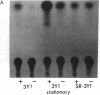
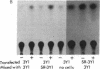
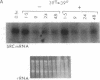
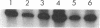Abstract
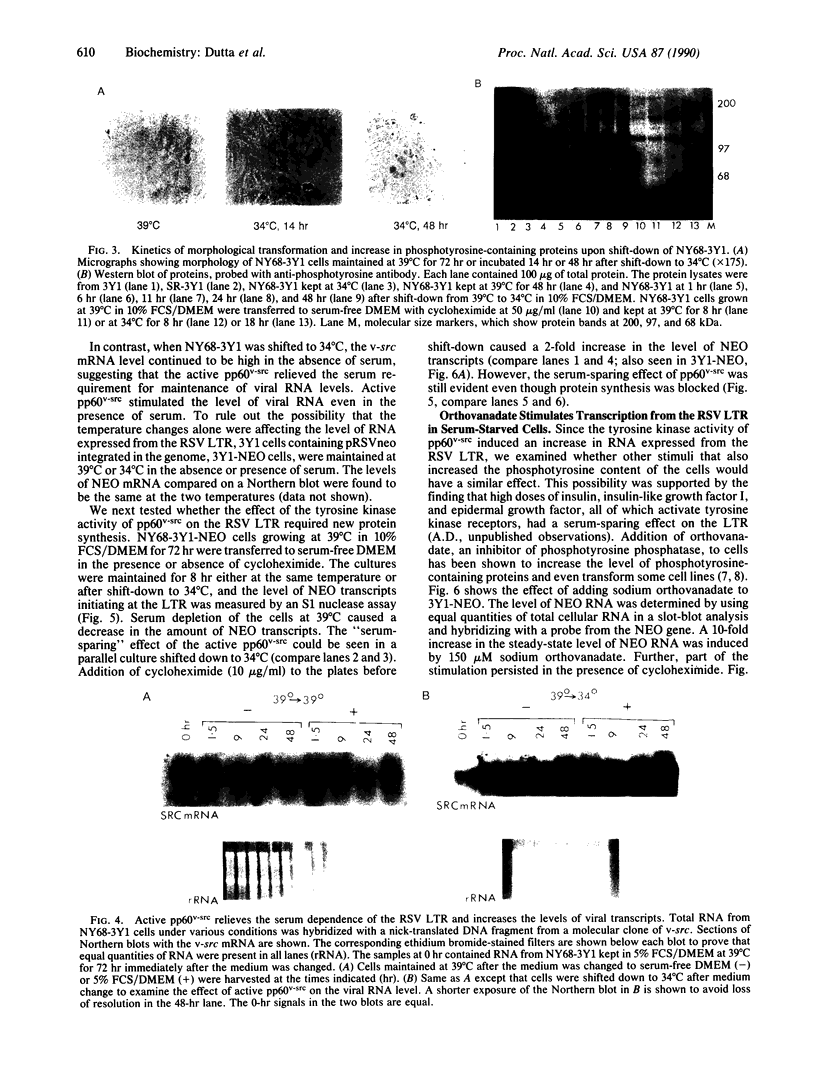
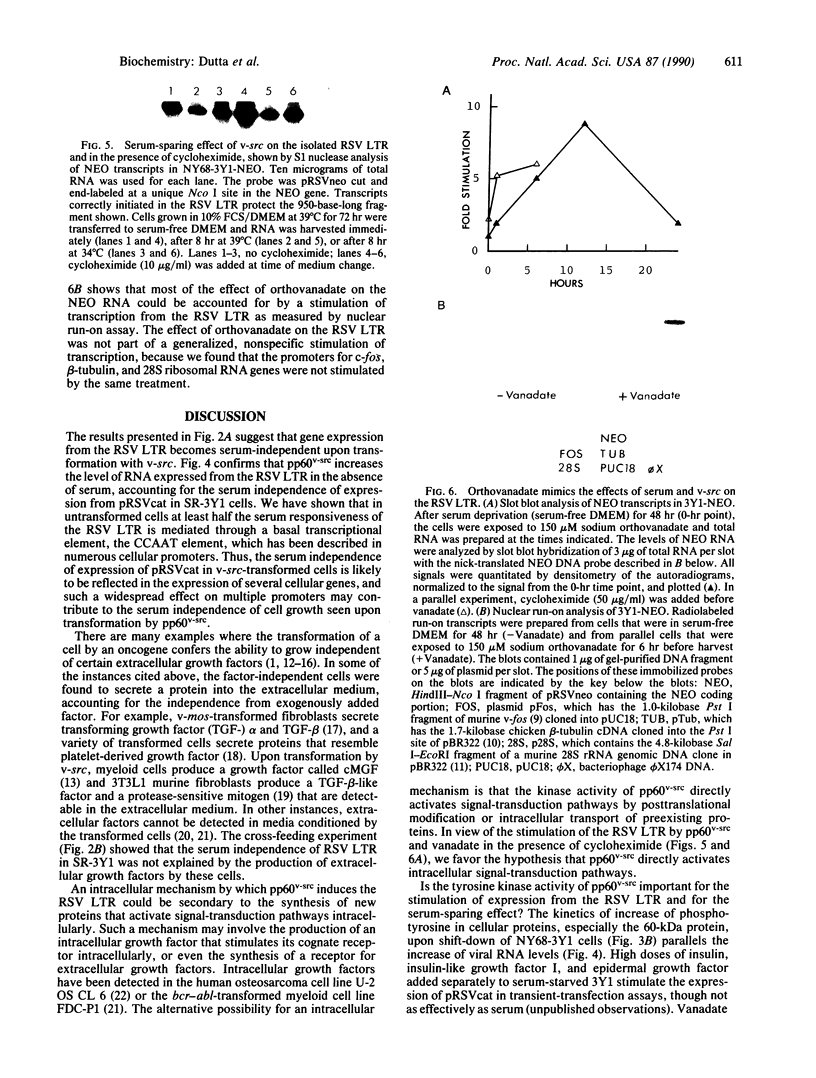
We found that transcription from the promoter in the long terminal repeat of Rous sarcoma virus in rat 3Y1 cells is dependent on the presence of serum in the culture. However, this serum dependence of transcription was relieved when 3Y1 cells were transformed by the oncogene v-src. Crossfeeding experiments showed no evidence for the production of a serum-substituting extracellular growth factor by the transformed cells. Using 3Y1 cells transformed with temperature-sensitive Rous sarcoma virus, we showed that the tyrosine kinase activity of pp60v-src was responsible for the serum-sparing effect on the level of RNA expressed from the viral promoter. Sodium orthovanadate, an inhibitor of phosphotyrosine phosphatases that nonspecifically elevates the level of phosphotyrosine-containing proteins in cells, stimulated transcription from the viral promoter. The effects of both pp60v-src and orthovanadate were resistant to cycloheximide. These results suggest that the serum independence of transcription from the viral promoter in v-src-transformed cells was probably due to the constitutive activation of intracellular growth-factor pathways by the tyrosine kinase activity of pp60v-src.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B., Leutz A., Graf T. Autocrine growth induced by src-related oncogenes in transformed chicken myeloid cells. Cell. 1984 Dec;39(3 Pt 2):439–445. doi: 10.1016/0092-8674(84)90451-3. [DOI] [PubMed] [Google Scholar]
- Anzano M. A., Roberts A. B., Smith J. M., Sporn M. B., De Larco J. E. Sarcoma growth factor from conditioned medium of virally transformed cells is composed of both type alpha and type beta transforming growth factors. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6264–6268. doi: 10.1073/pnas.80.20.