Abstract
Background
About 1% of adults suffer from pulmonary hypertension (PH). The various types of PH differ widely with respect to their incidence, clinical significance, and treatment.
Methods
Selective review of the literature in association with a consensus conference.
Results
Pulmonary hypertension is divided into five major categories. Those that are of particular clinical relevance are pulmonary arterial hypertension, chronic thromboembolic pulmonary hypertension, and pulmonary hypertension due to left heart and lung diseases. Ten drugs from five different substance classes are now available for the treatment of PH and are often given in combination. The treatment strategy is determined by risk stratification based on the severity of disease, along with the clinical phenotype and possible accompanying illnesses. The preferred treatment for chronic thromboembolic pulmonary hypertension is surgical pulmonary endarterectomy; inoperable patients are treated with drugs and endovascular interventions. PH due to left heart and lung diseases generally calls for specific treatment of pulmonary hypertension only if there is severe right-heart strain.
Conclusion
The diagnosis and treatment of severe forms of PH, in particular, pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension, are complex and are best carried out in close cooperation between the local physician and specialized centers.
The term pulmonary hypertension embraces a variety of diseases that have little in common apart from elevated blood pressure in the pulmonary circulation. Precise diagnostic classification of pulmonary hypertension is essential, not least for reasons of treatment and prognosis, because treatment options that are efficacious in some forms of pulmonary hypertension may be ineffective or even disadvantageous in other forms.
This CME article is based on a selective survey of the literature and also summarizes—and in some places supplements—the principal recommendations of both the European guidelines on pulmonary hypertension published in 2015 and the second Cologne Consensus Conference held on 16–18 June 2016. Pulmonary hypertension in childhood is not discussed.
Learning objectives
This article is intended to impart knowledge of:
The principal definitions and the classification of pulmonary hypertension
The cardinal symptoms of pulmonary hypertension and the diagnostic procedure in the case of clinical suspicion of pulmonary hypertension
The treatment principles in the main forms of pulmonary hypertension, the importance of specific treatment of particular forms of pulmonary hypertension, and the role of expert centers in the management of this group of diseases
Definitions and epidemiology
Definition of pulmonary hypertension.
Pulmonary hypertension is defined as resting mean pulmonary artery pressure of ≥ 25 mm Hg.
Pulmonary hypertension is not in itself a diagnosis, but solely a hemodynamic state characterized by resting mean pulmonary artery pressure (PAPm) of ≥ 25 mm Hg. The term pulmonary arterial hypertension describes a subgroup that is hemodynamically distinguished by precapillary pulmonary hypertension with elevated pulmonary vascular resistance (PVR), i.e., PAPm ≥ 25 mm Hg with normal pulmonary arterial wedge pressure (PAWP) ≤ 15 mm Hg and PVR >240 dyn × s × cm-5 (1, 2).
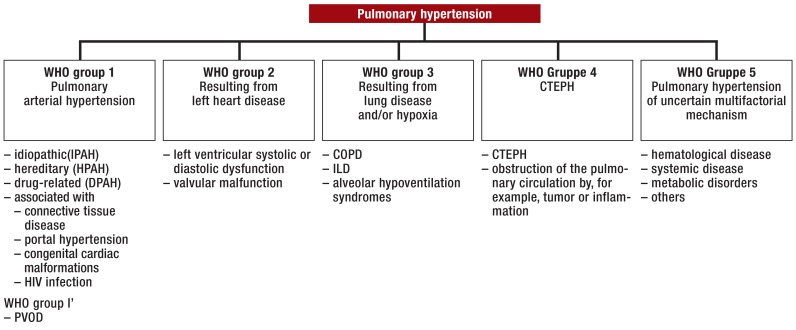
For a diagnosis of pulmonary arterial hypertension, not only must these criteria be fulfilled, but other forms of precapillary pulmonary hypertension must be excluded. This applies particularly to lung disease and chronic thromboembolic pulmonary hypertension, but also to left heart disease with normalised PAWP. Figure 1 shows a simplified form of the currently prevailing classification of pulmonary hypertension.
Figure 1.
The principal forms of pulmonary hypertension (modified from [1, 2])
COPD, Chronic obstructive lung disease; CTEPH, chronic thromboembolic pulmonary hypertension; HIV, human immunodeficiency virus; ILD, interstitial lung disease; PAH, pulmonary arterial hypertension; PVOD, pulmonary veno-occlusive disease
Pulmonary hypertension is by no means uncommon; on the contrary, it probably affects around 1% of the global population. In those over 65 years of age, the prevalence of pulmonary hypertension is thought to be around 10% (3). However, the various forms of pulmonary hypertension differ considerably in incidence and prevalence. In Germany, the incidence of pulmonary arterial hypertension in 2014 was 3.9 per 1 million adults; the prevalence was 25.9 per 1 million adults (4). In the same year the incidence of chronic thromboembolic pulmonary hypertension was 4 per 1 million adults (3).
While the epidemiological data on pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension are robust, the prevalence of other forms of pulmonary hypertension can only be estimated. One of the most frequent causes of pulmonary hypertension is left heart disease, which affects ca. 1.3 million people in Germany (www.gesundheitsforschung-bmbf.de/de/herzversagen.php; accessed on 12 June 2016). Around 50% of those with left heart disease develop pulmonary hypertension, which in 10% of cases takes a combined post- and precapillary form; therefore, up to 50 000 patients in Germany may suffer from severe pulmonary hypertension associated with left heart disease (3).
The second largest group of patients comprises those with lung disease, particularly chronic obstructive and fibrotic disease. Overall, the prevalence of pulmonary hypertension associated with lung disease is similar to that associated with left heart disease (3).
Definition of pulmonary arterial hypertension.
