Abstract
Background
The optimal approach to assess risk of venous thromboembolism (VTE) in hospitalized medical patients is unknown. We examined how well the Caprini risk assessment model (RAM) predicts VTE in hospitalized medical patients.
Methods
Between January 2011 and March 2014, VTE events and risk factors were collected from non-intensive care unit (ICU) medical patients hospitalized in facilities across Michigan. Following calculation of the Caprini score for each patient, mixed logistic spline regression was used to determine the predicted probabilities of 90-day VTE by receipt of pharmacologic prophylaxis across the Caprini risk continuum.
Results
A total of 670 (1.05%) of 63,548 eligible patients experienced a VTE event within 90 days of hospital admission. The mean Caprini risk score was 4.94 (range 0 - 28). Predictive modeling revealed a consistent linear increase in VTE for Caprini scores between 1-10; estimates beyond a score of 10 were unstable. Receipt of pharmacologic prophylaxis resulted in a modest decrease in VTE risk (odds ratio=0.85; 95% confidence interval 0.72 - 0.99, p = 0.04). However, the low overall incidence of VTE led to large estimates of numbers needed to treat in order to prevent a single VTE event. A Caprini cut-point demonstrating clear benefit of prophylaxis was not detected.
Conclusions
Although a linear association between the Caprini RAM and risk of VTE was noted, an extremely low incidence of VTE events in non-ICU medical patients was observed. The Caprini RAM was unable to identify a subset of medical patients who benefit from pharmacologic prophylaxis.
Introduction
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common cause of morbidity and mortality in hospitalized patients. While national guidelines endorse assessing VTE risk in hospitalized medical patients through use of various risk assessment models (RAMs),1-6 no accepted standard by which to perform this evaluation is currently available.7 Despite this fact, the Joint Commission and the Centers for Medicare and Medicaid Services have introduced a performance measure for VTE prophylaxis in hospitalized medical patients. This measure requires clinicians to either provide VTE prophylaxis, or document reasons for its omission.8
Originally developed for surgical patients, the Caprini RAM facilitates the derivation of VTE risk by summing individual risk factors so as to place patients into four categories: “low risk” (0-1 points), “moderate risk” (2 points), “high risk” (3-4 points), and “highest risk” (≥5 points).1 Because it is categorical and relatively easy to estimate, the Caprini RAM has been widely adopted and is increasingly applied to hospitalized medical patients.9-11 Yet, whether this tool adequately predicts VTE or identifies a risk threshold most likely to benefit from anticoagulation in this subset of patients is not known. This knowledge gap is important as US hospitals that currently use the Caprini RAM may not be effectively targeting or averting VTE events in medical populations.
A statewide quality collaborative aimed at preventing adverse events in hospitalized medical patients, the Michigan Hospital Medicine Safety Consortium (HMS) collects detailed data on VTE risk factors and outcomes across diverse Michigan hospitals.12 Using data from this collaborative, we conducted a retrospective study to assess the utility of the Caprini RAM in predicting risk of VTE in hospitalized medical patients.
Material and Methods
Study Setting and Participants
The HMS is a collaborative of 48 hospitals in Michigan dedicated to preventing adverse events in hospitalized medical patients through creation of a data registry and sharing of best practices. The setting and design of HMS have been previously described.12,13 Although voluntary, each hospital receives payments from Blue Cross Blue Shield of Michigan and Blue Care Network for participating in the consortium and for data collection.
Eligible cases include patients admitted to a medicine service for two or more days. Patients are excluded if they meet any of the following criteria: 1) under the age of 18; 2) pregnant; 3) any surgical procedure during the admission; 4) direct admission to an ICU; 5) direct admission for end-of-life or comfort care; 6) diagnosis of VTE in the 6 months prior to admission; 7) admitted for presumed VTE; 8) admitted under observation status; 9) re-admitted within 90 days of discharge from an admission included in the registry; or 10) receiving systemic anticoagulation.
Clinical data are collected through a standardized process at each hospital by trained medical record abstractors. Patients discharged from each participating hospital are sampled on an eight-day rolling cycle to avert bias in selecting cases for review.14 Data on the first 18 eligible cases discharged during each cycle are collected. Follow-up data are collected through both medical record review and direct telephone follow-up at 90 days post-hospital discharge. In the event a patient transfers to an ICU or palliative care, data collection is terminated; however, VTE events that may have contributed to such events were captured. Each hospital is audited on an annual basis by quality coordinators to ensure completeness and accuracy of data abstraction.
