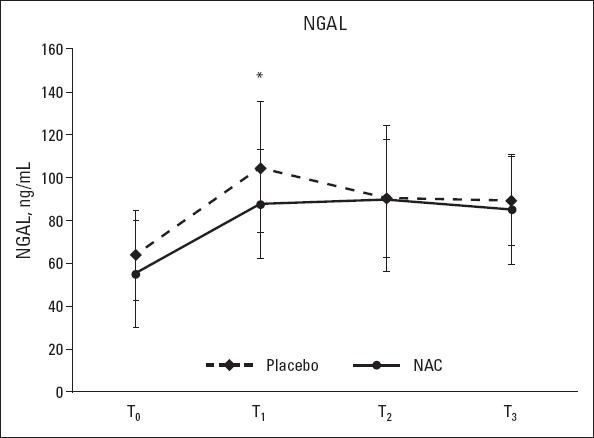
Serum NGAL levels at T0 (baseline), T1 (3 h postoperatively), T2 (12 h postoperatively), and T3 (24 h postoperatively). Student’s t-test was used for the comparison of groups; *: P<0.05
Figure 4.
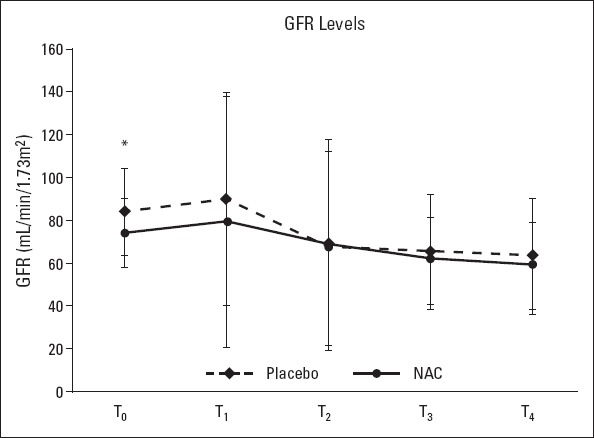
Estimated glomerular filtration rate (eGFR) at T0 (baseline), T1 (3 h postoperatively), T2 (12 h postoperatively), T3 (postoperative day 1), and T4 (postoperative day 2). Student’s t-test was used for the comparison of groups; *: P<0.05
With respect to hemodynamic parameters (Fig. 5), MAP before CPB and at the end of surgery was higher in the placebo group than in the NAC group (p<0.001). HR at the end of surgery was higher in the NAC group than in the placebo group (p<0.001). SaO2 before induction of anesthesia was higher in the placebo group than in the NAC group; however, during CPB and at the end of surgery, SaO2 was higher in the NAC group than in the placebo group (p<0.001). Furthermore, CVP was higher at all measurement time points (before induction of anesthesia, before CPB, and at the end of surgery) in the placebo group than in the NAC group (p<0.001).
Figure 5.
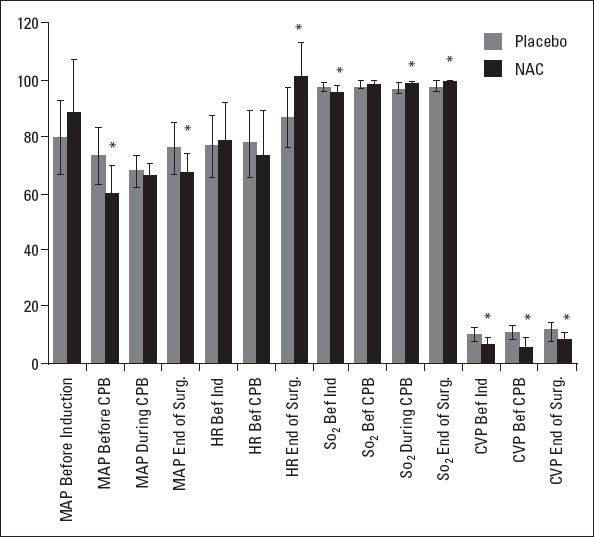
Intraoperative hemodynamic parameters. MAP, mean arterial pressure; HR, heart rate; SaO2, oxygen saturation; CVP, central venous pressure; *: P<0.001
One patient in the placebo group died on postoperative day 1 because of massive pulmonary arterial emboli diagnosed with CT angiography. Two patients in the NAC group were excluded from the study because of cutaneous eruptions (possible NAC allergy). There was no mortality in the NAC group. We added 3 new eligible patients to the study to bring the number to a total of 60. Furthermore, plasma creatinine levels of ≥1.5 mg/dL or >25% of the baseline value at any time during the study period were observed in 27% of patients in the NAC group and 37% of patients in the placebo group; the difference was statistically significant (p<0.05) (Table 2).
