Abstract
The development of a successful vaccine against HIV is a major global challenge. Antiretroviral therapy is the standard treatment against HIV-1 infection. However, only 46% of the eligible people received the therapy in 2015. Furthermore, suboptimal adherence poses additional obstacles. Therefore, there is an urgent need for an HIV-1 vaccine. The most promising clinical trial to date is Phase III RV144, which for the first time demonstrated the feasibility of vaccine-mediated immune protection against HIV-1. Nevertheless, its 31% efficacy and limited durability underscore major hurdles. Here, we discuss recent progress in HIV-1 vaccine development with a special emphasis on nanovaccines, which are at the forefront of efforts to develop a successful HIV-1 vaccine.
Keywords: : broadly neutralizing antibody, HIV, liposome, nanoparticle, vaccine
The development of a successful vaccine against HIV is a daunting challenge of global scale. It is the sixth leading cause of death worldwide with 1.1 million HIV-associated mortalities reported in 2015 according to the WHO [1]. HIV exploits immune activation and inflammation by replicating in activated CD4+ T cells, leading to their systemic depletion. Once the immune system is inundated, AIDS develops, and the body is unable to fight off opportunistic infections. Although 20% of HIV-infected patients are known to generate broadly neutralizing antibodies (bNAbs) against multiple strains of HIV, this process typically takes ≥2 years after the initial infection [2]. To preserve the immune functions at early stages of HIV infection and protect infected individuals from developing AIDS, it is critical that patients receive antiretroviral therapy (ART), a combination of drugs targeting the HIV life cycle [3]. However, the WHO and UNAIDS estimated that of the 25.7 million people in Africa eligible for ART in 2015, only 12.1 million people received HIV treatment [4]. In addition, strict adherence to complex ART regimens is a medical challenge, as one study suggested that subjects took only 71% of prescribed ART doses, with over 95% of patients reaching suboptimal adherence [5]. Therefore, there is an urgent need to develop a prophylactic vaccine against HIV.
The most promising HIV vaccine clinical trial to date is the RV144 Phase III trial conducted in Thailand [6,7]. That vaccine trial, which used viral vector priming and recombinant protein boosting, resulted in 31% efficacy, demonstrating for the first time that vaccine-mediated immune protection against HIV infection is feasible. However, this modest level of vaccine efficacy as well as its durability of protection must be drastically improved in order to have a meaningful impact on the global campaign against HIV [8]. There are currently two major hurdles facing the field of HIV vaccine development, namely: design and production of immunogens that can faithfully recapitulate and present conserved epitope(s), and development of an effective vaccine system that can deliver those immunogens to antigen-presenting cells (APCs) in lymphoid tissues and elicit robust adaptive immune responses. Successful immunogen design has proved challenging due to various evasion pathways of HIV. Within each HIV subtype, the amino acid sequences of the envelope glycoprotein (Env), which sits on the virion surface, vary from 4 to 30%, while the amino acid variation from HIV subtype to subtype can range from 20 to 36% [9]. In addition to the high mutation rate, many of the conserved Env sequences that are critical for binding to target cells and viral entry are buried under the dense glycan layer that severely restricts access to the epitopes, limiting its potential as an effective HIV immunogen [10].
As we discuss below, new breakthroughs in HIV-1 immunogen design have begun to address these issues and yielded promising HIV-neutralizing responses in preclinical models. Here, we highlight recent articles that integrate these findings with a generalizable vaccine approach designed to promote delivery of HIV-1 immunogens to APCs in lymphoid tissues and elicit concerted T-cell and B-cell immune responses. This special report is by no means a comprehensive overview of the HIV immunogen design and delivery approaches; the readers are referred to excellent reviews on these topics [2,10–15]. Here, we have emphasized the latest developments in the vaccine delivery technologies that are best suited for HIV vaccination, including nanocarrier-based strategies.
Live vaccine vectors for vaccination against HIV-1
Numerous vaccines based on inactivated or attenuated vectors, including vaccines against polio, measles, mumps, rubella, and yellow fever, have been successfully used worldwide [11]. However, this strategy does not work for HIV-1 due to its virulence and high mutation rate. As an alternative strategy, vaccines based on live vectors have been pursued widely over the last two decades. These live-vector-based vaccines use genetically attenuated pathogens to express viral antigens and stimulate the host immune system. Although live-vector-based vaccines can induce robust T-cell and B-cell immune responses, manufacturing of live organism-based vaccines as well as inherent antivector immunity observed in the general population are major obstacles to overcome [16–18]. A prime example of the latter is Merck and Co.'s (NJ, USA) STEP study, Phase II clinical trial that was stopped in 2007 [19]. In that trial, individuals at high risk of contracting HIV-1 were vaccinated with adenovirus type 5 (Ad5) vector expressing HIV-1 gag, pol and nef genes. The surprising result was that the HIV-1 infection rate actually increased for those who were seropositive for Ad5 [19]. Mechanistically, Benlahrech et al. have shown that among vaccines with pre-existing immunity to Ad5, vaccination promoted activation of Ad5-specific memory T cells and their subsequent trafficking to mucosal tissues, thus unintentionally providing a high frequency of target CD4+ T cells at the local site of HIV-1 entry [20]. With over 100 types of adenovirus identified, including 49 known to infect humans and 80% of children over the age of 10 months infected by at least one strain, the likelihood of exposure to multiple adenoviruses as an adult is almost certain [21,22]. Strategies based on live vectors need to overcome substantial hurdles associated with identification of live vector subtypes suitable for the general population as well as modification of the vector subtypes from prime to boost immunizations to avoid antivector immunity. Notably, a new HVTN 702 Phase II/III clinical trial has started in November 2016 in South Africa to test an updated version of the RV144 vaccine [23]. In order to increase the magnitude and duration of immune responses observed in the RV144 trial, the HVTN 702 trial will evaluate a canarypox vector-based vaccine called ALVAC-HIV and a two-component gp120 protein subunit vaccine formulated with the MF59 adjuvant, followed by a boost shot at the 1-year mark. The results are expected by 2021.