Ascertainment of Outcomes
The primary outcome was clinically suspected, image-confirmed hospital-associated VTE including proximal upper or proximal lower extremity DVT and PE. In order to be attributable to a hospital, we required that VTE events occur on or beyond the third day of the index hospitalization. Diagnosis of DVT required confirmation via Doppler ultrasound or venography whereas PE was confirmed by computed tomography (CT) scan, ventilation perfusion (V/Q) scan, or pulmonary angiography. VTE outcomes were assessed at 90 days post-hospital discharge from the index hospitalization. Medical record review at 90 days (including those discharged to home or post-acute settings) was completed for 100% of eligible patients in every hospital; telephone follow-up at 90 days was successfully completed for 58% of all patients.
Covariates of Interest
Detailed patient demographic, medical history, physical examination findings, laboratory and medication data were collected for all patients. Risk factors used to calculate the Caprini risk score were captured. Appropriate VTE prophylaxis was defined as receipt of any of the following treatments on day 1 and/or 2 of the index hospitalization: heparin 5,000 units BID; heparin 5,000 units TID; heparin 7,500 units TID (for morbid obesity); enoxaparin 40 mg daily; enoxaparin 30 mg daily (for creatinine clearance < 30 mL/min); enoxaparin 30 mg BID; dalteparin 5,000 units daily; or fondaparinux 2.5 mg daily.12,13
Statistical Analysis
Descriptive statistics were used to illustrate the percentage of patients with each Caprini risk factor. Bivariable logistic regression was used to calculate the odds of VTE for each individual risk factor. By summing points for risk factors specified in the Caprini RAM, a total VTE risk score for each patient was determined. Sensitivity and specificity values for each cut-point along the continuous Caprini RAM were estimated. To model an appropriate functional form of the continuous Caprini RAM, covariate, linear piecewise splines based on 6 knots forming the maximum number of unique quantiles of the data were generated. A mixed logistic regression model including the spline covariates, receipt of pharmacologic prophylaxis, and random intercept components for each hospital was fit. Predicted probabilities of 90-day VTE for the fixed effects of linear splines by receipt of pharmacologic prophylaxis were then estimated. All analyses were performed in Stata 13.0 (StataCorp, College Station, TX, USA).
Ethical and Regulatory Oversight
As the purpose of the HMS Consortium is to measure and improve the quality of existing medical practice, this project received a “not regulated” status by the University of Michigan Medical School’s Institutional Review Board.
Results
Between January 2011 and March 2014, data spanning 63,548 eligible patients across 48 Michigan hospitals were collected. The patients average age was 65.8 years and 35,264 (55.5%) were female. The average length of hospital stay was 4.5 days (median 4.0 days).
A VTE event occurred during the index hospitalization or within 90 days post hospital discharge in 670 (1.05%) patients: 412 (0.65%) patients had an isolated DVT, 185 (0.29%) had an isolated PE, and 73 (0.11%) had both a DVT and PE. While the vast majority of events were identified by medical record review, 44 (6.6%) of the events were confirmed via telephone follow-up. A total of 38,724 (60.9%) patients received pharmacologic prophylaxis on day 1 and/or day 2 of the index hospitalization. The overall rate of VTE among patients who received pharmacologic prophylaxis was not significantly different from those that did not receive this treatment (1.22 per 10,000 patient-days vs. 1.29 per 10,000 patient-days, p = 0.45).
Risk factors used to derive the Caprini RAM and results of the bivariable logistic regression analysis are displayed in Table 1. The presence of a central venous catheter on admission (odds ratio [OR] = 3.44, 95% confidence interval [CI] = 2.86 - 4.14), personal history of VTE (OR = 2.96, 95% CI = 2.41 - 3.63), family history of VTE (OR = 2.65, 95% CI = 1.49 - 4.74), admission or treatment of cancer in the past year (OR = 2.06, 95% CI = 1.76 - 2.41), and current immobility (OR = 1.61, 95% CI = 1.22 - 2.12) were among the strongest independent predictors of VTE (Table 1).
Table 1.