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C., Westermark B., Ek B., Heldin C. H. Coexpression of a PDGF-like growth factor and PDGF receptors in a human osteosarcoma cell line: implications for autocrine receptor activation. Cell. 1984 Dec;39(3 Pt 2):447–457. doi: 10.1016/0092-8674(84)90452-5. [DOI] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Vogel A., Ross R. Production of platelet-derived growth factor-like molecules and reduced expression of platelet-derived growth factor receptors accompany transformation by a wide spectrum of agents. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2396–2400. doi: 10.1073/pnas.81.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Curran T., Peters G., Van Beveren C., Teich N. M., Verma I. M. FBJ murine osteosarcoma virus: identification and molecular cloning of biologically active proviral DNA. J Virol. 1982 Nov;44(2):674–682. doi: 10.1128/jvi.44.2.674-682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley G. Q., Baltimore D. Transformation of an interleukin 3-dependent hematopoietic cell line by the chronic myelogenous leukemia-specific P210bcr/abl protein. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9312–9316. doi: 10.1073/pnas.85.23.9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère L., Gospodarowicz D. Effect of rous sarcoma virus transformation of rat-1 fibroblasts upon their growth factor and anchorage requirements in serum-free medium. Cancer Res. 1983 May;43(5):2121–2130. [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M., Grandori C., Hanafusa H. Phosphorylation of cellular proteins in Rous sarcoma virus-infected cells: analysis by use of anti-phosphotyrosine antibodies. Mol Cell Biol. 1988 Aug;8(8):3035–3042. doi: 10.1128/mcb.8.8.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan I. K., Adams J. M., Cory S. bcr-abl oncogene renders myeloid cell line factor independent: potential autocrine mechanism in chronic myeloid leukemia. Oncogene Res. 1988;3(4):387–399. [PubMed] [Google Scholar]
- Jove R., Hanafusa H. Cell transformation by the viral src oncogene. Annu Rev Cell Biol. 1987;3:31–56. doi: 10.1146/annurev.cb.03.110187.000335. [DOI] [PubMed] [Google Scholar]
- Kawai S. Transformation of rat cells by fusion-infection with Rous sarcoma virus. J Virol. 1980 Jun;34(3):772–776. doi: 10.1128/jvi.34.3.772-776.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarlund J. K. Transformation of cells by an inhibitor of phosphatases acting on phosphotyrosine in proteins. Cell. 1985 Jul;41(3):707–717. doi: 10.1016/s0092-8674(85)80051-9. [DOI] [PubMed] [Google Scholar]