Next-generation HIV-1 immunogens for eliciting bNAb responses
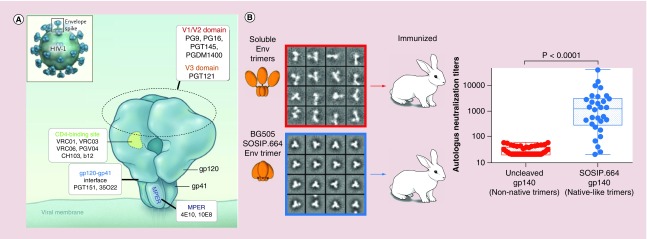
Env is translated as a heterodimer composed of the noncovalently bound gp120 and gp41 subunits and forms a functional unit when three Envs are displayed on the virion surface as a trimer (Figure 1A). The Env trimer is an attractive and logical target for HIV-1 vaccine development for two key reasons: it protrudes from the HIV-1 virion surface, making it accessible for antibody binding; and it is a part of the viral machinery needed for cellular targeting and entry, and consequently, antibodies directed against it could inhibit cellular uptake of virus. Promising HIV immunogens include BG505.SOSIP, structurally polymorphic HIV-1 gp120, Env-based epitope scaffolds, and HIV-1 glycans. In particular, BG505.SOSIP [24–28] self-assembles to form a trimer (Figure 1B), resembling the native Env trimer on HIV-1 virions. BG505.SOSIP trimer is recognized by a large panel of bNAbs, including PGV04, PGT145, PGT128, PGT151 and 2G12, but not by non-neutralizing antibodies against gp120 and gp41 epitopes on trimers, such as b6 and F240, respectively [24–28]. BG505.SOSIP trimer immunizations have been shown to generate HIV-1 neutralizing responses in preclinical models as discussed below. While the results presented so far have been very promising, it remains to be demonstrated whether vaccination with BG505.SOSIP trimer can generate bNAbs capable of neutralizing a wide spectrum of HIV variants.
Figure 1. . HIV-1 envelope glycoprotein and BG505.SOSIP envelope glycoprotein trimer.
(A) Native HIV-1 Env displayed on virion surface with epitopes for bNAb indicted. (B) BG505.SOSIP forms native-like Env trimers and induces significantly higher autologous tier 2 serum titers in rabbits, compared with uncleaved BG505 gp140 that exhibited an unstable, irregular trimeric configuration and generated weak autologous tier 2 serum titers.
bNAb: Broadly neutralizing antibody; Env: Envelope glycoprotein.
(A) Adapted with permission from [29]; (B) adapted with permission from [28].
Another class of immunogens uses the promising results from the RV144 Phase III trial. Analysis suggested that the modest protection observed was from V1V2-specific antibodies, a region of HIV-1 gp120 containing vulnerable sites recognized by bNAbs, such as PG9. The vulnerable site is structurally polymorphic and V1V2-scaffold immunogens have been designed to induce conformation-specific antibody responses in animal studies [30].
Lastly, HIV Env contains high-mannose clusters on its surface that interact with the broadly neutralizing anti-HIV-1 antibody 2G12, and chemically synthesized glycoconjugates may offer a promising platform for HIV vaccine development [31]. In particular, linear trimannosides and tetramannosides bind to 2G12 [32] and multivalent gold nanoparticles coated with self-assembled monolayers of synthetic oligomannosides inhibited the binding between 2G12 and gp120 with IC50 values in the micromolar range [33].
While HIV-1 immunogens will need continuous improvement and exploration, their vaccine delivery systems need to stimulate the immune system while protecting the immunogen without hindering the key epitopes. Although this is just one example, it highlights the vital need for the vaccine delivery systems, such as nanoparticles, to be developed alongside their complementary immunogen. As such, immunogens formulated into nanostructures that are breaking new grounds in HIV vaccine development will be discussed below.
Self-assembling ‘virus-like’ nanoparticles for presentation of HIV-1 antigens
Attempts at subunit vaccination with Env antigen are well documented [10,34–36]. In particular, recent studies have elegantly employed fusion proteins that spontaneously self-assemble into nanoparticles mimicking virus-like particles for multivalent display of Env-based immunogens, including germline targeting gp120 outer domains, Clade C ZM109-based V1V2 domain trimers, BG505 gp120 trimers, and BG505.SOSIP gp140 modeled trimers [37–42]. Specifically, protein core of various bacterial sources, including ferritin nanoparticles from Helicobacter pylori, lumazin synthase from Aquifex aeolicus, dihydrolipoyl acetyltransferase from Bacillus stearothermophilus, and E2 protein from Geobacillus stearothermophilus, were produced to display recombinant Env-based trimers at 8, 20 or 60 copies per nanoparticle in a native fashion [37–42]. Compared with soluble trimers, multivalent display of immunogens on these ‘virus-like’ protein nanoparticles enhanced binding affinity by retaining epitope and trimer conformation, and avidity due to the multivalent display of immunogens to many bNAbs, such as PG9, PGDM1400, VRC01, and PGT121, among others. In particular, BG505.SOSIP trimer displayed on ferritin nanoparticles induced higher HIV-neutralizing antibody responses in rabbits, compared with the soluble trimer vaccine group (∼3.2-fold increase in midpoint IC50 against tier 2 autologous virus, and ∼2.5-90-fold increase against a panel of six heterologous, tier 1 strains) [40]. These results suggest that Env protein presented in a native-like trimeric form on self-assembled protein-based particles may prime bNAb responses, while masking epitopes recognized by non-neutralizing antibodies and providing necessary T-cell help derived from bacterial antigen sources.