Mean pulmonary artery pressure ≥ 25 mm Hg, pulmonary arterial wedge pressure ≤ 15 mm Hg, and pulmonary vascular resistance >240 dyn × s × cm-5
Pulmonary arterial hypertension was originally thought to be a disease that mostly affected young women; however, the mean age of patients diagnosed with pulmonary arterial hypertension in Germany has risen steadily in recent years and is currently 65 years (4, 5). The reasons for this trend are complex, particularly since it cannot be assumed that the actual incidence of pulmonary arterial hypertension is increasing. Improvements in the quality of diagnosis are certainly behind the fact that many patients who not long ago would have been classified and treated as having cardiac insufficiency are now recognized to be suffering from pulmonary arterial hypertension. At the same time, many older patients in whom pulmonary arterial hypertension is diagnosed have cardiac or pulmonary comorbidities, a fact which often hampers precise classification. As a prominent example, up to 80% of patients with HFpEF (heart failure with preserved ejection fraction) develop a form of pulmonary hypertension (6) that is occasionally difficult to distinguish from “true” pulmonary arterial hypertension. This is the case when PAWP during treatment is in the normal range. In the absence of better terminology, pulmonary arterial hypertension in patients with significant cardiovascular risk factors is described as “atypical” to distinguish it from the “typical” pulmonary arterial hypertension in patients without significant cardiovascular risk factors or comorbidities (7). This differentiation may be of considerable relevance for treatment.
Despite the differing therapeutic implications, every form of pulmonary hypertension is clinically significant, because it is associated with increased symptoms and in nearly all cases with a higher risk of death (3). This is also true for the pulmonary hypertension in left heart disease or lung disease, although the consequences for treatment are not yet clear. The life expectancy of patients with pulmonary arterial hypertension has increased over the past three decades. The 3-year survival rate of this group is now 70 to 80% (5), compared with ca. 40% in the 1980s. The survival rates of patients with chronic thromboembolic pulmonary hypertension have also greatly improved. Before the introduction of effective treatment options the mortality associated with this disease was similar to that of pulmonary arterial hypertension, but properly treated patients now have a 3-year survival rate of 90% (8).
Symptoms and diagnosis of pulmonary hypertension
Incidence of pulmonary arterial hypertension.
The annual incidence of pulmonary arterial hypertension is around 3–10 new cases per 1 million adults.
The cardinal symptom of every form of pulmonary hypertension is progressive exercise dyspnea, often accompanied by fatigue and exhaustion. The symptoms are unspecific, so there is often a delay of many months or even years between onset of symptoms and diagnosis. With progression of the disease the symptoms become worse and new symptoms occur, e.g., dyspnea on bending down (bendopnea) and syncope, the latter particularly during or immediately after physical exertion. In patients with pulmonary hypertension, frequent syncope even on slight exertion clearly points to the presence of a life-threatening state associated with high mortality. In the event of cardiac decompensation the right cardiac filling pressures rise, with the typical triad of cervical venous congestion, ascites, and edema.
Physical examination of patients with compensated pulmonary hypertension often reveals no abnormalities. The most frequently occurring signs, often subtle, are peripheral or central cyanosis (often only, or more strongly, during exercise), a pronounced pulmonary valve component of the second heart sound, and a systolic flow murmur reaching its maximum at a left parasternal location in tricuspid valve insufficiency.
Common causes of pulmonary hypertension.
The most frequent causes of pulmonary hypertension are left heart disease and lung disease.
Early detection and precise classification of the disease are the essential goals of diagnosis in pulmonary hypertension. Together with physical examination, the basic diagnostic tests in every case of uncertain or progressive exercise dyspnea should include ECG and determination of brain natriuretic peptide (BNP) or the N-terminal fragment of its precursor (NT-proBNP). If both of these show no abnormality, pulmonary hypertension is highly unlikely to be present (9). Further diagnostic investigations are required only in the case of strong clinical suspicion of pulmonary hypertension or if the results of the above-mentioned tests are unclear. Pathologic ECG or BNP findings unequivocally indicate further cardiological investigation.
The crucial noninvasive procedure is generally echocardiography, which often arouses the first suspicion of pulmonary hypertension or right heart overload. Echocardiographic assessment of right ventricular pressure is frequently unreliable, but in combination with signs of right heart overload, echocardiography usually yields clear signs of pulmonary hypertension and thus indicates what kind of investigations should follow (1, 2).
Cardinal symptoms.
The cardinal symptoms of pulmonary hypertension are increasing exercise dyspnea, dyspnea on bending down, fatigue, exercise-induced syncope, and edema.
The diagnosis of pulmonary hypertension can be confirmed only by right heart catheterization. However, this invasive procedure is not indicated in all patients thought to have pulmonary hypertension. While the indication is indisputable in the case of suspected pulmonary arterial hypertension or chronic thromboembolic pulmonary hypertension, an invasive diagnostic procedure is usually not indicated in patients with chronic left heart disease or lung disease who show signs of pulmonary hypertension, because in most cases there would be no consequences for their treatment. The exceptions to this rule include patients planned for heart or lung transplantation and those with severe right heart overload or signs of severe pulmonary hypertension. This applies particularly in cases where the underlying disease is relatively mild and there is a discrepancy with the severity of the symptoms or that of the right heart overload. In the event of doubt the patient should be referred to a pulmonary hypertension center, particularly since right heart catheterization should in any case be carried out at a specialized institution. A list of pulmonary hypertension centers in Germany can be found on the website of the German self-help group pulmonale hypertonie e. v. (www.phev.de/professionals.html).
Echocardiography.
Echocardiography can reveal signs of pulmonary hypertension or right heart overload.
In patients with idiopathic, hereditary, or drug-related pulmonary arterial hypertension, right heart catheterization is accompanied by vasoreactivity testing to identify so-called responders who might benefit from treatment with high-dose calcium antagonists (1, 2).
Confirmation of diagnosis.
Right heart catheterization is necessary for confirmation of pulmonary hypertension.
An important follow-on investigation in patients with suspected or confirmed pulmonary hypertension is perfusion scintigraphy, to ensure that chronic thromboembolic pulmonary hypertension is not overlooked. Scintigraphy is thought to be more sensitive than angio-CT for this indication (1, 2). This may change with the universal introduction of dual-energy CT scanners, which as well as delivering conventional images also depict regional lung perfusion—without additional irradiation or contrast medium. However, this technique still requires further evaluation. Patients with signs of chronic thromboembolic pulmonary hypertension should be referred for further investigation to specialized centers, the only institutions equipped to decide on the best treatment in individual cases.