Table 2.
Number of patients (%) with acute kidney injury at any time point during the study
| Placebo (group I, n=30) | NAC (group II, n=30) | P | |
|---|---|---|---|
| Plasma creatinine levels of ≥1.5 mg/dL or >25% of baseline, % | 37 | 27 | 0.049 |
| Serum NGAL levels of >149 ng/mL, % | 4.17 | 0.83 | 0.099 |
| Renal replacement therapy, % | 3.33 | 0.00 | 0.321 |
Discussion
This randomized, double-blinded study demonstrated that I.V. NAC had some beneficial effects on renal functions in elderly patients undergoing CABG. In recent years, NAC has been found to have protective effects on oxidative stress-mediated organ injuries, particularly on renal function (13). According to the literature, there are some contradictory reports about the renoprotective effects of NAC. Consistent with our results, Fischer et al. (20) have demonstrated that NAC has some beneficial effects on renal function after cardiac surgery. However, in a study by Burns et al. (21), it was indicated that there was no difference between NAC and placebo with respect to the renal function of high-risk patients undergoing CABG. However, some methodological differences must be regarded when comparing these studies. The NAC doses were higher in our study than in the previous study, which used only creatinine as a biomarker for assessing renal function. In addition, we included elderly patients in our study, and older age is recognized as a risk factor for postoperative renal failure (4). Ristikankare et al. (1) performed the first study related to the dose and method of administration of NAC in order to observe the renoprotective roles of NAC during cardiac surgery with CPB. They selected high doses of NAC, similar to other studies that have shown the beneficial effects of NAC on hepatic injuries. Although the dose and method of administration of NAC in our study were the same as in the study of Ristikankare et al. (1), the results are contradictory.
AKI is one of the important complications of CABG that can lead to increased morbidity and mortality (22). Increased duration in the ICU or in the hospital due to AKI is also associated with the utilization of resources. Following cardiac surgery, elderly patients with CPB are particularly at a high risk of developing AKI. Because of the high prevalence of AKI and association with increased morbidity and mortality, renoprotective strategies and early diagnosis are essential.
Renal damage can emerge depending on many factors, such as the oxidative stress that can occur upon reperfusion in CPB (23). Renal dysfunction after cardiac surgery is frequently observed in patients, particularly in high-risk patients, even though there are common ways to control these risk factors. Therefore, effective treatments should be developed to cope with these postoperative problems. To achieve this, researchers have studied many antioxidant molecules and have indicated that only some molecules (such as NAC and superoxide dismutase) show a positive effect on the renal system and even improve its function (22).
As a thiol-modifying molecule and promoter of reduced glutathione (GSH), which is decreased during ischemia, NAC is known to be an oxidant scavenger (24). Some adverse events of NAC, such as nausea and vomiting, have been reported in a review by Adabağ et al. (25) Hypotension and diarrhea have also been reported in rare cases not needing inotropic or pressor medications. Anaphylactic reactions occur in approximately 3% cases and include urticarial rash, angioedema, bronchospasm, and hypotension. These reactions, however, are usually mild and respond to stopping the infusion and symptomatic treatment with antihistamines. However, systemic reactions may also occur, requiring treatment with intramuscular adrenaline and corticosteroids (26). Although there have been deaths associated with an overdose of NAC, none have been reported with normal treatment doses (27).
It has been stated that only higher doses of NAC can entirely show its antioxidant and anti-inflammatory effects (28). According to the literature, it has been shown that NAC has anti-inflammatory and antioxidant effects (such as TNF-a antagonism and inhibition of vascular cell adhesion molecule expression) that could prevent renal dysfunction (29). It has been found that NAC inhibits the oxidation and inflammatory responses in CPB (30) as well as oxidative stress-mediated proximal tubular damage in angiography (14).