Synthetic nanoparticles for presentation of HIV-1 antigens
While the results generated with self-assembling ‘virus-like’ particles are encouraging, there are potential concerns about antivector immunity associated with bacterial proteins as the core nanostructures, especially in the setting of multiple prime-boost immunizations. By contrast, synthetic polymeric nanoparticles and liposomal nanovesicles offer versatile platform technologies that can induce strong adaptive immune responses while avoiding antivector immunity and toxicity issues [43–45]. For example, polymeric particles based on biodegradable and biocompatible poly(lactic-co-glycolic) acid (PLGA) copolymer have been extensively investigated for vaccine delivery applications [46–50]. PLGA particles encapsulating HIV-1 peptide antigens administered via the intranasal route elicited Th1/Th2-balanced cellular immune responses in mucosal surfaces [46], while PLGA particles carrying HIV Env peptides administered via the oral route in mice conferred T-cell-mediated protection against viral infection at the rectal and vaginal mucosa [47]. Recently, Kasturi et al. have elegantly shown that PLGA nanoparticles co-loaded with TLR4 and TLR7/8 agonists can synergistically improve induction of antigen-specific antibody responses in nonhuman primates (NHPs) via triggering germinal center and plasma cell responses in lymphoid tissues [48]; in a subsequent study, the authors have shown that PLGA particles carrying TLR4 and TLR7/8 agonists admixed with soluble recombinant gp140 SIVmac239 Env and Gag p55 enhanced the magnitude and durability of humoral immune responses (6.5-fold and 4.7-fold higher antibody titers for protein + PLGA particles at weeks 27 and 42, compared with protein vaccine adjuvanted with Alum), and protected NHPs against repeated low-dose, intravaginal challenges with heterologous SIVsmE660 [49]. These results highlight the versatility of PLGA particle systems for delivery of peptide antigens and adjuvants.
Despite the advances in the design and synthesis of polymeric particle vaccines showcased above, it is yet very challenging to achieve multivalent presentation of complex immunogens, such as Env trimers, in their native configuration via polymeric particles. This is due to the loss of 3D structure and aggregation of immunogens during the synthesis of polymeric particles, which typically introduces organic solvents and high mechanical and/or chemical stresses to cargo materials [51]. New approaches to formulation of biologics into PLGA particles include a ‘self-healing encapsulation’ procedure that exploits the polymer's transition temperature to load antigens into preformed PLGA particles in an aqueous condition, thus avoiding the loss of their antigenicity and immunogenicity [52,53] [Bailey BA, Desai KH, Ochyl LJ, Ciotti SM, Moon JJ, Schwendeman SP. Self-encapsulating poly(lactic-co-glycolic acid) (PLGA) microspheres for intranasal vaccine delivery, Submitted Manuscript]. In addition, PLGA particles that can sustain antigen release over a long term and generate long-lasting immune responses may allow a single-dose vaccination [Bailey BA, Ochyl LJ, Schwendeman SP, Moon JJ. Towards a single-dose vaccination strategy with self-encapsulating PLGA microspheres, Submitted Manuscript]. Application of such innovative strategies in HIV vaccine design may bring significant advances.
On the other hand, recent studies have reported successful surface modification of recombinant Env trimers on synthetic lipid vesicles in an orientation-specific manner [54,55]. Using Ni-NTA-functionalized lipids, Env gp140 trimers with terminal polyhistidine tags (Histag) were anchored to the surfaces of interbilayer-crosslinked multilamellar vesicles (ICMVs). The average ICMV diameter was approximately 375 nm with each harboring on average 160 gp140 trimers for a mean inter-trimer distance of 33 nm [54]. In this instance, Env trimers were displayed greater than ten-times the amount seen on HIV virions while retaining a native orientation on the nanoparticle system designed for efficient delivery of antigens, stable antigen presentation and induction of germinal centers in vivo [54,56–58]. Immunizations with Env–ICMVs in mice-induced Th1/Th2-balanced Env-specific antibodies, IgG1 and IgG2c, expanded the breadth of serum antibodies to recognize peptide sequences of two additional Env regions, V2 and membrane-proximal external region (MPER), and increased their titers, compared with soluble protein formulated with a strong oil-in-water emulsion adjuvant [54]. Liposomes have also been employed to present a high density of Env JRFL.SOSIP trimers using the Histag-Ni-NTA strategy [55]. Env-modified liposomes with an average diameter of approximately 170 nm with an inter-trimer distance of approximately 12–14 nm were able to bind many different bNAbs, including VRC01, PGT145 and PGDM1400, but not non-NAbs, such as b6 and F104, and promoted activation of antigen-specific B cells and germinal center formation in rabbits. Neutralization analysis of immunized rabbit serum produced modest titers against the autologous, tier 2 pseudovirus [55].