Distribution of Caprini Risk Factors and Bivariable Associations with 90-day Venous Thromboembolism
| Risk factor | Caprini Points* |
n (%) | OR (95% CI) | p |
|---|---|---|---|---|
| Stroke | 5 | 3037 (4.78) | 0.39 (0.23 - 0.68) | 0.001 |
| Acute spinal cord injury or paralysis (<1 mo) | 5 | 1313 (2.07) | 1.16 (0.70 - 1.91) | 0.56 |
| Hip, pelvis, or leg fracture (<1 mo) | 5 | 551 (0.87) | 1.39 (0.69 -2.80) | 0.36 |
| Multiple trauma (<1 mo) | 5 | 491 (0.77) | 0.97 (0.40 -2.34) | 0.94 |
|
| ||||
| Age, ≥ 75 (y) | 3 | 22660 (35.66) | 1.27 (1.09 -1.48) | 0.003 |
| History of DVT/PE | 3 | 4069 (6.40) | 2.96 (2.41 - 3.63) | <0.001 |
| Family history of VTE | 3 | 441(0.69) | 2.65 (1.49 - 4.74) | 0.001 |
| History of thrombophilia** | 3 | 228 (0.36) | 1.68 (0.62 - 4.53) | 0.31 |
| Heparin-induced thrombocytopenia (HIT) | 3 | 83 (0.13) | 2.32 (0.57 - 9.46) | 0.24 |
|
| ||||
| Age, 61–74 (y) | 2 | 16763 (26.38) | 1.06 (0.89 - 1.25) | 0.52 |
| Positive history of cancer | 2 | 13862 (21.81) | 2.06 (1.76 - 2.41) | <0.001 |
| Immobilizing plaster cast | 2 | 138 (0.22) | 2.09 (0.66 - 6.58) | 0.21 |
| Patient confined to bed (>=72 h) | 2 | 2706 (4.26) | 1.79 (1.33 - 2.40) | <0.001 |
|
| ||||
| Age, 41–60 (y) | 1 | 17401 (27.38) | 0.84 (0.71 - 1.01) | 0.06 |
| Congestive heart failure | 1 | 5928 (9.33) | 1.08 (0.84- 1.39) | 0.55 |
| COPD or abnormal pulmonary function | 1 | 17904 (28.17) | 0.99 (0.84 - 1.18) | 0.95 |
| Inflammatory bowel disease | 1 | 2015 (3.17) | 0.79 (0.49- 1.29) | 0.35 |
| Severe lung disease (including pneumonia) | 1 | 12801 (20.14) | 1.28 (1.07 - 1.53) | 0.007 |
| Acute myocardial infarction | 1 | 1061 (1.67) | 0.80 (0.41 - 1.55) | 0.51 |
| Sepsis (<1 mo) | 1 | 6555 (10.32) | 1.06 (0.83 - 1.36) | 0.62 |
| Surgery (< 1 mo) | 1 | 1695 (2.67) | 1.91 (1.34 - 2.72) | <0.001 |
| Postpartum (<1 mo) | 1 | 41 (0.06) | 2.35 (0.32 - 17.11) | 0.40 |
| History of unexpected stillborn infant, recurrent spontaneous abortion (>=3), or premature birth |
1 | 90 (0.14) | 2.14 (0.53 - 8.70) | 0.29 |
| Varicose veins | 1 | 468 (0.74) | 1.22 (0.54 - 2.74) | 0.63 |
| Obesity (BMI > 25) | 1 | 39891 (62.77) | 0.94 (0.80 - 1.09) | 0.40 |
| Swollen legs (current) | 1 | 15707 (24.72) | 1.70 (1.45 - 1.99) | <0.001 |
| Central venous catheter present on admission | 1 | 5011 (7.89) | 3.44 (2.86 - 4.14) | <0.001 |
| Immobile/not ambulating*** | 1 | 3371 (5.30) | 1.61 (1.22 - 2.12) | 0.001 |
| Hormone replacement therapy or oral contraceptives | 1 | 710 (1.12) | 0.80 (0.36 - 1.79) | 0.58 |
Abbreviations: VTE, venous thromboembolism; HR, hazard ratio; CI, confidence interval; DVT, deep vein thrombosis; PE, pulmonary embolism; COPD, chronic obstructive pulmonary disease; BMI, body mass index.
Point assignment based on the Caprini risk score model.
History of thrombophilia includes positive Factor V Leiden, positive prothrombin, G20210A, positive lupus anticoagulant, other congenial or acquired thrombophilia.
Immobile/not ambulating defined as having at least one of the following: immobilizing plaster cast, paralysis, or bed rest for ≥ 72 hours prior to hospitalization.
Across all patients, the average Caprini risk score was 4.94 (SD = 2.92, range 0 - 28). Importantly, 60,726 (95.5%) patients had a Caprini score of 10 or less. The distribution of Caprini risk, rate of VTE and binary classification metrics for each Caprini cut-point are illustrated in Table 2.
Table 2.