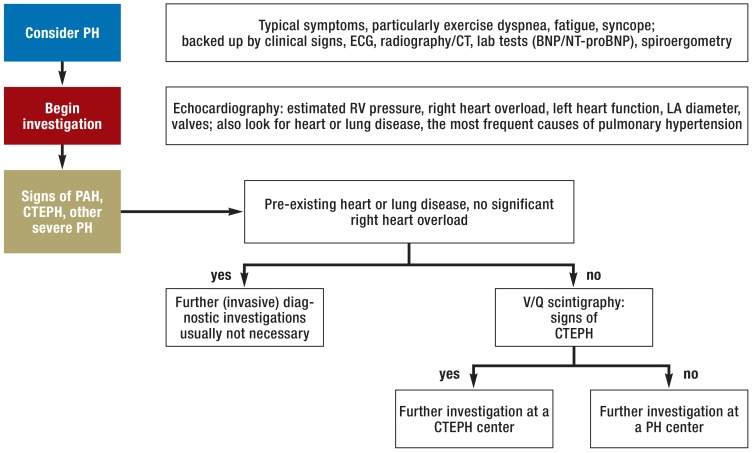
An algorithm for the initial diagnostic work-up in the case of suspected pulmonary hypertension is shown in Figure 2.
Figure 2.
Initial diagnostic procedure in suspected pulmonary hypertension
BNP, Brain natriuretic peptide; CT, computed tomography; CTEPH, chronic thromboembolic pulmonary hypertension; ECG, electrocardiography; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; LA, left atrium; NT-proBNP, N-terminal fragment of pro-brain natriuretic peptide; RV, right ventricle; V/Q, ventilation/perfusion
General treatment of pulmonary hypertension
The general treatment of pulmonary hypertension is predominantly symptomatic and depends on the type and severity of the disease and the patient’s requirements. The following recommendations are mostly based on expert consensus; apart from the rehabilitation measures, they are not backed up by randomized studies.
In analogy with the recommendations for patients with chronic obstructive pulmonary disease, oxygen therapy is indicated whenever there is manifest hypoxemia with arterial pO2 <60 mm Hg. Attention must also be paid to correcting nocturnal hypoxemia and exercise-induced hypoxemia in these patients. Any anemia or iron deficiency without anemia should be corrected. Venesection is also hardly ever indicated in patients with polycythemia. If at all, there is an indication in the presence of symptoms of hyperviscosity.
Diuretics are indicated in patients with signs of hyperhydration. The data for pulmonary hypertension are sparse; usually loop diuretics are used, often in combination with mineralocorticoid receptor antagonists. In some patients lymph drainage may be effective in supporting the drug treatment.
Chronic thromboembolic pulmonary hypertension.
Ventilation/perfusion scintigraphy is recommended for confirmation or exclusion of chronic thromboembolic pulmonary hypertension.
Anticoagulation is no longer recommended for general use; rather it is now restricted to patients with chronic thromboembolic pulmonary hypertension and those with comorbidities for which anticoagulation is indicated (1, 2, 10).
Specific rehabilitation measures and active physiotherapy help to improve the exercise capacity, quality of life, and cardiac function of patients with pulmonary hypertension (11).
Specific drug treatment of pulmonary arterial hypertension
Newly diagnosed pulmonary arterial hypertension should be treated swiftly and specifically. Patients with idiopathic, hereditary, or drug-related pulmonary arterial hypertension who are identified as responders on vasoreactivity testing during right heart catheterization (a fall in mean PAPm of more than 10 mm Hg to under 40 mm Hg without a decrease in cardiac output) are treated initially with calcium antagonists individually titrated to high doses (e.g., amlodipine 20 mg/day). In the most favorable case this leads to normalization or near-normalization of pulmonary hemodynamics. However, this form of treatment is an option in fewer than 5% of patients with pulmonary arterial hypertension and must be cautiously initiated and carefully monitored. Treatment with calcium antagonists is not indicated in patients with other forms of pulmonary (arterial) hypertension (1, 2). The practice of trying calcium antagonists without previous vasoreactivity testing is obsolete.
General treatment of pulmonary hypertension.
Rehabilitation measures and active physiotherapy help to improve the exercise capacity, quality of life, and cardiac function of patients with pulmonary hypertension.
Treatment of pulmonary arterial hypertension.
Specific treatment usually requires the combination of various medications and should be initiated at an expert center.
Drug treatment.
Ten medications from five different substance classes are currently licensed for the treatment of pulmonary arterial hypertension in Germany.
Ten drugs from five different substance classes are currently licensed for the treatment of pulmonary arterial hypertension in Germany (etable 1). These drugs are used singly or in combination. The treatment strategy should be determined at specialized centers. Pulmonary arterial hypertension remains an incurable illness. The goal of treatment is containment of the disease, i.e., stabilization of the patient at a satisfactory clinical level (WHO functional class I or II) without signs of right heart failure and ideally without disease progression. In one randomized study using initial combination therapy (12), this goal was achieved in 40% of the patients. The choice of medication depends partly on the severity of the pulmonary arterial hypertension. The current guidelines (1, 2) recommend classification into low-, intermediate-, and high-risk disease, based on the expected 1-year mortality (etable 2). Patients with newly diagnosed “typical” pulmonary arterial hypertension and low or intermediate risk receive initial or early combination treatment comprising an endothelin receptor antagonist (ERA) with a phosphodiesterase-5 (PDE5) inhibitor or a soluble guanylate cyclase (sGC) stimulator (12– 14). The recommended initial treatment for high-risk patients is a triple combination of an ERA, a PDE5 inhibitor or an sGC stimulator, and an intravenously administered prostacyclin analog.
eTable 1. Drugs licensed for the treatment of pulmonary arterial hypertension in Germany.