Instead of employing the intact Env antigen protein, some groups have employed short epitope peptides on liposomes [59–66]. The MPER, a functional domain of Env gp41, is highly conserved and is a target epitope of several bNAbs, such as 4E10 and 10E8 [10]. However, free soluble MPER peptide, which does not retain the conformation seen when associated with a membrane surface, is poorly immunogenic [67–69]. To overcome this limitation, Hanson et al. have designed a liposomal system embedded with palmitoylated MPER peptides in order to present MPER peptides in the context of lipid layers in a manner mimicking the native virus [63,65]. They evaluated three key properties of the vaccine formulation to enhance immunogenicity of MPER peptides: physicochemical properties of liposomal carriers, inclusion of molecular adjuvants and incorporation of CD4+ T-cell help. The results showed that anti-MPER antibody responses were enhanced in vivo when the liposomal vaccine platform was composed of high-melting-temperature lipids with an average liposome diameter of 150–200 nm; incorporated with MPLA and cyclic di-GMP (a TLR4 agonist and a STING agonist, respectively); loaded with CD4+ T-cell helper peptide, HIV30; and decorated with high surface densities of MPER with a mean distance of approximately 10–15 nm between peptides [63,65]. Optimized MPER/HIV30/MPLA-liposomes adjuvanted with separate cyclic di-GMP-containing liposomes triggered germinal center B-cell differentiation and promoted strong antibody responses in mice, characterized by maintenance of ten-fold higher IgG titers for approximately 100 days after the final boost, compared with the MPER liposome plus soluble cdGMP control group [65]. Despite the promising IgG titer responses, the immune sera did not neutralize HIV. It is notable that in a parallel study [60], the binding epitope of anti-MPER sera IgG was altered by modifying the immunodominant tryptophan residue-680 in MPER to alanine (W680A) or by introducing a covalent transmembrane domain into MPER peptide, thus shifting the IgG antibody recognition from the C-terminus toward the N-terminal end and central region of the MPER. While these studies have underscored the impact and benefits of nanoparticle-mediated delivery of HIV-1 immunogens, they also highlight the critical need for further refinements of the immunogen design and the vaccine carrier itself.
siRNA-based nanotherapeutics to prevent HIV-1 infection
Although many prophylactic vaccine approaches use protein immunogens, a complementary strategy involves silencing the very receptor that mediates viral entry into cells. This strategy uses siRNA-based knockdown of target protein [70,71]. In order for CCR5-tropic HIV-1 to deliver its genome via virus–cell fusion, HIV-1 needs to interact with CD4 and co-receptor CCR5. Therefore, individuals lacking expression of functional CCR5 are resistant to HIV-1 infection, with the Berlin patient being the prime example [72]. Toward the goal of knocking down CCR5 in leukocytes, Kim et al. have developed liposomes decorated with antibodies against LFA-1, an integrin found on leukocytes [73]. Bone marrow, liver, thymus (BLT) mice reconstituted with human immune cells via engraftment of human hematopoietic stem cells, liver tissue and thymus-gland tissue were administered with a single dose of LFA-1-targeted liposomes carrying siRNA against CCR5, leading to significant reduction in CCR5 mRNA levels among leukocytes for 10 days. Upon HIV challenge, BLT mice treated with siRNA liposomes maintained CD4 T-cell count and reduced plasma viral load by two orders of magnitude, compared with the control group, which showed a 31% drop in CD4 T cells and succumbed to HIV-1 infection [73]. As LFA-1 engagement has been shown to trigger recruitment of the microtubule organizing center in HIV-1-infected primary CD4 T cells and plays a crucial role during viral dissemination between T cells [74], delivery strategies targeted to immune cells via LFA-1 may halt viral spread. Another potential avenue for innovation would be to knock out CCR5 using genome-editing complexes, such as ZFN, CRISPR/Cas9 or TALENs, which would address the transient impact of siRNA-based therapeutics and maintain long-term immunity against HIV-1 [75].
Conclusion & future perspective
HIV vaccine development faces numerous challenges that need to be addressed stemming from the mutative nature of HIV. One major hurdle involves the design and production of an effective HIV-1 immunogen. While there are many HIV-1 immunogens being designed, BG505.SOSIP, in particular, has achieved tier-1 and autologous tier-2 neutralization in preclinical studies and is paving the way toward a new generation of HIV-1 vaccines designed to induce bNAb responses. It remains to be seen whether the new immunogen can achieve broad-spectrum tier-2 neutralizing antibody responses against multiple strains of HIV-1. In this respect, we argue that nanovaccines formulated with HIV-1 immunogens, including BG505.SOSIP, may be able to provide comprehensive protection against HIV-1. The benefits of using nanoparticles for prophylactic HIV-1 vaccination include safety profile afforded by the use of biocompatible biomaterials as well as vaccine dose titration; protection of HIV-1 antigens from enzymatic degradation, thus increasing the in vivo stability of immunogens and their interactions with APCs; improved targeting to APC-enriched lymphoid tissues; enhancement of phagocytosis and processing of HIV-1 antigens; and multivalent presentation of antigens for optimal interaction with B cells [76], induction of germinal centers and sustained IgG production. Notably, a number of nanoparticle formulations have been US FDA-approved and commercialized, including Janssen Products, LP's Doxil® (doxorubicin-loaded liposomes for treatment of ovarian cancer) and Merrimack's Onivyde (irinotecan-loaded liposomes for treatment of pancreatic cancer, FDA-approved in 2015) [77]. Clearly, translating these advances to nanovaccines would allow us to expand our arsenal of vaccine-delivery vehicles. However, unlike therapeutic-loaded nanoparticles, a prophylactic vaccine against HIV-1 requires a more detailed intervention. As illustrated in this special report, there are an array of immunogens, delivery vehicles and immunization schemes that should be carefully considered for induction of bNAbs or the blocking of viral entry. Furthermore, integration of HIV-1 immunogen design with innovations in de novo protein interface design, exemplified by recent reports of self-assembling 60-subunit and 120-subunit protein nanostructures with icosahedral symmetry, may produce significant progress in the HIV-1 vaccine field [78–80].