Caprini Score Distribution and non-Parametric Binary Classification Metrics for Venous Thromboembolism
| Caprini score |
n | VTE, n(%) | Sensitivity | Specificity | LR+ | LR− | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
| 0 | 1427 | 1(0.07) | 100.00% | 0.00% | 1 | 0 | ||
| 1 | 4183 | 16(0.38) | 99.85% | 2.27% | 1.0217 | 0.0658 | 0.01077 | 0.99930 |
| 2 | 7158 | 29 (0.41) | 97.46% | 8.90% | 1.0698 | 0.2853 | 0.01127 | 0.99697 |
| 3 | 9268 | 61 (0.66) | 93.13% | 20.23% | 1.1676 | 0.3393 | 0.01229 | 0.99640 |
| 4 | 9782 | 96 (0.98) | 84.03% | 34.88% | 1.2903 | 0.4579 | 0.01356 | 0.99514 |
| 5 | 8418 | 102 (1.21) | 69.70% | 50.28% | 1.4019 | 0.6026 | 0.01472 | 0.99362 |
| 6 | 7124 | 79 (1.11) | 54.48% | 63.51% | 1.4928 | 0.7168 | 0.01566 | 0.99242 |
| 7 | 5349 | 101 (1.89) | 42.69% | 74.71% | 1.6879 | 0.7671 | 0.01767 | 0.99189 |
| 8 | 3819 | 61 (1.60) | 27.61% | 83.06% | 1.6296 | 0.8716 | 0.01707 | 0.99080 |
| 9 | 2586 | 39 (1.51) | 18.51% | 89.03% | 1.6875 | 0.9153 | 0.01766 | 0.99034 |
| 10 | 1612 | 39 (2.42) | 12.69% | 93.08% | 1.8342 | 0.9380 | 0.01917 | 0.99010 |
| 11 | 1031 | 18 (1.75) | 6.87% | 95.59% | 1.5551 | 0.9744 | 0.01630 | 0.98972 |
| 12 | 611 | 7 (1.15) | 4.18% | 97.20% | 1.4905 | 0.9859 | 0.01563 | 0.98960 |
| 13 | 394 | 9 (2.28) | 3.13% | 98.16% | 1.7004 | 0.9868 | 0.01780 | 0.98959 |
| 14 | 298 | 5 (1.68) | 1.79% | 98.77% | 1.4550 | 0.9943 | 0.01527 | 0.98952 |
| 15 | 181 | 3 (1.66) | 1.04% | 99.24% | 1.3658 | 0.9972 | 0.01434 | 0.98949 |
| 16 | 128 | 1 (0.78) | 0.60% | 99.52% | 1.2389 | 0.9988 | 0.01303 | 0.98947 |
| 17 | 64 | 2 (3.13) | 0.45% | 99.72% | 1.5997 | 0.9983 | 0.01676 | 0.98947 |
| 18 | 50 | 0 (0) | 0.15% | 99.82% | 0.8232 | 1.0003 | 0.00870 | 0.98945 |
| 19 | 30 | 1 (3.33) | 0.15% | 99.90% | 1.4664 | 0.9995 | 0.01538 | 0.98946 |
| 20 | 18 | 0 (0) | 0.00% | 99.94% | 0 | 1.0006 | 0 | 0.98945 |
| 21 | 5 | 0 (0) | 0.00% | 99.97% | 0 | 1.0003 | 0 | 0.98945 |
| 22 | 4 | 0 (0) | 0.00% | 99.98% | 0 | 1.0002 | 0 | 0.98945 |
| 23 | 2 | 0 (0) | 0.00% | 99.99% | 0 | 1.0001 | 0 | 0.98946 |
| 24 | 2 | 0 (0) | 0.00% | 99.99% | 0 | 1.0001 | 0 | 0.98946 |
| 25 | 0 | 0 (0) | - | - | - | - | - | - |
| 26 | 2 | 0 (0) | 0.00% | 99.99% | 0 | 1.0001 | 0 | 0.98946 |
| 27 | 1 | 0 (0) | 0.00% | 100.00% | 0 | 1 | 0 | 0.98946 |
| 28 | 1 | 0 (0) | 0.00% | 100.00% | 0 | 1 | 0 | 0.98946 |
Abbreviations: VTE, venous thromboembolism; LR+, positive likelihood ratio; LR-, negative likelihood ratio; PPV, positive predictive value; NPV, negative predictive value.
Binary classification metrics based on cut-points ≥ to the Caprini score represented in each row.
Across all hospitals, rates of pharmacologic prophylaxis increased with Caprini score. For example, 35% of hospitalized patients with a Caprini score of 0 received VTE prophylaxis compared to 63% of patients with a Caprini score of 5. However, after a score of 5, rates of pharmacologic prophylaxis remained unchanged up to the maximum observed score of 28. Despite these overall trends, substantial variation in rates of prophylaxis across individual institutions was observed (Figure 1).