| Substance group | Substance | Approved dosage and route of administration | Reference(s) |
|
Endothelin receptor antagonists |
Ambrisentan | 1 × 5 mg/day or 1 × 10 mg/day orally | (12, 28) |
| Bosentan | Initial dosage 2 × 62.5 mg/day, Target dosage 2 × 125 mg/day orally |
(29– 31) | |
| Macitentan | 1 × 10 mg/day orally | (13) | |
| PDE5 inhibitors | Sildenafil | 3 × 20 mg/day orally | (32) |
| Tadalafil | 1 × 40 mg/day orally | (12, 33) | |
|
Stimulator of soluble guanylate cyclase |
Riociguat | Initial dosage 3 × 1.0 mg/day, Target dosage 3 × 2.5 mg/day orally |
(34, 35) |
| Prostacyclin analogs | Epoprostenol (classic form and thermostable variant) |
Individual dosing, target dosage usually 20–50 ng/kg/min IV |
(36) |
| Iloprost | 2.5 µg or 5 µg 6–9 ×/day by inhalation |
(37) | |
| Treprostinil | Individual dosing, target dosage usually 20–50 ng/kg/min IV or SC |
(38) | |
|
Prostacyclin receptor agonist |
Selexipag | Individual dosing, initial dosage 2 × 200 µg/day, maximum dosage 2 × 1600 µg/day |
(39) |
eTable 2. Risk stratification for patients with pulmonary arterial hypertension (modified from [1, 2]).
|
Prognostic parameter (estimated 1-year mortality) |
Slight risk (<5%) | Intermediate risk (5–10%) | High risk (>10%) |
|
Clinically manifest right heart failure |
No | No | Yes |
| Progression of symptoms | No | Slow | Rapid |
| Syncope | None | Occasionally, orthostatic or on unaccustomed physical exertion |
Frequent, even on minor physical exertion |
| WHO functional class | I/II | III | IV |
| Six-minute walking distance | >440 m | 165–440 m | <165 m |
| Spiroergometry | Peak VO2 >15 mL/min/kg (>65% ref.); VE/VCO2 slope <36 |
Peak VO2 11–15 mL/min/kg (>65% ref.); VE/VCO2 slope 36–44 |
Peak VO2 <11 ml/min/kg (>65% ref.); VE/VCO2 slope >44 |
| Serum BNP/NT-proBNP level | BNP <50 ng/l NT-proBNP <300 ng/l |
BNP 50–300 ng/L NT-proBNP 300–1400 ng/L |
BNP >300 ng/L NT-proBNP >1400 ng/L |
|
Cardiac imaging (echocardiography, cMRT) |
RA surface area <18 cm2 No pericardial effusion |
RA surface area 18–26 cm2 No or minimal pericardial effusion |
RA surface area >26 cm2 Pericardial effusion |
| Hemodynamics | RA <8 mm hg CI >2.5 L/min/m2 SvO2 >65% |
RA 8–14 mm Hg CI 2.0–2.4 L/min/m2 SvO2 60–65% |
RA >14 mm Hg CI <2.0 l/min/m2 SvO2 <60% |
BNP, Brain natriuretic peptide; CI, cardiac index; NT-proBNP, N-terminal fragment of pro-brain natriuretic peptide; peak VO2, highest value of maximal oxygen uptake; RA, right atrium; SvO2, mixed venous oxygen saturation; VE/CO2 slope, ventilatory equivalent for CO2; ref., of reference value
The patient’s reaction to treatment is usually verified after 4 to 12 weeks and then at intervals of 3 to 6 months. How the treatment continues depends on the individual response. If the patient has not achieved the primary treatment goal, i.e., attainment of the low-risk category (etable 2), after the initial treatment, the next step is dual or triple combination treatment. A potential further option, switching from a PDE5 inhibitor to riociguat, is currently being evaluated (RESPITE; clinicaltrials.gov identifier NCT02007629).
Treatment goals.
Pulmonary arterial hypertension is an incurable illness. The goal of treatment is containment of the disease, i.e., stabilization of the patient at a satisfactory clinical level without signs of right heart failure and ideally without disease progression.
Treatment recommendation.
The recommended initial treatment for high-risk patients is a triple combination of an ERA, a PDE5 inhibitor or an sGC stimulator, and an intravenously administered prostacyclin analog.
If the treatment response still remains inadequate, evaluation for lung transplantation should be initiated without delay, because such patients may decompensate rapidly and without warning. Although nowadays the majority of patients with pulmonary arterial hypertension do not require transplantation, this measure is indispensable for those who are not helped by medication. Combined heart and lung transplantation is necessary only in exceptional cases, because right heart function is almost always restored to normal after lung transplantation (15). The outcome of lung transplantation has steadily improved in recent years, to the point where experienced centers now report 1-year survival rates of >90% (16).
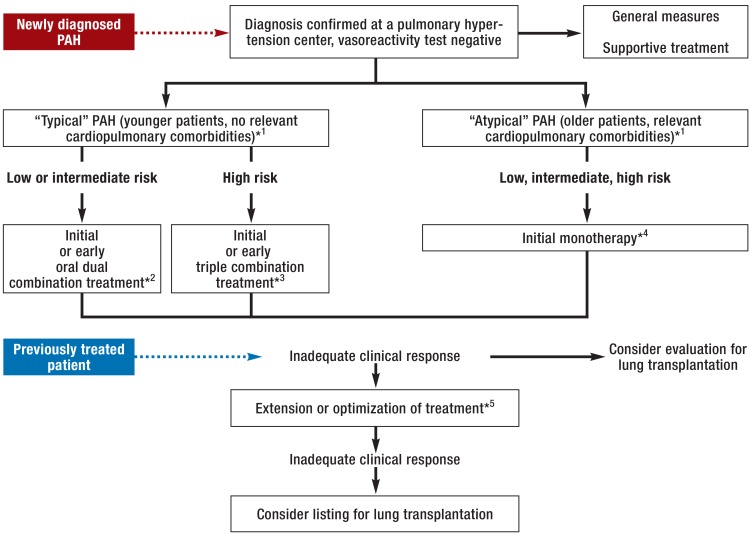
The management of patients with “atypical” pulmonary arterial hypertension (table) is less standardized. The majority of these patients are first treated with a single agent, usually a PDE5 inhibitor (7). The further procedure depends on the response and on the individual circumstances; owing to the lack of data, no general recommendations can be given for these patients. On grounds of age and comorbidities most patients with “atypical” pulmonary arterial hypertension are usually not candidates for lung transplantation. Figure 3 shows the currently valid treatment algorithm for patients with pulmonary arterial hypertension.