In summary, we believe that the field of nanovaccines is positioned to make major progress in HIV vaccine development – a common theme among the vaccine formulations that we have highlighted here. While numerous questions and hurdles are yet to be addressed in this challenging and rapidly evolving field of research, the expanding immunogen arsenal under development, coupled with concerted efforts to design and translate nanovaccines for a successful HIV-1 vaccine, may provide a breakthrough in the near future.
Executive summary.
The standard treatment for HIV-1 infection is antiretroviral therapy (ART), which has met with significant challenges in Africa, including limited access and adherence, thus pointing to the need for development of a prophylactic vaccine against HIV-1.
Merck's STEP Study elucidated the potential risks of viral vectors such as pre-existing immunity and subsequent trafficking of T cells to sites of HIV-1 entry.
BG505.SOSIP is a promising HIV-1 immunogen that resembles the native envelope glycoprotein (Env) trimer on the virion surface and is recognized by a large panel of broadly neutralizing antibodies (bNAbs).
Nanoparticle delivery systems must be developed alongside HIV-1 immunogens to enhance immune stimulation and protect presented epitopes.
Multivalent display of Env on protein-based nanoparticles enhances its binding affinity and avidity to many bNAbs.
Env presented in a native-like trimeric form on self-assembled protein nanoparticles may prime bNAb responses while masking epitopes recognized by non-NAbs and providing T-cell help from bacterial antigens.
Synthetic nanoparticles displaying HIV-1 immunogens can elicit strong adaptive immunity and avoid antivector immunity.
Genomic and proteomic tools could be applied via nanoparticles to achieve immunity against HIV-1 infection.
The field of nanomedicine is positioned to make major progress in HIV-1 vaccine development.
Footnotes
Financial & competing interest disclosure
This work was supported in part by NIH 1R01-AI127070, NIH 1R01-EB022563, NIH 1R01-CA210273 and NIH UL1TR000433. JJ Moon is a Young Investigator supported by the Melanoma Research Alliance (348774), NSF CAREER Award (1553831) and DoD/CDMRP Peer Reviewed Cancer Research Program (W81XWH-16-1-0369). Opinions’ interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. JJ Moon is an inventor on patents related to the ICMV technology, and these patents have been licensed to Vedantra Pharmaceuticals, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.World Health Organization. The 10 Leading Causes of Death in the World, 2000 and 2012. World Health Organization; Geneva, Switzerland: 2014. www.who.int/mediacentre/factsheets/fs310/en/ [Google Scholar]
- 2.Kwong PD, Mascola JR, Nabel GJ. Broadly neutralizing antibodies and the search for an HIV-1 vaccine: the end of the beginning. Nat. Rev. Immunol. 2013;13(9):693–701. doi: 10.1038/nri3516. [DOI] [PubMed] [Google Scholar]
- 3.Broder S. The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antiviral Res. 2010;85(1):1–18. doi: 10.1016/j.antiviral.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global AIDS Update 2016. www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf
- 5.Golin CE, Liu H, Hays RD, et al. A prospective study of predictors of adherence to combination antiretroviral medication. J. Gen. Intern. Med. 2002;17(10):756–765. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 7.Haynes BF, Gilbert PB, Mcelrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 2012;366(14):1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JH, Rerks-Ngarm S, Excler JL, Michael NL. HIV vaccines: lessons learned and the way forward. Curr. Opin. HIV AIDS. 2010;5(5):428–434. doi: 10.1097/COH.0b013e32833d17ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korber B, Gaschen B, Yusim K, Thakallapally R, Kesmir C, Detours V. Evolutionary and immunological implications of contemporary HIV-1 variation. Br. Med. Bull. 2001;58:19–42. doi: 10.1093/bmb/58.1.19. [DOI] [PubMed] [Google Scholar]
- 10.Burton DR, Hangartner L. Broadly neutralizing antibodies to HIV and their role in vaccine design. Annu. Rev. Immunol. 2016;34:635–659. doi: 10.1146/annurev-immunol-041015-055515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plotkin SA. Vaccines: past, present and future. Nat. Med. 2005;11(Suppl. 4):S5–S11. doi: 10.1038/nm1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Migueles SA, Connors M. Success and failure of the cellular immune response against HIV-1. Nat. Immunol. 2015;16(6):563–570. doi: 10.1038/ni.3161. [DOI] [PubMed] [Google Scholar]