Figure 1. Hospital Rates of VTE Prophylaxis by Caprini Score.
The predicted prophylaxis rate averaged over hospitals is shown by the black line with 95% confidence intervals based on the linear spline random effects model. The observed prophylaxis rate by hospital is shown with the light gray circles and the predicted empirical Bayes mean prophylaxis rate by hospital is shown with the small x’s. Overall rates of prophylaxis plateau after a Caprini score of 5.
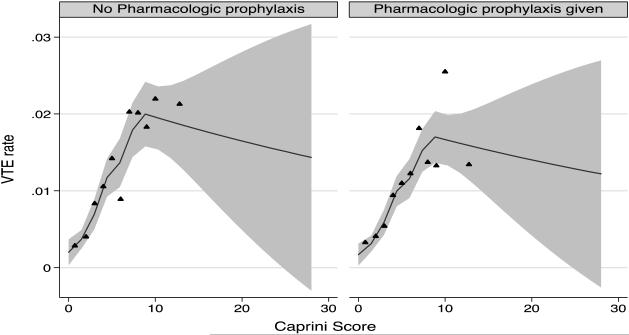
Results from the multivariable logistic spline regression are presented in Figure 2. Results suggest a consistent linear increase in the risk of VTE from Caprini scores of 0 to 10. The risk of VTE appeared to plateau after a score of 10. However, only 2,822 (4.5%) patients in our study had Caprini values >10; thus relatively few VTE events (n=46) were observed in these very high-risk patients (Table 2). Controlling for the piecewise linear spline fit to the Caprini RAM, a borderline decrease in the odds of VTE with pharmacologic prophylaxis was noted (OR = 0.85, 95%CI 0.72 - 0.99, p = 0.04). However, upon examining individual Caprini cut-points between 1 and 10, we were unable to detect a threshold where benefit from receipt of prophylaxis was statistically significant (Table 3).
Figure 2. Predicted 90-day VTE by Caprini Score and Receipt of Pharmacologic Prophylaxis.
The predicted 90-day mean VTE rate averaged over hospitals is shown for those with and without pharmacologic prophylaxis with 95% CI. The relationship to Caprini score is modeled as a piecewise linear spline using knots based on the unique quantiles in the data. The triangles are a binned scatterplot of the raw data representing a non-parametric way of displaying the relationship between Caprini score and VTE.
Table 3.
Number Needed to Treat to Prevent a Single VTE Event by Caprini Score
| Caprini Score |
Event rate without pharmacologic prophylaxis |
Event rate with pharmacologic prophylaxis |
ARR (95% CI) | NNT |
|---|---|---|---|---|
| ≥1 | 1.13% | 1.04% | 0.08% (−0.08% to 0.26%) | 1179 |
| ≥2 | 1.21% | 1.08% | 0.13% (−0.05% to 0.31%) | 759 |
| ≥3 | 1.35% | 1.16% | 0.19% (−0.01% to 0.40%) | 519 |
| ≥4 | 1.48% | 1.29% | 0.20% (−0.04% to 0.44%) | 511 |
| ≥5 | 1.62% | 1.39% | 0.22% (−0.05% to 0.52%) | 447 |
| ≥6 | 1.69% | 1.50% | 0.19% (−0.14% to 0.54%) | 534 |
| ≥7 | 2.03% | 1.62% | 0.41% (−0.02% to 0.86%) | 247 |
| ≥8 | 2.03% | 1.53% | 0.50% (−0.06% to 1.06%) | 199 |
| ≥9 | 2.04% | 1.61% | 0.42% (−0.21% to 1.13%) | 237 |
| >10 | 2.15% | 1.78% | 0.37% (−0.49% to 1.23%) | 269 |
Abbreviations: VTE, venous thromboembolism; ARR, absolute risk reduction; NNT, number needed to treat, CI, confidence interval
As the 95% CIs for the ARR at each Caprini cut-point extends from a negative number (treatment may harm) to a positive number (treatment may benefit), there is uncertainty regarding benefit, harm, or lack of effect at all Caprini score values.
Discussion
In this study of over 60,000 hospitalized medical patients across 48 Michigan hospitals, we found that the Caprini RAM was linearly associated with risk of VTE up to a score of 10. Once the Caprini score exceeded 10, the relationship between estimated risk and VTE events was unclear due to a paucity of VTE events and patients in these strata. In patients that developed VTE, a personal or family history of VTE, cancer, immobility, and presence of a central venous catheter on admission were among the strongest covariates associated with VTE. After multivariable analysis of the Caprini RAM, we found a borderline 15% decrease in the odds of VTE with pharmacologic prophylaxis. Even if this modest risk reduction were causally related to prophylaxis, the very low overall rate of VTE observed in this study and very high numbers needed to treat at each Caprini cut-point prompts questions regarding the overall benefits of VTE prophylaxis in non-ICU medical patients.