Table. Criteria for “atypical” pulmonary arterial hypertension (based on the recommendations of the second Cologne Consensus Conference).
| Hemodynamic ‧profile | Corresponds to that of other forms of PAH,i.e., precapillary PH with elevated PVR |
| Phenotypic ‧characteristics | Predominantly older patients (mostly >65 years);risk profile or comorbidities as in patients with left heart ‧disease or lung disease |
| Cardiac phenotype | At least three of the following risk factors:hypertension, coronary heart disease, diabetes mellitus, ‧obesity (BMI >30 kg/m2); further ‧characteristics including left atrial enlargement, atrial fibrillation |
| Pulmonary ‧phenotype | Normal or near-normal whole-body plethysmography, no clinically significant alterations of lung ‧parenchyma on chest CT, DLCO <45 % of reference value, often hypoxemia |
BMI, Body mass index; CT, computed tomography; DLCO, diffusion capacity of lungs for carbon monoxide; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; PVR, pulmonary vascular resistance
Figure 3.
Treatment algorithm for pulmonary arterial hypertension based on the European guidelines and the Cologne Consensus Conference
PAH, pulmonary hypertension
*1 The phenotype determines whether PAH is classified as typical or atypical; age alone is not a sufficient criterion, but the older the patient, the greater the likelihood of comorbidity and risk factors for cardiopulmonary disease (hypertonia, coronary heart disease, diabetes mellitus, obesity, etc.).
*2 Initiation immediately, or within 3 months of diagnosis, of combination treatment with endothelin receptor antagonists plus phosphodiesterase-5 inhibitors or stimulators of soluble guanylate cyclase.
*3 Initiation immediately, or within 3 months of diagnosis, of combination treatment with endothelin receptor antagonists plus phosphodiesterase-5 inhibitors or stimulators of soluble guanylate cyclase plus a prostacyclin derivative.
*4 In these often elderly patients, who present with cardiac and/or pulmonary comorbidity, the efficacy and tolerance of drugs for PAH have been less extensively investigated; as this is particularly true fo combination treatments, starting with monotherapy is recommended.
*5 Individual adjustment of treatment: in “typical” PAH, if it seems appropriate, further escalation of the combination treatment to include prostacyclin derivatives; consider SC/IV prostacyclin; consider switch from phosphodiesterase-5 inhibitor to sGC stimulator; in “atypical” PAH,decide case by case; moreover, optimize supportive treatment for all patients, including rehabilitation measures.
Treatment of pulmonary hypertension in left heart disease and lung disease
Atypical pulmonary arterial hypertension.
The management of patients with “atypical” pulmonary arterial hypertension is less standardized. The majority of these patients are first treated with a single agent, usually a PDE5 inhibitor.
The basic principles of the treatment of pulmonary hypertension in left heart disease and in lung disease are practically identical. None of the drugs licensed for the treatment of pulmonary arterial hypertension (etable 1) have any proven effect in patients with pulmonary hypertension on the basis of left heart disease or lung disease, so their use cannot be recommended in these indications. The randomized controlled multicenter studies conducted to test the action of drugs for pulmonary arterial hypertension in these groups of patients have all been negative, i.e., there was no sign of efficacy or the drug was actually harmful (17– 21). The potential risk entailed in using drugs for pulmonary arterial hypertension in patients with left heart disease or lung disease was emphasized by the recent discontinuation of a phase-II study of riociguat in patients with pulmonary hypertension based on fibrotic lung disease owing to signs of an elevated risk of mortality in the riociguat group (RISE-IIP, clinicaltrial.gov NCT02138825). The occasional patients with simultaneous left heart or lung disease and severe pulmonary (arterial) hypertension in whom the underlying disease does not explain the extent of pulmonary hypertension or right heart overload constitute an exception to the recommendation not to use pulmonary arterial hypertension drugs. The background to this recommendation is the observation that pulmonary arterial hypertension is increasingly also being diagnosed in the elderly, a category at high risk of other cardiopulmonary diseases. For example, the prevalence of chronic obstructive pulmonary disease in the over-70 age group is ca. 20% (22). Therefore, the same rate of chronic obstructive pulmonary disease can be expected in persons older than 70 years with “true” pulmonary arterial hypertension. The same is true for other common cardiopulmonary diseases. Differentiation between pulmonary hypertension due to left heart disease or lung disease and “true” pulmonary arterial hypertension coexisting with left heart or lung disease may be difficult and is a task for the experienced specialist. The therapeutic consequences are far-reaching, because only in the former case will specific treatment of pulmonary arterial hypertension be initiated.