- 13.Shin SY. Recent update in HIV vaccine development. Clin. Exp. Vaccine Res. 2016;5(1):6–11. doi: 10.7774/cevr.2016.5.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glass JJ, Kent SJ, De Rose R. Enhancing dendritic cell activation and HIV vaccine effectiveness through nanoparticle vaccination. Expert Rev. Vaccines. 2016;15(6):719–729. doi: 10.1586/14760584.2016.1141054. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Chen C. Role of nanotechnology in HIV/AIDS vaccine development. Adv. Drug Deliv. Rev. 2016;103:76–89. doi: 10.1016/j.addr.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Zak DE, Andersen-Nissen E, Peterson ER, et al. Merck Ad5/HIV induces broad innate immune activation that predicts CD8(+) T-cell responses but is attenuated by preexisting Ad5 immunity. Proc. Natl Acad. Sci. USA. 2012;109(50):E3503–E3512. doi: 10.1073/pnas.1208972109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brave A, Ljungberg K, Wahren B, Liu MA. Vaccine delivery methods using viral vectors. Mol. Pharm. 2007;4(1):18–32. doi: 10.1021/mp060098+. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JA, Barouch DH, Baden LR. Nonreplicating vectors in HIV vaccines. Curr. Opin. HIV AIDS. 2013;8(5):412–420. doi: 10.1097/COH.0b013e328363d3b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the STEP Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benlahrech A, Harris J, Meiser A, et al. Adenovirus vector vaccination induces expansion of memory CD4 T cells with a mucosal homing phenotype that are readily susceptible to HIV-1. Proc. Natl Acad. Sci. USA. 2009;106(47):19940–19945. doi: 10.1073/pnas.0907898106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doerfler W. Adenoviruses. In: Baron S, editor. Medical Microbiology. University of Texas Medical Branch, Department of Microbiology; TX, USA: 1996. [Google Scholar]
- 22.Trojnar Z, Ciepiela O, Demkow UA. The prevalence of IgG and IgA against adenoviruses in serum of children aged 1126 months, hospitalised in the Clinical Paediatric Hospital in Warsaw, Poland. Cent. Eur. J. Immunol. 2014;39(1):91–95. doi: 10.5114/ceji.2014.42131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Institute of Health. First new HIV vaccine efficacy study in seven years has begun. www.nih.gov/news-events/news-releases/first-new-hiv-vaccine-efficacy-study-seven-years-has-begun
- 24.Sanders RW, Derking R, Cupo A, et al. A next-generation cleaved, soluble HIV-1 Env Trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog. 2013;9(9):e1003618. doi: 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ringe RP, Sanders RW, Yasmeen A, et al. Cleavage strongly influences whether soluble HIV-1 envelope glycoprotein trimers adopt a native-like conformation. Proc. Natl Acad. Sci. USA. 2013;110(45):18256–18261. doi: 10.1073/pnas.1314351110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Julien JP, Cupo A, Sok D, et al. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science. 2013;342(6165):1477–1483. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyumkis D, Julien JP, De Val N, et al. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science. 2013;342(6165):1484–1490. doi: 10.1126/science.1245627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanders RW, Van Gils MJ, Derking R, et al. HIV-1 vaccines. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science. 2015;349(6244):aac4223. doi: 10.1126/science.aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caskey M, Klein F, Nussenzweig MC. Broadly neutralizing antibodies for HIV-1 prevention or immunotherapy. N. Engl. J. Med. 2016;375(21):2019–2021. doi: 10.1056/NEJMp1613362. [DOI] [PubMed] [Google Scholar]
- 30.Jiang X, Totrov M, Li W, et al. Rationally designed immunogens targeting HIV-1 gp120 V1V2 induce distinct conformation-specific antibody responses in rabbits. J. Virol. 2016;90(24):11007–11019. doi: 10.1128/JVI.01409-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernardi A, Jimenez-Barbero J, Casnati A, et al. Multivalent glycoconjugates as anti-pathogenic agents. Chem. Soc. Rev. 2013;42(11):4709–4727. doi: 10.1039/c2cs35408j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enriquez-Navas PM, Marradi M, Padro D, Angulo J, Penades S. A solution NMR study of the interactions of oligomannosides and the anti-HIV-1 2G12 antibody reveals distinct binding modes for branched ligands. Chemistry. 2011;17(5):1547–1560. doi: 10.1002/chem.201002519. [DOI] [PubMed] [Google Scholar]
- 33.Marradi M, Di Gianvincenzo P, Enriquez-Navas PM, et al. Gold nanoparticles coated with oligomannosides of HIV-1 glycoprotein gp120 mimic the carbohydrate epitope of antibody 2G12. J. Mol. Biol. 2011;410(5):798–810. doi: 10.1016/j.jmb.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 34.Vancott TC, Kaminski RW, Mascola JR, et al. HIV-1 neutralizing antibodies in the genital and respiratory tracts of mice intranasally immunized with oligomeric gp160. J. Immunol. 1998;160(4):2000–2012. [PubMed] [Google Scholar]
- 35.Lamalle-Bernard D, Munier S, Compagnon C, et al. Coadsorption of HIV-1 p24 and gp120 proteins to surfactant-free anionic PLA nanoparticles preserves antigenicity and immunogenicity. J. Control. Release. 2006;115(1):57–67. doi: 10.1016/j.jconrel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Liu Y, Chen Z, et al. Surface-engineered gold nanorods: promising DNA vaccine adjuvant for HIV-1 treatment. Nano Lett. 2012;12(4):2003–2012. doi: 10.1021/nl300027p. [DOI] [PubMed] [Google Scholar]
- 37.Jardine J, Julien JP, Menis S, et al. Rational HIV immunogen design to target specific germline B cell receptors. Science. 2013;340(6133):711–716. doi: 10.1126/science.1234150. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Self-assembling nanoparticles presenting 60-mer copies of VRC01 germline targeting immunogens activate rare VRC01-class precursors in VRC01 gH knock-in mice.