Our study adds to the current literature in a number of important ways. First, a limited number of studies have evaluated the ability of the Caprini RAM to predict VTE in hospitalized medical patients. For example, an earlier version of the Caprini RAM was assessed in a retrospective case-control study at a single center using discharge billing codes and chart review. The authors reported greater than a 2-fold increase in the odds of VTE risk with increasing Caprini scores.11 However, this single-center study was limited by a small number of cases (65 patients with a VTE event). A more recent retrospective Chinese study assessed the Caprini RAM in medical and surgical patients who developed image-confirmed VTE.15 While the Caprini RAM was noted to be a practical and effective tool to predict VTE risk, the analysis was notably limited by lack of a concurrent control group without hospital-associated VTE. Through use of a large, multi-site sample of non-surgical, non-ICU, medical patients, and state-of-the-art analyses examining the association between the continuous Caprini RAM and 90-day VTE events, our work circumvents many of these limitations and advances the science in novel ways.
Second, our analysis helps shed light on the applicability of group-based VTE prophylaxis strategies in hospitalized medical patients. Although this approach is recommended in surgical populations (e.g. orthopedic and trauma surgery) as it is less cumbersome and easier to operationalize than estimating individual patient risk-scores16, our data suggest that it may not be useful in non-surgical, general medical patients with very low VTE rates. Even among patients with Caprini scores ≥ 5 who did not receive pharmacologic prophylaxis, the 90-day rate of VTE was less than 2.0 per 10,000 patient-days. This low observed rate of VTE is likely specific to this patient population; namely, non-ICU and non-surgical patients which may inherently be at lower risk of VTE. However, it is important to emphasize that this patient group accounts for most inpatients across US hospitals and a number of studies have also reported similarly low rates of VTE in this subset.17,18 Given these data, group-based VTE prophylaxis strategies may not be of value to hospitalized medical patients who are heterogeneous and generally at lower risk for VTE. Additionally, because rates of VTE are so low, our findings raise questions regarding existing VTE prevention strategies that often advocate for routine use of pharmacologic prophylaxis in hospitalized medical patients.2,19-21
Third, in assessing the overall association between increasing Caprini risk score and VTE, we modeled for a flexible fit of the data but did not find any substantial deviations from a relatively linear relationship between increasing Caprini risk and VTE incidence. This finding is clinically important and highlights robust VTE risk incidence levels in a large cohort of general medical patients across the continuum of the Caprini RAM. However, owing to low overall rates of VTE and a linear risk-relationship, we were not able to identify a clear Caprini threshold that effectively isolates a patient sub-group that may benefit from pharmacologic VTE prophylaxis. While we did find an overall reduced odds of VTE among patients treated with pharmacologic prophylaxis this modest reduction translates into relatively high numbers needed to treat (NNT) for VTE prophylaxis in medical patients. For instance, administration of pharmacologic prophylaxis to nearly 500 non-surgical, non-ICU, medical patients with Caprini scores ≥ 5 would be needed to prevent a single VTE event according to our analysis. While the observational design of this study does not protect against the possibility that physicians may choose to administer prophylaxis for reasons other than the risk factors that comprise the Caprini RAM, our findings raise questions regarding the applicability of the Caprini RAM in determining which medical patients warrant prophylaxis.
How then should providers operationalize the Caprini RAM for the care of hospitalized medical patients? Our results highlight the importance of balancing the benefits of preventing VTE against the risks associated with anticoagulation (e.g. increased bleeding risk, cost, and patient discomfort). While a Caprini score subset most likely to benefit from prophylaxis was not discernable in our analysis, an approach that restricts prophylaxis to medical patients with a Caprini score ≥ 5 (with an associated NNT of ~500 patients) may be an acceptable strategy to some. Importantly, this threshold will vary and should be adjusted according to the patient’s risk/benefit ratio. In our study, approximately 20% of hospitalized medical patients had Caprini scores < 5 and would thus not have received pharmacologic prophylaxis. Regardless of which Caprini value is selected for hospitalized medical patients, our data show that the overall prevalence of VTE is low and that the oft-quoted saying "less may be more" might be highly appropriate for this patient cohort.