Chronic thromboembolic pulmonary hypertension
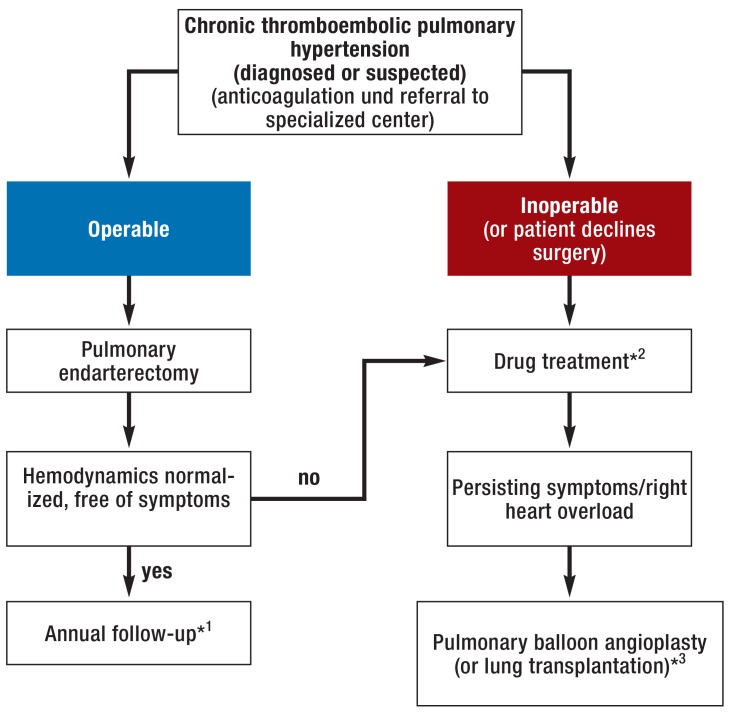
The treatment of chronic thromboembolic pulmonary hypertension is distinct from that of the other forms of pulmonary hypertension (figure 4). The preferred treatment is surgical pulmonary endarterectomy (1, 2), currently performed on a regular basis at three centers in Germany (Bad Nauheim, Hanover, and Homburg). This operation was developed in San Diego, California in the 1970s and the results have improved with the passage of time. The perioperative mortality at experienced centers is now 2 to 4% (8, 23). In nearly all cases, pulmonary endarterectomy yields substantial hemodynamic and clinical improvement (8, 23). In around 50% of cases the pulmonary blood pressure is restored to normal; the majority of the remaining patients end up with slight residual pulmonary hypertension that does not require treatment (8). In around 20% of patients treated with pulmonary endarterectomy, however, the residual pulmonary hypertension is clinically significant and has to be treated (8).
Figure 4.
Procedure in patients with chronic thromboembolic pulmonary hypertension (modified from [27, 40])
*1 Clinical examination and echocardiography are usually sufficient
*2 Currently only riociguat is licensed for the treatment of these patients
*3 Lung transplantation is seldom necessary in patients with CTEPH
The decision regarding operability should logically be made at a center for chronic thromboembolic pulmonary hypertension, where the most suitable treatment procedure is determined in regular multidisciplinary discussions of the clinical and hemodynamic findings together with CT and angiography. Advanced age and relevant comorbidities do not per se contraindicate surgical pulmonary endarterectomy.
Treatment of chronic thromboembolic pulmonary hypertension.
Surgical pulmonary endarterectomy is the treatment of choice.
Nowadays around 50 to 70% of patients with chronic thromboembolic pulmonary hypertension are operable (24). Riociguat is a licensed drug treatment for nonoperable patients and those with residual pulmonary hypertension after pulmonary endarterectomy (25, 26). In the event that riociguat does not achieve the desired improvement, the currently valid guidelines recommend the use of other medications for pulmonary arterial hypertension, although these have not been licensed for this indication (1, 2). In the past few years, moreover, some centers for chronic thromboembolic pulmonary hypertension have been evaluating pulmonary balloon angioplasty as a new interventional treatment option. This procedure can be used to recanalize obliterated pulmonary vessels at the peripheral, i.e., subsegmental level (27). The results to date are encouraging. For the time being, however, this method should be restricted to experienced centers for chronic thromboembolic pulmonary hypertension.
Summary
The management options for patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension have broadened considerably in recent years. This has made treatment more successful, but also more complex. For the essentially more widespread forms of pulmonary hypertension, observed above all in patients with left heart disease or lung disease, the sole established option is treatment of the underlying disease. A small proportion of these patients develop severe pulmonary hypertension that may occasionally resemble pulmonary arterial hypertension. The best treatment for this group of patients currently has to be determined on an individual basis. Like any serious, life-threatening rare disease, pulmonary arterial hypertension, chronic thromboembolic pulmonary hypertension, and other severe forms of pulmonary hypertension should be diagnosed and treated at specialized centers.
Treatment of pulmonary hypertension in left heart or lung disease.
In these forms of pulmonary hypertension the use of medications for pulmonary arterial hypertension is indicated only in exceptional cases.
Lung transplantation.
Lung transplantation is a valuable option in patients with pulmonary arterial hypertension resistant to treatment. These patients should be referred to specialized centers in good time.
Further information on CME.
This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education. Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Medical Associations of the German federal states (Länder). CME points of the Medical Associations can be acquired only through the Internet, not by mail or fax, by the use of the German version of the CME questionnaire. See the following website: cme.aerzteblatt.de.
Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the cme.aerzteblatt.de website under “meine Daten” (“my data”), or upon registration. The EFN appears on each participant’s CME certificate.
This CME unit can be accessed until 30 April 2017, and earlier CME units until the dates indicated:
“Treatment Options in Hepatitis C” (Issue 1–2/2017) until 2 April 2017, and
“the Differential Diagnosis of Dyspnea” (Issue 49/2016) until 5 March 2017.
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the most appropriate answer.
Question 1
Pulmonary arterial hypertension is characterized by which of the following hemodynamic criteria?
Systolic pulmonary artery pressure (PAPs) >40 mm Hg
Mean pulmonary artery pressure (PAPm) ≥ 25 mm Hg at rest and >30 mm Hg on exercise; pulmonary arterial wedge pressure (PAWP) ≤ 15 mm Hg
Resting PAPm ≥ 20 mm Hg, PAWP ≤ 15 mm Hg, pulmonary vascular resistance (PVR) >160 dyn × s × cm-5
Resting PAPm ≥ 25 mm Hg, PAWP ≤ 15 mm Hg, PVR >240 dyn× s × cm-5
Resting PAPm ≥ 30 mm Hg, PAWP ≤ 15 mm Hg, PVR >320 dyn × s × cm-5
Question 2
What is the approximate annual incidence of pulmonary arterial hypertension in Germany?
1–2 per 1 million adults
3–10 per 1 million adults
11–20 per 1 million adults
20–50 per 1 million adults
50–100 per 1 million adults
Question 3
Which imaging procedure is particularly recommended to confirm or rule out a thromboembolic origin in patients with known or suspected pulmonary hypertension?