It is very important to protect both cardiac and renal functions, and therefore, tubular necrosis with CABG needs to be diagnosed and treated as soon as possible. The small human NGAL protein (25 kDa), known as a lipocalin family member, was first identified in 2003 as a renal gene activated in the AKI animal model. The NGAL protein is mostly produced in neutrophils and renal proximal tubules, whereas prostate and epithelial cells of the respiratory system contain comparatively lower levels of the NGAL protein. Human NGAL protein is known to be highly produced under stress conditions such as infection, ischemia, and inflammation, as well as in cancer. NGAL can be practically quantified in blood and urine because of its small size and non-degradable structure (31). According to experimental studies performed in animal models, NGAL is the first gene activated upon ischemia and nephrotoxic damage. Therefore, it can be regarded as an AKI marker (32).
In this study, blood NGAL levels in each group increased considerably during the first 3 h postoperatively. Blood NGAL levels were found to be significantly higher in the placebo group than in the NAC group at 3 h postoperatively. The levels in both groups were almost equal at 12 h postoperatively and remained constant thereafter, although the values were still higher than the baseline levels. On the other hand, serum creatinine levels in each group were increased at 12 h postoperatively, which is later than the time point at which NGAL levels increased. Serum creatinine levels in the placebo group remained the same after 12 h postoperatively, but decreased almost to the baseline levels in the NAC group. The difference in creatinine levels between the placebo and NAC groups was significant at 24 h postoperatively. It is possible to state that measurement of NGAL protein is more useful than other kidney function tests for the early detection of renal dysfunction. Early identification of AKI is very important for preventing organ loss (32). In a previous study, NGAL protein was regarded as a marker for all types of AKI. The study detected NGAL protein in the urine even though there was no alteration in the renal function upon CABG (33).
A limited number of studies have examined the relationship between specific intraoperative CPB parameters and AKI risk. Hypotension is not a unique factor related to AKI during CPB (34). It has been stated that when CPB takes a longer time, it could have a detrimental effect on renal function and can trigger organ damage (35). Despite the vital role of CPB, only a limited number of studies have mentioned this deleterious feature of CPB hemodynamics. Kanji et al. (36) have shown that AKI is closely related to delta MAP (preoperative MAP minus the average MAP during CPB) or low CPB flow in high-risk patients during heart surgery. According to our results, MAP values of the placebo group were lower than those of the NAC group before induction of anesthesia, whereas the values were higher in the placebo group than in the NAC group before CPB, during CPB, and at the end of surgery. Therefore, we can state that the placebo patients had an advantage with respect to MAP. Nevertheless, a large number of placebo patients showed high serum creatinine or NGAL levels at each time point during the study period, indicating a renoprotective effect of NAC.
It has been suggested that higher BMI is associated with a greater decline in kidney function in young adults with preserved GFR at baseline (37). Although the average BMI in our NAC group was higher than that in the placebo group, renal functions were preserved in the NAC group compared with the placebo group. These results may support the evidence of a renoprotective effect of NAC.
In our study, SaO2 was higher in the placebo group before induction of anesthesia; however, it was observed to be higher in the NAC group before CPB, during CPB, and at the end of surgery. In a study designed by Haase et al. (38), it was suggested that the hemoglobin level during CPB is an independent risk factor for AKI but SaO2 levels did not support this association. Therefore, it is not accurate to relate the low levels of creatinine and NGAL in the NAC group to higher SaO2 levels compared with placebo patients.
Study limitations
One limitation of our study was the relatively small number of patients. Secondly, postoperative CVP of ≥14 cm H2O is one of the important risk factors for AKI after cardiac surgery (39). When we looked at the CVP values of the 2 groups in this study, the placebo group had higher CVP values and this might be a confounding effect for the difference in the renal function tests after CABG.
Conclusion
In summary, the present study reveals that large NAC infusion doses can be useful in preventing the aggravation of AKI in elderly patients undergoing CABG with CPB. Conventional renal function tests such as serum creatinine, blood urea nitrogen, and urine output measurements may not detect the development of acute kidney dysfunction in the early postoperative period. The renoprotective effect of NAC in the early postoperative period, as determined by measurements of blood NGAL levels, was significant.