- Langer-Safer P. R., Lehrman S. R., Skalka A. M. v-src inhibits differentiation via an extracellular intermediate(s). Mol Cell Biol. 1985 Oct;5(10):2847–2850. doi: 10.1128/mcb.5.10.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathey-Prevot B., Nabel G., Palacios R., Baltimore D. Abelson virus abrogation of interleukin-3 dependence in a lymphoid cell line. Mol Cell Biol. 1986 Nov;6(11):4133–4135. doi: 10.1128/mcb.6.11.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa M., Mayer B. J., Takeya T., Yamamoto T., Toyoshima K., Hanafusa H., Kawai S. Two independent mutations are required for temperature-sensitive cell transformation by a Rous sarcoma virus temperature-sensitive mutant. J Virol. 1985 Dec;56(3):743–749. doi: 10.1128/jvi.56.3.743-749.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees-Jones R. W., Goldfarb M., Goff S. P. Abelson murine leukemia virus induces platelet-derived growth factor-independent fibroblast growth: correlation with kinase activity and dissociation from full morphologic transformation. Mol Cell Biol. 1989 Jan;9(1):278–287. doi: 10.1128/mcb.9.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. L., Levy D. B., Yannoni Y., Erikson R. L. Identification of a phorbol ester-repressible v-src-inducible gene. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1178–1182. doi: 10.1073/pnas.86.4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemeier D. C., Tilghman S. M., Leder P. Purification and cloning of a mouse ribosomal gene fragment in coliphage lambda. Gene. 1977;2(3-4):173–191. doi: 10.1016/0378-1119(77)90016-6. [DOI] [PubMed] [Google Scholar]
- Waneck G. L., Keyes L., Rosenberg N. Abelson virus drives the differentiation of Harvey virus-infected erythroid cells. Cell. 1986 Jan 31;44(2):337–344. doi: 10.1016/0092-8674(86)90768-3. [DOI] [PubMed] [Google Scholar]
- Yonemoto W., Filson A. J., Queral-Lustig A. E., Wang J. Y., Brugge J. S. Detection of phosphotyrosine-containing proteins in polyomavirus middle tumor antigen-transformed cells after treatment with a phosphotyrosine phosphatase inhibitor. Mol Cell Biol. 1987 Feb;7(2):905–913. doi: 10.1128/mcb.7.2.905. [DOI] [PMC free article] [PubMed] [Google Scholar]