Our study has several limitations. First, this is an observational study subject to inherent biases including unmeasured confounding, selection, and ascertainment bias. Thus, our findings should be not be interpreted as causal. While we compare patients within similar Caprini risk categories, the selection effects described above could theoretically lead to a smaller treatment effect than would be seen if patients were randomized to pharmacologic prophylaxis. Second, use of graduated compression stockings or intermittent pneumatic compression devices was not incorporated into this analysis. Although published data regarding the efficacy of such mechanical prophylaxis strategies in hospitalized medical patients is limited, if use of these methods is both widespread and effective, it is possible they may lower VTE rates and change the relationship between Caprini RAM and outcome. Third, we did not take into consideration contraindications to pharmacologic prophylaxis (e.g. active bleeding). Although most contraindications are unlikely to exert an influence on VTE risk, some contraindications (e.g. thrombocytopenia or coagulopathy) may represent a marker for advanced illness and thus confound our analysis. Fourth, post-discharge medical record follow-up was limited to the discharging hospital and affiliated clinics. Therefore, it is possible that some VTE events may have occurred at other institutions and were missed after hospital discharge. Still, all patients had complete medical record review at 90-days and telephone follow-up was completed for 58% of patients. Finally, although we assigned points for various VTE risk factors in accordance with weights established in the Caprini RAM,1 some risk factors (e.g. stroke) had weights assigned that were counter to the bivariable associations observed in our data. However, as our intent was to assess the performance of the Caprini RAM as specified rather than derive new weights based on findings, our approach is appropriate in this regard.
Despite these limitations, our study has several strengths. To our knowledge, this is the largest study that examines the performance of an established RAM to predict VTE in non-surgical, non-ICU medical patients. As our data were collected through review of individual medical records by trained abstractors in a standardized fashion and represent real-world patients across diverse hospital settings, our findings have a high degree of generalizability and importance for US hospitals. Furthermore, our findings add to mounting evidence that suggests most hospitalized medical patients are at low risk of VTE. Use of pharmacologic prophylaxis in this cohort may expose patients to risk without direct benefit, with large NNTs illustrative of this phenomenon. This highlights that strategies advocating for broad use of VTE prophylaxis may be misaligned for hospitalized medical patients, regardless of how risk is quantified in this subgroup. Reconsideration of such policies that take into account the underlying low incidence, risk, and heterogeneity of this large subset of patients appears necessary.
In conclusion, we found a linear association between the Caprini RAM and incidence of VTE in hospitalized medical patients. However, the overall incidence of VTE in this population was extremely low. The utility of the Caprini RAM in determining a risk threshold above which there is clear benefit of administering prophylaxis thus appears limited in non-surgical, non-ICU, medical patients. Despite being among the largest proportion of hospitalized patients, general medical patients have been largely underrepresented in VTE trials leading to approaches that, while well intentioned, may have prompted excessive pharmacologic prophylaxis in this subset. Reconsideration of the clinical risks and benefits encouraging use of VTE prophylaxis in this patient population appears necessary.
Footnotes
Author Contributions:
PJG and MTG contributed equally as lead authors and take responsibility for the content of the manuscript, including the data and analysis.
PJG, MTG and TPH had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
PJG, MTG, VC, SJB, TPH, and SAF contributed to the study design, data analysis and interpretation, writing and final approval of the manuscript.
Conflict of Interest Disclosures:
PJG reports receiving royalties from Wiley Publishing and compensation for expert witness testimony.
MTG has no conflicts of interest to disclose.
VC has no conflicts of interest to disclose.
SJB reports receiving compensation as a consultant for Blue Care Network and the Michigan Department of Community Health, honoraria for various talks, and grants from Blue Cross Blue Shield of Michigan, Michigan Health and Hospital Association, Department of Veterans Affairs, National Institutes of Health, and the Agency for Healthcare Research and Quality.
TPH reports receiving grant funding as an investigator or co-investigator from the Department of Veterans Affairs, National Institutes of Health, and the Agency for Healthcare Research and Quality.
SAF reports receiving compensation for consultancies for the Institute for Healthcare Improvement and the Society of Hospital Medicine; royalties from Wiley Publishing; honoraria for various talks at hospitals as a visiting professor; grants from the Centers for Disease Control and Prevention Foundation, Blue Cross Blue Shield of Michigan, Michigan Health and Hospital Association, and the Agency for Healthcare Research and Quality; and compensation for expert witness testimony.
Role of the Funder/Sponsor:
Blue Cross Blue Shield of Michigan and Blue Care Network supported data collection at each participating site and funded the data coordinating center but had no role in study concept, interpretation of findings, or preparation, review, or final approval of the manuscript.