Acknowledgments:
The authors acknowledge with gratitude the cooperation of people who collected and managed the database of our institution. This study was supported by the Afyon Kocatepe University Scientific Research Projects unit.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Author contributions: Concept- M.A., A.S.K., E.D.B., N.Ö.K.; Design- M.A., G.Ç.; Supervision- M.A., G.Ç.; Funding-H.B.K., M.E., E.D.B., N.Ö.K., A.S.K.; Materials- H.B.K., M.E., E.D.B., N.Ö.K., A.S.K.; Data collection &/or processing – H.B.K., M.E., E.D.B., O.T.D., F.A.; Analysis and/or interpretation– M.A., O.T.D., F.A.; Literature search- M.A., F.A., G.Ç.; Writing – M.A., O.T.D.; Critical review- M.A., O.T.D., E.D.B.
References
- 1.Ristikankare A, Kuitunen T, Kuitunen A, Uotila L, Vento A, Suojaranta-Ylinen R, et al. Lack of renoprotective effect of i.v. N-acetylcysteine in patients with chronic renal failure undergoing cardiac surgery. Br J Anaesth. 2006;97:611–6. doi: 10.1093/bja/ael224. [DOI] [PubMed] [Google Scholar]
- 2.Wijeysundera DN, Karkouti K, Dupuis JY, Rao V, Chan CT, Granton JT, et al. Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. JAMA. 2007;297:1801–9. doi: 10.1001/jama.297.16.1801. [DOI] [PubMed] [Google Scholar]
- 3.Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med. 1998;128:194–203. doi: 10.7326/0003-4819-128-3-199802010-00005. [DOI] [PubMed] [Google Scholar]
- 4.Conlon PJ, Stafford-Smith M, White WD, Newman MF, King S, Winn MP, et al. Acute renal failure following cardiac surgery. Nephrol Dial Transplant. 1999;14:1158–62. doi: 10.1093/ndt/14.5.1158. [DOI] [PubMed] [Google Scholar]
- 5.Moshkovitz Y, Paz Y, Shabtai E, Cotter G, Amir G, Smolinsky AK, et al. Predictors of early and overall outcome in coronary artery bypass without cardiopulmonary bypass. Eur J Cardiothorac Surg. 1997;12:31–9. doi: 10.1016/s1010-7940(97)00129-2. [DOI] [PubMed] [Google Scholar]
- 6.McCord JM, Fridovich I. The biology and pathology of oxygen radicals. Ann Intern Med. 1978;89:122–7. doi: 10.7326/0003-4819-89-1-122. [DOI] [PubMed] [Google Scholar]
- 7.Kiefer P, Vogt J, Radermacher P. From mucolytic to antioxidant and liver protection: new aspects in the intensive care unit career of N-acetylcysteine. Crit Care Med. 2000;28:3935–6. doi: 10.1097/00003246-200012000-00037. [DOI] [PubMed] [Google Scholar]
- 8.Dimari J, Megyesi J, Udvarhelyei N, Price P, Davis R, Safirstein R. N-acetylcysteine ameliorates ischemic renal failure. Am J Physiol. 1997;272:292–8. doi: 10.1152/ajprenal.1997.272.3.F292. [DOI] [PubMed] [Google Scholar]
- 9.Tariq M, Morais C, Sobki S, Al Sulaiman M, Al Khader A. N-acetylcysteine attenuates cyclosporin-induced nephrotoxicity in rats. Nephrol Dial Transplant. 1999;14:923–9. doi: 10.1093/ndt/14.4.923. [DOI] [PubMed] [Google Scholar]
- 10.Mazzon E, Britti D, De Sarro A, Caputi AP, Cuzzocrea S. Effect of N-acetylcysteine on gentamicin-mediated nephropathy in rats. Eur J Pharmacol. 2001;424:75–83. doi: 10.1016/s0014-2999(01)01130-x. [DOI] [PubMed] [Google Scholar]
- 11.Conesa EL, Valero F, Nadal JC, Fenoy FJ, López B, Arregui B, et al. N-acetyl-L-cysteine improves renal medullary hypoperfusion in acute renal failure. Am J Physiol Regul Integr Comp Physiol. 2001;281:730–7. doi: 10.1152/ajpregu.2001.281.3.R730. [DOI] [PubMed] [Google Scholar]
- 12.Birck R, Krzossok S, Markowetz F, Schnülle P, van der Woude FJ, et al. Acetylcysteine for prevention of contrast nephropathy: meta-analysis. Lancet. 2003;362:598–603. doi: 10.1016/S0140-6736(03)14189-X. [DOI] [PubMed] [Google Scholar]