- 38.Krebs SJ, Mcburney SP, Kovarik DN, et al. Multimeric scaffolds displaying the HIV-1 envelope MPER induce MPER-specific antibodies and cross-neutralizing antibodies when co-immunized with gp160 DNA. PLoS ONE. 2014;9(12):e113463. doi: 10.1371/journal.pone.0113463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jardine JG, Ota T, Sok D, et al. HIV-1 VACCINES. Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen. Science. 2015;349(6244):156–161. doi: 10.1126/science.aac5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sliepen K, Ozorowski G, Burger JA, et al. Presenting native-like HIV-1 envelope trimers on ferritin nanoparticles improves their immunogenicity. Retrovirology. 2015;12:82. doi: 10.1186/s12977-015-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• BG505.SOSIP trimers multimerized on ferritin nanoparticles enhance HIV-1 neutralizing antibody responses, compared with soluble protein.
- 41.He L, De Val N, Morris CD, et al. Presenting native-like trimeric HIV-1 antigens with self-assembling nanoparticles. Nat. Commun. 2016;7:12041. doi: 10.1038/ncomms12041. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Multivalent display of Env trimers on self-assembling nanoparticles leads to avid recognition by bNAbs and allows robust stimulation of B cells carrying the VRC01 B-cell receptors.
- 42.Sok D, Briney B, Jardine JG, et al. Priming HIV-1 broadly neutralizing antibody precursors in human Ig loci transgenic mice. Science. 2016;353(6307):1557–1560. doi: 10.1126/science.aah3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sahdev P, Ochyl LJ, Moon JJ. Biomaterials for nanoparticle vaccine delivery systems. Pharm. Res. 2014;31(10):2563–2582. doi: 10.1007/s11095-014-1419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuai R, Ochyl LJ, Schwendeman A, Moon JJ. Lipid-based nanoparticles for vaccine applications. In: Jo H, Jun HW, Shin H, editors. Biomedical Engineering: Convergence Technologies. Elsevier B.V.; 2015. [Google Scholar]
- 45.Fan Y, Sahdev P, Ochyl LJ, J Akerberg J, Moon JJ. Cationic liposome–hyaluronic acid hybrid nanoparticles for intranasal vaccination with subunit antigens. J. Control. Release. 2015;208:121–129. doi: 10.1016/j.jconrel.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohan T, Sharma C, Bhat AA, Rao DN. Modulation of HIV peptide antigen specific cellular immune response by synthetic alpha- and beta-defensin peptides. Vaccine. 2013;31(13):1707–1716. doi: 10.1016/j.vaccine.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 47.Zhu Q, Talton J, Zhang G, et al. Large intestine-targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection. Nat. Med. 2012;18(8):1291–1296. doi: 10.1038/nm.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasturi SP, Skountzou I, Albrecht RA, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470(7335):543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kasturi SP, Kozlowski PA, Nakaya HI, et al. Adjuvanting an SIV vaccine with TLR ligands encapsulated in nanoparticles induces persistent antibody responses and enhanced protection in TRIM5alpha restrictive macaques. J. Virol. 2016 doi: 10.1128/JVI.01844-16. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pavot V, Climent N, Rochereau N, et al. Directing vaccine immune responses to mucosa by nanosized particulate carriers encapsulating NOD ligands. Biomaterials. 2016;75:327–339. doi: 10.1016/j.biomaterials.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 51.Jiang W, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv. Drug Deliv. Rev. 2005;57(3):391–410. doi: 10.1016/j.addr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Reinhold SE, Desai KG, Zhang L, Olsen KF, Schwendeman SP. Self-healing microencapsulation of biomacromolecules without organic solvents. Angew. Chem. Int. Ed. Engl. 2012;51(43):10800–10803. doi: 10.1002/anie.201206387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desai KG, Schwendeman SP. Active self-healing encapsulation of vaccine antigens in PLGA microspheres. J. Control. Release. 2013;165(1):62–74. doi: 10.1016/j.jconrel.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pejawar-Gaddy S, Kovacs JM, Barouch DH, Chen B, Irvine DJ. Design of lipid nanocapsule delivery vehicles for multivalent display of recombinant Env trimers in HIV vaccination. Bioconjug. Chem. 2014;25(8):1470–1478. doi: 10.1021/bc5002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ingale J, Stano A, Guenaga J, et al. High-density array of well-ordered HIV-1 spikes on synthetic liposomal nanoparticles efficiently activate B cells. Cell Rep. 2016;15(9):1986–1999. doi: 10.1016/j.celrep.2016.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Synthetic liposomes surface-decorated with a high density of Env trimers efficiently activate Env-specific B cells, enhance germinal center formation and induce neutralizing antibody responses against the autologous HIV-1 tier-2 strain.