Ventilation/perfusion scintigraphy
Contrast-enhanced computed tomography of the lungs
High-resolution computed tomography of the lungs
Magnetic resonance imaging of the pulmonary vessels
Right heart catheterization
Question 4
Which of the following is one of the usual general measures in patients with pulmonary hypertension?
Oxygen treatment to lower the pulmonary artery pressure
Anticoagulation, provided there are no contraindications
Strict avoidance of physical activity and exercise
Venesection from hematocrit of 50% to improve blood viscosity
Treatment of iron deficiency with or without anemia
Question 5
Which of these symptoms or findings in patients with pulmonary arterial hypertension is associated with a high risk of death?
WHO functional class II
Repeated syncope on slight physical exertion
Six-minute walking distance ≤ 350 m
Maximal oxygen uptake 13 mL/min/kg
Cardiac index 2.5 l/min/m2
Question 6
When is a trial of treatment with calcium antagonists indicated in pulmonary hypertension?
In newly diagnosed pulmonary arterial hypertension
In all forms of pulmonary hypertension
In patients with idiopathic pulmonary arterial hypertension and positive vasoreactivity test during right heart catheterization
In all forms of pulmonary hypertension with a positive vasoreactivity test during right heart catheterization
In the presence of arterial hypertonia
Question 7
What treatment should be given to an elderly patient with atypical pulmonary arterial hypertension and cardiopulmonary comorbidities?
Pulmonary endarterectomy
Lung transplantation
Early dual combination treatment
Initial monotherapy
Early triple combination treatment
Question 8
What, together with physical examination and ECG, is an essential diagnostic procedure in the case of exercise dyspnea of unknown origin?
V/Q scintigraphy
Right heart catheterization
Magnetic resonance imaging of the heart
Angio-CT
Determination of BNP or NT-proBNP
Question 9
What proportion of patients with chronic thromboembolic pulmonary hypertension are operable?
10 to 30%
20 to 40%
30 to50%
40 to60%
50 to 70%
Question 10
In which patients with pulmonary arterial hypertension should lung transplantation be considered?
In patients over 75 years of age
In patients whose quality of life is impaired by multiple comorbidities
In patients in whom a 3-month trial of PDE5 inhibitors has been unsuccessful
In patients in whom combination treatment has not achieved a sufficient response
In patients who have an accompanying disorder of left heart function
Acknowledgments
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
Prof. Hoeper has received payments for consultancy from Actelion, Bayer, Gilead, GSK, MSD, and Pfizer.
Prof. Ghofrani has received payments for consultancy, payments for expert reviewing, reimbursement of attendance fees and costs for travel and accommodation, and payments for preparation of scientific lectures from Actelion, Bayer, Gilead, GSK, MSD, Novartis, Pfizer, and United Therapeutics. He has received third-party funding from Actelion, Bayer, Novartis, and Pfizer. He has received funds for the conduct of clinical studies from Actelion, Bayer, Gilead, GSK, Novartis, Pfizer, and United Therapeutics.
Prof. Grünig has received payments for consultancy and reimbursement of congress attendance fees and costs for travel and accommodation from Actelion, Bayer, and GSK. He has received funds for the conduct of commissioned clinical studies from Actelion, Bayer, MSD, GSK, Gilead, and United Therapeutics. He received support for the conduct of a research project of his own initiation from Actelion, Bayer, and GSK.
Dr. Klose has received payments for consultancy from GSK, Pfizer, Actelion, Bayer, OMT, and United Therapeutics. He has received reimbursement of attendance fees and costs for travel and accommodation from Actelion and Bayer. He has received payments for lectures from Actelion, Bayer, GSK, and OMT. He received funds for the conduct of a research project of his own initiation from Actelion, GSK, and Bayer.
Prof. Olschewski has received payments for consultancy from Actelion, Bayer, Gilead, GSK, Novartis, Pfizer, and Bellerophon. Congress attendance fees have been reimbursed by Boehringer and Menarini. He has received reimbursement of costs for travel and accommodation from Actelion and Bayer and payments for scientific lectures from Actelion, Bayer, GSK, and Novartis. He received support for the conduct of a research project of his own initiation from Roche, Boehringer, and Actelion.
Prof. Rosenkranz has received payments for lectures from Actelion, Bayer, GSK, Gilead, Novartis,Pfizer, and United Therapeutics. He has received reimbursement of congress attendance fees from Actelion and Bayer and of travel and accommodation costs from Actelion, Bayer, and United Therapeutics. He has received payments for the conduct of commissioned clinical studies from Actelion, Bayer, GSK, Gilead, Novartis, Pfizer, and United Therapeutics. He received support for the conduct of a research project of his own initiation from Actelion, Bayer, Novartis, and United Therapeutics.