- 13.Tepel M, Van der Giet M, Schawarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180–4. doi: 10.1056/NEJM200007203430304. [DOI] [PubMed] [Google Scholar]
- 14.Drager LF, Andrade L, Barros de Toledo JF, R M Laurindo F, César LAM, et al. Renal effects of N-acetylcysteine in patients at risk for contrast nephropathy: decrease in oxidant stress-mediated renal tubular injury. Nephrol Dial Transplant. 2004;19:1803–7. doi: 10.1093/ndt/gfh261. [DOI] [PubMed] [Google Scholar]
- 15.Bellomo R, Kellum JA, Ronco C. Defining acute renal failure: physiological principles. Intensive Care Med. 2004;30:33–7. doi: 10.1007/s00134-003-2078-3. [DOI] [PubMed] [Google Scholar]
- 16.Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114:5–14. doi: 10.1172/JCI22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel M, Sachan R, Gangwar R, Sachan P, Natu S. Correlation of serum neutrophil gelatinase-associated lipocalin with acute kidney injury in hypertensive disorders of pregnancy. Int J Nephrol Renovasc Dis. 2013;6:181–6. doi: 10.2147/IJNRD.S45523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gormley SM, McBride WT, Armstrong MA, Young IS, McClean E, MacGowan SW, et al. Plasma and urinary cytokine homeostasis and renal dysfunction during cardiac surgery. Anesthesiology. 2000;93:1210–6. doi: 10.1097/00000542-200011000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Balkanay OO, Göksedef D, Ömeroğlu SN, İpek G. The doserelated effects of Dexmedetomidine on renal functions and serum neutrophil gelatinase-associated lipocalin values after coronary artery bypass grafting: a randomized, triple-blind, placebo-controlled study. Interact Cardiovasc Thorac Surg. 2015;20:209–14. doi: 10.1093/icvts/ivu367. [DOI] [PubMed] [Google Scholar]
- 20.Fischer UM, Tossios P, Mehlhorn U. Renal protection by radical scavenging in cardiac surgery patients. Curr Med Res Opin. 2005;21:1161–4. doi: 10.1185/030079905X53289. [DOI] [PubMed] [Google Scholar]
- 21.Burns KE, Chu MW, Novick RJ, Fox SA, Gallo K, Martin CM, et al. Perioperative N-acetylcysteine to prevent renal dysfunction in high-risk patients undergoing CABG surgery: a randomized controlled trial. JAMA. 2005;294:342–50. doi: 10.1001/jama.294.3.342. [DOI] [PubMed] [Google Scholar]
- 22.Santana-Santos E, Marcusso ME, Rodrigues AO, Queiroz FG, Oliveira LB, Rodrigues AR, et al. Strategies for prevention of acute kidney injury in cardiac surgery: an integrative review. Rev Bras Ter Intensiva. 2014;26:183–92. doi: 10.5935/0103-507X.20140027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellomo R, Auriemma S, Fabbri A, D’Onofrio A, Katz N, McCullough PA, et al. The pathophysiology of cardiac surgery-associated acute kidney injury (CSA-AKI) Int J Artif Organs. 2008;31:166–78. doi: 10.1177/039139880803100210. [DOI] [PubMed] [Google Scholar]
- 24.Tossios P, Bloch W, Huebner A, Raji MR, Dodos F, Klass O, et al. N-Acetylcysteine prevents reactive oxygen species-mediated myocardial stress in patients undergoing cardiac surgery: results of a randomized, double-blind, placebo-controlled clinical trial. J Thorac Cardiovasc Surg. 2003;126:1513–20. doi: 10.1016/s0022-5223(03)00968-1. [DOI] [PubMed] [Google Scholar]
- 25.Adabağ AS, Ishani A, Bloomfield HE, Ngo AK, Wilt TJ. Efficacy of N-acetylcysteine in preventing renal injury after heart surgery: a systematic review of randomizedtrials. Eur Heart J. 2009;30:1910–7. doi: 10.1093/eurheartj/ehp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey B, McGuigan MA. Management of anaphylactoid reactions to N-acetylcysteine. Ann Emerg Med. 1998;31:710–5. doi: 10.1016/s0196-0644(98)70229-x. [DOI] [PubMed] [Google Scholar]