Previous Presentation of Information:
Results previously presented at the Society of Hospital Medicine Annual Meeting, Las Vegas, NV, March 26, 2014
References
- 1.Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51(2-3):70–78. doi: 10.1016/j.disamonth.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Maynard GA, Morris TA, Jenkins IH, et al. Optimizing prevention of hospital-acquired venous thromboembolism (VTE): prospective validation of a VTE risk assessment model. J Hosp Med. 2010;5(1):10–18. doi: 10.1002/jhm.562. [DOI] [PubMed] [Google Scholar]
- 3.Spyropoulos AC, Anderson FA, FitzGerald G, et al. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140(3):706–714. doi: 10.1378/chest.10-1944. [DOI] [PubMed] [Google Scholar]
- 4.Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352(10):969–977. doi: 10.1056/NEJMoa041533. [DOI] [PubMed] [Google Scholar]
- 5.Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua prediction score. J Thromb Haemost. 2010;8:2450–7. doi: 10.1111/j.1538-7836.2010.04044.x. [DOI] [PubMed] [Google Scholar]
- 6.Woller SC, Stevens SM, Jones JP, et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947–954. doi: 10.1016/j.amjmed.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Guyatt G, Akl EA, Crowther M, et al. Introduction to the 9th edition: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice. Chest. 2012;141(2 suppl):48s–52s. doi: 10.1378/chest.11-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Joint Commission Venous thromboembolism core measure set. Available at: http://www.jointcommission.org/core_measure_sets.aspx. Accessed September 7, 2014.
- 9.Caprini JA. Individual risk assessment is the best strategy for thromboembolic prophylaxis. Dis Mon. 2010;56:552–559. doi: 10.1016/j.disamonth.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Soomro Q, Yousuf N, Bhutto AA, Abro HA, Memon AA. Venous Thromboembolism (VTE): Risk assessment in hospitalized patients. J Coll Physicians Surg Pak. 2014;7:455–458. [PubMed] [Google Scholar]
- 11.Zakai NA, Wright J, Cushman M. Risk factors for venous thrombosis in medical inpatients: validation of a thrombosis risk score. J Thromb Haemost. 2004;2(12):2156–2161. doi: 10.1111/j.1538-7836.2004.00991.x. [DOI] [PubMed] [Google Scholar]
- 12.Flanders SA, Greene MT, Grant P, Kaatz S, Paje D, Lee B, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: A cohort study. JAMA Intern Med. 2014;174(10):1577–1584. doi: 10.1001/jamainternmed.2014.3384. [DOI] [PubMed] [Google Scholar]
- 13.Greene MT, Flanders SA, Woller SC, Bernstein SJ, Chopra V. The association between PICC use and venous thromboembolism in upper and lower extremities. Am J Med. 2015 May 1; doi: 10.1016/j.amjmed.2015.03.028. pii: S0002-9343(15)00319-8. doi: 10.1016/j.amjmed.2015.03.028. [Epub ahead of print] PMID: 25940453. [DOI] [PubMed] [Google Scholar]
- 14.American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP®): American College of Surgeons. 2013 [accessed September 30, 2014]. Available from: http://site.acsnsqip.org/
- 15.Zhou H, Peng L, Yan Y, et al. Validation of the Caprini risk assessment model in Chinese hospitalized patients with venous thromboembolism. Thrombosis Research. 2012;130:735–740. doi: 10.1016/j.thromres.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133:381–453. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 17.Lederle FA, Zylla D, MacDonald R, Wilt TJ. Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602–615. doi: 10.7326/0003-4819-155-9-201111010-00008. [DOI] [PubMed] [Google Scholar]
- 18.Rothberg MB, Lindenauer PK, Lahti M, Pekow PS, Selker HP. Risk factor model to predict venous thromboembolism in hospitalized medical patients. J Hosp Med. 2011;6(4):202–209. doi: 10.1002/jhm.888. [DOI] [PubMed] [Google Scholar]
- 19.Agency for Healthcare Research and Quality Preventing hospital-acquired venous thromboembolism: a guide for effective quality improvement. 2008 http://www.ahrq.gov/professionals/quality-patient-safety/patient-safety-resources/resources/vtguide/index.html. Accessed January 5, 2015.
- 20.Maynard GA. Medical admission order sets to improve deep vein thrombosis prevention: a model for others or a prescription for mediocrity? J Hosp Med. 2009;4(2):77–80. doi: 10.1002/jhm.423. [DOI] [PubMed] [Google Scholar]
- 21.Cohen AT, Alikhan R, Arcelus JI, et al. Assessment of venous thromboembolism risk and the benefits of thromboprophylaxis in medical patients. Thromb Haemost. 2005;94(4):750–759. [PubMed] [Google Scholar]