- 56.Moon JJ, Suh H, Bershteyn A, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat. Mater. 2011;10(3):243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc. Natl Acad. Sci. USA. 2012;109(4):1080–1085. doi: 10.1073/pnas.1112648109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li AV, Moon JJ, Abraham W, et al. Generation of effector memory T cell-based mucosal and systemic immunity with pulmonary nanoparticle vaccination. Sci. Transl. Med. 2013;5(204):204ra130. doi: 10.1126/scitranslmed.3006516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matyas GR, Wieczorek L, Beck Z, et al. Neutralizing antibodies induced by liposomal HIV-1 glycoprotein 41 peptide simultaneously bind to both the 2F5 or 4E10 epitope and lipid epitopes. AIDS. 2009;23(16):2069–2077. doi: 10.1097/QAD.0b013e32832faea5. [DOI] [PubMed] [Google Scholar]
- 60.Kim M, Song L, Moon J, et al. Immunogenicity of membrane-bound HIV-1 gp41 membrane-proximal external region (MPER) segments is dominated by residue accessibility and modulated by stereochemistry. J. Biol. Chem. 2013;288(44):31888–31901. doi: 10.1074/jbc.M113.494609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Venditto VJ, Watson DS, Motion M, Montefiori D, Szoka FC., Jr Rational design of membrane proximal external region lipopeptides containing chemical modifications for HIV-1 vaccination. Clin. Vaccine Immunol. 2013;20(1):39–45. doi: 10.1128/CVI.00615-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Venditto VJ, Wieczorek L, Molnar S, et al. Chemically modified peptides based on the membrane-proximal external region of the HIV-1 envelope induce high-titer, epitope-specific nonneutralizing antibodies in rabbits. Clin. Vaccine Immunol. 2014;21(8):1086–1093. doi: 10.1128/CVI.00320-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hanson MC, Abraham W, Crespo MP, et al. Liposomal vaccines incorporating molecular adjuvants and intrastructural T-cell help promote the immunogenicity of HIV membrane-proximal external region peptides. Vaccine. 2015;33(7):861–868. doi: 10.1016/j.vaccine.2014.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Apellaniz B, Nieva JL. The use of liposomes to shape epitope structure and modulate immunogenic responses of peptide vaccines against HIV MPER. Adv. Protein Chem. Struct. Biol. 2015;99:15–54. doi: 10.1016/bs.apcsb.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 65.Hanson MC, Crespo MP, Abraham W, et al. Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. J. Clin. Invest. 2015;125(6):2532–2546. doi: 10.1172/JCI79915. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The membrane-proximal external region peptide displayed on liposomes generates durable antibody responses and germinal center formation in draining lymph nodes.
- 66.Donius LR, Cheng Y, Choi J, et al. Generation of long-lived bone marrow plasma cells secreting antibodies specific for the HIV-1 gp41 membrane-proximal external region in the absence of polyreactivity. J. Virol. 2016;90(19):8875–8890. doi: 10.1128/JVI.01089-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun ZY, Oh KJ, Kim M, et al. HIV-1 broadly neutralizing antibody extracts its epitope from a kinked gp41 ectodomain region on the viral membrane. Immunity. 2008;28(1):52–63. doi: 10.1016/j.immuni.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 68.Kim M, Sun ZY, Rand KD, et al. Antibody mechanics on a membrane-bound HIV segment essential for GP41-targeted viral neutralization. Nat. Struct. Mol. Biol. 2011;18(11):1235–1243. doi: 10.1038/nsmb.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song L, Sun ZY, Coleman KE, et al. Broadly neutralizing anti-HIV-1 antibodies disrupt a hinge-related function of gp41 at the membrane interface. Proc. Natl Acad. Sci. USA. 2009;106(22):9057–9062. doi: 10.1073/pnas.0901474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mamo T, Moseman EA, Kolishetti N, et al. Emerging nanotechnology approaches for HIV/AIDS treatment and prevention. Nanomedicine (Lond.) 2010;5(2):269–285. doi: 10.2217/nnm.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swamy MN, Wu H, Shankar P. Recent advances in RNAi-based strategies for therapy and prevention of HIV-1/AIDS. Adv. Drug Deliv. Rev. 2016;103:174–186. doi: 10.1016/j.addr.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hutter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 2009;360(7):692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 73.Kim SS, Peer D, Kumar P, et al. RNAi-mediated CCR5 silencing by LFA-1-targeted nanoparticles prevents HIV infection in BLT mice. Mol. Ther. 2010;18(2):370–376. doi: 10.1038/mt.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Administration of LFA-1-targeted liposomes carrying siRNA against CCR5 silences CCR5 among leukocytes and reduces viremia after HIV-1 challenge in humanized bone marrow liver thymic mice.
- 74.Starling S, Jolly C. LFA-1 engagement triggers T cell polarization at the HIV-1 virological synapse. J. Virol. 2016;90(21):9841–9854. doi: 10.1128/JVI.01152-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang W, Ye C, Liu J, Zhang D, Kimata JT, Zhou P. CCR5 gene disruption via lentiviral vectors expressing Cas9 and single guided RNA renders cells resistant to HIV-1 infection. PLoS ONE. 2014;9(12):e115987. doi: 10.1371/journal.pone.0115987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheng W. The density code for the development of a vaccine? J. Pharm. Sci. 2016;105(11):3223–3232. doi: 10.1016/j.xphs.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Passero FC, Jr, Grapsa D, Syrigos KN, Saif MW. The safety and efficacy of Onivyde (irinotecan liposome injection) for the treatment of metastatic pancreatic cancer following gemcitabine-based therapy. Expert Rev. Anticancer Ther. 2016;16(7):697–703. doi: 10.1080/14737140.2016.1192471. [DOI] [PubMed] [Google Scholar]
- 78.Hsia Y, Bale JB, Gonen S, et al. Design of a hyperstable 60-subunit protein icosahedron. Nature. 2016;535(7610):136–139. doi: 10.1038/nature18010. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• De novo protein design of self-assembling protein nanostructures with icosahedral symmetry.
- 79.Bale JB, Gonen S, Liu Y, et al. Accurate design of megadalton-scale two-component icosahedral protein complexes. Science. 2016;353(6297):389–394. doi: 10.1126/science.aaf8818. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• De novo protein design of self-assembling protein nanostructures with icosahedral symmetry.
- 80.Huang PS, Boyken SE, Baker D. The coming of age of de novo protein design. Nature. 2016;537(7620):320–327. doi: 10.1038/nature19946. [DOI] [PubMed] [Google Scholar]; •• De novo protein design of self-assembling protein nanostructures with icosahedral symmetry.