- 27.Appelboam AV, Dargan PI, Knighton J. Fatal anaphylactoid reaction to N acetylcysteine: caution in patients with asthma. Emerg Med J. 2002;19:594–5. doi: 10.1136/emj.19.6.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadowska AM, Manuel-y-Keenoy B, Vertongen T, Schippers G, Radomska-Lesniewska D, Heytens E, et al. Effect of N-acetylcysteine on neutrophil activation markers in healthy volunteers: in vivo and in vitro study. Pharmacol Res. 2006;53:216–25. doi: 10.1016/j.phrs.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Meldrum DR, Donnahoo KK. Role of TNF in mediating renal insufficiency following cardiac surgery: evidence of a postbypass cardiorenal syndrome. J Surg Res. 1999;85:185–99. doi: 10.1006/jsre.1999.5660. [DOI] [PubMed] [Google Scholar]
- 30.Sucu N, Cinel I, Ünlü A, Aytaçoğlu B, Tamer L, Koçak Z, et al. N-acetylcysteine for preventing pump-induced oxidoinflammatory response during cardiopulmonary bypass. Surg Today. 2004;34:237–42. doi: 10.1007/s00595-003-2699-8. [DOI] [PubMed] [Google Scholar]
- 31.Antonucci E, Lippi G, Ticinesi A, Pigna F, Guida L, Morelli I, et al. Neutrophil gelatinase-associated lipocalin (NGAL): a promising biomarker for the early diagnosis of acute kidney injury (AKI) Acta Biomed. 2014;85:289–94. [PubMed] [Google Scholar]
- 32.Shapiro NI, Trzeciak S, Hollander JE, Birkhahn R, Otero R, Osborn TM, et al. A prospective, multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Crit Care Med. 2009;37:96–104. doi: 10.1097/CCM.0b013e318192fd9d. [DOI] [PubMed] [Google Scholar]
- 33.Leino K, Hynynen M, Jalonen J, Salmenperä M, Scheinin H, Aantaa R. Renal effects of dexmedetomidine during coronary artery bypass surgery: a randomized placebo-controlled study. BMC Anesthesiol. 2011;11:9. doi: 10.1186/1471-2253-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartels C, Gerdes A, Babin-Ebell J, Beyersdorf F, Boeken U, Doenst T, et al. Cardiopulmonary bypass: Evidence or experience based? J Thorac Cardiovasc Surg. 2002;124:20–7. doi: 10.1067/mtc.2002.121506. [DOI] [PubMed] [Google Scholar]
- 35.Palomba H, de Castro I, Neto AL, Lage S, Yu L. Acute kidney injury prediction following elective cardiac surgery: AKICS Score. Kidney Int. 2007;72:624–31. doi: 10.1038/sj.ki.5002419. [DOI] [PubMed] [Google Scholar]
- 36.Kanji HD, Schulze CJ, Hervas-Malo M, Wang P, Ross DB, Zibdawi M, et al. Difference between pre-operative and cardiopulmonary bypass mean arterial pressure is independently associated with early cardiac surgery-associated acute kidney injury. J Cardiothorac Surg. 2010;5:71. doi: 10.1186/1749-8090-5-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grubbs V, Lin F, Vittinghoff E, Shlipak MG, Peralta CA, Bansal N, et al. Body mass index and early kidney function decline in young adults: a longitudinal analysis of the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Kidney Dis. 2014;63:590–7. doi: 10.1053/j.ajkd.2013.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haase M, Bellomo R, Story D, Letis A, Klemz K, Matalanis G, et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol Dial Transplant. 2012;27:153–60. doi: 10.1093/ndt/gfr275. [DOI] [PubMed] [Google Scholar]
- 39.Harel Z, Chan CT. Predicting and preventing acute kidney injury after cardiac surgery. Curr Opin Nephrol Hypertens. 2008;17:624–8. doi: 10.1097/MNH.0b013e32830f4590. [DOI] [PubMed] [Google Scholar]