References
- 1.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 2.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 3.Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. Lancet Respir Med. 2016;4:306–322. doi: 10.1016/S2213-2600(15)00543-3. [DOI] [PubMed] [Google Scholar]
- 4.Hoeper MM, Huscher D, Pittrow D. Incidence and prevalence of pulmonary arterial hypertension in Germany. Int J Cardiol. 2016;203:612–613. doi: 10.1016/j.ijcard.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Hoeper MM, Huscher D, Ghofrani HA, et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol. 2013;168:871–880. doi: 10.1016/j.ijcard.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Rosenkranz S, Gibbs JS, Wachter R, De Marco T, Vonk-Noordegraaf A, Vachiery JL. Left ventricular heart failure and pulmonary hypertension. Eur Heart J. 2016;37:942–954. doi: 10.1093/eurheartj/ehv512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Opitz C, Hoeper MM, Gibbs JSR, et al. Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum? J Am Coll Cardiol. 2016;68:368–378. doi: 10.1016/j.jacc.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 8.Cannon JE, Su L, Kiely DG, et al. Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: results from the United Kingdom national cohort. Circulation. 2016;133:1761–1771. doi: 10.1161/CIRCULATIONAHA.115.019470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonderman D, Wexberg P, Martischnig AM, et al. A noninvasive algorithm to exclude pre-capillary pulmonary hypertension. Eur Respir J. 2011;37:1096–1103. doi: 10.1183/09031936.00089610. [DOI] [PubMed] [Google Scholar]
- 10.Olsson KM, Delcroix M, Ghofrani HA, et al. Anticoagulation and survival in pulmonary arterial hypertension: results from the comparative, prospective registry of newly initiated therapies for pulmonary hypertension (COMPERA) Circulation. 2014;129:57–65. doi: 10.1161/CIRCULATIONAHA.113.004526. [DOI] [PubMed] [Google Scholar]
- 11.Ehlken N, Lichtblau M, Klose H, et al. Exercise training improves peak oxygen consumption and haemodynamics in patients with severe pulmonary arterial hypertension and inoperable chronic thrombo-embolic pulmonary hypertension: a prospective, randomized, controlled trial. Eur Heart J. 2016;37:35–44. doi: 10.1093/eurheartj/ehv337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galie N, Barbera JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373:834–844. doi: 10.1056/NEJMoa1413687. [DOI] [PubMed] [Google Scholar]
- 13.Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369:809–818. doi: 10.1056/NEJMoa1213917. [DOI] [PubMed] [Google Scholar]
- 14.Lajoie AC, Lauziere G, Lega JC, et al. Combination therapy versus monotherapy for pulmonary arterial hypertension: a meta-analysis. Lancet Respir Med. 2016;4:291–305. doi: 10.1016/S2213-2600(16)00027-8. [DOI] [PubMed] [Google Scholar]
- 15.Mandich Crovetto D, Alonso Charterina S, Jimenez Lopez-Guarch C, et al. Multidetector computed tomography shows reverse cardiac remodeling after double lung transplantation for pulmonary hypertension. Radiologia. 2016;58:277–282. doi: 10.1016/j.rx.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Tudorache I, Sommer W, Kuhn C, et al. Lung transplantation for severe pulmonary hypertension-awake extracorporeal membrane oxygenation for postoperative left ventricular remodelling. Transplantation. 2015;99:451–458. doi: 10.1097/TP.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 17.Hoendermis ES, Liu LC, Hummel YM, et al. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. Eur Heart J. 2015;36:2565–2573. doi: 10.1093/eurheartj/ehv336. [DOI] [PubMed] [Google Scholar]
- 18.Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, Hunninghake GW. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363:620–628. doi: 10.1056/NEJMoa1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corte TJ, Keir GJ, Dimopoulos K, et al. Bosentan in pulmonary hypertension associated with fibrotic idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2014;190:208–217. doi: 10.1164/rccm.201403-0446OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goudie AR, Lipworth BJ, Hopkinson PJ, Wei L, Struthers AD. Tadalafil in patients with chronic obstructive pulmonary disease: a randomised, double-blind, parallel-group, placebo-controlled trial. The Lancet Respiratory Medicine. 2014;2:293–300. doi: 10.1016/S2213-2600(14)70013-X. [DOI] [PubMed] [Google Scholar]
- 22.Raherison C, Girodet PO. Epidemiology of COPD. Eur Respir Rev. 2009;18:213–221. doi: 10.1183/09059180.00003609. [DOI] [PubMed] [Google Scholar]
- 23.Madani MM, Auger WR, Pretorius V, et al. Pulmonary endarterectomy: recent changes in a single institution’s experience of more than 2,700 patients. Ann Thorac Surg. 2012;94:97–103. doi: 10.1016/j.athoracsur.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124:1973–1981. doi: 10.1161/CIRCULATIONAHA.110.015008. [DOI] [PubMed] [Google Scholar]
- 25.Ghofrani HA, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369:319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 26.Simonneau G, D’Armini AM, Ghofrani HA, et al. Predictors of long-term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: data from the CHEST-2 open-label, randomised, long-term extension trial. Lancet Respir Med. 2016;4:372–380. doi: 10.1016/S2213-2600(16)30022-4. [DOI] [PubMed] [Google Scholar]
- 27.Hoeper MM, Madani MM, Nakanishi N, Meyer B, Cebotari S, Rubin LJ. Chronic thromboembolic pulmonary hypertension. The Lancet Respiratory Medicine. 2014;2:573–582. doi: 10.1016/S2213-2600(14)70089-X. [DOI] [PubMed] [Google Scholar]
- 28.Galie N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117:3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 29.Galie N, Rubin L, Hoeper M, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet. 2008;371:2093–2100. doi: 10.1016/S0140-6736(08)60919-8. [DOI] [PubMed] [Google Scholar]
- 30.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 31.McLaughlin V, Channick RN, Ghofrani HA, et al. Bosentan added to sildenafil therapy in patients with pulmonary arterial hypertension. Eur Respir J. 2015;46:405–413. doi: 10.1183/13993003.02044-2014. [DOI] [PubMed] [Google Scholar]
- 32.Galie N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 33.Galie N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 34.Ghofrani HA, Galie N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369:330–340. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 35.Ghofrani HA, Grimminger F, Grunig E, et al. Predictors of long-term outcomes in patients treated with riociguat for pulmonary arterial hypertension: data from the PATENT-2 open-label, randomised, long-term extension trial. Lancet Respir Med. 2016;4:361–367. doi: 10.1016/S2213-2600(16)30019-4. [DOI] [PubMed] [Google Scholar]
- 36.Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension The primary pulmonary hypertension study group. N Engl J Med. 1996;334:296–302. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 37.Olschewski H, Simonneau G, Galie N, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322–329. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 38.Simonneau G, Barst RJ, Galie N, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:800–804. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 39.Sitbon O, Channick R, Chin KM, et al. Selexipag for thetreatment of pulmonary arterial hypertension. N Engl J Med. 2015;373:2522–2533. doi: 10.1056/NEJMoa1503184. [DOI] [PubMed] [Google Scholar]
- 40.Olsson KM, Meyer B, Hinrichs J, Vogel-Claussen J, Hoeper MM, Cebotari S. Chronic thromboembolic pulmonary hypertension. Dtsch Arztebl Int. 2014;111:856–862. doi: 10.3238/arztebl.2014.0856. [DOI] [PMC free article] [PubMed] [Google Scholar]